Abstract
The neural correlates of successful retrieval on tests of word stem recall and recognition memory were compared. In the recall test, subjects viewed word stems, half of which were associated with studied items and half with unstudied items, and for each stem attempted to recall a corresponding study word. In the recognition test, old/new judgments were made on old and new words. The neural correlates of successful retrieval were identified by contrasting activity elicited by correctly endorsed test items. Old > new effects common to the two tasks were found in medial and lateral parietal and right entorhinal cortex. Common new > old effects were identified in medial and left frontal cortex, and left anterior intra‐parietal sulcus. Greater old > new effects were evident for cued recall in inferior parietal regions abutting those demonstrating common effects, whereas larger new > old effects were found for recall in left frontal cortex and the anterior cingulate. New > old effects were also found for the recall task in right lateral anterior prefrontal cortex, where they were accompanied by old > new effects during recognition. It is concluded that successful recall and recognition are associated with enhanced activity in a common set of recollection‐sensitive parietal regions, and that the greater activation in these regions during recall reflects the greater dependence of that task on recollection. Larger new > old effects during recall are interpreted as reflections of the greater opportunity for iterative retrieval attempts when retrieval cues are partial rather than copy cues. Hum Brain Mapp, 2012. © 2011 Wiley Periodicals, Inc.
Keywords: episodic memory, recognition memory, cued recall, fMRI, parietal cortex, recollection
INTRODUCTION
The past few years have seen a surge in the use of event‐related functional magnetic resonance imaging (fMRI) to investigate the neural correlates of episodic memory retrieval. The bulk of these studies have focused on the cortical correlates of “retrieval success,” operationalized by contrasting the activity elicited by different classes of test item in tests of recognition memory or one of its variants [e.g., correctly recognized versus correctly rejected items, or studied items endorsed as “Remembered” versus “Known”; for reviews see Rugg and Henson,2002; Skinner and Fernandes,2007; Spaniol et al.,2009]. Retrieval success (or “old/new”) effects have consistently been identified in a variety of cortical regions, including medial and lateral parietal cortex and several regions of prefrontal cortex (PFC). Importantly, it has been proposed that success effects in lateral parietal cortex vary according to whether recognition is accompanied by retrieval of episodic details about the study episode (“recollection”), or is instead based solely on an acontextual sense of “familiarity” [Vilberg and Rugg,2008a]. Specifically, whereas effects in left superior parietal cortex in the vicinity of the intra‐parietal sulcus (IPS) are insensitive to whether or not a test item is judged old on the basis of recollection or familiarity, effects in a more inferior left lateral parietal region centered on the angular gyrus (BA 39) are evident only for recollected items [e.g., Vilberg and Rugg,2008a; Wheeler and Buckner,2004]. These latter effects co‐vary with the amount of information recollected, but appear largely insensitive to the nature of the recollected information [Vilberg and Rugg,2007,2008b; see also Guerin and Miller,2009], suggesting that this region may support the amodal or heteromodal representation of recollected content [Vilberg and Rugg,2008a].
Analogous dissociations have also been reported in left PFC between lateral inferior and anterior regions that demonstrate generic “old/new” effects, and more superior prefrontal regions where effects appear to be selectively associated with recollection [Vilberg and Rugg,2007,2008b; Yonelinas et al.,2005]. By contrast, effects in right dorsolateral and lateral anterior PFC have been linked to “post‐retrieval” processes that support the monitoring and evaluation of the outcome of a retrieval attempt [Fletcher and Henson,2001; Rugg et al.,2002; but see also Dobbins and Han,2006].
The brief review above is sufficient to indicate that significant progress has been made in identifying the cortical correlates of successful episodic retrieval. This progress has been achieved however almost exclusively through studies that investigated retrieval success as it is operationalized in variants of recognition memory tests. As we have noted previously [Vilberg and Rugg,2008a], there is a dearth of knowledge about retrieval success effects associated with other types of memory test. Thus, the generality of the findings from studies of recognition memory, including, but not limited to, proposals about the cortical regions engaged during successful recollection, largely remains to be established. For example, if the locus of retrieval success effects in inferior lateral parietal cortex was found to vary according to type of memory test, the proposal that this region plays a generic role in episodic retrieval would be called into question.
Here, we describe an event‐related fMRI study in which retrieval success effects were compared between tests of recognition memory and word‐stem cued recall. We elected to investigate cued recall both because of its historical importance as a test of explicit memory in behavioral and neuropsychological studies and because it was employed in several early functional neuroimaging studies that investigated retrieval processing using blocked rather than event‐related designs, including two studies that attempted to identify retrieval success effects [Allan et al.,2000; Rugg et al.,1998; see below]. Recall is thought to be largely supported by the same processes that support recollection‐based recognition [e.g., Yonelinas,2002]. Thus, significant overlap between the two tests in retrieval success effects might be expected. Consistent with this expectation, the sole prior event‐related fMRI study of cued recall [Schott et al.,2005] reported that, relative to word stems completed with study items that were misclassified as “new,” stems eliciting completions correctly endorsed as “old” elicited enhanced activity in regions associated in other studies with recollection‐based recognition memory [Skinner and Fernandes,2007; Spaniol et al.,2009; Vilberg and Rugg,2008a], including bilateral medial and lateral parietal cortex and the hippocampus.
Other findings suggest however that retrieval success effects in recognition and cued recall tests may not overlap entirely. In an early event‐related study that employed ERPs rather than fMRI, Allan and Rugg [1997] reported that the scalp distributions of the old/new ERP effects elicited in the two tests differed, with cued recall effects demonstrating a more diffuse distribution. In a subsequent positron emission tomography (PET) study, Rugg et al. [1998]; [see also Allan et al.,2000] attempted to identify the neural correlates of successful recall and recognition by contrasting test blocks containing either a high proportion of or no retrieval cues corresponding to studied items. In both tasks, contrasts between test blocks associated with high versus zero probability of retrieval revealed enhanced activity in medial and lateral parietal cortex. These “retrieval success effects” were of greater magnitude in the recognition test in lateral parietal regions, but were larger for cued recall in medial parietal cortex. In addition, a double dissociation between the two tests was evident in right lateral anterior PFC (BA 10): activity in this region was greater in high than zero old item blocks for recognition, whereas the reverse was the case for cued recall. Rugg et al. [1998] conjectured that these latter findings reflected the role of this PFC region in monitoring and evaluating the outcome of retrieval attempts. They argued that each recognition test item was used to probe memory once only, meaning that, on average, more information was returned when memory was probed with a studied than an unstudied item. By contrast, because word stems can be used to generate several candidate study items, stems that initially failed to elicit successful recall were employed in iterative retrieval attempts, leading to greater monitoring demands for stems corresponding to unstudied than to studied items.
In summary, prior findings provide only limited evidence about the degree to which retrieval success effects differ according to whether memory is tested with recognition or cued recall. The findings of the PET study of Rugg et al. [1998] are perhaps the most informative in this regard, but run foul of the difficulties in distinguishing between item‐ and state‐related effects that bedevil blocked functional neuroimaging designs [e.g., Rugg,1998]. And although there are no such difficulties of interpretation in the event‐related fMRI study of Schott et al. [2005], a recognition test was not employed in that study, and hence retrieval success effects for cued recall and recognition cannot be directly compared.
In the present study, which is based on the ERP experiment of Allan and Rugg [1997], we used fMRI to directly compare the neural correlates of retrieval success in a recognition memory and a cued recall task, holding study conditions constant and equating the response demands of the two tasks as closely as possible. At issue is the extent to which successful retrieval in the two tasks engages overlapping neural regions. In light of the shared dependence of cued recall and recognition memory on recollection we expected to find overlap between the neural correlates of successful recall and recognition in regions—such as left inferior parietal cortex—identified as recollection‐sensitive in previous studies that employed tests of recognition. We further expected, however, that these recollection effects would be larger in the cued recall task. This follows from the assumption that a smaller proportion of successful recognition trials will be recollection‐based than in the case of recall, reflecting that fact that while correct recognition judgments can be made on the basis of either recollection or familiarity, recall depends almost exclusively on recollection. In addition to regions where retrieval success effects overlap, we were also interested in identifying regions where the two classes of effect dissociate. One region where such a dissociation was predicted was right dorsolateral PFC where, on the basis of prior PET findings, and subsequent evidence pointing to a role for this region in post‐retrieval monitoring, we expected that the opportunity afforded by the cued recall task for iterative retrieval attempts would lead to more activity in this region when recall was unsuccessful than when it succeeded [cf. Rugg et al.,1998].
METHODS
Subjects
Subjects were right‐handed, native English speakers aged between 18 and 30 years. A total of 23 individuals (11 female) took part in the experiment. All were right‐handed, with normal or corrected‐to‐normal vision, no known history of neurological disease, and no other contraindications for MRI. Informed consent was obtained from each subject prior to participation in accordance with UCI Institutional Review Board guidelines. Four subjects were excluded from all analyses due to excessive motion artifact (greater than 3 mm).
Stimuli
Critical stimuli were drawn from a pool of 560 words [based on the words originally employed by Rugg et al.,1998] in which the first three letters of each word were unique to the pool but were shared with at least four other words that were not included in the pool [words were five to nine letters long with a mean written frequency between 1 and 536 counts per million according to Kucera and Francis,1967]. Allocation of words to experimental conditions was randomized on a subject‐specific basis. For each subject, two study blocks were created containing 60 words each. Recognition test lists were composed of 60 studied words and 30 unstudied words. Cued recall test lists were composed of 90 unique, three‐letter word stems created from 60 studied words and 30 unstudied words. An additional 24 words were selected from the stimulus pool to be used in a practice session, and another 12 were used as buffers in the study and test blocks.
All stimuli were presented individually in a white font on a black background. Words subtended a maximum horizontal visual angle of 6.4°. Two buffer trials were added to the beginning and end of each study list, and to the beginning of each test list.
Procedure
Subjects completed a short practice session outside of the scanner prior to beginning the first study session. The practice session consisted of two study‐test blocks of 9 and 12 trials each, respectively. One block employed a recognition test, and the other employed a cued recall test. The study task was held constant across blocks. Subjects received instructions on how to perform both study and test tasks prior to beginning the first study practice. After practice, participants were informed that they would undergo two study‐test cycles just as in the practice session, with one recognition and one cued recall test. Participants were then positioned in the scanner and remained there for the duration of the two study‐test cycles.
All stimuli were presented in central vision during both study and test. Study trials consisted of the presentation of a white fixation cross for 500 ms, followed by a red fixation cross for 200 ms, followed by a word for 2,000 ms, and finally another white fixation cross for 1,000 ms. Participants were instructed to evaluate whether each word was concrete or abstract and respond according to a four point scale where 1 = very concrete, 2 = somewhat concrete, 3 = somewhat abstract, and 4 = very abstract. Abstract judgments were made with the index and middle fingers of one hand, while concrete judgments were made with the index and middle fingers of the other hand.
Each participant was pseudo‐randomly assigned an ordering of the two test blocks such that across all participants, half received the cued recall test first. In each test block, old and new items were pseudo‐randomly interspersed such that no more than four trials of a given type (old or new) occurred in succession. All test trials began with a white fixation cross for 500 ms followed by a red fixation cross for 200 ms, followed by a word or a word stem for 1,000 ms (depending on the test task). Then, a variable duration (3,000–7,500 ms) white fixation cross was presented, followed by a speech prompt (the word “speak!”) for 1,000 ms, followed by another variable duration (2,300–6,800 ms) white fixation cross. These variable duration fixations were used to ensure that vocalization only occurred during the “silent” period of a volume acquisition (see fMRI Data Acquisition), and were set such that the interval between the onset of successive test items was always 12 s. The interval between the onset of the test item and the speech prompt varied across trials between 4 and 8.5 s.
In the recognition task, participants were instructed to judge whether the test word was studied or unstudied, to wait for the speak prompt, and then to recite the word aloud and state whether it was “new” or “old” to indicate their recognition judgment. In the cued recall task, instructions were to attempt to complete the test tem with a studied word, or if this was not possible, to complete the stem with the first word that came to mind. As in the recognition task, participants were instructed to wait until the “speak!” prompt and then to recite the completion and say whether it was old or new. Verbal responses were recorded via a scanner‐compatible microphone.
fMRI Data Acquisition
High‐resolution T1‐weighted anatomical images (240 × 240 matrix, 1 mm isotropic voxels) and blood oxygenation level‐dependent (BOLD), T2*‐weighted echoplanar functional images (SENSE factor of 2, flip angle 70°, 80 × 80 matrix, FOV = 24 cm, TE = 30 ms) were acquired using a 3T Philips Achieva MRI scanner equipped with an eight channel receiver head coil (Philips Medical Systems, Andover, MA). Three‐hundred and twenty functional volumes were acquired during each test session. Each volume comprised 30 slices oriented parallel to the AC‐PC line (thickness 3 mm, 1 mm inter‐slice gap, 3 mm isotropic voxels) acquired in an ascending sequence. The first 5 vol of each session were discarded to allow equilibration of tissue magnetization. Volumes were acquired with an acquisition time (TA) of 1,479 ms and a repetition time (TR) of 3,500 ms. Combined with the 12‐s interval between successive item onsets, this TR gave an effective sampling rate of the hemodynamic response of ∼2 Hz across every seven trials of each test phase. The timing of successive trials was structured so as to ensure that each speech prompt onset immediately after data acquisition, providing a 2‐s window for a vocal response prior to acquisition of the succeeding volume. Thus, the fMRI data were not compromised by motion artifact associated with vocalization [see Henson et al.,2002].
fMRI Data Analysis
Statistical Parametric Mapping (SPM5, Wellcome Department of Imaging Neuroscience, London, UK), run under Matlab R2006a (The Mathworks, USA) was used for fMRI data analysis. Functional imaging time series were subjected to realignment, reorientation, spatial normalization to a standard EPI template [based on the Montreal Neurological Institute (MNI) reference brain; Cocosco et al.,1997], resampling into 2 mm isotropic voxels using nonlinear basis functions [Ashburner and Friston,1999], and smoothing with an 8 mm FWHM Gaussian kernel. Analysis was performed using a General Linear Model (GLM) in which a delta function was used to model neural activity at stimulus onset. To model the BOLD response this function was convolved with a canonical hemodynamic response function [HRF; Friston et al.,1998] and a delayed HRF, generated by shifting the canonical HRF 2 s later in time. The delayed HRF was orthogonalized with respect to the canonical function such that variance common to both functions was allocated to the canonical HRF [Andrade et al.,1999]. The delayed HRF was included to capture effects that onset or extended beyond the time‐course encompassed by the canonical HRF. For the recognition test block, five event types (hits, correct rejections, speech onset cues, and events of no interest such as buffer trials, and trials with incorrect or omitted responses) were modeled. For the cued recall block, five event‐types were again modeled: stems associated with correct recall (stems corresponding to studied items that were correctly completed and endorsed as old), failure to recall (studied stems completed with new words and endorsed as new), correct rejections (stems corresponding to unstudied words endorsed as new), speech onset cues, and events of no interest (because of their rarity, studied stems correctly completed but endorsed as new were included in this category). For each test block, the model also included as covariates the across‐scan mean and six regressors representing motion‐related variance (three for rigid‐body translation and three for rotation). For each voxel, the image time‐series was high‐pass filtered to 1/128 Hz and scaled to a grand mean of 100 across voxels and scans. An AR(1) model was used to estimate and correct for nonsphericity of the error covariance [Friston et al.,2002]. The GLM was used to obtain parameter estimates representing the activity elicited by the events of interest. Only those analyses based on trials associated with correct memory judgments (hits, stems associated with correct recall and correct rejections) are reported.
An uncorrected statistical threshold of P < 0.001 (one‐sided), combined with a cluster extent threshold of 20 contiguous voxels, was employed for the principal unidirectional contrasts [the present extent threshold, applied to SPMs based on 2 × 2 × 2 mm3 voxels, is slightly more stringent than the five voxel extent threshold employed in prior studies in which SPMs were based on 3 × 3 × 3 mm3 voxels, e.g., Vilberg et al.,2008b,2009]. Coordinates of significant effects are reported in MNI space. Localization of these effects was accomplished by visual inspection of the outcome of the relevant SPM projected onto a representative subject's anatomical image, guided by reference to the Anatomy Toolbox v1.5 [Eickhoff et al.,2005,2006,2007] and the MSU utility associated with SPM5 (http://www.fil.ion.ucl.ac.uk/spm/ext/#MSU). For the purpose of visualization of the findings, Caret software [Van Essen et al.,2001; version 5.613] was used to map cortical effects onto inflated fiducial brains via average fiducial mapping (AFM) onto the PALS‐B12 atlas [Van Essen,2002,2005] in SPM5 space. Note that AFM only illustrates the location of effects that are estimated to intersect with the surface of the fiducial brain.
RESULTS
Behavioral Data
Mean performance indices for the two tasks are summarized in Table I. As is evident from the table, subjects correctly completed just under half of the stems corresponding to studied words in the cued recall task, endorsing the overriding majority (95%) of these completions as “old.” By contrast, only around 10% of incorrect completions received an “old” endorsement. A similar false alarm rate was evident for completions to unstudied stems. Table I also shows that recognition memory judgments were highly accurate, with a mean Pr (pHit‐pFalse Alarm) of 0.82.
Table I.
Mean performance indices for the cued recall and recognition tasks
| Stem completed with old word | Stem completed with new word | |||
|---|---|---|---|---|
| Response | “Old” | “New” | “Old” | “New” |
| Cued Recall | ||||
| Old stem | 0.457 (0.023) | 0.024 (0.005) | 0.058 (0.010) | 0.462 (0.022) |
| New stem | — | — | 0.146 (0.026) | 0.854 (0.026) |
| Recognition | ||||
| Old word | 0.916 (0.014) | 0.084 (0.014) | — | — |
| New word | 0.096 (0.022) | 0.904 (0.022) | — | — |
Note: Column headings correspond to subject responses. Standard error is given in parentheses. Note that recognition results are displayed with respect to whether old or new responses were given by subjects as no stems were completed in this task.
fMRI Data
Neural correlates of retrieval success were identified in the recognition task by the contrast between test items correctly endorsed as old or new [hits and correct rejections (CRs)]. For the cued recall task, “hits” were defined as word stems that were both correctly completed with a studied item and endorsed as such. Two different categories of “correct rejection” trial are available in this task: stems corresponding to studied words completed with an unstudied item and then endorsed as “new,” and stems corresponding to unstudied words for which novel completions were correctly judged new. To maintain as close a correspondence with the recognition task as possible, we describe here the outcome of contrasts that employed the second of these two categories of correct rejections. Almost identical outcomes were obtained when the analyses presented below were repeated using the alternate category.
Canonical HRF
Effects common to recognition and cued recall
Retrieval success (old > new) effects common to the two tasks were identified by exclusively masking the main effect of retrieval success (recognition hit + cued recall hit > recognition correct rejection + cued recall correct rejection; P < 0.001) with the F contrast for the task × retrieval success interaction (P < 0.05) so as to remove voxels where effects in the two tasks differed in magnitude. As is illustrated in Figure 1 and documented in Table II, the procedure identified clusters in bilateral medial and lateral parietal cortex, as well as in a small region of right entorhinal cortex. Regions demonstrating the reverse (new > old) effect were identified with an analogous procedure. This contrast revealed effects common to the two tasks in medial and left ventrolateral PFC, as well as in left anterior intraparietal sulcus (Fig. 1 and Table II).
Figure 1.
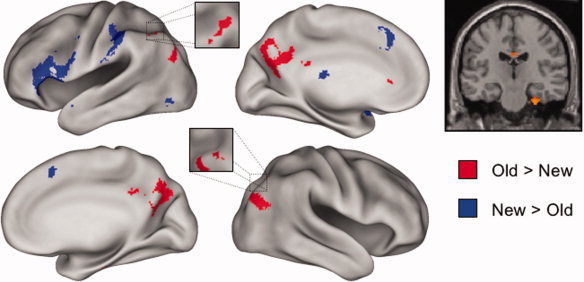
Left: Regions demonstrating old > new and new > old effects common to the recall and recognition tasks are displayed in red and blue, respectively, on the left and right lateral and medial hemispheres of an inflated fiducial brain (see Methods). Right: The common old > new effect in the right medial temporal lobe is displayed on a section at y = −20 through the canonical single‐subject T1‐weighted image. Effects are thresholded for display purposes at P < 0.0025.
Table 2.
Main effects across the cued recall and recognition tasks (canonical HRF)
| Region | BA | HM | Location | Peak Z (# vox) |
|---|---|---|---|---|
| Old > New | ||||
| Medial temporal lobe (entorhinal cortex) | 36 | R | 26 −22 −32 | 4.09 (37) |
| Precuneus/posterior cingulate | 7/31 | L/R | 16 −68 30 | 5.62 (1,065) |
| Intraparietal sulcus | 7/40 | L | −42 −64 52 | 3.67 (40) |
| Inferior parietal cortex | 39/19 | L | −46 −78 34 | 3.96 (36) |
| 39/19 | R | 40 −80 24 | 4.27 (179) | |
| New > Old | ||||
| Medial prefrontal cortex | 8 | L | −6 30 44 | 4.66 (170) |
| Inferior frontal gyrus | 44/45 | L | −50 28 12 | 4.26 (1,173) |
| Postcentral gyrus/inferior parietal cortex | 2/40 | L | −52 −34 48 | 4.32 (274) |
| Inferior/middle occipital gyrus | 19 | L | −40 −74 −6 | 3.47 (21) |
Task × retrieval success interactions
Regions where the magnitude of the effects varied according to task were identified by interaction contrasts, the outcomes of which are listed in Table III and illustrated in Figure 2. To elucidate these interactions, each side of the interaction contrast was inclusively masked with each of the corresponding simple effects (thresholded at P < 0.05). Thus, the interaction contrast of Cued Recall (old > new) > Recognition (old > new) was masked by the old > new effect for cued recall and, separately, by the new > old effect for recognition, and analogously for the Recognition (old > new) > Cued Recall (old > new) contrast. In the case of the first of these interaction contrasts, essentially every voxel where the contrast was significant also demonstrated a reliable old > new effect for cued recall. By contrast, no voxels demonstrated the reverse effect (new > old) for recognition. Thus, it can be concluded that this side of the interaction contrast was driven exclusively by larger retrieval success effects in the cued recall than in the recognition task.
Table 3.
Task × old/new interaction effects (canonical and delayed HRF)
| Region | BA | HM | Location | Peak Z (# vox) |
|---|---|---|---|---|
| Canonical HRF | ||||
| (Cued recall Old > New) > (Recognition Old > New) | ||||
| Middle frontal gyrus | 8 | L | −44 18 46 | 3.66 (35) |
| Superior temporal gyrus/angular gyrus | 39 | L | −52 −62 28 | 3.47 (27) |
| Inferior parietal cortex | 40/39 | R | 48 −60 30 | 4.01 (195) |
| 39/19 | L | −38 −70 46 | 3.77 (127) | |
| (Recognition Old > New) > (Cued Recall Old > New) | ||||
| Middle frontal gyrus | 9/46 | R | 34 46 34 | 3.93 (196) |
| Cingulate gyrus | 32 | L | −8 14 54 | 4.32 (165) |
| Inferior frontal gyrus | 6/9 | R | 38 4 26 | 4.21 (134) |
| Superior parietal cortex | 7 | L | −28 −62 54 | 3.90 (29) |
| Superior/medial parietal cortex | 7 | R | 24 −74 52 | 3.72 (33) |
| Lingual gyrus | 18 | R | 14 −70 −4 | 3.64 (128) |
| Delayed HRF | ||||
| (Recognition Old > New) > (Cued Recall Old > New) | ||||
| Inferior frontal gyrus | 46/47 | R | 48 38 12 | 3.82 (38) |
| 9/46 | L | −52 26 20 | 4.53 (648) | |
| 47 | L | −44 24 −6 | 3.96 (93) | |
| Inferior/middle frontal gyrus | 9 | L | −56 10 36 | 4.39 (65) |
| Anterior cingulate | 32 | L | −4 24 42 | 4.41 (202) |
| Intraparietal sulcus | 7 | L | −28 −58 34 | 3.57 (24) |
| Fusiform gyrus | 18 | L | −34 −86 −18 | 4.17 (210) |
Figure 2.
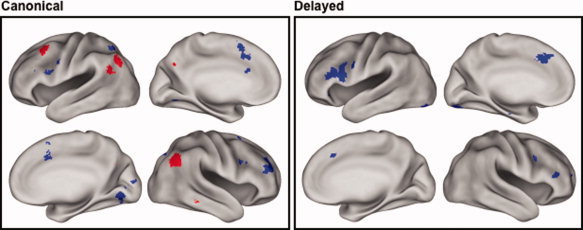
Regions where old > new and new > old effects varied in magnitude according to task. Left: Effects as identified by analyses employing the canonical HRF. Right: Effects as identified by analyses employing the delayed HRF. Red: Regions where Cued Recall (old > new) > Recognition (old > new). Blue: Regions where Recognition (old > new) > Cued Recall (old > new). Effects are displayed at P < 0.0025 for display purposes. Lateral and medial views of the inflated brain are shown for each hemisphere.
The outcome of the analogous inclusive masking procedures for the other side of the interaction [Recognition (old > new) > Cued Recall (old > new)] revealed new > old effects for cued recall in every region identified by the interaction contrast. In addition, however, there were two clusters in right PFC that also demonstrated reliable old > new effects for Recognition. Thus, outside of the right PFC, this side of the interaction was driven by greater new > old effects for cued recall. Within the right PFC, however, the interaction constituted a crossover effect, such that the same clusters demonstrated opposite effects in the two tasks (see Fig. 3).
Figure 3.
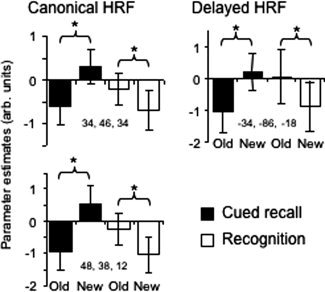
Left: Peak parameter estimates (arbitrary units) from voxels identified by the canonical HRF showing a crossover interaction between the tasks in old/new effects in right lateral prefrontal cortex. Right: Peak parameter estimates from a voxel in left fusiform cortex identified by the delayed HRF showing a crossover interaction.
Delayed HRF
The foregoing analyses were repeated with the orthogonalized delayed HRF. For the common effects, no additional regions were identified. In the case of the interaction contrasts, the Recognition (old > new) > Cued Recall (old > new) contrast gave rise to no significant clusters. However, the other side of the contrast [Cued Recall (old > new) > Recognition (old > new)] revealed clusters in several regions that were not identified in the canonical analysis, most notably, in left and right IFG and left posterior fusiform cortex (see Table III). Inclusive masking revealed that the interaction effects in the left fusiform and right IFG took the form of crossover interactions between the two tasks (see Fig. 3 for parameter estimates from the left fusiform cluster), whereas the interaction in the left IFG was due to the presence of new > old effects for Cued Recall in the absence of any effects for Recognition.
DISCUSSION
Behavioral Performance
Performance on the two retrieval tests was very similar to that reported by Allan and Rugg [1997]. Subjects correctly completed just less than half of the stems corresponding to studied words and went on to endorse the great majority of these as old. By contrast, only a minority of incorrectly completed stems were so endorsed. Thus, successful retrieval of study words was almost invariably associated with explicit memory for the words (indeed, if recall hit rate is defined relative to correctly completed stems, performance was very similar to that on the recognition test; pHit—pFalseAlarm of 0.80 and 0.82, respectively). In keeping with prior proposals [Allan et al.,1998; Jacoby,1998], we assume that on the majority of trials successful recall was mediated by direct retrieval of the associated study word rather than by an indirect “generate‐recognize” strategy. As discussed below, however, we further assume that when direct retrieval failed (as was invariably the case for the stems corresponding to unstudied words), subjects fell back on an iterative generate‐recognize strategy until candidate completions were exhausted.
fMRI Findings
Unless explicitly noted, discussion of the fMRI data pertains to the findings identified with the canonical HRF.
Retrieval success effects common to the two tasks were identified in medial and lateral parietal cortex, as well as a small region of right entorhinal cortex. The loci of the parietal effects overlap regions identified in numerous prior studies of recognition memory [Cabeza et al.,2008; Vilberg and Rugg,2008a]. Thus, in the present study, retrieval success effects were identified in the left mid‐IPS (peak −42, −64, 52) in close proximity to the center of mass (−38, −62, −46) of the effects associated with familiarity‐driven recognition that were identified in the review of Vilberg and Rugg [2008a]. Additionally, retrieval success effects common to the two tasks were evident bilaterally in more inferior parietal regions in the vicinity of the angular gyrus, close to the center of mass of the effects associated with recollection [Vilberg and Rugg,2008a]. Thus, the present results add to the evidence that, at least at the spatial scale afforded by fMRI, retrieval success effects in these parietal regions are shared between tests of recognition and cued recall.
As was noted in the Introduction and above, it has been proposed that retrieval success effects in superior and inferior regions of left lateral parietal cortex [mid‐IPS (BA7/40) and angular gyrus (BA39), respectively] are functionally dissociable. According to one proposal [Cabeza,2008; Cabeza et al.,2008; Ciaramelli et al.,2008], retrieval‐related activity in left IPS is a reflection of the top‐down allocation of attentional resources to a retrieval cue, and co‐varies with the amount of “effort” associated with its processing. Thus, cue‐related activity is predicted to be greater when retrieval (or an attempt to retrieve) is effortful relative to when it is relatively fluent [Cabeza et al.,2008]. The present finding that word stems associated with successful recall elicited greater activity in the left mid‐IPS than new stems is difficult to reconcile with this proposal: as noted above (see below, also), it is highly likely that stems for which recall failed were subjected to more effortful processing than stems that elicited successful recall. Thus, the present findings converge with other recent results to suggest that the sensitivity of the left mid‐IPS to successful retrieval is not the result of a confound between retrieval success and such factors as retrieval effort [see also Vilberg and Rugg,2009]. Rather, old > new effects in this region appear to reflect processes engaged specifically by successful retrieval, regardless of whether retrieval is based on familiarity‐driven recognition or the processes supporting recollection‐driven recognition and recall. As discussed previously [Donaldson et al.,2010; Suzuki et al., in press; Vilberg and Rugg,2009; Wagner et al.,2005], one possibility is that the mid‐IPS supports a “mnemonic accumulator” that tracks the amount of evidence that a retrieval cue corresponds to a studied item, regardless of whether the evidence derives from familiarity or recollection [cf. Wixted,2007].
Whereas the mid‐IPS demonstrated task‐invariant retrieval success (old > new) effects, task‐invariant new > old effects were evident in a more anterior region of the IPS (y coordinates for the two regions were −64 and −34, respectively). Together with the success effects in the mid‐IPS, the reversed anterior effects constitute compelling evidence for the functional heterogeneity of retrieval processing within the IPS. Intriguingly, the present new > old effect in the anterior IPS (peak voxel at −52, −34, 48) overlaps a region where retrieval success effects were found to vary according to the relative probability of old vs. new recognition test items [Vilberg and Rugg,2009; peak at −48, −27, 54]. Together with this prior result, the current findings suggest that it is the anterior rather than the mid‐IPS where activity reflects strategic adjustments to cue processing contingent on the outcome of a retrieval attempt [cf. Cabeza et al.,2008].
The only regions to exhibit greater retrieval success effects for cued recall than recognition were bilateral inferior lateral parietal cortex and left superior PFC. The parietal regions correspond well with inferior parietal regions that have consistently been identified as recollection‐sensitive in studies of recognition memory [Vilberg and Rugg,2008a; see above]. Indeed, both the left and right parietal regions which demonstrated greater effects for cued recall survived small volume corrections (P < 0.01) within 5‐mm radii spheres located at the centers of mass of the recollection effects identified in the above‐cited review. Similarly, the superior PFC region is in the vicinity of a left superior PFC region that demonstrated recollection‐selective success effects in a prior study [Vilberg and Rugg,2007; small volume correction P < 0.01 within a 5‐mm radius sphere centered at −36, 21, 51]. A plausible account of these findings is that recall is more dependent on recollection than is recognition. By this account, whereas recognition could succeed on the basis of familiarity even when recollection failed, this was not the case for recall. Hence, the present findings reflect differences between the two tests in the proportions of trials where successful retrieval was associated with recollection of the study episode as well as, possibly, differences in the amount of information recollected in response to the two types of retrieval cue [cf. Vilberg and Rugg,2007].
In addition to midline and lateral parietal cortex, retrieval success effects common to the two tests were also evident in right entorhinal cortex. Although the right‐sided lateralization of this MTL effect is reminiscent of prior PET findings of right‐lateralized medial temporal activity in association with successful cued recall [Schacter et al.,1996; Squire et al.,1992], the present asymmetry is more apparent than real: reducing the threshold of the primary contrast to P < 0.005 was sufficient to reveal a sizeable cluster (33 voxels) in the homologous region of the left MTL. Whereas old > new effects in the MTL are reported only rarely in studies of simple recognition memory [in contrast to effects associated with contrasts between test items recognized on the basis of recollection vs. familiarity, see Wais,2008, for review], the present findings are easily accommodated by the widely‐held view that the MTL plays a key role in episodic memory retrieval.
In addition to the anterior IPS region discussed above, new > old effects common to the two tests were also identified in medial and, more prominently, left VLPFC. The analyses based on the delayed HRF indicated that, in both regions, these effects were more sustained in the cued recall test. These findings likely point to a role for these PFC regions in the processing of retrieval cues in service of controlled memory search. Notably, Dobbins et al. [2002] implicated an overlapping left VLPFC region in cue processing in the context of a source recollection task, arguing that this region supported such processes as selecting among competing representations activated by the cue and using the selected representation to probe memory. By this account, the present left VLPFC new > old effect reflects the fact that these cue‐processing operations were more sustained and, perhaps, recruited additional attentional resources, when an initial retrieval attempt was unsuccessful. The more sustained effects associated with cued recall presumably reflect the greater scope for iterative retrieval attempts afforded by word stems rather than copy cues (see below).
In a replication of prior PET findings [Rugg et al.,1998], retrieval effects in right lateral anterior PFC demonstrated a crossover interaction, with reliable new > old effects for cued recall accompanied by old > new effects for recognition. As was outlined in the Introduction, Rugg et al. [1998] argued that this crossover effect reflected the role of the right lateral anterior PFC in monitoring the outcome of a retrieval attempt [cf. Fletcher and Henson,2001]. Rugg et al. proposed that copy cues offered only limited opportunity for iterative retrieval attempts, whereas word stems allow as many attempts as there are potential stem completions. Hence, monitoring is required in recognition tests primarily when a test item gives rise to successful retrieval, but in cued recall it is required each time a different candidate completion is employed as a memory probe. The present findings can easily be accommodated by this account. It should be noted, however, that the “monitoring hypothesis” of right lateral PFC engagement during episodic retrieval has been challenged by an alternative account proposing that engagement of these regions is proportional to the number of internal decisions made in response to a stimulus event such as a memory test item [Dobbins and Han,2006]. In the absence of evidence about the relative number of internal decisions elicited by old and new items in recognition and cued recall tests it is not possible to assess how well this alternative account might accommodate the present findings.
A final noteworthy difference between retrieval‐related activity in the two tests was identified in bilateral extrastriate visual cortex, where new > old effects were evident for cued recall only. Analogous effects have previously been reported in extrastriate regions in the indirect test of word stem completion [e.g., Schacter et al.,1996; Squire et al.,1992], and the effects are widely regarded as a neural correlate of priming. Schott et al. [2005] reported that new > old effects in fusiform cortex were equally evident for correctly completed stems regardless of whether the completions were correctly endorsed as old or misclassified as “new.” Therefore it seems unlikely that the present effects reflect engagement of retrieval processes supporting explicit memory for studied items. It is unclear why similar priming effects were not evident for the recognition test items. Nor is it clear why recognition should have been associated with effects in the opposite direction (old > new) in left fusiform cortex (see Fig. 3).
In conclusion, the present findings constitute direct evidence for the engagement in medial and lateral parietal cortex of a common set of processes associated with successful recognition and cued recall. Hence the findings emphasize the generality of parietal “retrieval success effects,” and highlight the need for a theoretical perspective that extends beyond the compass of recognition memory when accounting for these effects. In addition, the findings point to important differences in the neural correlates of recognition and cued recall that likely reflect differences in how copy and partial retrieval cues are employed to probe memory.
REFERENCES
- Allan K, Rugg MD ( 1997): An event‐related potential study of explicit memory on tests of cued recall and recognition. Neuropsychologia 35: 387–397. [DOI] [PubMed] [Google Scholar]
- Allan K, Wilding EL, Rugg MD ( 1998): Electrophysiological evidence for dissociable processes contributing to recollection. Acta Psychol 98: 231–252. [DOI] [PubMed] [Google Scholar]
- Allan K, Dolan RJ, Fletcher PC, Rugg MD ( 2000): The role of the right anterior prefrontal cortex in episodic retrieval. NeuroImage 11: 217–227. [DOI] [PubMed] [Google Scholar]
- Andrade A, Paradis A, Rouquette S, Poline J ( 1999): Ambiguous results in functional neuroimaging data analysis due to covariate correlation. NeuroImage 10: 483–486. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ ( 1999): Nonlinear spatial normalization using basis functions. Hum Brain Mapp 7: 254–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R ( 2008): Role of parietal regions in episodic memory retrieval: The dual attentional processes hypothesis. Neuropsychologia 46: 1813–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Ciaramelli E, Olson IR, Moscovitch M ( 2008): The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci 9: 63–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramelli E, Grady C, Moscovitch M ( 2008): Top‐down and bottom‐up attention to memory: A hypothesis (AtoM) on the role of the posterior parietal cortex in memory retrieval. Neuropsychologia 46: 1828–1851. [DOI] [PubMed] [Google Scholar]
- Cocosco C, Kollokian V, Kwan RS, Evans A ( 1997): Brainweb: Online interface to a 3D MRI simulated brain database. NeuroImage 5: S425. [Google Scholar]
- Dobbins IG, Han S ( 2006): Isolating rule versus evidence‐based prefrontal activity during episodic and lexical discrimination: A functional magnetic resonanceimaging investigation of detection theory distinctions. Cereb Cortex 16: 1614–1622. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD ( 2002): Executive control during episodic retrieval: Multiple prefrontal processes subserve source memory. Neuron 35: 989–996. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Wheeler ME, Petersen SE ( 2010): Remembering the source: Dissociating frontal and parietal contributions to episodic memory. J Cogn Neurosci 22: 377–391. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K ( 2005): A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Heim S, Zilles K, Amunts K ( 2006): Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage 32: 570–582. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans A, Zilles K, Amunts K ( 2007): Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage 36: 511–521. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Henson RN ( 2001): Frontal lobes and human memory: Insights from functional neuroimaging. Brain 124: 849–881. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Fletcher PC, Josephs O, Holmes A, Rugg MD, Turner R ( 1998): Event‐related fMRI: Characterizing differential responses. NeuroImage 7: 30–40. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J ( 2002): Classical and Bayesian inference in neuroimaging: Applications. NeuroImage 16: 484–512. [DOI] [PubMed] [Google Scholar]
- Guerin SA, Miller MB ( 2009): Lateralization of the parietal old/new effect: An event‐related fMRI study of recognition memory for words and faces. NeuroImage 44: 232–242. [DOI] [PubMed] [Google Scholar]
- Henson RN, Shallice T, Josephs O, Dolan RJ ( 2002): Functional magnetic resonance imaging of proactive interference during spoken cued recall. NeuroImage 17: 543–558. [PubMed] [Google Scholar]
- Jacoby LL ( 1998): Invariance in automatic influences of memory: Toward a user's guide for the process‐dissociation procedure. J Exp Psych 24: 3–26. [DOI] [PubMed] [Google Scholar]
- Kucera H, Francis WN ( 1967): Computational Analysis of Present‐Day American English. Providence: Brown University Press. [Google Scholar]
- Rugg MD ( 1998): Convergent approaches to electrophysiological and hemodynamic investigations of memory. Hum Brain Mapp 6: 394–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA ( 2002): Episodic memory retrieval: An (event‐related) functional neuroimaging perspective In: Parker AE, Wilding EL, Bussey T, editors. The Cognitive Neuroscience of Memory Encoding and Retrieval. London: Psychology Press; pp 83–89. [Google Scholar]
- Rugg MD, Fletcher PC, Allan K, Frith CD, Frackowiak RS, Dolan RJ ( 1998): Neural correlates of memory retrieval during recognition memory and cued recall. NeuroImage 8: 262–273. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RN ( 2002): The neural basis of episodic memory: Evidence from functional neuroimaging. Philos Trans R Soc Lond B Biol Sci 357: 1097–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner EI, Fernandez MA ( 2007): Neural correlates of recollection and familiarity: A review of neuroimaging and patient data. Neuropsychologia 45: 2163–2179. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Alpert NM, Savage CR, Rauch SL, Albert MS ( 1996): Conscious recollection and the human hippocampal formation: Evidence from positron emission tomography. Proc Natl Acad Sci USA 93: 321–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott BH, Henson RN, Richardson‐Klavehn A, Becker C, Thomas V, Heinze HJ, Duzel E ( 2005): Redefining implicit and explicit memory: The functional neuroanatomy of priming, remembering, and control of retrieval. Proc Natl Acad Sci 102: 1257–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaniol J, Davidson PS, Kim AS Han H, Moscovitch M, Grady CL ( 2009): Event‐related fMRI studies of episodic encoding and retrieval: Meta‐analyses using activation likelihood estimate. Neuropsychologia 47: 1765–1779. [DOI] [PubMed] [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME ( 1992): Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proc Natl Acad Sci USA 89: 1837–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Johnson JD, Rugg MD: Decrements in hippocampal activity with item repetition during continuous recognition: An fMRI study. J Cogn Neurosci (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC ( 2002): Windows on the brain. The emerging role of atlases and databases in neuroscience. Curr Opin Neurobiol 12: 574–579. [DOI] [PubMed] [Google Scholar]
- Van Essen DC ( 2005): A population‐average, landmark‐ and surface‐based (PALS) atlas of human cerebral cortex. Neuroimage 28: 635–662. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Dickson J, Harwell J, Hanlon D, Anderson CH, Drury HA ( 2001): An integrated software system for surface‐based analyses of cerebral cortex. JAMIA 41: 1359–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD ( 2007): Dissociation of the neural correlates of recognition memory according to familiarity, recollection, and amount of recollected information. Neuropsychologia 45: 2216–2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD ( 2008a): Memory retrieval and the parietal cortex: A review of evidence from a dual‐process perspective. Neuropsychologia 46: 1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD ( 2008b): Functional significance of retrieval‐related activity in lateral parietal cortex: Evidence from fMRI and ERPs. Hum Brain Mapp 30: 1490–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD ( 2009): An investigation of the effects of relative probability of old and new test items on the neural correlates of successful and unsuccessful source memory. NeuroImage 45: 562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL ( 2005): Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci 9: 445–453. [DOI] [PubMed] [Google Scholar]
- Wais PE ( 2008): fMRI signals associated with memory strength in the medial temporal lobes: A meta‐analysis. Neuropsychologia 46: 3185–3196. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL ( 2004): Functional‐anatomic correlates of remembering and knowing. NeuroImage 21: 1337–1349. [DOI] [PubMed] [Google Scholar]
- Wixted JT ( 2007): Dual‐process theory and signal‐detection theory of recognition memory. Psychol Rev 114: 152–176. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP ( 2002): The nature of recollection and familiarity: A review of 30 years of research. J Mem Lang 46: 441–517. [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD ( 2005): Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci 25: 3002–3008. [DOI] [PMC free article] [PubMed] [Google Scholar]


