Abstract
Purpose
To determine whether an association exists between various components of metabolic syndrome (diabetes mellitus (DM), systemic arterial hypertension (HTN), hyperlipidemia, and obesity) and open-angle glaucoma (OAG) in a large, diverse group of individuals throughout the United States.
Design
Longitudinal cohort study.
Participants
All beneficiaries age ≥40 years continuously enrolled in a managed care network who had ≥1 visit to an eye care provider were identified from 2001–2007.
Methods
Billing codes were used to identify individuals with OAG and those with components of metabolic syndrome. Cox regression was used to determine the hazard of developing OAG in enrollees with individual components or combinations of components of metabolic syndrome, with adjustment for sociodemographic factors, systemic medical conditions, and other ocular diseases.
Main Outcome Measures
Hazard of developing OAG.
Results
Of the 2,182,315 enrollees who met inclusion criteria, 54,558 (2.5%) had OAG. After adjustment for confounding factors, those with DM (hazard ratio (HR)=1.35 [95% confidence interval (CI): 1.21–1.50]) or HTN (HR=1.17 [95% CI: 1.13–1.22]) alone, or in combination, (HR=1.48 [95% CI: 1.39–1.58]) had an increased hazard of developing OAG relative to persons with neither of these conditions. By contrast, persons with hyperlipidemia alone had a 5% decreased hazard of OAG (HR=0.95 [95% CI: 0.91–0.98]). Comorbid hyperlipidemia attenuated the increased hazard between HTN (HR=1.09 [95% CI 1.05–1.12]) or DM (HR=1.13 [95% CI 1.05–1.21]) and OAG.
Conclusion
Given the increasing prevalence of metabolic disorders in the United States, this study furthers our understanding of risk factors associated with OAG and helps identify persons who may be at risk for this condition.
Introduction
Open angle glaucoma (OAG) is a leading cause of irreversible blindness worldwide.1 In the United States (US) alone, there are over 2.2 million individuals with this disease. Given the aging of the US population, estimates of glaucoma are expected to rise in the coming decades.1 Since OAG is often an asymptomatic disease until late in the course, understanding the risk factors associated with OAG development or progression will help clinicians identify which patients would most benefit from screening and careful monitoring for the disease.
Known risk factors for OAG include elevated intraocular pressure (IOP), positive family history of OAG, increased age, and non-white race. There is conflicting evidence in the literature as to whether components of metabolic syndrome, including obesity, hypertension (HTN), diabetes mellitus (DM), and hyperlipidemia, increase or decrease the risk of OAG. Several large population-based studies have demonstrated that HTN2–8 and DM7–14 are each independently associated with OAG. Other studies have shown no association between DM and OAG15–20 or between HTN and OAG.16,17,19,20 Oh and colleagues demonstrated an association between hyperlipidemia and elevated IOP,21 and Jaen-Diaz and colleagues have demonstrated an association between hyperlipidemia and OAG.22 Many Americans have multiple components of metabolic syndrome.23 Thus, interpreting the relationship between metabolic syndrome and OAG from the majority of existing studies in the literature can be challenging because many do not adequately adjust for how one metabolic syndrome component may be affecting the contribution of other components to the risk of developing OAG.
Data from the 1988–2000 National Health and Nutrition Examination Survey (NHANES), a cross-sectional health survey, showed that approximately 22% of Americans over the age of 18 met criteria for metabolic syndrome.23 Given the significant rise in HTN, DM, hyperlipidemia, and obesity in recent years in the US, and the fact that even children and adolescents are affected by the obesity epidemic,24 it is essential for eye care providers to obtain a better understanding of the relationship between metabolic syndrome and chronic eye diseases including OAG.
The purpose of this study is to assess the relationship between the various components of the metabolic syndrome and OAG in a large, diverse sample of individuals enrolled in a managed care network throughout the United States. This study seeks to understand whether certain components, singly, or in combination, impact the likelihood of developing OAG, after adjustment for sociodemographic factors, ocular and other systemic medical conditions.
Methods
Data Source
The i3 InVision Data Mart database (Ingenix, Eden Prairie, MN) contains detailed records of all beneficiaries in a national managed care network throughout the United States. The dataset contains all individuals with one or more International Classification of Diseases, Ninth Revision (ICD-9CM)25 codes for eye-related diagnoses (360–379.9), one or more Current Procedural Terminology (CPT)26 codes for any eye-related visits, diagnostic, or therapeutic procedures (65091–68899 or 92002–92499), or any other claim adjudicated by an ophthalmologist or optometrist from January 1, 2001 through December 31, 2007. For each enrollee, we had access to all medical claims for ocular and non-ocular conditions and sociodemographic information including age, sex, race, education level and household net worth.
Participants and Sample Selection
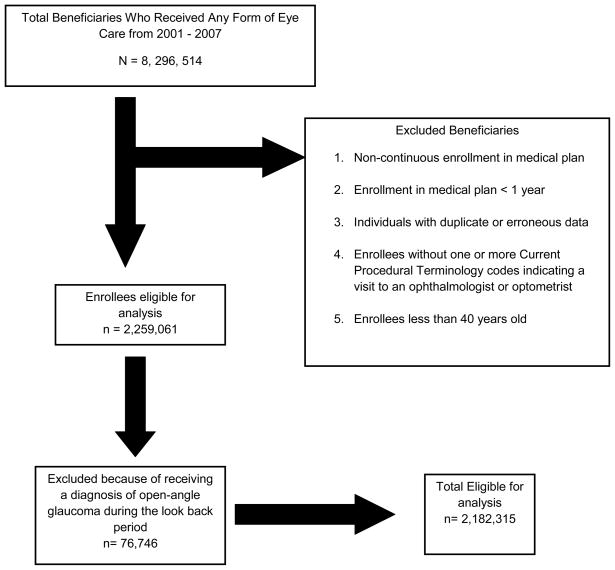
Individuals were included in the analysis if they met the following criteria: continuous enrollment in the medical plan for at least one year, one or more visits to an eye care provider (ophthalmologist or optometrist) and age ≥ 40 years. (Figure 1) Individuals were excluded if they received an OAG diagnosis during the first year they were enrolled in the plan, in order to exclude non-incident cases. Beneficiaries were identified as having OAG if they had one or more of the following ICD-9CM codes: 365.1, 365.10, 365.11, 365.12, and 365.15. Those coded with only suspected glaucoma who never received a diagnosis of OAG were not included. ICD-9CM diagnosis codes were used to identify individuals with the following components of metabolic syndrome: HTN, DM, hyperlipidemia, and obesity (Table 1, available at http://aaojournal.org).
Figure 1.
Selection of Eligible Enrollees for the Analysis
Table 1.
International Classification of Disease Codes
| Condition | ICD-9CM Codes |
|---|---|
| Age-related macular degeneration | 362.50, 362.51, 362.52, 362.57 |
| Cataract | 366, 366.0, 366.00, 366.01, 366.02, 366.03, 366.04, 366.09, 366.1, 366.10, 366.12, 366.13, 366.14, 366.15, 366.16, 366.17, 366.18, 366.19, 366.41, 366.45 |
| Diabetes mellitus | 250.0, 250.00, 250.01, 250.02, 250.03, 250.1, 250.10, 250.11, 250.12, 250.13, 250.2, 250.20, 250.21, 250.22, 250.23, 250.3, 250.30, 250.31, 250.32, 250.33, 250.4, 250.40, 250.41, 250.42, 250.43, 250.5, 250.50, 250.51, 250.52, 250.53, 250.5, 250.50, 250.51, 250.52, 250.53, 250.6, 250.60, 250.61, 250.62, 250.63, 250.7, 250.70, 250.71, 250.72, 250.73, 250.8, 250.80, 250.81, 250.82, 250.83, 250.9, 250.90, 250.91, 250.92, 250.93, 362.01, 362.92, 362.03, 362.04, 362.05, 362.06, 362.07 |
| Diabetic retinopathy | 362.01, 362.92, 362.03, 362.04, 362.05, 362.06, 362.07 |
| Diabetic Complications | 250.4, 250.40, 250.41, 250.42, 250.43, 250.5, 250.50, 250.51, 250.52, 250.53, 250.6, 250.60, 250.61, 250.62, 250.63, 250.7, 250.70, 250.71, 250.72, 250.73 |
| Hyperlipidemia | 272, 272.0, 272.1, 272.2, 272.3, 272.4, 272.5, 272.6, 272.7, 272.8, 272.9 |
| Hypertension | 401, 401.0, 401.1, 401.9, 405, 405.0, 405.1, 405.01, 405.09, 405.11, 405.19, 405.9, 405.91, 405.99, 362.11, 402, 402.0, 402.00, 402.01, 402.1, 402.10, 402.11, 402.9, 402.90, 402.91, 403, 403.0, 403.00, 403.01, 403.1, 403.10, 403.11, 403.9, 403.90, 403.91, 404.0, 404.00, 404.01, 404.02, 404.03, 404.1, 404.10, 404.11, 404.12, 404.13, 404.9, 404.90, 404.91, 404.92, 404.93 |
| Hypertensive Complications | 362.11, 402, 402.0, 402.00, 402.01, 402.1, 402.10, 402.11, 402.9, 402.90, 402.91, 403, 403.0, 403.00, 403.01, 403.1, 403.10, 403.11, 403.9, 403.90, 403.91, 404.0, 404.00, 404.01, 404.02, 404.03, 404.1, 404.10, 404.11, 404.12, 404.13, 404.9, 404.90, 404.91, 404.92, 404.93 |
| Hypotension | 458, 458.0, 458.1, 485.2, 485.21, 458.29, 458.8, 458.9 |
| Migraine | 346, 346.0, 346.00, 346.01, 346.1, 346.10, 346.11, 346.2, 346.20, 346.21, 346.8, 346.80, 346.81, 346.9, 346.90, 346.91 |
| Narrow-angle glaucoma | 365.02, 365.2, 365.21, 365.22, 365.23, 365.61, 365.73 |
| Obesity | 278.0, 278.00, 278.01, 278.02 |
| Open-angle glaucoma | 365.1, 365.10, 365.11, 365.12, 365.15 |
| Pseudophakia or aphakia | V43.1, 379.3, 379.31 |
| Sleep apnea syndrome | 327.2, 327.20, 327.21, 327.23, 327.27, 327.29, 780.51, 780.53, 780.57 |
ICD-9CM-International Classification of Diseases, Ninth Revision, Clinical Modification
Analyses
Statistical analyses were performed by using SAS software, version 9.2 (SAS Institute, Cary, NC). Participant characteristics were summarized for the entire sample using means and standard deviations for continuous variables and frequencies and percentages for categorical variables. Cox regression with delayed entry was used to estimate the hazard of developing OAG associated with each of the metabolic syndrome components. We used the first year each beneficiary was enrolled in the medical plan as a look back period. Individuals who received one or more OAG diagnoses during this look back period were excluded from the analysis since we were unable to determine with any certainty whether they had the condition before enrollment in the medical plan or whether they were first diagnosed with this condition during the look back period. All beneficiaries were followed in the model from the index date (one year after entry into the plan) until they either developed the outcome of interest (OAG), or were censored. Censoring occurred at age of disenrollment or end of study period (December 31, 2007). Initially, single-variable models were run to test potential predictors individually. Multivariable models were adjusted for age (the time axis), sex, race, education level, household net worth, region of residence at the time of medical plan enrollment, cataract, pseudophakia or aphakia, macular degeneration, diabetic retinopathy, systemic hypotension, sleep apnea syndrome, migraine headache, and the Charlson comorbidity index (a measure of overall health)27. Additional Cox regression models were performed to assess whether the severity of DM and HTN affected the association between each of these conditions and OAG, adjusting for the above-mentioned covariates. Individuals with DM and HTN were each stratified into two groups, those with and without complications, based on ICD-9 billing codes. For all analyses, p-values of < 0.05 were considered statistically significant. Finally, a sensitivity analysis was performed to determine whether the association between the main predictor variables and OAG were affected if we changed our definition of OAG from requiring at least one ICD-9 code for OAG to requiring at least two codes for OAG.
Since all the data were de-identified, the University of Michigan determined that this study was exempt from requiring Institutional Review Board approval.
Results
A total of 2,182,315 beneficiaries met the study inclusion criteria (Figure 1). Among those in the study, the mean (± standard deviation [SD]) age was 54.5 (± 10.3) years. There were 1,238,266 females (57%) and the racial distribution included 1,485,866 whites (86.7%), 73,084 blacks (4.3%), 96,293 Latinos (5.6%), 42,650 Asian Americans (2.5%), and 15,488 individuals of other races (0.9%) (Table 2). Overall, 55,090 individuals (2.5%) received at least one OAG diagnosis during their time in the medical plan. A total of 605,322 enrollees (27.7%) had no HTN, DM or hyperlipidemia and 1,576,993 enrollees (72.3%) had at least one of these conditions. There were 22,500 individuals (1.0%) with DM alone, 198,398 enrollees with HTN alone (9.1%), and 378,771 individuals (17.4%) with hyperlipidemia alone. Two of these conditions were present in 633,709 individuals (29.0%) and 343,615 beneficiaries (15.8%) had all 3 conditions. 202,816 beneficiaries (9.3%) were considered obese (Table 3).
Table 2.
Demographic Characteristics of Study Population
| Demographic Characteristics | With OAG | Without OAG |
|---|---|---|
| Study Population (N) | 55,090 | 2,127,225 |
| Age | ||
| Mean (SD) | 59.7(11.2) | 54.3 (10.2) |
| Range | 40–87 | 40–87 |
| Sex | ||
| Male, N (%) | 25,644 (46.6%) | 918,185 (43.2%) |
| Female, N (%) | 29,442 (53.4%) | 1,208,824 (56.8%) |
| Race | ||
| White, N (%) | 36,705 (79.8%) | 1,449,461 (86.9%) |
| Black, N (%) | 4,319 (9.4%) | 68,765 (4.1%) |
| Latino, N (%) | 3,142 (6.8%) | 93,151 (5.6%) |
| Asian-American, N (%) | 1,422 (3.1%) | 41,228 (2.5%) |
| Other, N (%) | 417 (0.9%) | 15,071 (0.9%) |
OAG, Open-Angle Glaucoma; N, Number; %, Percent; SD, Standard Deviation
Table 3.
Prevalence of Open-Angle Glaucoma Stratified by Metabolic Syndrome Component
| Metabolic Syndrome Component | Beneficiaries with OAG N (%) | Beneficiaries without OAG N (%) | Total Beneficiaries with Each Metabolic Syndrome Component, N | Proportion of Total Beneficiaries with Each Metabolic Syndrome Component, % |
|---|---|---|---|---|
| None of the three conditions | 8538 (1.4%) | 596.784 (98.6%) | 605,322 | 27.7% |
| HTN Only | 5,330 (2.7%) | 193,068 (97.3%) | 198,398 | 9.1% |
| DM Only | 506 (2.2%) | 21,994 (97.8%) | 22,500 | 1.0% |
| Hyperlipidemia Only | 7,023 (1.9%) | 371,748 (98.1%) | 378,771 | 17.4% |
| DM + HTN | 1,699 (3.8%) | 42,523 (96.2%) | 44,222 | 2.0% |
| DM + Hyperlipidemia | 1,320 (2.4%) | 53,070 (97.6%) | 54,390 | 2.5% |
| HTN + Hyperlipidemia | 16,651 (3.1%) | 518,446 (96.9%) | 535,097 | 24.5% |
| DM + HTN + Hyperlipidemia | 14,023 (4.1%) | 329,592 (95.9%) | 343,615 | 15.8% |
| Total beneficiaries with ≥ 1 component of metabolic syndrome | 46,552 (3.0%) | 1,530,441 (97.0%) | 1,576,993 | 72.3% |
| Obesity | 6,283 (3.1%) | 196,533 (96.9%) | 202,816 | 9.3% |
OAG = Open-Angle Glaucoma; HTN = Hypertension; DM = Diabetes Mellitus.
Association Between Components of Metabolic Syndrome and OAG
Table 3 shows the frequency of OAG among persons with HTN, DM, hyperlipidemia, as well as different combinations of these conditions. Before adjustment for covariates, those with DM or HTN were found to have a significantly increased hazard of developing OAG while those with hyperlipidemia had a significantly decreased hazard of developing OAG. After adjustment for sociodemographic factors, other ocular and systemic conditions, obesity, and general health status, individuals with DM alone demonstrated a 35% increased hazard of developing OAG (adjusted hazard ratio [HR]=1.35 [95% confidence interval (CI) 1.21–1.50]), and those with HTN alone had a 17% increased hazard of developing OAG (adjusted HR=1.17 [95% CI 1.13–1.22]). For persons with HTN along with DM, the hazard was greater than the hazard of each of these conditions individually (adjusted HR=1.48 [95%CI 1.39–1.58]). Individuals with DM, whether alone or in combination with any other component of metabolic syndrome, had an increased hazard of developing OAG relative to those who had any other combination of metabolic syndrome components. In contrast, those with hyperlipidemia alone showed a 5% decreased hazard of developing OAG (adjusted HR=0.95 [95% CI 0.91–0.98]), and hyperlipidemia attenuated the effect of DM by 17% (adjusted HR=0.83 [95% CI 0.74–0.94]) and of HTN by 7% (adjusted HR=0.93 [95% CI 0.89–0.96]) on the hazard of developing OAG. Persons with all 3 of these conditions had a 26% increased hazard of developing OAG (adjusted HR=1.26 [95% CI 1.22–1.31]) relative to those with none of these conditions. (Table 4 and Figure 2)
Table 4.
Unadjusted and Adjusted Hazard Ratios of Developing Open-Angle Glaucoma
| Metabolic Syndrome Components | Unadjusted Hazard Ratio of developing OAG [95% Confidence Interval] | P-value | Adjusted Hazard Ratio of developing OAG [95% Confidence Interval] | P-value |
|---|---|---|---|---|
| HTN only | 1.26 [1.22–1.31] | <0.0001 | 1.17 [1.13–1.22] | <0.0001 |
| DM only | 1.47 [1.34–1.61] | <0.0001 | 1.35 [1.21–1.50] | <0.0001 |
| Hyperlipidemia only | 0.94 [0.91–0.98] | 0.0005 | 0.95 [0.91–0.98] | 0.0038 |
| Obesity | 1.14 [1.11–1.17] | <0.0001 | ||
| Obesity/Sex Interaction | ||||
| Female & Obesea | 1.06 [1.02–1.10] | 0.0107 | ||
| Male & Obeseb | 0.98 [0.94–1.03] | 0.5080 | ||
| DM + HTN | 1.79 [1.69–1.89] | <0.0001 | 1.48 [1.39–1.58] | <0.0001 |
| DM + Hyperlipidemia | 1.28 [1.21–1.36] | <0.0001 | 1.13 [1.05–1.21] | 0.0008 |
| HTN + Hyperlipidemia | 1.15 [1.12–1.18] | <0.0001 | 1.09 [1.05–1.12] | <0.0001 |
| DM + HTN + Hyperlipidemia | 1.52 [1.48–1.57] | <0.0001 | 1.26 [1.22–1.31] | <0.0001 |
Multivariable analysis adjusted for age, sex, race, education level, household net worth, region of residence at the time of enrollment in the medical plan, cataract, pseudophakia or aphakia, macular degeneration, diabetic retinopathy, systemic hypotension, sleep apnea, migraine headache, the Charlson comorbidity index, and each of the other metabolic syndrome variables.
Reference group, non-obese females
Reference group, non-obese males
OAG = Open-Angle Glaucoma; HTN = Hypertension; DM = Diabetes Mellitus.
HTN = Hypertension; DM = Diabetes Mellitus; OAG = Open-Angle Glaucoma; ICD-9 CM, International Classification of Disease Codes, Ninth Edition, Clinical Modification
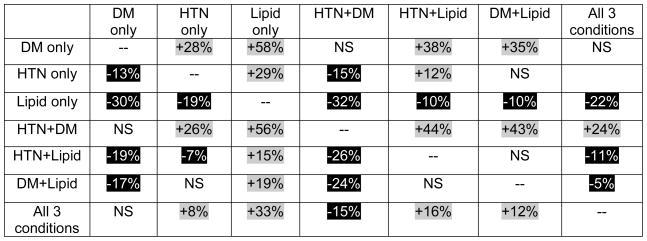
Figure 2.
The hazard of developing open-angle glaucoma, comparing various components of metabolic syndrome. Comparisons of different components of metabolic syndrome with one another. In the table, a given cell reports the percentage increased or decreased hazard of developing OAG in persons with the metabolic syndrome component(s) listed on the left as compared with individuals who have the metabolic syndrome component(s) listed above. For example, individuals with hypertension only have a 29% increased hazard of developing OAG relative to those with hyperlipidemia only.
Gray highlighting depicts increased hazard; Black highlighting depicts decreased hazard. HTN = Hypertension; DM = Diabetes Mellitus; Lipid = Hyperlipidemia; NS = non-significant.
Severity of Hypertension or Diabetes Mellitus on Hazard of Developing OAG
Next, we created a separate Cox regression model to assess the relationship between severity of HTN or DM on the hazard of developing OAG, with adjustment for confounding factors. Compared with those who do not have HTN, individuals with uncomplicated HTN had a 15% (adjusted HR=1.15 [95% CI 1.12–1.18]) increased hazard of developing OAG and those with complications caused by HTN (hypertensive heart disease, nephropathy, retinopathy) had a 19% (adjusted HR=1.19 [95% CI 1.15–1.24]) increased hazard of developing OAG. When comparing persons with HTN without complications to those who have complications from HTN, those with complicated HTN had a 4% (adjusted HR=1.04 [95% CI 1.01–1.07]) increased hazard of developing OAG. Relative to individuals without DM, persons with DM without complications had an 18% (adjusted HR=1.18 [95% CI 1.15–1.22]) increased hazard of developing OAG and those with complications caused by DM (retinopathy, neuropathy, nephropathy) had a 17% (adjusted HR=1.17 [95% CI 1.12–1.23]) increased hazard of developing OAG. No significant difference was found in the hazard of developing OAG when comparing those with DM who had or did not have complications with one another (HR=0.99 [95% CI 0.95–1.04]) (Table 5, available at http://aaojournal.org).
Table 5.
Severity of Metabolic Syndrome Components on the Hazard of Developing Open-Angle Glaucoma
| Severity of DM or HTN | Adjusted Hazard Ratio of Developing OAG | P-Value |
|---|---|---|
| [95% Confidence Interval] | ||
| HTN | ||
| Complicated HTNa | 1.19 [1.15–1.24] | <0.0001 |
| Uncomplicated HTNa | 1.15 [1.12–1.18] | <0.0001 |
| Complicated HTNb | 1.04 [1.01–1.07] | 0.009 |
| DM | ||
| Complicated DMc | 1.17[1.12–1.23] | <0.0001 |
| Uncomplicated DMc | 1.18 [1.12–1.23] | <0.0001 |
| Complicated DMd | 0.99 [0.95–1.04] | 0.700 |
Multivariable analysis adjusted for age, sex, race, education level, household net worth, region of residence at the time of enrollment in the medical plan, cataract, pseudophakia or aphakia, macular degeneration, diabetic retinopathy, systemic hypotension, sleep apnea, migraine headache, the Charlson comorbidity index, and each of the other metabolic syndrome variables
Reference group, beneficiaries without hypertension
Reference group, beneficiaries with uncomplicated hypertension
Reference group, beneficiaries without diabetes mellitus
Reference group, beneficiaries with uncomplicated diabetes mellitus
Complicated hypertension was defined by ICD-9 CM codes for such diseases such as hypertensive nephropathy, retinopathy, and heart disease
Complicated diabetes was defined by ICD-9 CM codes for such diseases as diabetic retinopathy, neuropathy and nephropathy
HTN = Hypertension; DM = Diabetes Mellitus; OAG = Open-Angle Glaucoma; ICD-9 CM, International Classification of Disease Codes, Ninth Edition, Clinical Modification
Association Between Obesity and OAG
The frequency of OAG among obese individuals (3.1%) was significantly higher than that among non-obese individuals (2.5%) (p<0.0001). In the univariable model, obese individuals had a 14% increased hazard of OAG (unadjusted HR=1.14, [95% CI 1.11–1.17]) relative to non-obese individuals. In the multivariable Cox regression model, when assessing the relationship between obesity and OAG, an interaction with sex was observed. After adjustment for other covariates, compared to non-obese females, obese females had a 6% increased hazard of developing OAG (adjusted HR=1.06 [95% CI 1.02–1.10]). Compared with non-obese males, males who were obese had no significant increased hazard of developing OAG (adjusted HR=0.98 [95% CI 0.94–1.03]) (Table 4).
Impact of Demographic and Socioeconomic Factors on the Relationship Between Metabolic Syndrome and OAG
All racial minorities had an increased hazard of developing OAG compared with whites. Blacks had a 119%% increased hazard (adjusted HR= 2.19 [95% CI 2.11–2.27], Latinos had a 38% increased hazard (adjusted HR= 1.38 [95% CI 1.33–1.44], and Asian-Americans had a 51% increased hazard (adjusted HR=1.51 [95% CI 1.42–1.60] of developing OAG relative to whites. (Table 6)
Table 6.
Impact of Race, Education Level and Household Net Worth on Hazard of Developing Open-Angle Glaucoma
| N | Hazard Ratio | 95% CI | |
|---|---|---|---|
| Race | |||
| White | 1,485,866 | Reference | |
| Black | 73,084 | 2.19 | 2.11–2.27 |
| Latino | 96,293 | 1.38 | 1.33–1.44 |
| Asian American | 42,650 | 1.51 | 1.42–1.60 |
| Other | 15,488 | 1.24 | 1.12–1.37 |
| Net Worth | |||
| ≤$25,000 | 130,220 | Reference | |
| $25,000–49,000 | 55,286 | 0.97 | 0.92–1.04 |
| $50,000–74,000 | 53,226 | 0.94 | 0.88–1.00 |
| $75,000–99,000 | 71,496 | 0.91 | 0.86–0.97 |
| $100,000–124,000 | 144,433 | 0.88 | 0.84–0.92 |
| $125,000–249,000 | 285,307 | 0.85 | 0.82–0.89 |
| $250,000–499,000 | 523,825 | 0.82 | 0.79–0.85 |
| $500,000–749,000 | 247,628 | 0.78 | 0.75–0.82 |
| $750,000–999,000 | 96,307 | 0.80 | 0.76–0.85 |
| ≥$1,000,000 | 139,963 | 0.81 | 0.77–0.86 |
| Education | |||
| Less than high school | 23,589 | Reference | |
| High School diploma | 605,810 | 0.85 | 0.79–0.91 |
| Some college | 699,410 | 0.83 | 0.78–0.89 |
| College diploma | 485,790 | 0.82 | 0.76–0.88 |
| Advanced degree | 5083 | 0.68 | 0.54–0.85 |
Multivariable analysis adjusted for each of the components of metabolic syndrome, age, sex, race, education level, household net worth, region of residence at the time of enrollment in the medical plan, cataract, pseudophakia or aphakia, macular degeneration, diabetic retinopathy, systemic hypotension, sleep apnea, migraine headache, the Charlson comorbidity index.
CI = Confidence Interval
Since the development of metabolic syndrome is closely related to lifestyle, we wanted to assess the relationship between measures of socioeconomic status (household net worth, education level) on the hazard of developing OAG after adjustment for metabolic syndrome components and other possible confounders. Multivariable analysis revealed a decreased hazard of developing OAG with increasing levels of education and with increasing levels of household net worth. Compared to those without a high school diploma, those with a high school diploma had a 15% decreased hazard of developing OAG (adjusted HR=0.85 [95% CI 0.79–0.91]). Compared to beneficiaries with a household net worth <$25,000, those with a net worth of $75,000 to $100,000 had a 4% (adjusted HR=0.96 [95% CI 0.86–0.96]) decreased hazard of developing OAG and those with a net worth $500,000 to $749,999 had a 19% (HR=0.81 [95% CI 0.72–0.85] P<0.0001) decreased hazard of developing OAG. (Table 6)
Sensitivity Analyses
Changing our definition of OAG from requiring only one OAG diagnosis to requiring at least two OAG diagnoses to classify an enrollee with OAG did not significantly change the observed relationships between OAG and metabolic syndrome components.
Furthermore, since beta blocker use can affect the lipid profile, we re-analyzed the data including oral beta blocker use as a covariate in the models and the results did not materially change. (data not shown).
Discussion
By following a large national cohort of beneficiaries longitudinally, and adjusting for important confounding variables, this study demonstrated that both DM and HTN are independently associated with development of OAG. In this study, those with DM alone had a 35% increased hazard of developing OAG and persons with HTN alone had 17% increased hazard of developing OAG. Individuals with both DM and HTN had an even higher hazard (48%) of developing OAG than each condition individually. By comparison, individuals with hyperlipidemia alone had a 5% reduced hazard of developing OAG and the increased hazard of OAG associated with DM and HTN were attenuated by the presence of hyperlipidemia.
The only other study that we are aware of that has examined combinations of components of metabolic syndrome is the Singapore Malay Eye Study, which looked at combinations of components of metabolic syndrome in a population of 3,280 adults in Singapore.18 These authors controlled for age, sex, education level, smoking status, central corneal thickness and diabetes treatment and found no association between any component alone or in combination with OAG. However, these investigators did not control for the possibility of confounding by one metabolic syndrome component on other components. There are numerous studies (discussed below) that have looked at the association between individual components of metabolic syndrome and OAG. When comparing the findings from the present analysis to those studies, it is important to keep in mind that in addition to differences in the extent to which these studies controlled for potential confounding factors (including other components of metabolic syndrome), many of these studies employed a cross-sectional study design. In the current analysis, individuals are followed longitudinally over time to assess for incident diagnoses of OAG and each of the components of metabolic syndrome, making direct comparisons with the existing literature challenging.
Diabetes
Our results confirm the findings of a recent meta-analysis by Bonovas and colleagues which revealed a 27–50% increase in the risk of developing OAG in persons with DM14. The following population-based studies also showed that DM confers an increased risk of developing OAG: the Blue Mountain Eye Study,10 the Beaver Dam Eye Study,9 the Framingham Eye Study7, the Los Angeles Latino Eye Study11 and a longitudinal population-based study of women in the Nurses’ Health Study.13 Several other studies have demonstrated conflicting results,15–20 which may be attributed to differences in sociodemographic characteristics of the study populations, the extent to which other components of metabolic syndrome were adjusted for in the analyses, and the methods used to identify the presence or absence of DM and OAG. It is certainly possible that individuals who have DM are more likely to have an eye exam than the general population and are therefore more likely to be diagnosed with OAG.
There are several theories about possible mechanisms by which DM increases one’s risk of developing OAG. DM is a systemic disease which is known to cause widespread vascular, autonomic, and endothelial dysfunction. Vascular dysfunction of the small blood vessels feeding the optic nerve may result in glaucomatous damage to the optic nerve and retinal nerve fiber layer; oxidative damage from diabetes may lead to a similar end result.28 In addition, we do not know if any of the medications used to treat DM may play a role in the increased hazard of OAG.
Hypertension
Our results support the findings of many studies in the literature that have demonstrated that HTN is a risk factor for OAG.2–8 In our multivariable models, individuals with HTN alone, HTN and DM, HTN and hyperlipidemia, or all 3 of these conditions all had an increased hazard of developing OAG relative to individuals with no components of metabolic syndrome. Moreover, individuals with more severe HTN had a slightly higher hazard of experiencing OAG relative to those with less severe disease.
There are several proposed mechanisms explaining the relationship between HTN and OAG. One theory suggests that elevated blood pressure results in increased ciliary artery perfusion, resulting in increased aqueous production, which, in turn, increases the risk of developing OAG.2,29 Another theory contends that many persons with HTN have arteriolosclerotic damage and stiffening of the small end vessels feeding the optic nerve, which may predispose them to experiencing glaucomatous optic neuropathy.30 A third possibility includes damage to the optic nerve from decreased perfusion pressure caused during periods of episodic systemic hypotension related to blood pressure-lowering medication usage.
Hyperlipidemia
We found that individuals with hyperlipidemia had a reduced hazard of developing OAG relative to those with no components of metabolic syndrome. It is unclear whether it is the condition itself, the medications used to treat hyperlipidemia, or both, which reduce the hazard of developing OAG. However, there is some evidence, suggesting that treatments for hyperlipidemia may reduce the risk of developing OAG.31–33 A case-control study by McGwin and colleagues found that among individuals taking statins or other cholesterol-lowering medications for at least 23 months, there was a reduced risk of OAG as compared with age-matched controls.32 Basic science research in a trabecular meshwork cell culture model revealed that statins can increase aqueous outflow facility.34 Statins have also been shown to have neuroprotective effects in the setting of ischemia in the brain35 and in an ischemia-reperfusion rat model of the retina.36 Additional studies are warranted to better understand the relationship between hyperlipidemia, cholesterol-lowering medications, and OAG.
Obesity
Several studies have demonstrated a relationship between obesity and elevated IOP,37–41 yet few have looked at the relationship between obesity and OAG. Among studies specifically assessing the relationship between obesity and OAG, one study found an association42 while others have not.16,43 In the present analysis, the relationship between OAG and obesity had significant interplay with sex. Compared with non-obese women, obese women had a 6% increased hazard of developing OAG while there was no significant effect observed when comparing obese to non-obese men. These findings are similar to those from a small observational study by Zang and Wynder of 396 hospitalized patients (80 women and 316 men) in which the diagnosis of OAG was twice as likely in women with a BMI >27.5, but BMI level in men had no impact on the likelihood of an OAG diagnosis.42 Comparing the findings from these various studies with those from the current analysis are difficult due to differences in study design, differences in the study populations, and differences in the definitions of obesity and OAG used.
There are several theories as to why obesity may be associated with OAG. One hypothesis is that increased orbital fat and increased blood viscosity increases the episcleral venous pressure and reduces the outflow facility of aqueous, which results in increased IOP. This hypothesis has been confirmed clinically with several studies finding that obesity correlates with increased IOP.37–41 However, this theory, alone, does not explain why only obese women would have an increased hazard of OAG. A second hypothesis relating obesity to OAG is that the hyperleptinemia, which accompanies obesity45 may lead to increased oxidative stress.45 Studies have shown that patients with OAG have higher levels of oxidative damage in their trabecular meshwork than controls.46,47 Of interest, is the fact that women have higher circulating leptin levels than men.48 Additional investigation is needed to better understand the relationship between obesity, sex, and OAG.
Socioeconomic Status, Metabolic Syndrome and OAG
Despite the fact that everyone in this analysis had some form of medical insurance, after adjustment for possible confounders we found that increasing household net worth and higher educational status significantly decreased the hazard of developing OAG. While others have noted a link between lower socioeconomic status and metabolic syndrome,49 the relationship between these factors and development of OAG is unclear. Differences in health behaviors, environmental exposures, and psychological stressors such as community and interpersonal violence, all of which are more common among individuals who are socioeconomically disadvantaged, have been found to play a role in the development of a number of other chronic diseases. Future work is required to better understand the complex relationship between socioeconomic status and OAG.
Study Strengths and Limitations
There are several strengths to using claims data to study the association between components of metabolic syndrome and OAG. Our sample size is considerably larger than other studies in the literature and subjects come from diverse communities throughout the United States. The large sample size enables adjustment for numerous potential confounding variables. Diagnoses are made by medical professionals instead of relying on patient self-report, which some have shown to be inaccurate. (Patty LE, et al. Validity of self-reported diagnosis and treatment among Latinos in the Los Angeles Latino Eye Study. Poster presented at Association of Research and Vision in Ophthalmology meeting, May 6, 2010, Fort Lauderdale, Florida.)
There are several limitations to using claims data for this analysis. Due to fact that our data source was completely de-identified, we were unable to verify in enrollees’ medical records whether each beneficiary did indeed have each medical and ocular conditions of interest. Claims data do not include information on relevant clinical parameters such as smoking status, systolic and diastolic blood pressure, BMI, or laboratory values, which, ideally, we would have liked to include in the models. Our study identified obesity based on billing codes. Most other studies use BMI to determine obesity, and so a direct comparison is difficult. The prevalence of obesity may be underestimated as medical providers may be diagnosing obesity using billing codes among only those who are morbidly obese. It was beyond the scope of the current analysis to study the influence of medications used to treat HTN, DM, and hyperlipidemia on OAG or whether bariatric surgery for obesity may affect the study findings. Finally, it is important to recognize that all of the enrollees in this study had some form of commercial insurance. Therefore, the findings from our study may not apply to other groups including patients who are uninsured or underinsured.
In conclusion, given that approximately one-fifth of the US population has metabolic syndrome and both metabolic syndrome23 and OAG increase in prevalence with age, our data suggest that as the US population ages, the disease burden of OAG may increase in the coming years. Given the increasing prevalence of metabolic disorders in the US, this study furthers our understanding of risk factors associated with OAG and helps identify persons who may be at risk for this condition.
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (JDS:1K23EY019511-01); American Glaucoma Society Clinician Scientist Grant (JDS), Blue Cross Blue Shield of Michigan Foundation (JDS), Research to Prevent Blindness (DCM)
Footnotes
The authors have no proprietary interest in any material discussed in this manuscript
Presented in part at: the Association of Research and Vision in Ophthalmology meeting, May 4, 2009, Fort Lauderdale, Florida.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–93. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bulpitt CJ, Hodes C, Everitt MG. Intraocular pressure and systemic blood pressure in the elderly. Br J Ophthalmol. 1975;59:717–20. doi: 10.1136/bjo.59.12.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dielemans I, Vingerling JR, Algra D, et al. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population: the Rotterdam Study. Ophthalmology. 1995;102:54–60. doi: 10.1016/s0161-6420(95)31054-8. [DOI] [PubMed] [Google Scholar]
- 4.Tielsch JM, Katz J, Sommer A, et al. Hypertension, perfusion pressure, and primary open-angle glaucoma: a population-based assessment. Arch Ophthalmol. 1995;113:216–21. doi: 10.1001/archopht.1995.01100020100038. [DOI] [PubMed] [Google Scholar]
- 5.Bonomi L, Marchini G, Marraffa M, et al. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–93. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 6.Wilson MR, Hertzmark E, Walker AM, et al. A case-control study of risk factors in open angle glaucoma. Arch Ophthalmol. 1987;105:1066–71. doi: 10.1001/archopht.1987.01060080068030. [DOI] [PubMed] [Google Scholar]
- 7.Kahn HA, Milton RC. Alternative definitions of open-angle glaucoma: effect on prevalence and associations in the Framingham Eye Study. Arch Ophthalmol. 1980;98:2172–7. doi: 10.1001/archopht.1980.01020041024003. [DOI] [PubMed] [Google Scholar]
- 8.Zafra Perez JJ, Villegas Perez MP, Canteras Jordana M, Miralles De Imperial J. Intraocular pressure and prevalence of occult glaucoma in a village of Murcia [in Spanish] Arch Soc Esp Oftalmol. 2000;75:171–8. [PubMed] [Google Scholar]
- 9.Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes: the Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–7. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 10.Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:712–8. doi: 10.1016/s0161-6420(97)30247-4. [DOI] [PubMed] [Google Scholar]
- 11.Chopra V, Varma R, Francis BA, et al. Los Angeles Latino Eye Study Group. Type 2 diabetes mellitus and the risk of open-angle glaucoma: the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–32. doi: 10.1016/j.ophtha.2007.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz J, Sommer A. Risk factors for primary open angle glaucoma. Am J Prev Med. 1988;4:110–4. [PubMed] [Google Scholar]
- 13.Pasquale LR, Kang JH, Manson JE, et al. Prospective study of type 2 diabetes mellitus and risk of primary open angle glaucoma in women. Ophthalmology. 2006;113:1081–6. doi: 10.1016/j.ophtha.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 14.Bonovas S, Peponis V, Filioussi K. Diabetes mellitus as a risk factor for primary open-angle glaucoma: a meta-analysis. Diabet Med. 2004;21:609–14. doi: 10.1111/j.1464-5491.2004.01173.x. [DOI] [PubMed] [Google Scholar]
- 15.de Voogd S, Ikram MK, Wolfs RC, et al. Is diabetes mellitus a risk factor for open-angle glaucoma? The Rotterdam Study. Ophthalmology. 2006;113:1827–31. doi: 10.1016/j.ophtha.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 16.Leske MC, Connell AM, Wu SY, et al. Barbados Eye Study Group. Risk factors for open-angle glaucoma: the Barbados Eye Study. Arch Ophthalmol. 1995;113:918–24. doi: 10.1001/archopht.1995.01100070092031. [DOI] [PubMed] [Google Scholar]
- 17.Quigley HA, West SK, Rodriguez J, et al. The prevalence of glaucoma in a population-based study of Hispanic subjects: Proyecto VER. Arch Ophthalmol. 2001;119:1819–26. doi: 10.1001/archopht.119.12.1819. [DOI] [PubMed] [Google Scholar]
- 18.Tan GS, Wong TY, Fong CW, Aung T. Diabetes, metabolic abnormalities, and glaucoma: the Singapore Malay Eye Study. Arch Ophthalmol. 2009;127:1354–61. doi: 10.1001/archophthalmol.2009.268. [DOI] [PubMed] [Google Scholar]
- 19.Wormald RP, Basauri E, Wright LA, Evans JR. The African Caribbean Eye Survey: risk factors for glaucoma in a sample of African Caribbean people living in London. Eye (Lond) 1994;8:315–20. doi: 10.1038/eye.1994.64. [DOI] [PubMed] [Google Scholar]
- 20.Kaimbo Wa Kaimbo D, Missotten L. Risk factors for open-angle glaucoma in 260 Black subjects in Congo. Bull Soc Belge Ophtalmol. 1998;267:29–34. [PubMed] [Google Scholar]
- 21.Oh SW, Lee S, Park C, Kim DJ. Elevated intraocular pressure is associated with insulin resistance and metabolic syndrome. Diabetes Metab Res Rev. 2005;21:434–40. doi: 10.1002/dmrr.529. [DOI] [PubMed] [Google Scholar]
- 22.Jaen-Diaz JI, Sanz Alcolea I, López De Castro F, et al. Glaucoma and ocular hypertension in primary care [in Spanish] Aten Primaria. 2001;28:23–30. doi: 10.1016/S0212-6567(01)78891-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 24.Steinberger J, Daniels SR, Eckel RH, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–47. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- 25.Physician ICD-9-CM 2006: International Classification of Diseases. 9th revision, clinical modification. Vol. 1. Chicago, IL: AMA Press; 2006. [Google Scholar]
- 26.CPT 2006. Current Procedural Terminology. Chicago, IL: AMA Press; 2006. Professional ed. [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Szaflik JP, Rusin P, Zaleska-Zmijewska A, et al. Reactive oxygen species promote localized DNA damage in glaucoma-iris tissues of elderly patients vulnerable to diabetic injury. Mutat Res. 2010;697:19–23. doi: 10.1016/j.mrgentox.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 29.Shiose Y, Kawase Y. A new approach to stratified normal intraocular pressure in a general population. Am J Ophthalmol. 1986;101:714–21. doi: 10.1016/0002-9394(86)90776-2. [DOI] [PubMed] [Google Scholar]
- 30.Wolf S, Arend O, Sponsel WE, et al. Retinal hemodynamics using scanning laser ophthalmoscopy and hemorheology in chronic open-angle glaucoma. Ophthalmology. 1993;100:1561–6. doi: 10.1016/s0161-6420(93)31444-2. [DOI] [PubMed] [Google Scholar]
- 31.Leung DY, Li FC, Kwong YY, et al. Simvastatin and disease stabilization in normal tension glaucoma: a cohort study. Ophthalmology. 2010;117:471–6. doi: 10.1016/j.ophtha.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 32.McGwin G, Jr, McNeal S, Owsley C, et al. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004;122:822–6. doi: 10.1001/archopht.122.6.822. [DOI] [PubMed] [Google Scholar]
- 33.De Castro DK, Punjabi OS, Bostrom AG, et al. Effect of statin drugs and aspirin on progression in open-angle glaucoma suspects using confocal scanning laser ophthalmoscopy. Clin Experiment Ophthalmol. 2007;35:506–13. doi: 10.1111/j.1442-9071.2007.01529.x. [DOI] [PubMed] [Google Scholar]
- 34.Song J, Deng PF, Stinnett SS, et al. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005;46:2424–32. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- 35.Vaughan CJ, Delanty N. Neuroprotective properties of statins in cerebral ischemia and stroke. Stroke. 1999;30:1969–73. doi: 10.1161/01.str.30.9.1969. [DOI] [PubMed] [Google Scholar]
- 36.Honjo M, Tanihara H, Nishijima K, et al. Statin inhibits leukocyte-endothelial interaction and prevents neuronal death induced by ischemia-reperfusion injury in the rat retina. Arch Ophthalmol. 2002;120:1707–13. doi: 10.1001/archopht.120.12.1707. [DOI] [PubMed] [Google Scholar]
- 37.Klein BE, Klein R, Linton KL. Intraocular pressure in an American community: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–8. [PubMed] [Google Scholar]
- 38.Mori K, Ando F, Nomura H, et al. Relationship between intraocular pressure and obesity in Japan. Int J Epidemiol. 2000;29:661–6. doi: 10.1093/ije/29.4.661. [DOI] [PubMed] [Google Scholar]
- 39.Yoshida M, Ishikawa M, Kokaze A, et al. Association of life-style with intraocular pressure in middle-aged and older Japanese residents. Jpn J Ophthalmol. 2003;47:191–8. doi: 10.1016/s0021-5155(02)00666-4. [DOI] [PubMed] [Google Scholar]
- 40.Wu SY, Leske MC Barbados Eye Study Group. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol. 1997;115:1572–6. doi: 10.1001/archopht.1997.01100160742012. [DOI] [PubMed] [Google Scholar]
- 41.Lee JS, Choi YR, Lee JE, et al. Relationship between intraocular pressure and systemic health parameters in the Korean population. Korean J Ophthalmol. 2002;16:13–9. doi: 10.3341/kjo.2002.16.1.13. [DOI] [PubMed] [Google Scholar]
- 42.Zang EA, Wynder EL. The association between body mass index and the relative frequencies of diseases in a sample of hospitalized patients. Nutr Cancer. 1994;21:247–61. doi: 10.1080/01635589409514323. [DOI] [PubMed] [Google Scholar]
- 43.Gasser P, Stumpfig D, Schotzau A, et al. Body mass index in glaucoma. J Glaucoma. 1999;8:8–11. [PubMed] [Google Scholar]
- 44.Considine RV, Sinha MK, Heiman ML, et al. Serum imunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–5. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 45.Bouloumie A, Marumo T, Lafontan M, Busse R. Leptin induces oxidative stress in human endothelial cells. FASEB J. 1999;13:1231–8. [PubMed] [Google Scholar]
- 46.Sacca SC, Pascotto A, Camicione P, et al. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–63. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 47.Izzotti A, Sacca SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–46. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 48.Wong SL, DePaoli AM, Lee JH, Mantzoros CS. Leptin hormonal kinetics in the fed state: effects of adiposity, age, and gender on endogenous leptin production and clearance rates. J Clin Endocrinol Metab. 2004;89:2672–7. doi: 10.1210/jc.2003-031931. [DOI] [PubMed] [Google Scholar]
- 49.Loucks EB, Magnusson KT, Cook S, et al. Socioeconomic position and the metabolic syndrome in early, middle, and late life: evidence from NHANES 1999–2002. Ann Epidemiol. 2007;17:782–90. doi: 10.1016/j.annepidem.2007.05.003. [DOI] [PubMed] [Google Scholar]