Abstract
Background
A key immunological feature of food allergy (FA) is the presence of a T-helper-2 (Th2)-type cytokine bias. Ligation of the invariant natural killer T cell (iNKT) T cell receptor (TCR) by sphingolipids (SL) presented via the CD1d molecule leads to copious secretion of Th2-type cytokines. Major food allergens (e.g. milk, egg) are the richest dietary source of SL (food-SL). Nonetheless, the role of iNKTs in FA is unknown.
Objective
To investigate the role of iNKTs in FA and to assess whether food-SL-CD1d complexes can engage the iNKT-TCR and induce iNKT cell functions.
Methods
Peripheral blood mononuclear cells from 15 children allergic to cow's milk (FA-MA), 12 children tolerant to cow's milk but with allergy to egg (FA-NMA) and 13 healthy controls were incubated with α-galactosylceramide (αGal), cow's milk-sphingomyelin-[SM] or hen's egg-ceramide-[CE]. iNKTs were quantified and their cytokine production and proliferation were assessed. Human CD1d tetramers loaded with milk-SM or egg-CE were used to determine food-SL binding to the iNKT-TCR.
Results
Milk-SM, but not egg-CE, can engage the iNKT-TCR and induce iNKT-proliferation and Th2-type cytokine secretion. FA-children, especially those with MA, had significantly fewer peripheral blood (PB) iNKTs and their iNKTs exhibited a greater Th2-response to αGal and milk-SM compared to iNKTs of healthy controls.
Conclusion
iNKTs from FA-children, especially those with MA, are reduced in number and exhibit a Th2-bias in response to αGal and milk-SM. These data suggest a potential role for iNKTs in FA.
Clinical Implications
Milk-SM activate PB-iNKTs to produce Th2-cytokines and this effect is greater in FA-MA-children. Hence, SL contained in milk may promote an iNKT cell-mediated-Th2-type-cytokine bias that facilitates sensitization to food allergens.
Keywords: Food allergy, invariant natural killer T cells, sphingolipids
Introduction
Food allergy (FA) is highly prevalent in children and on occasion can cause fatal allergic reactions1. It is postulated that in FA the presence of T helper 2 type (Th2)-cytokines contributes to the breakdown of oral tolerance2-4. Th2-type cytokines can be produced by a variety of cells including invariant natural killer T cells (iNKTs)3,5. Indeed, iNKTs, when appropriately stimulated, promote a Th2-response, IgE production and subsequent sensitization to protein antigens6,7. Although iNKTs have pleiotropic roles in multiple diseases8,9, the role of iNKTs in FA has not been previously reported.
As opposed to conventional T cells that recognize peptides bound to highly polymorphic major histocompatibility complex (MHC) molecules, iNKTs recognize sphingolipids (SL) presented by CD1d, a non-polymorphic MHC-class I-like molecule, via a semi-invariant Vα24-Vβ11 T cell receptor (TCR). All iNKTs proliferate in response to α-galactosylceramide (αGal)8. Only a limited number of endogenous or bacterial SL are known to activate iNKTs8,10. Following engagement of their invariant TCR by SL, iNKTs rapidly upregulate co-stimulatory molecules and secrete Th1- and Th2-type cytokines. Thus, iNKTs may act similarly to innate immune cells to influence the activation and polarization of naïve T-cells during induction of adaptive responses.
The major food allergens in pediatric-FA are egg and milk1. Interestingly, these foods are also the richest dietary source of SL, such as sphingomyelin (SM), which have the potential to act as ligands of the iNKT-TCR11. It is estimated that the average individual consumes approximately 0.3 to 0.4 grams of SL every day11. For example, dairy products contain 70-400 μg of SM per gram12. We undertook the current investigation to examine the role of iNKTs in the pathogenesis of FA. Here we show that a subset of iNKTs in normal individuals recognizes and responds to cow's milk-derived SM (milk-SM), as demonstrated by increased proliferation and Th2-type cytokine production. Interestingly, the iNKT Th2-type cytokine response to milk-SM in children with FA, especially cow's milk allergy (FA-MA), is more pronounced, suggesting a role for iNKTs in the pathogenesis of FA. We hypothesize that milk-SM could promote a Th2-skewed environment in predisposed individuals by inducing iNKT cells to secrete Th2 type cytokines.
Materials and Methods
Study Subjects
Twenty-seven children with milk and/or egg allergy were recruited from the Allergy Clinic at The Children's Hospital of Philadelphia (CHOP), including 15 children with cow's milk allergy (FA-MA) (13 males, 2 females, average age ± SD = 5.4 ± 2.5 years, Table E1), 12 children tolerant to milk but with allergy to egg (FA-NMA) (10 males, 2 females, average age ± SD = 4.1 ± 1.8 years, Table E2). To be eligible for study, patients needed to have all of the following characteristics: 1) positive skin prick testing and/or presence of specific IgE in the serum to milk and/or egg; 2) positive food challenge test or recurrence of allergic reaction following accidental exposure to either milk and/or egg; 3) clinical stability on a diet excluding the offending food. Thirteen children without a history of FA, asthma or allergic rhinitis (Non-FA-children) were recruited from the primary care clinics at CHOP (10 males, 3 females, average age ± SD = 4.38 ± 3.7 years). All investigations were approved by the CHOP Internal Review Board. (See additional Methods information in the online repository).
Reagents
Hen's egg-ceramide (Egg-CE, 860051P-endotoxin-free) and cow's milk-SM (860063P-endotoxin-free) were purchased from Avanti Polar Lipids (Alabaster, AL). αGal was from Alexis Biochemicals (Farmingdale, NY). Human CD1d tetramers were unloaded (hCD1d) or loaded with the αGal analogue PBS57 (PBS57-hCD1d), milk-SM (milk-SM-hCD1d) or egg-CE (egg-CE-hCD1d) and were provided by the MHC-Tetramer Core Facility, Emory University (Atlanta, GA). Anti-Vα24 and anti-Vβ11 Abs were from Coulter-Immunotech (Marseille, France).
Culture
PBMCs were resuspended in complete medium (CM) (AIM-V; 10% FCS; rhIL-2 [40 U/ml]) in the presence of milk-SM (500ng/ml), egg-CE (500ng/ml), αGal (500ng/ml) or DMSO. After 5 days, half of the CM was replaced with CM-supplemented with rhIL-7 (5ng/ml) and rhIL-15 (10ng/ml)13,14. iNKTs were also purified from human CD3+ positive cells by cell sorting (95-99% purity). (See additional Methods information in the online repository).
Flow Cytometry
iNKTs were defined as CD3+/Vα24+/Vβ11+ or CD3+/PBS57-hCD1d+ by flow cytometry (see Methods information and Figure E1 in the online repository for gating strategy used). The percentage of iNKTs was expressed as those cells staining positive and compared with cells stained similarly using isotype-matched control antibodies or unloaded hCD1d tetramers. Stained cells were collected using the FACScalibur Flow Cytometry System (Becton Dickinson, San Jose, CA). Data were analyzed using FlowJo software (Tree Star, Ashland, OR).
Measurement of iNKT proliferation
(See Methods information in the online repository).
RT-PCR analyses
(See Methods information in the online repository).
Statistical Analysis
(See Methods information in the online repository).
Results
FA-MA children have fewer PB-iNKTs
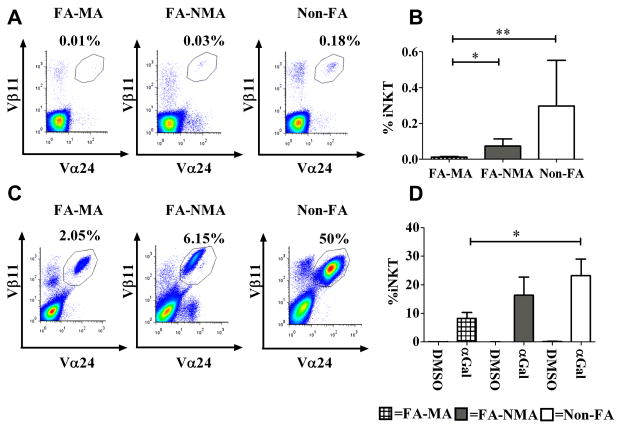
In many human disorders, iNKTs are reduced in number and/or function, suggesting a role for this lineage in disease pathogenesis8, 10. To determine whether iNKTs are involved in FA, we measured the iNKT percentage (%) and absolute number (#) in 15 children with milk allergy (FA-MA; Table E1), 12 children who were tolerant to milk but had allergy to egg (FA-NMA; Table E2) and 13 healthy controls (non-FA). In non-FA-children, the percentage and # of CD3+/Vα24+/Vβ11+ iNKTs was consistent with those reported previously (mean % ± SEM 0.29 ± 0.25, median % 0.02, range 0.001-3.6%; mean #/mL ± SEM 7,982 ± 6542; median absolute #/mL 1200, range 0-86,400)13,15-17. Similar results were obtained regardless of whether we identified iNKTs based on Vα24/Vβ11 positivity or reactivity with PBS57-hCD1d tetramers (not shown). The % and #/mL of iNKTs in FA-MA children (mean % ± SEM 0.01 ± 0.003, median % 0.01, range 0.001-0.04%; mean #/mL ± SEM 171.2 ± 81.78; median absolute #/mL 57, range 0-933) were significantly lower compared to FA-NMA children (mean % ± SEM 0.07 ± 0.04, median % 0.017, range 0.01-0.36%; mean #/mL ± SEM 735 ± 2324; median absolute #/mL 735, range 214-28543) and to non-FA controls (Figure 1A,B and Figure E2).
Figure 1. FA-MA children have significantly fewer PB- iNKTs that respond to αGal.
Fresh PBMCs from 15 FA-MA, 12 FA-NMA and 13 Non-FA-children were stained ex vivo for iNKTs (CD3+Vα24+Vβ11+) (A, B) and then cultured for 10 days with αGal or DMSO (C, D). (A, C) Dot plot of a representative experiment. (B, D) Mean levels of iNKT cells expressed as CD3+ cells. *p<0.02; **p<0.002.
To investigate whether the lower number of iNKTs observed in children with FA, particularly those with FA-MA, was due to a reduced proliferative capacity, we examined iNKT responsiveness to a 10 day in vitro culture with the potent iNKT cell agonist αGal. iNKTs from FA-MA children expanded in response to αGal, although the percentages of expanded iNKTs in αGal-stimulated PBMC cultures were lower in FA-MA children and mirrored the low initial levels of iNKTs observed in fresh peripheral blood samples (Figure 1C-D, Figure E3). The responsiveness of iNKTs from FA-MA children to αGal was confirmed by measuring the expression of the activation marker CD25 on αGal-stimulated cells. αGal induced upregulation of CD25 equivalently in all study groups (Figure E4). Therefore, although iNKTs are reduced in number in FA-MA patients, they do not appear to differ in their responsiveness to αGal.
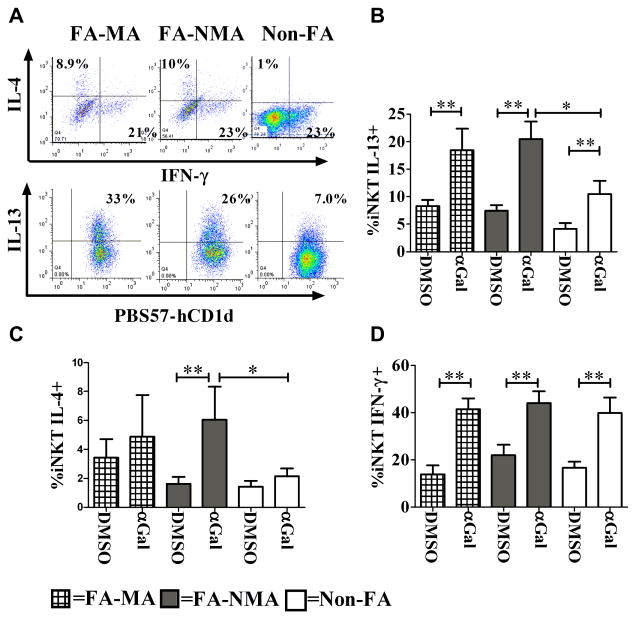
iNKTs from FA-children produce more Th2-type cytokines
iNKTs have the capacity to produce Th1- and Th2-type cytokines, but in some diseases they exhibit Th2-skewing8,18,19. We questioned whether iNKTs from FA-children might exhibit a Th2-skewed cytokine profile, as has been observed for the T cells of FA-patients1. To test this hypothesis, we measured Th1- and Th2-type cytokine production by αGal-expanded iNKTs following short-term stimulation with PMA/ionomycin. We observed that a greater percentage of iNKTs from FA-children, compared to controls, expressed IL-4 or IL-13 after αGal expansion and secondary stimulation, although such difference reached statistical significance only in children with FA-NMA. iNKTs from children with FA-MA have a tendency even when unstimulated to produce more IL-4 compared to the other groups of children although this didn't reach staticals significance (p=0.06). Such tendency was not enhanced by αGal expansion (Figure 2A-C; Figure E5A-F). In contrast, production of IFNγ was similar in the iNKTs of all patient cohorts (Figure 2A,D; Figure E5G-I). Collectively, these data indicate that iNKTs from FA-children produce a Th2-skewed cytokine profile in response to αGal.
Figure 2. Th2-skewed cytokine responses of iNKTs from children with FA compared to non-allergic controls.
PBMCs from 15 FA-MA, 12 FA-NMA and 13 Non-FA-children were cultured for 10 days with αGal or DMSO, then stimulated for 4 hours with PMA and ionomycin and stained for intracellular cytokines. (A) Dot plot of a typical experiment. (B, C, D) Mean percentages of iNKTs staining for IL-13, IL-4 or IFNγ. *p<0.05; **p<0.002.
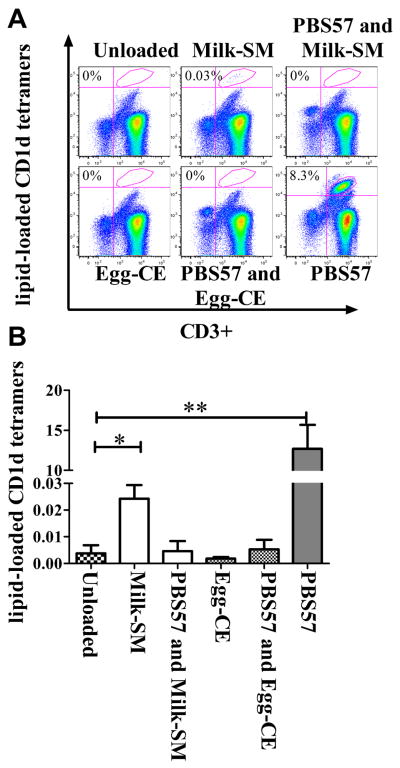
hCD1d tetramers loaded with milk-SM specifically bind to iNKTs
SL are abundant in milk and egg20. Since the iNKTs from FA-children exhibited quantitative and qualitative differences compared to the iNKTs of controls, we questioned whether milk- and/or egg-derived SL might drive iNKT functions. To address this possibility, we first examined whether food-SL-loaded-hCD1d tetramers could bind to the invariant TCR present on normal iNKTs. PBMCs from 7 normal adult donors (ND) were expanded with αGal and stained using hCD1d loaded with αGal, milk-SM or egg-CE. We choose these two food-SL as they differ in their head group (i.e. substituents at the 1-hydroxyl position (1-OH) position) of the SL21,22. Indeed, the iNKT TCR is capable of recognizing the head group of lipid antigens, ensuring specificity of antigen recognition and strength of signal. Hence, we hypothesized that milk-SM, which has a phosphocholine residue at the 1-OH position, but not egg-CE, which has only hydrogen in this position, might be able to activate iNKTs22. We observed that milk-SM-loaded-but not egg-CE-loaded-hCD1d tetramers stained a statistically higher number of PB-iNKTs previously expanded with αGal compared to unloaded-hCD1d tetramers (Figure 3A-B; Figure E6). Milk-SM-tetramer–TCR interactions were blocked by pre-incubation of cells with non-fluorescently conjugated PBS57-hCD1d tetramers, confirming that the milk-SM-tetramers were recognizing the iNKT invariant TCR and not binding non-specifically to another structure on the surface of the iNKT cells. Milk-SM-loaded-hCD1d recognized a mean % ± SEM of iNKTs of 0.02±0.005 (median% 0.027) and a mean absolute #/mL ± SEM of 4220 ± 1170 (median absolute #/mL 3044). This is in contrast to unloaded tetramers which recognized a mean % ± SEM of iNKTs of 0.0037 ± 0.003 (median% 0.00044) and a mean absolute #/mL ± SEM of 432 ± 304 (median absolute #/mL 82) (Figure 3A-B; Figure E6). This observation suggests that at least a small population of iNKTs from ND has the capacity to bind milk-SM-loaded-hCD1d tetramers.
Figure 3. Human CD1d tetramers loaded with milk-SM specifically bind to iNKTs.
αGal-expanded PBMCs were stained with lipid-loaded hCD1d tetramers and anti-CD3 antibodies, or where indicated, were blocked by pre-incubation with non-fluorescently conjugated PBS57-hCD1d tetramers and then washed and stained with food-lipid-loaded-hCD1d tetramers and anti-CD3 antibodies. (A) Dot plot of a representative experiment. (B) Mean percentage of CD3+ cells stained with the indicated tetramers from 7 ND. *p<0.05; ** p<0.005.
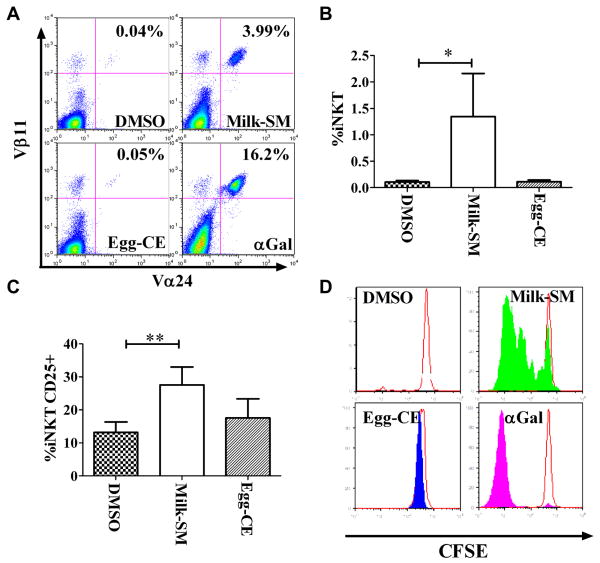
Milk-SM induces iNKT proliferation
To determine whether recognition of SL in the context of CD1d leads to TCR-mediated iNKT activation, we cultured PBMCs from 25 ND in the presence of different lipids and assessed for iNKT expansion, CD25 expression and iNKT proliferation. We observed that αGal or milk-SM, but not egg-CE or DMSO, induced an increase in the % and # of iNKTs (Figure 4A-B; Figure E7A-G). Milk-SM, but not egg-CE, also induced CD25 expression on iNKTs, although less robustly than αGal (Figure 4C; Figure E7H). Finally, to confirm that the expansion of iNKTs following culture with αGal or food-SL was the result of iNKT proliferation, CFSE-labeled iNKTs were stimulated with different lipids. αGal and milk-SM, but not egg-CE or DMSO, induced the proliferation of iNKTs, as determined by the dilution of CFSE (Figure 4D). Collectively, these data reveal that naturally occurring milk-SM can engage the iNKT-TCR, presumably via its interaction with CD1d on APCs, and thereby lead to iNKT expansion and activation.
Figure 4. Milk-SM induces iNKT activation and expansion.
PMBCs were cultured for 10 days with the indicated reagents. (A) Dot plot of a representative experiment. (B) Mean percentage of iNKTs following culture with lipids averaged from 25 ND. (C) Mean percentage of CD25+ iNKTs averaged from 25 ND. (D) CFSE-labeled PBMCs were cultured with the indicated reagents. After 5 days, CFSE-content in CD3+PBS57-hCD1d+ cells was assessed by flow cytometry (n=3 experiments). A representative experiment is shown. *p<0.03; **p<0.001.
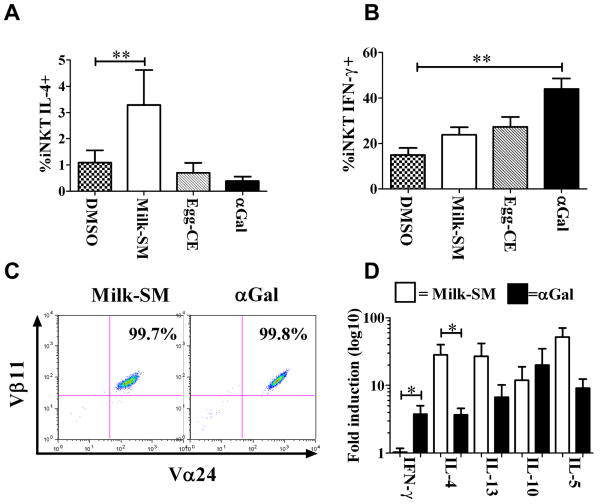
More milk-SM-expanded iNKTs make IL-4 than αGal-expanded iNKTs
IFNγ expression requires a longer and stronger iNKT TCR stimulation than IL-4 expression5. The weaker proliferation of iNKTs after milk-SM stimulation (Figure 4) suggests that milk-SM may bind only weakly to the iNKT TCR. Therefore, we hypothesized that milk-SM would preferentially drive the production of IL-4 by iNKTs. To test this, we stimulated PBMCs that had been pre-cultured with different lipids with PMA/ionomycin, and measured the percentage of iNKTs producing IL-4 or IFNγ. Consistent with our hypothesis, a greater percentage and number of milk-SM-expanded iNKTs expressed IL-4 (Figure 5A; Figure E8A-C) compared to αGal-expanded iNKTs, which preferentially produced IFNγ (Figure 5B; Figure E8D-F).
Figure 5. More milk-SM-activated iNKTs produce IL-4 compared to iNKTs activated by αGal.
PBMCs from 25 ND were cultured for 10 days with the indicated reagents and stimulated for 4h with PMA/ionomycin. (A, B) Mean percentage of iNKTs expressing IL-4 or IFNγ. (C) Expansion of sort-enriched iNKTs cultured for 2 weeks with APC pre-pulsed with the indicated reagents. (D) Cytokine-mRNA expression of sorted iNKTs reported as relative expression of milk-SM or αGal vs. DMSO treated iNKTs (average from 5 different donors). *p<0.05; ** p<0.005.
To determine whether this cytokine response was due to direct milk-SM-mediated iNKT cell activation as opposed to activation of other lineages within the PBMC cultures that then influenced iNKT cytokine responses, we cultured FACS-purified iNKTs from 5 ND with DMSO-, milk-SM- or αGal-pulsed APCs (Figure 5C). After 14 days (the time necessary for iNKTs to sufficiently expand to provide enough cells for analysis) we measured the levels of mRNA encoding different cytokines in non-adherent cells. In these assays, we observed statistically higher levels of mRNA encoding IL-4 and reduced levels of mRNA encoding IFNγ in iNKTs cultured in the presence of milk-SM compared to iNKTs cultured in the presence of αGal (Figure 5D). We also observed a higher, but not statistically significant, increase in the level of IL-13- and IL-5-encoding mRNA in the iNKTs from milk-SM-APC cultures. To assess the production of cytokines themselves, we sort-purified iNKTs from 5 ND and cultured them for 72 hours with antigen-loaded APCs. Subsequently, we measured the levels of cytokines secreted into the supernatants. Although we were not able to detect IL-4 under any of the conditions used, we observed lower levels of IFNγ and higher levels of IL-5 in the supernatants of iNKTs cultured with milk-SM- as opposed to αGal-pulsed APCs (Figure E8G,H). These data suggest that iNKTs can indeed respond to the physiological presentation of milk-SM by APCs. Furthermore, milk-SM preferentially induce a Th2-skewed cytokine profile by human-PB-iNKTs.
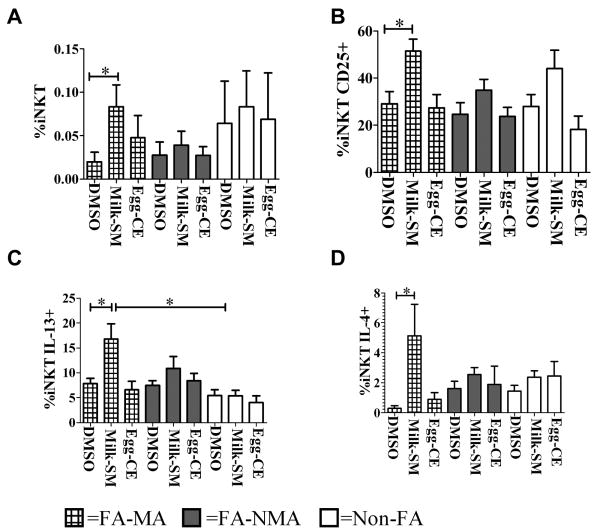
Milk-SM-stimulated iNKTs expand more in FA-MA children
Because milk-SM activate ND-derived iNKTs to produce Th2 cytokines, we examined whether this effect would be greater in iNKTs from FA-children, who have a genetic predisposition to produce stronger Th2-responses23. Thus, we cultured PBMCs from FA- and non-FA-children for 10 days in the presence of milk-SM, egg-CE or DMSO. We observed that milk-SM, but not egg-CE, induced a greater increase in iNKT % and # in FA-MA children compared to FA-NMA or non-FA-children (Figure 6A; Figure E9A-C). Similarly, milk-SM upregulated CD25 expression more in iNKTs from FA-MA children compared to the other two groups studied (Figure 6B; Figure E9D-E). Furthermore, when iNKTs from FA-MA children were cultured in the presence of milk-SM and then stimulated with PMA/ionomycin, we observed more IL-13 and IL-4, and less IFNγ production, suggesting a stronger Th2-bias in cytokine production, compared to the other studied groups (Figure 6C-D; Figure E10A-D). Together, these observations demonstrate that iNKTs from FA-MA children preferentially expand in response to milk-SM and that expanded iNKTs selectively produce Th2-type cytokines, suggesting that milk-SM might activate more iNKTs to produce Th2-type cytokines in certain individuals, such as those with FA to milk.
Figure 6. iNKTs from FA-MA children expand more and have a stronger Th2 response to milk-SM compared to those from Non-FA-children.
PMBCs from 15 FA-MA, 12 FA-NMA and 13 Non-FA-children were cultured for 10 days with food-SL or DMSO. (A, B) Mean percentage of total iNKTs and CD25+ iNKTs when cultured with the indicated reagents. (C, D) Mean percentage of iNKTs staining for IL-13 or IL-4. *p<0.005.
Discussion
In this study, we evaluated the response of iNKTs to food-SL in controls and FA-children. Our results indicate that there are quantitative and qualitative differences between the iNKT cell populations in controls and FA-children, especially in those with FA-MA, suggesting a possible role for iNKTs in the pathogenesis of FA. Here we demonstrate that FA-MA children exhibit a 30-fold reduction in the percentage and a 15-fold reduction in # of PB iNKTs compared to non-FA-children. The number of PB iNKTs has been used as an indicator of iNKT involvement in human disease14,24,25. In conditions with low PB iNKT cell numbers, there are conflicting reports regarding iNKT cell function14,24-26. To assess whether iNKTs in FA-MA children have impaired function, iNKTs were stimulated with the potent iNKT cell agonist αGal in vitro. Despite lower starting percentages and absolute numbers, the iNKTs from FA-MA children exhibited responsiveness to αGal that was similar to control iNKTs. From these experiments, we conclude that αGal-induced iNKT proliferation and activation are preserved in FA-MA children. We also observed that the iNKTs from FA-children, expecially from those tolerant to milk, exhibited a skewed production of cytokines following expansion in the presence of αGal. Specifically, more iNKTs produced IL-4 and IL-13 in FA-patients compared to non-FA controls, although such difference reached statistical significance only in FA-NMA children. These results mirror the findings previously reported in patients with asthma27. Thus, our results indicate a possible role for iNKTs in the development of FA through Th2-type cytokine skewing.
The ligands that drive iNKT cell expansion and cytokine production in non-infectious diseases remain to be identified. Exposure of self-lipids due to lung inflammation or glycolipids from inhaled pollens has been postulated, but never demonstrated, to activate iNKTs in patients with asthma. All iNKTs proliferate in response to αGal, which is itself not believed to be a relevant physiological ligand in human diseases8. A small number of other ligands can activate iNKTs8,29-31. Interestingly, all these ligands are SL. SL are widespread components found in eukaryotic cells, where they are sequestered (i.e. not antigenic) in cell membranes12,32. SL are common in many foods in our diet. Notably, the foods richest in SL (milk and egg) also represent the most common triggers of childhood FA1. Food-SL inhibit colorectal cancer and in mice increase both Th1- and Th2-cytokine secretion in the small intestine by a mechanism that has not yet been clarified20,33,34. Thus, it remains possible that food-SL could activate iNKTs.
To examine whether food-SL specifically bind to the iNKT-TCR, hCD1d tetramers were loaded with milk-SM or egg-CE. Our experiments indicate that the iNKT-TCR was able to bind milk-SM upon CD1d presentation, with a frequency of 300-fold fewer iNKTs compared to PBS57-loaded tetramers. When we cultured PBMCs from ND in presence of food–SL, we saw that milk-SM could expand iNKTs, albeit to a lesser extent than αGal. This finding mirrors the percentage of food-SM responsive iNKTs measured by tetramers and indicates that the iNKTs detected by tetramer analysis were not anergic.
Because we studied human iNKTs, which are relatively rare in the PB, in the present work we were not able to carry out an exhaustive specificity analysis, using many compounds related or unrelated to the ones tested. However, the fact that iNKTs failed to recognize egg-CE is indicative of some degree of specificity. We hypothesize that the difference in efficacy among different food-SL in activating iNKTs may result from the diversity of the substituents at the 1-OH-position of the SL21,22. Indeed, milk-SM, which has a phosphocholine residue at the 1-OH-position, appears to be a stronger activator of iNKTs compared to egg-CE, which has only hydrogen in this position. Moreover, the length of the tail could explain why Gumperz35 failed to find consistent activation of iNKTs using a synthetic-SM which contained a C18:1 acyl chain; our milk-derived-SM is a mix of different sphingomyelin constituents with the main component being C23:0 and the remainder being C16:0, C18:0, C20:0, C20:4, C22:0, C24:0, and C24:1.
The molecular characteristics of SL influence the strength of signaling through the iNKT-TCR and determine distinct cytokine secretion patterns. Indeed IFNγ expression requires stronger and longer iNKT simulation than does IL-45. From our data, it appears that milk-SM is a weak ligand of the iNKT-TCR. Therefore, we hypothesized that it would drive an IL-4 response from iNKTs. To test this hypothesis, we measured iNKT Th1 (IFNγ) and Th2 (IL-4, IL-13) cytokine expression after expansion with food-SL and stimulation with PMA/ionomycin. We recognize that this stimulation is not physiologic; however, the low numbers of iNKTs, even after milk-SM expansion, mandated the use of such an approach. Using this method, we found that more ND milk-SM responsive iNKTs secreted IL-4 compared to cells activated by αGal. To validate these data, we sorted iNKTs from 5 ND and expanded them using milk-SM pulsed APC. We subsequently assessed the levels of mRNA for Th1/Th2-type cytokines and confirmed that iNKTs expanded with milk-SL exhibit more transcripts encoding Th2-type cytokines even without PMA/ionomycin stimulation.
Given the ability of milk-SM to specifically activate iNKTs to produce IL-4, we hypothesized that this effect would be greater in FA-children, as they may have a genetic predisposition to produce stronger Th2 responses23. In this study, we showed that SL contained in milk activate iNKTs to preferentially express Th2-cytokines in FA-MA children. This effect was different from the one observed with αGal stimulation, which induced more Th2 cytokine production in FA-NMA, suggesting a more specific Th2 response to milk derived SM in children with FA-MA. The iNKT response we observed with milk-SM is typical of a Th2-biased response. Although we do not have any information about the downstream immunological effects of the food-reactive iNKTs observed in this work, one could postulate that iNKTs stimulated with milk-SM favor a Th2-biased environment and thereby promote IgE class switching during the humoral response to milk3. This effect could be stronger early on in life when the adaptive immune system is not well developed and could revert later in life when the T-regulatory population develops and children outgrow food allergy. There is indeed evidence to suggest that iNKTs can provide T-cell help to B-cells resulting in enhanced immunoglobulin secretion37. In addition, primary sensitization to protein antigens is promoted by iNKTs when an iNKT agonist is co-administered with the allergen6.
In summary, we demonstrate here that iNKTs are reduced in number in FA-MA, and to a lesser extent in FA-NMA patients, and that both subsets of iNKTs exhibit Th2-skewed responses following activation with αGal. Of note, iNKTs from FA-MA, but not FA-NMA patients, demonstrate a similar skewing of cytokine responses following exposure to milk-SM. Thus, iNKTs may play a role in FA, and more specifically in FA-MA pathogenesis, by recognizing specific food-SL and serving to create a Th2-skewed environment. Further studies are needed to address why PB-iNKTs are lower in FA-MA patients and whether the observed Th2-cytokine bias is reversible upon acquisition of food tolerance. Finally, we will need to investigate whether the observed characteristics in our study are specifically linked to FA-MA or to atopic individuals in general. The results, however, define a novel mechanism of promoting atopic inflammatory responses after exposure to allergen-derived physiological ligands. This may present the opportunity for specific therapeutic modulation of this interaction to interfere with the immunologic pathophysiology underlying the present FA pandemic.
Acknowledgments
We thank Drs. Michael Brenner and Patrick Brennan from the Division of Rheumatology, Immunology and Allergy, Brigham and Women's Hospital, Boston, MA for their constructive comments. We also thank Dr. Kathleen Sullivan and Kelly Maurer from the Children's Hospital of Philadelphia, Allergy and Immunology Division for their support.
Funding Sources: NIH K12HD043245-06, CTRC Junior Investigator Pilot Grant Program (JIPGP). This project was supported by Grant Number UL1-RR-024134 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Abbreviations
- αGal
α-galactosylceramide
- Abs
antibodies
- milk-SM
cow's milk sphingomyelin
- Non-FA-children
children without FA
- FA-MA
children with allergy to milk
- FA-NMA
children who are tolerant to milk but have allergy to egg
- FA
food allergy
- PBS57hCD1d
human CD1d tetramers loaded with PBS57
- food-SL
food-derived sphingolipids
- egg-CE
hen's egg ceramide
- IFNγ
interferon gamma
- IL-4
interleukin 4
- IL-13
interleukin 13
- iGb3
isoglobotrihexosylceramide
- iNKT
invariant natural killer T
- PB
peripheral blood
- PBMCs
peripheral blood mononuclear cells
- PIM4
phosphatidylinositolmannoside
- PE
Phycoerythrin
- SL
sphingolipids
- Th1
T helper cell type 1
- Th2
T helper cell type 2
- hCD1d
unloaded human Cd1d tetramers
Footnotes
Conflict of Interest: The authors have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–19. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Chehade M, Mayer L. Oral tolerance and its relation to food hypersensitivities. J Allergy Clin Immunol. 2005;115:3–12. doi: 10.1016/j.jaci.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Berin MC, Shreffler WG. T(H)2 adjuvants: implications for food allergy. J Allergy Clin Immunol. 2008;121:1311–20. doi: 10.1016/j.jaci.2008.04.023. [DOI] [PubMed] [Google Scholar]
- 4.Beyer K, Castro R, Birnbaum A, Benkov K, Pittman N, Sampson HA. Human milk-specific mucosal lymphocytes of the gastrointestinal tract display a TH2 cytokine profile. J Allergy Clin Immunol. 2002;109:707–13. doi: 10.1067/mai.2002.122503. [DOI] [PubMed] [Google Scholar]
- 5.Oki S, Tomi C, Yamamura T, Miyake S. Preferential T(h)2 polarization by OCH is supported by incompetent NKT cell induction of CD40L and following production of inflammatory cytokines by bystander cells in vivo. Int Immunol. 2005;17:1619–29. doi: 10.1093/intimm/dxh342. [DOI] [PubMed] [Google Scholar]
- 6.Kim JO, Kim DH, Chang WS, Hong C, Park SH, Kim S, et al. Asthma is induced by intranasal coadministration of allergen and natural killer T-cell ligand in a mouse model. J Allergy Clin Immunol. 2004;114:1332–8. doi: 10.1016/j.jaci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Bendelac A, Hunziker RD, Lantz O. Increased interleukin 4 and immunoglobulin E production in transgenic mice overexpressing NK1 T cells. J Exp Med. 1996;184:1285–93. doi: 10.1084/jem.184.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 9.Kim EY, Battaile JT, Patel AC, You Y, Agapov E, Grayson MH, et al. Persistent activation of an innate immune response translates respiratory viral infection into chronic lung disease. Nat Med. 2008;14:633–40. doi: 10.1038/nm1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen NR, Garg S, Brenner MB. Antigen Presentation by CD1 Lipids, T Cells, and NKT Cells in Microbial Immunity. Adv Immunol. 2009;102:1–94. doi: 10.1016/S0065-2776(09)01201-2. [DOI] [PubMed] [Google Scholar]
- 11.Symolon H, Schmelz EM, Dillehay DL, Merrill AH., Jr Dietary soy sphingolipids suppress tumorigenesis and gene expression in 1,2-dimethylhydrazine-treated CF1 mice and ApcMin/+ mice. J Nutr. 2004;134:1157–61. doi: 10.1093/jn/134.5.1157. [DOI] [PubMed] [Google Scholar]
- 12.Berra B, Colombo I, Sottocornola E, Giacosa A. Dietary sphingolipids in colorectal cancer prevention. Eur J Cancer Prev. 2002;11:193–7. doi: 10.1097/00008469-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Gumperz JE, Miyake S, Yamamura T, Brenner MB. Functionally distinct subsets of CD1d-restricted natural killer T cells revealed by CD1d tetramer staining. J Exp Med. 2002;195:625–36. doi: 10.1084/jem.20011786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179:3452–62. doi: 10.4049/jimmunol.179.6.3452. [DOI] [PubMed] [Google Scholar]
- 15.Lee PT, Putnam A, Benlagha K, Teyton L, Gottlieb PA, Bendelac A. Testing the NKT cell hypothesis of human IDDM pathogenesis. J Clin Invest. 2002;110:793–800. doi: 10.1172/JCI15832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araki M, Kondo T, Gumperz JE, Brenner MB, Miyake S, Yamamura T. Th2 bias of CD4+ NKT cells derived from multiple sclerosis in remission. Int Immunol. 2003;15:279–88. doi: 10.1093/intimm/dxg029. [DOI] [PubMed] [Google Scholar]
- 17.Marsh RA, Villanueva J, Kim MO, Zhang K, Marmer D, Risma KA, et al. Patients with X-linked lymphoproliferative disease due to BIRC4 mutation have normal invariant natural killer T-cell populations. Clin Immunol. 2009;132:116–23. doi: 10.1016/j.clim.2009.03.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9:582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 19.Taniguchi M, Harada M, Kojo S, Nakayama T, Wakao H. The regulatory role of Valpha14 NKT cells in innate and acquired immune response. Annu Rev Immunol. 2003;21:483–513. doi: 10.1146/annurev.immunol.21.120601.141057. [DOI] [PubMed] [Google Scholar]
- 20.Vesper H, Schmelz EM, Nikolova-Karakashian MN, Dillehay DL, Lynch DV, Merrill AH., Jr Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J Nutr. 1999;129:1239–50. doi: 10.1093/jn/129.7.1239. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–44. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, et al. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 2005;6:819–26. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 23.Sampson HA. Food allergy. Part 1: immunopathogenesis and clinical disorders. J Allergy Clin Immunol. 1999;103:717–28. doi: 10.1016/s0091-6749(99)70411-2. [DOI] [PubMed] [Google Scholar]
- 24.Tupin E, Nicoletti A, Elhage R, Rudling M, Ljunggren HG, Hansson GK, et al. CD1d-dependent activation of NKT cells aggravates atherosclerosis. J Exp Med. 2004;199:417–22. doi: 10.1084/jem.20030997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lisbonne M, Diem S, de Castro Keller A, Lefort J, Araujo LM, Hachem P, et al. Cutting edge: invariant V alpha 14 NKT cells are required for allergen-induced airway inflammation and hyperreactivity in an experimental asthma model. J Immunol. 2003;171:1637–41. doi: 10.4049/jimmunol.171.4.1637. [DOI] [PubMed] [Google Scholar]
- 26.Thomas SY, Lilly CM, Luster AD. Invariant natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:2613–6. doi: 10.1056/NEJMc066189. author reply-6. [DOI] [PubMed] [Google Scholar]
- 27.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354:1117–29. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 28.Gausling R, Trollmo C, Hafler DA. Decreases in interleukin-4 secretion by invariant CD4(-)CD8(-)V alpha 24J alpha Q T cells in peripheral blood of patients with relapsing-remitting multiple sclerosis. Clin Immunol. 2001;98:11–7. doi: 10.1006/clim.2000.4942. [DOI] [PubMed] [Google Scholar]
- 29.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Exp Med. 2003;198:173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sriram V, Du W, Gervay-Hague J, Brutkiewicz RR. Cell wall glycosphingolipids of Sphingomonas paucimobilis are CD1d-specific ligands for NKT cells. Eur J Immunol. 2005;35:1692–701. doi: 10.1002/eji.200526157. [DOI] [PubMed] [Google Scholar]
- 31.Fischer K, Scotet E, Niemeyer M, Koebernick H, Zerrahn J, Maillet S, et al. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc Natl Acad Sci U S A. 2004;101:10685–90. doi: 10.1073/pnas.0403787101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huwiler A, Kolter T, Pfeilschifter J, Sandhoff K. Physiology and pathophysiology of sphingolipid metabolism and signaling. Biochim Biophys Acta. 2000;1485:63–99. doi: 10.1016/s1388-1981(00)00042-1. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez E, Gil A, Garcia-Olivares E, Rueda R. Weaning induces an increase in the number of specific cytokine-secreting intestinal lymphocytes in mice. Cytokine. 2000;12:1267–70. doi: 10.1006/cyto.2000.0706. [DOI] [PubMed] [Google Scholar]
- 34.Vazquez E, Gil A, Rueda R. Dietary gangliosides positively modulate the percentages of Th1 and Th2 lymphocyte subsets in small intestine of mice at weaning. Biofactors. 2001;15:1–9. doi: 10.1002/biof.5520150101. [DOI] [PubMed] [Google Scholar]
- 35.Fox LM, Cox DG, Lockridge JL, Wang X, Chen X, Scharf L, et al. Recognition of lysophospholipids by human natural killer T lymphocytes. PLoS Biol. 2009;7:e1000228. doi: 10.1371/journal.pbio.1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Galli G, Nuti S, Tavarini S, Galli-Stampino L, De Lalla C, Casorati G, et al. CD1d-restricted help to B cells by human invariant natural killer T lymphocytes. J Exp Med. 2003;197:1051–7. doi: 10.1084/jem.20021616. [DOI] [PMC free article] [PubMed] [Google Scholar]