Abstract
Purpose
To investigate in The Standard Care versus COrticosteroid for REtinal Vein Occlusion (SCORE) Study: 1) incidences of neovascular events and retinal capillary nonperfusion (abbreviated as “nonperfusion”), and their relationship with treatment groups; 2) neovascular incidences by nonperfusion status; and 3) pertinent baseline factors for their potential risk for neovascular events.
Design
Two multi-center, randomized clinical trials: one evaluating participants with central retinal vein occlusion (CRVO) and the other evaluating participants with branch retinal vein occlusion (BRVO).
Participants
At 36 months, data were available for 81 participants with CRVO and 128 with BRVO.
Intervention
Standard care (observation or grid photocoagulation) versus 1 mg or 4 mg intravitreal triamcinolone.
Main Outcome Measures
Neovascularization of the iris (NVI), neovascular glaucoma (NVG), disc or retinal neovascularization (NVD/NVE), pre-retinal or vitreous hemorrhage (PRH/VH), and nonperfusion.
Results
Cumulative 36-month incidences for CRVO and BRVO eyes, respectively, were: 8.5% and 2.4% for NVI or NVG; 8.8 % and 7.6% for NVD/NVE or PRH/VH. There were no differences in incidences of neovascular events or risk of nonperfusion when comparing the 3 treatment groups within diseases. For CRVO at 36 months, 16.6% of eyes with ≥ 5.5 disc areas of nonperfusion vs. 4.0% of eyes with < 5.5 disc areas of nonperfusion developed NVG (P= 0.0003); for BRVO at 36 months, 14.6% versus 2.4% developed NVD/NVE (P < 0.0001). Nonperfusion was the only significant baseline factor for neovascularization in BRVO, with the risk of a neovascular event increasing with greater disc areas of nonperfusion, and the highest risk noted at ≥ 5.5 disc areas.
Conclusions
In the SCORE Study, triamcinolone treatment was not associated with lower incidences of neovascular events or nonperfusion status compared with observation or grid photocoagulation. Cumulative 36-month incidences for most neovascular events were significantly higher for nonperfused than perfused eyes. Greater baseline disc areas of nonperfusion increased the risk of neovascularization in BRVO but not CRVO eyes, possibly due to obscuration of retinal capillary details caused by dense hemorrhage at baseline for CRVO eyes. Increased risk of neovascularization was noted below the historical threshold of 10 disc areas of nonperfusion for retinal vein occlusion.
Central retinal vein occlusion (CRVO) and branch retinal vein occlusion (BRVO) are major causes of vision loss on a global scale.1-4 The Beaver Dam Eye Study showed that retinal vein occlusion was responsible for severe vision loss in 12% of the eyes over a 15-year period.3 The Standard Care versus COrticosteroid for REtinal Vein Occlusion Study (SCORE) was a multi-center, prospective, randomized, phase III study funded by the National Eye Institute. It consisted of two separate clinical trials designed to investigate the safety and efficacy of standard care (SC) versus intravitreal triamcinolone acetonide injections for treating vision loss associated with macular edema in eyes with CRVO or BRVO, respectively (the SCORE-CRVO trial and the SCORE-BRVO trial).5 The primary results of the SCORE Study demonstrated that intravitreal triamcinolone, an angiostatic corticosteroid, is superior to observation for treating vision loss associated with macular edema secondary to CRVO for patients similar to those enrolled into the SCORE-CRVO trial (7%, 27%, and 26%, achieved the primary outcome of a gain in visual acuity letter score of 15 or more from baseline to 12 months in the observation, 1 mg, and 4 mg groups, respectively).6 In the SCORE-BRVO trial, intravitreal triamcinolone was found to be equivalent to grid photocoagulation in terms of the percentage of eyes achieving the primary outcome (29%, 26%, and 27% for observation/grid photocoagulation, 1 mg, and 4 mg groups respectively) at 12 months, and for decreasing macular edema measured by optical coherence tomography (OCT).7 The clinical effect of triamcinolone is thought to be related to its inhibition of vascular endothelial growth factor (VEGF) and other mediators for reducing macular edema.6-12 Previous studies have also reported the anti-angiogenic effects of triamcinolone through its inhibition of VEGF and other mediators, e.g., stromal cell-derived factor 1 (SDF-1α), a potent chemokine that promotes both physiologic vascular remodeling as well as pathologic neovascularization.13-19 Inhibition of these proangiogenic and inflammatory mediators may occur via both transcriptional and posttranscriptional mechanisms.20,21 The current study compares the incidence of neovascular events in eyes receiving standard care versus 1 mg or 4 mg triamcinolone acetonide in the SCORE Study. Participants in the SCORE-CRVO trial assigned to standard care (SC) were observed; participants in the SCORE-BRVO trial assigned to SC were treated with grid photocoagulation in eyes without dense macular hemorrhage, and in eyes with dense macular hemorrhage, photocoagulation was deferred until the hemorrhage cleared sufficiently to permit grid photocoagulation to be performed.
The cumulative incidences of neovascular events compared among the 3 treatment groups in this study included those for neovascularization of the iris (NVI), neovascular glaucoma (NVG), disc or retinal neovascularization (NVD/NVE), and pre-retinal or vitreous hemorrhage (PRH/VH). Based on stereoscopic fluorescein angiograms evaluated at the University of Wisconsin Fundus Photograph Reading Center (Reading Center), the incidence of retinal capillary nonperfusion was evaluated among the 3 groups. Cumulative 36-month incidences for neovascular events were also compared according to retinal perfusion status over follow-up (< 5.5 disc areas at all visits versus ≥ 5.5 disc areas of retinal capillary nonperfusion at any visit). Finally, multiple baseline factors were assessed for their potential predictive value for neovascular events in the CRVO and BRVO trials in the SCORE Study.
METHODS
The SCORE Study design and methods, described in detail in previous SCORE reports 5-7,22,23 are summarized here in brief. The protocol and consent forms for this randomized multicenter clinical study were approved by either a clinical site’s institutional review board or a centralized institutional review board (Jaeb Center for Health Research, Tampa, Florida), whichever was applicable. Data were obtained from 66 clinical sites in the CRVO trial and 75 clinical sites in the BRVO trial across the United States. All neovascular events (NVI, NVG, NVD, NVE, PRH, and VH) were recorded independently at the clinical sites and the Reading Center. An independent data and safety monitoring committee appointed by the National Eye Institute provided data and safety monitoring oversight. The study adhered to the tenets of the Declaration of Helsinki. Written Health Insurance Portability and Accountability Act-compliant informed consents were obtained from all participants prior to screening for eligibility.
Eligibility criteria and examination procedures
Detailed descriptions of the study population and the randomization, examination, and treatment procedures can be found in previous SCORE Study reports.5-7,22,23 Major ocular eligibility criteria have been published in a previous SCORE report.5 Stereoscopic fundus photography, fluorescein angiography, and OCT were obtained by certified photographers at baseline and repeated at specified intervals as outlined in previously published reports.5-7,22,23
Reading Center grading of neovascular events and area of retinal capillary nonperfusion
In the SCORE Study grading system of stereoscopic fundus photographs,24 new vessels were defined as vessels that were clearly on the disc or retinal surface, or located further anteriorly in the vitreous cavity. New vessels on the disc (NVD) were those that either: a) originated and resided within 1 disc-diameter of the disc margin; or b) originated outside of 1 disc-diameter of the disc margin, and extended within ½ disc-diameter of the disc margin in the absence of other new vessels fitting the description of a). New vessels on the retina that did not fit the criteria for NVD were considered new vessels elsewhere (NVE). The grading scale for both NVD and NVE was as follows: “Absent”, “Questionable”, “Definite” and “Cannot grade”. Pre-retinal hemorrhage (PRH) included “boat-shaped” hemorrhage and round, oval, or linear patches of hemorrhage immediately anterior to the retina or under the internal limiting membrane. Hemorrhage located further anteriorly, including hemorrhage on or within fibrovascular proliferation, was considered vitreous hemorrhage (VH). In the event of difficulty discerning between PRH and VH, the grader was required to decide on the more appropriate of the two choices, instead of assigning a grade of “Questionable” to both PRH and VH.
Fluorescein angiograms, graded according to the Early Treatment Diabetic Retinopathy Study (ETDRS) fluorescein angiogram grading protocol,25 were performed on study participants at baseline, and at Months 4, 12, and 24. Disc areas of retinal capillary nonperfusion assessed from fluorescein angiograms referred to the portions of the fundus devoid of retinal arterioles and/or capillaries, and were detected by angiographic characteristics such as a “pruned” appearance of adjacent arterioles and a darker appearance of the choroid. The area shown for the entire fundus for each study eye was used for analysis of retinal capillary loss, ranging from 0 to 210 disc areas.5,24 The area of retinal capillary nonperfusion was quantified at each visit. A single grader assessed all visits and had access to previous angiograms and grading data when assessing follow-up angiograms of the same patients. This longitudinal system of assessing angiograms allowed the graders to overcome differences in quality of images over subsequent visits. The inability to grade capillary loss was often attributed to substantial posterior segment hemorrhage, shown by the finding that eyes with ungradable images had a mean of 4.5 disc areas of retinal hemorrhage compared with 2.6 disc areas of retinal hemorrhage among eyes with a calculated capillary loss (P < 0.0001).5,24,26 For the purposes of this report, retinal capillary perfusion status over follow-up was defined as < 5.5 disc areas at all visits (labeled perfused) versus ≥ 5.5 disc areas of retinal capillary nonperfusion at any visit (labeled non-perfused).
Data for neovascular events
Incident disc and retinal neovascularization and pre-retinal and vitreous hemorrhage event data were identified by masked graders from fundus photographs sent by the clinical sites to the Reading Center when study participants reported for regular 4-month visits through Month 36. About 10% of reading center image data was missing, either because the graders could not assess the image or because the image was expected but not available. All these cases were recoded to “Absent”, so that only codes of “Absent” and “Definite” remained for visits (up to 36 months) while the patient was still in the study. Data that were missing due to the common closeout were treated as censored in this analysis. Based on the common closeout, study participants had potential follow-up of at least 12 months and at most 36 months. About 2% of individuals who had neovascularization at baseline were excluded from the analysis of incidence.
Incident NVG and NVI event data analyzed in this report were derived from the clinical sites, where investigators reported these events on an adverse event form as part of routine safety monitoring. All adverse events in the SCORE Study were coded per the Medical Dictionary for Regulatory Activities (MedDRA), and those with preferred terms of glaucoma, NVI or neovascularization were identified for further investigation. Once this subset was identified, one of us (CC) reviewed the MedDRA terms and adverse event narratives provided by the clinical site to classify the events and define which were appropriate for the analyses reported herein.
Statistical analyses
Data from the Reading Center reflected status of eyes documented during the 4-month examinations at the clinical sites, and were analyzed by life-table methods, with the log-rank test used to compare groups statistically. Clinical site data were derived from adverse event reports, and thus reflected the exact dates that the events in question were reported, so that waiting time analyses were performed by Kaplan-Meier methodology. The results of these analyses, referred to as “cumulative incidences”, are presented as values of the survival function at specific time points and expressed as percents. The log-rank test was used to compare groups statistically. Analyses investigating the effect of baseline predictors on hazard rates of neovascular events were performed by univariate and multivariate (considering all the factors) Cox regression analyses, which estimate the increase in hazard associated with a predictor. We use the hazard as a measure of risk in these analyses. The analyses were performed separately for NVI or NVG based on data from the clinical sites, and for NVD/NVE or PRH/VH based on data from the Reading Center. The following baseline factors were evaluated for potential predictive values for a neovascular event in the SCORE-CRVO trial and the SCORE-BRVO trial, respectively: observation/grid photocoagulation versus 1 mg triamcinolone, observation/grid photocoagulation versus 4 mg triamcinolone, 1 mg triamcinolone versus 4 mg triamcinolone, age, intraocular pressure, gender, pseudophakia, diabetes, systemic hypertension, coronary disease, visual acuity, prior macular edema, disc area of retinal hemorrhage, and disc area of retinal capillary nonperfusion. To further investigate the relationship between retinal capillary nonperfusion and neovascular events, additional exploratory analyses were performed, including modeling disc area of retinal capillary nonperfusion as a time-varying covariate in multivariate Cox regression analyses, and examining the risk of neovascular events based on levels of retinal capillary nonperfusion observed during follow-up (e.g., ≥ 5.5 disc areas). Note that this latter analysis does not consider the temporal order of the retinal capillary nonperfusion and neovascular events. Due to the multitude of statistical tests performed predisposing for Type-1 errors, only P values of ≤ 0.001 are considered to be significant for all analyses of this study. All analyses were performed in SAS 9.2 (2008, Cary, North Carolina).
RESULTS
Enrollment into the SCORE Study occurred from November 4, 2004 to February 29, 2008. At baseline, 271 participants were enrolled in the SCORE-CRVO trial and 411 participants were enrolled in the SCORE-BRVO trial.8 In the SCORE-CRVO trial, approximately 45% were women and more than 90% were Caucasian. In the SCORE-BRVO trial, 50% of the participants were women and more than 88% were Caucasian. The average age of the participants was 68 years in the SCORE-CRVO trial and 67 years in the SCORE-BRVO trial. Most participants were phakic (80%, 82%) and hypertensive (71%, 69%) in the SCORE-CRVO trial and the SCORE-BRVO trial, respectively. In the SCORE Study, the common closeout allowed at least 12 months of follow-up on all participants, which then resulted in approximately 50% of the participants completing 24 months and 30% completing 36 months of follow-up. Specifically, follow-up data were available for 238 participants and 369 participants at 12 months, 151 participants and 238 participants at 24 months, and 81 participants and 128 participants at 36 months for the SCORE-CRVO trial and the SCORE-BRVO trial, respectively
Incidence of NVI and NVG
The 36-month cumulative incidences of NVI and NVG based on Kaplan-Meier Survival analysis on data from the clinical sites for the observation/grid laser photocoagulation, 1mg triamcinolone, and 4 mg triamcinolone groups, respectively, are listed in Tables 1 and 2 (available at http://aaojournal.org). The overall 36-month incidences of NVI and NVG were 3.2% and 5.8% for the SCORE-CRVO trial, respectively. They were 0.3% and 2.2% for the SCORE-BRVO trial, respectively. The cumulative incidences for NVI or NVG at 12 and 36 months were 6.1 % and 8.5 %, respectively in the SCORE-CRVO trial and 1.3% and 2.4% in the SCORE-BRVO trial (Table 3). There was a lack of significant difference in the cumulative incidences of NVI or NVG among the 3 study groups for the SCORE-CRVO trial (Figure 1; p=0.31, Log-rank test) and the SCORE-BRVO trial (p=0.18, Log-rank test).
Table 3.
Iris Neovascularization or Neovascular Glaucoma by Treatment Groups*
| CRVO | BRVO | ||||||
|---|---|---|---|---|---|---|---|
| Month | Month | ||||||
| 12 | 24 | 36 | 12 | 24 | 36 | ||
| Observation / grid photocoagulation |
Risk set | 75 | 45 | 15 | 120 | 79 | 22 |
| Cumulative Events | 2 | 3 | 3 | 1 | 1 | 1 | |
| Percent** | 2.4 | 4.2 | 4.2 | 0.8 | 0.8 | 0.8 | |
| 1 mg | Risk set | 76 | 48 | 16 | 121 | 78 | 26 |
| Cumulative Events | 9 | 10 | 10 | 1 | 2 | 2 | |
| Percent** | 9.9 | 11.8 | 11.8 | 0.8 | 1.9 | 1.9 | |
| 4 mg | Risk set | 72 | 43 | 13 | 119 | 68 | 15 |
| Cumulative Events | 5 | 7 | 7 | 3 | 4 | 5 | |
| Percent** | 5.8 | 9.4 | 9.4 | 2.2 | 3.1 | 4.8 | |
| Overall | Risk set | 223 | 136 | 44 | 360 | 225 | 63 |
| Cumulative Events | 16 | 20 | 20 | 5 | 7 | 8 | |
| Percent** | 6.1 | 8.5 | 8.5 | 1.3 | 1.9 | 2.4 | |
CRVO = central retinal vein occlusion; BRVO = branch retinal vein occlusion.
P =0.31, for CRVO eyes, 0.18 for BRVO eyes for comparison of SC, 1mg, and 4 mg groups, P =0.0002 for overall comparison of CRVO and BRVO eyes, respectively (Log-Rank tests).
“Percent” stands for the value of the Kaplan-Meier survival function expressed as a percent.
Figure 1.
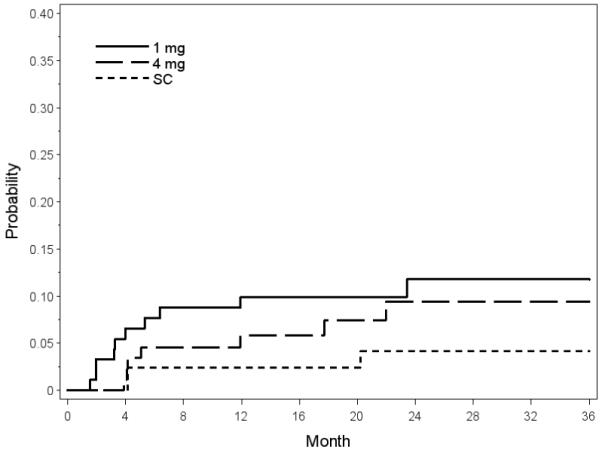
Plot of Kaplan-Meier Survival analysis on neovascularization of the iris or neovascular glaucoma (NVI/NVG) shows the lack of significant difference in the corresponding neovascular events among the 3 treatment groups (standard care [SC], 1 mg triamcinolone, and 4 mg triamcinolone) in the Standard Care versus COrticosteroid for REtinal Vein Occlusion-Central Retinal Vein Occlusion (SCORE-CRVO) trial.
Incidence of NVD/NVE and PRH/VH
The 36-month incidences of NVD/NVE and PRH/VH based on life-table analysis on data from the Reading Center for the observation/grid photocoagulation, 1mg triamcinolone, and 4mg triamcinolone groups, respectively, are listed in Tables 4 and 5 (available at http://aaojournal.org). The overall 36-month incidences of NVD/NVE and PRH/VH were 3.6% and 7.6% for the SCORE-CRVO trial, respectively. They were 5.8% and 3.8% for the SCORE-BRVO trial, respectively. The cumulative incidences for NVD/NVE or PRH/VH at 12 and 36 months were 2.9% and 7.6%, respectively in the SCORE-BRVO trial and 2.8% and 8.8% in the SCORE-CRVO trial (Table 6). There was a lack of significant difference in the cumulative incidences of NVD/NVE or PRH/VH among the 3 study groups for the SCORE-CRVO trial (p-0.70, Log-rank test) and the SCORE-BRVO trial (Figure 2; p=0.08, Log-rank test).
Table 6.
Disc or Retinal Neovascularization or Pre-retinal or vitreous Hemorrhage* by Treatment Groups
| CRVO | BRVO | ||||||
|---|---|---|---|---|---|---|---|
| Month | Month | ||||||
| 12 | 24 | 36 | 12 | 24 | 36 | ||
| Observation / grid photocoagulation |
Risk set | 74 | 55 | 27 | 115 | 88 | 48 |
| Cumulative Events | 3 | 5 | 5 | 9 | 11 | 13 | |
| Percent** | 3.7 | 3.7 | 7.5 | 6.3 | 8.8 | 10.2 | |
| 1 mg | Risk set | 83 | 59 | 29 | 117 | 86 | 49 |
| Cumulative Events | 6 | 8 | 8 | 3 | 7 | 8 | |
| Percent** | 2.3 | 7.1 | 10.5 | 1.6 | 4.4 | 8.4 | |
| 4 mg | Risk set | 80 | 55 | 28 | 121 | 87 | 47 |
| Cumulative Events | 2 | 4 | 5 | 1 | 3 | 4 | |
| Percent** | 2.3 | 5.6 | 8.4 | 0.8 | 2.6 | 4.1 | |
| Overall | Risk set | 237 | 169 | 84 | 353 | 261 | 144 |
| Cumulative Events | 11 | 17 | 18 | 13 | 21 | 25 | |
| Percent** | 2.8 | 5.5 | 8.8 | 2.9 | 5.3 | 7.6 | |
CRVO = central retinal vein occlusion; BRVO = branch retinal vein occlusion.
P =0.70 for CRVO eyes, 0.08 for BRVO eyes for comparison of SC, 1 mg, and 4 mg groups. P =0.76 for overall comparison of CRVO and BRVO eyes, respectively (Log-Rank tests).
“Percent” stands for the value of the Life-table analysis expressed as a percent
Figure 2.
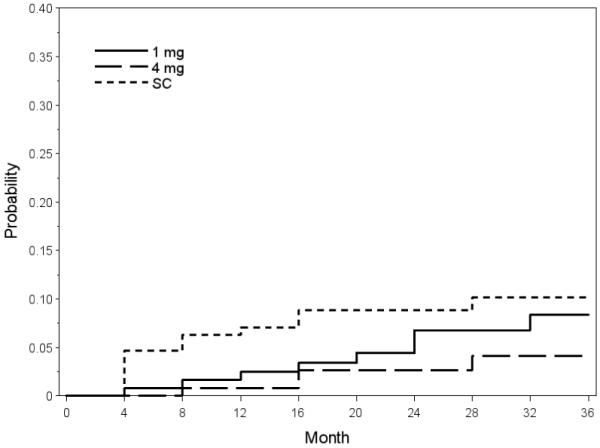
Plot of Life-table analysis on disc or retinal neovascularization or pre-retinal or vitreous hemorrhage (NVD/NVE or PRH/VH) shows the lack of significant difference in the corresponding neovascular events among the 3 treatment groups (standard care [SC], 1 mg triamcinolone, and 4 mg triamcinolone) in the Standard Care versus COrticosteroid for REtinal Vein Occlusion-Branch Retinal Vein Occlusion (SCORE-BRVO) trial.
Prediction analyses
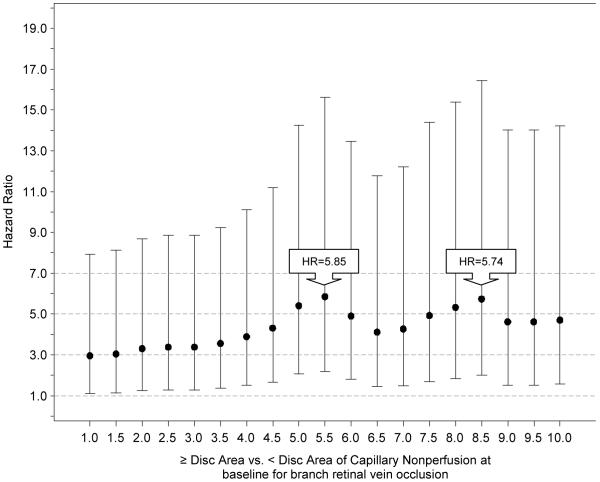
For the SCORE-CRVO trial, neither the univariate nor multivariate Cox regression analyses showed any baseline factor significantly associated with a neovascular event. For the SCORE-BRVO trial, univariate analysis showed larger disc areas of retinal capillary nonperfusion to be a significant baseline factor for a neovascular event (P=0.0004). Multivariate analysis, with all the baseline factors included in the model, also showed larger disc areas of retinal capillary nonperfusion to be the only significant baseline factor associated with a neovascular event (P <0.0001) in the SCORE-BRVO trial, with a 9% increase in the hazard of a neovascular event for every one disc-area of increase in retinal capillary nonperfusion at baseline (Table 7). For the SCORE-BRVO trial, exploratory analyses based on Cox regression models were performed to better understand the hazard of a neovascular event based on discrete cut-points of retinal capillary nonperfusion. Figure 3 shows the hazard ratios with 95% confidence intervals for these specific cut-points that range from ≥1.0 disc area (compared with < 1.0) to ≥10.0 disc areas (compared with < 10) in increments of 0.5 disc areas. The peak of the hazard ratios corresponds to 5.5 disc areas of retinal capillary nonperfusion at baseline, where the hazard of a neovascular event was 5.86 times higher for eyes with a baseline retinal capillary nonperfusion of greater than or equal to 5.5 disc areas compared with those less than 5.5 disc areas of retinal capillary nonperfusion (p=0.0004).
Table 7.
Summary of Retinal Capillary Nonperfusion Results in the Multivariate Cox Regression Analyses
| BRVO | CRVO | |||
|---|---|---|---|---|
| HR | P-Value | HR | P-Value | |
| Retinal capillary nonperfusion as a baseline predictor | ||||
| Continuous, per 1 DA increase | 1.09 | <0.0001 | 0.64 | 0.35 |
| ≥ 5.5 (relative to < 5.5 DA) DA | 5.86 | 0.0004 | * | * |
| Retinal capillary nonperfusion as time-varying covariate | ||||
| Continuous, per 1 DA increase | 1.05 | 0.0003 | 1.04 | 0.007 |
| ≥ 5.5 DA (relative to < 5.5 DA) | 3.81 | 0.0007 | 1.13 | 0.85 |
Unable to fit model
CRVO = central retinal vein occlusion; BRVO = branch retinal vein occlusion. HR=Hazard ratio.; DA=Disc Area.
Figure 3.
Plot from exploratory analyses based on Cox regression models showing the hazard ratios (HR) with 95% confidence intervals for specific cut-points to assess the hazard of a neovascular event, whereby each specific disc area or greater of retinal capillary nonperfusion compared with less than that specific disc area of retinal capillary nonperfusion at baseline (x-axis) is plotted against the corresponding hazard ratio (y-axis); the cut-points of capillary retinal capillary nonperfusion ranged from ≥1.0 disc area (compared with < 1.0) to ≥10.0 disc areas (compared with < 10) in increments of 0.5 disc areas in the Standard Care versus COrticosteroid for REtinal Vein Occlusion-Branch Retinal Vein Occlusion (SCORE-BRVO) trial.
Further investigation of the relationship between retinal capillary nonperfusion and neovascular events was performed by treating disc areas of retinal capillary nonperfusion as a time-varying covariate from baseline and throughout the course of the study in the multivariate Cox regression models (retinal capillary nonperfusion preceding neovascularization). In this analysis for the SCORE-BRVO trial, there was a 5% increase in hazard of a neovascular event for every one disc area of increase in retinal capillary nonperfusion with retinal capillary nonperfusion treated as a continuous variable (p=0.0003; Table 7), and a 3.81 times increase in hazard for a neovascular event increase when comparing eyes with ≥ 5.5 disc areas versus < 5.5 disc areas of retinal capillary nonperfusion with the retinal capillary nonperfusion treated as a binary variable (p=0.0007; Table 8). For the SCORE-CRVO trial, despite the lack of statistical significance in baseline predictive analyses, the multivariate analysis with retinal capillary nonperfusion as a time-varying continuous variable showed a trend towards an increase in hazard for a neovascular event for every disc area increase in retinal capillary nonperfusion (HR=1.04, p=0.007; Table 7), although this did not meet our level of statistical significance that has been set lower than the traditional 0.05 due to the performance of multitude of statistical tests in this study.
Table 8.
Summary of 36-month Incidence of Neovascular Events by Retinal Capillary Nonperfusion Status
| Trial | Source | Neovascular event |
Nonperfused eyes* (≥ 5.5 Disc areas) |
Perfused eyes ** (<5.5 Disc areas) |
P-Value (log-rank test) |
||
|---|---|---|---|---|---|---|---|
| Number of events |
36-month cumulative incidence (%) |
Number of events |
36-month cumulative incidence (%) |
||||
| CRVO | Reading Center |
NVD/NVE or PRH/VH |
8 | 24.1 | 10 | 6.1 | 0.0002 |
| NVD/NVE | 6 | 17.2 | 2 | 1.1 | <0.0001 | ||
| PRH/VH | 7 | 22.6 | 8 | 4.9 | 0.0004 | ||
| Clinical Sites |
NVG or NVI | 9 | 23.6 | 11 | 5.9 | <0.0001 | |
| NVG | 6 | 16.6 | 8 | 4.0 | 0.0003 | ||
| NVI | 4 | 10.7 | 3 | 1.9 | 0.0012 | ||
| BRVO | Reading Center |
NVD/NVE or PRH/VH |
16 | 17.8 | 9 | 3.6 | <0.0001 |
| NVD/NVE | 14 | 14.6 | 6 | 2.4 | <0.0001 | ||
| PRH/VH | 7 | 8.7 | 6 | 1.9 | 0.04 | ||
| Clinical Sites |
NVG or NVI | 2 | 2.1 | 6 | 2.6 | 0.82 | |
| NVG | 2 | 2.1 | 5 | 2.2 | 0.99 | ||
| NVI | 0 | 0.0 | 1 | 0.4 | 0.52 | ||
BRVO = branch retinal vein occlusion; CRVO = central retinal vein occlusion; NVD/NVE = Disc or retinal neovascularization; PRH/VH = Pre-retinal or vitreous hemorrhage; NVG = neovascular glaucoma; NVI = iris neovascularization.
Retinal capillary nonperfusion at any time during 24 months of follow-up
Retinal capillary perfusion was present during 24 months of follow-up
Retinal capillary nonperfusion status
The 24-month cumulative incidences of the presence of 5.5 or more disc areas of retinal capillary nonperfusion over follow-up ranged from 11.3% to 15.3% within the 3 treatment groups (P =0.75, Log-rank test) for CRVO eyes and 13.8% to 22.9% within the 3 treatment groups for BRVO eyes (P =0.43, Log-rank test) (data not shown in the tables).
Table 8 shows that, for CRVO eyes at 36 months, 16.6% of the nonperfused eyes (i.e., ≥ 5.5 disc areas of retinal capillary nonperfusion at anytime over followup) versus 4.0% of the perfused eyes (i.e., < 5.5 disc areas of retinal capillary nonperfusion at anytime over follow up) developed NVG (P=0.0003, Log-rank test), and 23.6% of the nonperfused eyes versus 5.9% of the perfused eyes developed NVI or NVG (P <0.0001, Log-rank test). In addition, 17.2% of the nonperfused eyes versus 1.1% of the perfused eyes developed NVD/NVE (P <0.0001, Log-rank test), 22.6% of the nonperfused eyes versus 4.9% of the perfused eyes developed PRH/VH (P =0.0004, Log-rank test), and 24.1% of the nonperfused eyes versus 6.1% of the perfused eyes developed NVD/NVE or PRH/VH (P =0.0002, Log-rank test).
For the BRVO eyes at 36 months, 14.6% of the nonperfused eyes versus 2.4% of the perfused eyes developed NVD/NVE (P <0.0001, Log-rank test), and 17.8% of the nonperfused eyes versus 3.6% of the perfused eyes developed NVD/NVE or PRH/VH (P <0.0001, Log-rank test), Table 7. There were no significant differences in the incidence of PRH/VH, NVI, or NVG between the perfused and nonperfused BRVO eyes.
DISCUSSION
The cumulative incidences of NVD/NVE and PRH/VH in the SCORE Study are based on data derived from standardized FA and FP images analyzed by trained graders at the Reading Center using a consistent methodology).24 This is consistent with the methodology utilized in the Branch Vein Occlusion Study (BVOS).27 In contrast, the cumulative incidences of NVI and NVG in the SCORE Study are based on data obtained at the clinical sites, similar to the strategy utilized in the Central Vein Occlusion Study (CVOS),28 since these ocular conditions are detected optimally in a clinical setting.
Consistent with clinical experience, the 36-month cumulative incidences for NVI, NVG, and NVI or NVG associated with eyes in the SCORE-CRVO trial were significantly higher than those associated with eyes in the SCORE-BRVO trial (P= 0.005, P= 0.006, and P =0.0002, respectively, Log-rank test). However, there were no significant differences in the 36-month cumulative incidences for NVD/NVE, PRH/VH, and NVD/NVE or PRH/VH when comparing CRVO with BRVO eyes.
The cumulative 12 and 36-month incidences of 6.1% and 8.5% respectively, for NVI or NVG in the SCORE-CRVO trial (Table 4) are lower than those reported by the CVOS. In the CVOS, the cumulative 36-month incidence of NVI was 16%. The CVOS also showed that for perfused eyes, 15% at 4 months, and 34% at 36 months developed retinal capillary nonperfusion.28 The cumulative 12 and 36-month incidences of 2.9% and 7.6% of NVD/NVE and PRH/VH in the SCORE-BRVO trial are also lower than the incidences reported in previous studies on BRVO. For instance, Michels and Gass29 found that 24% of their patients with a BRVO developed retinal neovascularization in their study, and the BVOS27 reported the development of retinal neovascularization or vitreous hemorrhage in 22% of the control eyes and 12% of the eyes treated with photocoagulation. Reasons for the lower incidences of neovascular events in the SCORE Study compared to the previous clinical trials cited above are unknown, but there are multiple potential explanations. For instance, the eligibility criteria for the SCORE Study were more restrictive than the CVOS. For the latter trial, eyes with a wide-range of vision and retinal capillary perfusion status were enrolled at baseline, whereas primarily perfused eyes with relatively good vision at baseline (best-corrected Snellen acuities from 20/40 to 20/400) were enrolled into the SCORE Study. As a result, there were likely more CRVO eyes with a better retinal capillary perfusion status in the SCORE Study in comparison to the eyes in the CVOS at baseline. Therefore, the lower cumulative incidences of neovascular events in the SCORE Study than in the CVOS might be expected. In the case of the BVOS, the majority of the eyes were followed for more than 3 years. In contrast, the SCORE Study followed study participants for at most 3 years. Therefore, the higher cumulative incidences of NVE in the BVOS in comparison to the SCORE Study might be expected. In addition, it is known that the baseline characteristics of different study populations for different clinical trials frequently vary from study to study on the same condition. The 14-year temporal disparity between the CVOS and the SCORE-CRVO trial and the 23-year difference between the BVOS and the SCORE-BRVO trial likely further increases the differences in baseline ocular and systemic features and the subsequent risk of neovascular events in the study populations. Regarding the assessment for NVE in both the SCORE-CRVO and BRVO trials, it is conceivable that peripheral retinal neovascular lesions located beyond the standardized photographic fields could have been missed, potentially underestimating the incidence of NVE in the SCORE Study. However, a similar limitation would be associated with the BVOS that utilized a comparable standardized methodology for assessing NVE.
Analyses in this report show the lack of substantial reduction in the cumulative incidences of the neovascular events by intravitreal triamcinolone in comparison to observation/laser photocoagulation for CRVO and BRVO eyes with characteristics similar to the participants enrolled in the SCORE Study when given at the specific doses of this study (1 mg or 4 mg), despite the known anti-angiogenic properties of triamcinolone (Figures 1 and 2; Tables 3 and 6; and Tables 1, 2, 4, and 5 [available at http://aaojournal.org]). The SCORE Study protocol encouraged frequent retreatment. Thus, as a result of the frequent retreatment, the treatment effects were likely maximized and the participants in the study were unlikely to be undertreated. It is noteworthy that the data derived from both the clinical sites as well as the data derived from the Reading Center in the SCORE Study showed no significant differences among the three treatment groups (observation/grid laser photocoagulation, 1 mg triamcinolone, and 4 mg triamcinolone) in the incidences of neovascular events for CRVO and BRVO eyes.
Consistent with previous studies on retinal vein occlusion,27,28 the presence of retinal capillary nonperfusion during the course of the disease was significantly associated with neovascular events in the SCORE Study, particularly for NVI and NVG in the SCORE-CRVO trial, and for NVD/NVE and PRH/VH in the SCORE-BRVO and the SCORE-CRVO trials (Tables 8). Although some of the analyses performed did not establish a temporal relationship between retinal capillary nonperfusion and the development of neovascular events, the most plausible explanation is that increased ischemia in eyes with larger areas of retinal capillary nonperfusion increases the risk for the development of neovascular events. In the SCORE-CRVO trial, 26.9% of the eyes with ≥ 10 disc areas of retinal capillary nonperfusion and 23.6 % of the eyes with ≥ 5.5 disc areas of retinal capillary nonperfusion in comparison to 6.6% of eyes with < 10 disc areas of retinal capillary nonperfusion and 5.9% of eyes with < 5.5 disc areas of retinal capillary nonperfusion, respectively, developed NVI or NVG at 36 months. These results are lower than, but consistent with, the corresponding incidences in the CVOS, which showed that 35% of the study eyes classified as nonperfused (≥ 10 disc areas of retinal capillary nonperfusion) or indeterminate and 10% classified as perfused (< 10 disc areas of retinal capillary nonperfusion) developed NVI, respectively.28 It should be pointed out that the retinal capillary nonperfusion status for the CVOS was established at baseline, while the retinal capillary nonperfusion status for the SCORE Study was assessed throughout the study. Similarly, in the SCORE-BRVO trial, 17.8% of the retinal capillary nonperfused eyes in contrast to only 3.6% of retinal capillary perfused eyes developed NVD/NVE or PRH/VH (Table 8). For those eyes that developed retinal neovascularization or vitreous hemorrhage in the BVOS, 31% of control eyes and 19.2% of treated eyes were retinal capillary nonperfused in comparison to only 11.1% of control eyes and 5.7% of treated eyes that were retinal capillary perfused.27 The possible reasons for the differences are elaborated in detail above.
In the SCORE-CRVO trial, univariate and multivariate Cox regression analyses showed no significant baseline factors predictive of a neovascular event. For the SCORE-BRVO trial, larger disc areas of retinal capillary nonperfusion was the only baseline factor associated with an increased the risk of a neovascular event and was noted in both the univariate and multivariate Cox regression analyses.
Historically, a threshold of 10-disc areas of retinal capillary nonperfusion has been generally accepted as a cut-off value for increased risk of neovascular events associated with CRVO eyes.28 However, a precise threshold of retinal capillary nonperfusion associated with an increased risk for a neovascular event in retinal venous occlusion has not been validated in the context of a multi-center clinical trial. The SCORE Study has confirmed an increased risk of a neovascular events with the historical threshold of 10 disc areas or greater of retinal capillary nonperfusion for eyes with retinal venous occlusion, consistent with similar findings in the CVOS.28 Further, exploratory analyses showed a lack of an absolute cut-off in the disc areas of retinal capillary non-perfusion associated with the risk of neovascular events for eyes with retinal vein occlusion in the SCORE Study (Table 7). Rather, analyses in BRVO eyes showed a linear relationship when analyzing baseline area of retinal capillary nonperfusion as a continuous variable. When examining a wide-range of discrete cut-points of retinal capillary nonperfusion, an increased hazard of a neovascular event was noted for retinal capillary nonperfusion as low as 1 disc area, far below the historical threshold of 10 disc areas, and its highest point was observed at approximately 5.5 disc areas (Figure 3, Table 7). Using this estimated peak cut-point, the baseline predictor analysis showed a 5.9 times increase in the hazard of a neovascular event for an eye with baseline retinal capillary nonperfusion of 5.5 disc areas or greater of retinal capillary nonperfusion in comparison to an eye with less than 5.5 disc areas of retinal capillary nonperfusion (p=0.004), (Figure 3, Table 7). Thus, our study shows that the peak of the disc areas of retinal capillary nonperfusion that may serve as a useful cut-off point to distinguish between low and high risks of neovascularization is likely below the historical value of 10 disc areas of retinal capillary nonperfusion for the BRVO eyes, although the wide-range on the confidence intervals associated with the cut-points (Figure 3) limits the certainty of this conclusion.
Regarding the SCORE-CRVO trial, the baseline predictor analysis failed to show an increased risk in retinal capillary nonperfusion for the threshold of 5.5 disc areas and with retinal capillary nonperfusion as a continuous baseline measure. Although the precise reason for this lack of significance in baseline retinal capillary nonperfusion is unknown, it may be related to obscuration of the full extent of the area of retinal capillary nonperfusion by dense retinal hemorrhage at baseline for CRVO eyes as pointed out in a previously published paper of the SCORE Study.26 Thus, dense retinal hemorrhage at baseline prevented accurate assessment of the disc areas of retinal capillary nonperfusion as a baseline predictive factor for a neovascular event in the SCORE-CRVO trial. However, in the Cox regression analysis treating retinal capillary nonperfusion as a continuous time-varying covariate beyond baseline, there was a 4% increase in the risk for a neovascular event for every one disc area of increase in retinal capillary nonperfusion in the SCORE-CRVO trial, a finding that was of borderline statistical significance (p=0.007), (Table 7). This finding may be attributable to the gradual clearance of the dense retinal hemorrhage during subsequent follow-up visits that allowed a more accurate assessment of the full extent of the retinal capillary nonperfusion for these eyes. When analyzing retinal capillary nonperfusion as a binary variable based on any retinal capillary nonperfusion over follow up thereby removing the temporal relationship between retinal capillary nonperfusion and neovascularization (retinal capillary nonperfusion preceding neovascularization), the threshold of 5.5 disc areas or greater of retinal capillary nonperfusion was also found to be highly correlated with multiple neovascular events for the SCORE-CRVO trial, similar to the same Cox multivariate regression analysis for the SCORE-BRVO trial (Table 8).
In conclusion, the results of this report have implications for future planning of prospective clinical trials for investigating neovascular events associated with retinal vein occlusion. For such trials, data analyses involving both clinical sites and a centralized reading center are indicated, since both are important sources for capturing data for different neovascular events. Despite the anti-angiogenic properties of triamcinolone, there is a lack of significant reduction in the incidences of neovascular events associated with intravitreal triamcinolone therapy at the doses utilized in the SCORE Study. For eyes with either CRVO or BRVO, treatment group assignment did not have an effect on the changes in retinal capillary perfusion status from baseline. Lastly, the SCORE Study has shown a linear relationship between the risk of neovascularization and retinal capillary nonperfusion in a continuous fashion, so that the larger the disc areas of retinal capillary nonperfusion, the higher the risk of neovascularization. Such a correlation was found to be applicable to a wide-range of disc areas of retinal capillary nonperfusion at baseline below the historical threshold of 10 disc areas and highest at 5.5 disc areas or greater in retinal capillary nonperfusion in the SCORE-BRVO trial. These results are not as compelling in CRVO eyes, possibly due to obscuration of retinal capillary details by dense hemorrhage at baseline for accurate assessment of capillary status. This may have implications for the planning of future clinical trials in retinal vein occlusion.
PRÉCIS.
There were no differences in incidence of neovascular events and perfusion status among standard care and intravitreal triamcinolone groups; increased risk of neovascularization was noted below the historical threshold of 10 disc areas of nonperfusion.
Supplementary Material
Acknowledgments
Supported by the National Eye Institute (National Institutes of Health, Department of Health and Human Services) grants 5U10EY014351, 5U10EY014352, and 5U10EY014404. Support also provided in part by Allergan, Inc through donation of investigational drug and partial funding of site monitoring visits and secondary data analyses.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors has a financial interest in the subject matter of this article.
REFERENCES
- 1.Branch Vein Occlusion Study Group Argon laser photocoagulation for macular edema in branch vein occlusion. Am J Ophthalmol. 1984;98:271–82. doi: 10.1016/0002-9394(84)90316-7. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Smith W, Chang A. Prevalence and associations of retinal vein occlusion in Australia: the Blue Mountains Eye Study. Arch Ophthalmol. 1996;114:1243–7. doi: 10.1001/archopht.1996.01100140443012. [DOI] [PubMed] [Google Scholar]
- 3.Klein R, Moss SE, Meuer SM, Klein BE. The 15-year cumulative incidence of retinal vein occlusion: the Beaver Dam Eye Study. Arch Ophthalmol. 2008;126:513–8. doi: 10.1001/archopht.126.4.513. [DOI] [PubMed] [Google Scholar]
- 4.Klein R, Klein BE, Lee KE, et al. Changes in visual acuity in a population over a 15-year period: the Beaver Dam Eye Study. Am J Ophthalmol. 2006;142:539–49. doi: 10.1016/j.ajo.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 5.Scott IU, VanVeldhuisen PC, Oden NL, et al. SCORE Study Investigator Group SCORE Study report 1: Baseline associations between central retinal thickness and visual acuity in patients with retinal vein occlusion. Ophthalmology. 2009;116:504–12. doi: 10.1016/j.ophtha.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study report 5. Arch Ophthalmol. 2009;127:1101–14. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.SCORE Study Research Group A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with standard care to treat vision loss associated with macular edema secondary to branch retinal vein occlusion: the Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study report 6. Arch Ophthalmol. 2009;127:1115–28. doi: 10.1001/archophthalmol.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott IU, Ip MS. It’s time for a clinical trial to investigate intravitreal triamcinolone for macular edema due to retinal vein occlusion: the SCORE Study [letter] Arch Ophthalmol. 2005;123:581–2. doi: 10.1001/archopht.123.4.581. [DOI] [PubMed] [Google Scholar]
- 9.Greenberg PB, Martidis A, Rogers AH, et al. Intravitreal triamcinolone acetonide for macular oedema due to central retinal vein occlusion [letter] Br J Ophthalmol. 2002;86:247–8. doi: 10.1136/bjo.86.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ip MS, Gottlieb JL, Kahana A, et al. Intravitreal triamcinolone for the treatment of macular edema associated with central retinal vein occlusion. Arch Ophthalmol. 2004;122:1131–6. doi: 10.1001/archopht.122.8.1131. [DOI] [PubMed] [Google Scholar]
- 11.Cekic O, Chang S, Tseng JJ, et al. Intravitreal triamcinolone injection for treatment of macular edema secondary to branch retinal vein occlusion. Retina. 2005;25:851–5. doi: 10.1097/00006982-200510000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Degenring RF, Kamppeter B, Kreissig I, Jonas JB. Morphological and functional changes after intravitreal triamcinolone acetonide for retinal vein occlusion. Acta Ophthalmol Scand. 2003;81:548–50. [PubMed] [Google Scholar]
- 13.Brooks HL, Jr, Caballero S, Jr, Newell CK, et al. Vitreous levels of vascular endothelial growth factor and stromal-derived factor 1 in patients with diabetic retinopathy and cystoid macular edema before and after intraocular injection of triamcinolone. Arch Ophthalmol. 2004;122:1801–7. doi: 10.1001/archopht.122.12.1801. [DOI] [PubMed] [Google Scholar]
- 14.Butler JM, Guthrie SM, Koc M, et al. SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005;115:86–93. doi: 10.1172/JCI22869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lima e Silva R, Shen J, Hackett SF, et al. The SDF-1/CXCR4 ligand/receptor pair is an important contributor to several types of ocular neovascularization. FASEB J. 2007;21:3219–30. doi: 10.1096/fj.06-7359com. [DOI] [PubMed] [Google Scholar]
- 16.Arimura N, Otsuka H, Yamakiri K, et al. Vitreous mediators after intravitreal bevacizumab or triamcinolone acetonide in eyes with proliferative diabetic retinopathy. Ophthalmology. 2009;116:921–6. doi: 10.1016/j.ophtha.2008.12.024. [DOI] [PubMed] [Google Scholar]
- 17.Grant MB, May WS, Caballero S, et al. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–12. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 18.Ratajczak MZ, Zuba-Surma E, Kucia M, et al. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20:1915–24. doi: 10.1038/sj.leu.2404357. [DOI] [PubMed] [Google Scholar]
- 19.Ki-I Y, Arimura N, Noda Y, et al. Stromal-derived factor-1 and inflammatory cytokines in retinal vein occlusion. Curr Eye Res. 2007;32:1065–72. doi: 10.1080/02713680701758727. [DOI] [PubMed] [Google Scholar]
- 20.Mukaida N, Morita M, Ishikawa Y, et al. Novel mechanism of glucocorticoid-mediated gene repression: nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. J Biol Chem. 1994;269:13289–95. [PubMed] [Google Scholar]
- 21.Nie M, Corbett L, Knox AJ, Pang L. Differential regulation of chemokine expression by peroxisome proliferator-activated receptor gamma agonists: interactions with glucocorticoids and beta2-agonists. J Biol Chem. 2005;280:2550–61. doi: 10.1074/jbc.M410616200. [DOI] [PubMed] [Google Scholar]
- 22.Manual of Policies and Procedures (MOPP) for the Standard Care vs. COrticosteroid for REtinal Vein Occlusion (SCORE) Study, Version 4.0. National Eye Institute; Bethesda, MD: 2008. pp. 4-1–4-8.pp. 6-1–6-10. NTIS PB2008-106870. AQ: provide specific inclusive pagination for material being cited from this report
- 23.Protocol for the Standard Care vs. COrticosteroid for REtinal Vein Occlusion (SCORE) Study, Version 7.0. National Eye Institute; Bethesda, MD: 2008. pp. 24–47.pp. 54–65. NTIS PB2008-113743. AQ: provide specific inclusive pagination for material being cited from this report
- 24.Blodi BA, Domalpally A, Scott IU, et al. SCORE Study Research Group Standard Care vs. Corticosteroid for Retinal Vein Occlusion (SCORE) Study system for evaluation of stereoscopic color fundus photographs and fluorescein angiograms: SCORE Study report 9. Arch Ophthalmol. 2010;128:1140–5. doi: 10.1001/archophthalmol.2010.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Early Treatment Diabetic Retinopathy Study Research Group Classification of diabetic retinopathy from fluorescein angiograms: ETDRS report number 11. Ophthalmology. 1991;98:807–22. [PubMed] [Google Scholar]
- 26.Ip MS, Oden NL, Scott IU, et al. SCORE Study Investigator Group SCORE Study report 3: study design and baseline characteristics. Ophthalmology. 2009;116:1770–7. doi: 10.1016/j.ophtha.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Branch Vein Occlusion Study Group Argon laser scatter photocoagulation for prevention of neovascularization and vitreous hemorrhage in branch vein occlusion: a randomized clinical trial. Arch Ophthalmol. 1986;104:34–41. doi: 10.1001/archopht.1986.01050130044017. [DOI] [PubMed] [Google Scholar]
- 28.Central Vein Occlusion Study Group Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115:486–91. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 29.Michels RG, Gass JD. The natural course of retinal branch vein obstruction. Trans Am Acad Ophthalmol Otolaryngol. 1974;78:OP166–77. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.