Abstract
We have shown previously that withaferin A (WA), a promising anticancer constituent of Ayurvedic medicine plant Withania somnifera, inhibits growth of MCF-7 and MDA-MB-231 human breast cancer cells in culture and MDA-MB-231 xenografts in vivo by causing apoptosis. However, the mechanism of WA-induced apoptosis is not fully understood. The present study was designed to systematically determine the role of tumor suppressor p53 and estrogen receptor-α (ER-α) in proapoptotic response to WA using MCF-7, T47D, and ER-α overexpressing MDA-MB-231 cells as a model. WA treatment resulted in induction as well as increased Ser15 phosphorylation of p53 in MCF-7 cells, but RNA interference of this tumor suppressor gene conferred modest protection at best against WA-induced apoptosis. WA-mediated growth inhibition and apoptosis induction in MCF-7 cells were significantly attenuated in the presence of 17β-estradiol (E2). Exposure of MCF-7 cells to WA resulted in a marked decrease in protein levels of ER-α (but not ER-β) and ER-α regulated gene product pS2, and this effect was markedly attenuated in the presence of E2. WA-mediated down-regulation of ER-α protein expression correlated with a decrease in its nuclear level, suppression of its mRNA level, and inhibition of E2-dependent activation of ERE2e1b-luciferase reporter gene. Ectopic expression of ER-α in the MDA-MB-231 cell line conferred partial but statistically significant protection against WA-mediated apoptosis, but not G2/M phase cell cycle arrest. Collectively, these results indicate that WA functions as an anti-estrogen, and the proapoptotic effect of this promising natural product is partially attenuated by p53 knockdown and E2-ER-α.
Keywords: Breast Cancer, Estrogen Receptor-α, Withaferin A, Chemoprevention
INTRODUCTION
Despite significant progress towards screening efforts and targeted therapies, breast cancer is still a leading cause of cancer-related deaths among women worldwide [1,2]. Li-Fraumeni syndrome, atypical hyperplasia of the breast, late age at first full-term pregnancy, early menarche, late menopause, and family history are some of the risk factors associated with breast cancer [3–5]. Novel strategies for prevention of breast cancer are clinically appealing because many of the risk factors linked with this disease (e.g., family history) are not modifiable. Natural products continue to garner interest for identification of potential cancer chemopreventive and therapeutic agents [6,7].
Medicinal plant Withania somnifera (also known as Ashwagandha or Indian winter cherry) has been used safely for centuries in Indian Ayurvedic medicine practice for treatment of different ailments. A formulation of Withania somnifera is available over the counter in the United States as a dietary supplement. Some of the known pharmacological actions of Withania somnifera include modulation of immune function [8], protection against ischemia and reperfusion injury [9], neuroprotective effect on 6-hydroxydopamine-induced Parkinson symptoms in rats [10], anti-bacterial effects [11], and anti-inflammatory effects [12]. Withania somnifera inhibited nuclear factor κB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells [13].
Research over the past decade has identified bioactive compounds with anticancer activity in Withania somnifera [14–29]. Withaferin A (WA) is one such naturally-occurring constituent of Withania somnifera with effects against cancer cells in culture and in vivo. For example, WA was shown to be a radiosensitizer and growth inhibitor of a mouse melanoma and Ehrlich ascites carcinoma in vivo [14,15]. WA-mediated suppression of angiogenesis, alteration of cytoskeletal architecture, and inhibition of proteasomal activity has also been documented [19–21]. WA treatment resulted in suppression of IκB kinase beta phosphorylation concomitant with inhibition of its kinase activity [18]. WA was shown to trigger Par-4-dependent apoptosis in human prostate cancer cells [22]. In U937 human leukemia cells, WA-induced apoptosis correlated with inhibition of Akt phosphorylation [26]. WA-induced apoptosis in leukemia cells of lymphoid and myeloid origin was associated with activation of p38 mitogen-activated protein kinase [27]. WA was shown to target heat shock protein 90 in pancreatic cancer cells [28].
We showed previously that WA inhibited growth of cultured human breast cancer cells (MCF-7 and MDA-MB-231) and MDA-MB-231 xenografts in vivo by causing apoptosis [24]. On the other hand, a spontaneously immortalized and non-tumorigenic human mammary epithelial cell line (MCF-10A) was significantly more resistant to growth inhibition and apoptosis induction by WA compared with breast cancer cells [24]. The mechanism underlying differential sensitivity of normal versus cancerous mammary cells to WA is unclear, but proapoptotic response to this agent in MCF-7 and MDA-MB-231 cells was accompanied by FOXO3a-dependent induction of Bim protein level [24]. Furthermore, knockdown of FOXO3a and Bim proteins conferred statistically significant protection against WA-induced apoptosis [24]. We also found that while WA treatment inhibited constitutive (MDA-MB-231) as well as interleukin-6-inducible (MCF-7 and MDA-MB-231) activation of STAT3 (Signal Transducer and Activator of Transcription 3), this transcription factor was largely dispensable for proapoptotic response to WA [29].
The present study was designed to determine the role of p53 and estrogen receptor-α (ER-α) in proapoptotic response to WA using MCF-7, T47D, and MDA-MB-231 cells. This was a worthy mechanistic objective based on following considerations: (a) p53 is a known regulator of apoptosis [30]; (b) ER-α is a well-recognized target for chemoprevention of human breast cancer; (c) selective estrogen receptor modulators (e.g., tamoxifen and raloxifene) are clinically effective against ER-α-positive tumors [31,32]; (d) clinical trials and laboratory studies have identified ER-α as a possible determinant of chemotherapy response [33,34]; and (f) WA has structural similarity to steroid backbone of estradiol.
MATERIALS AND METHODS
Reagents
WA (structure is shown in Figure 1A) was purchased from Chromadex (Irvine, CA). 17β-estradiol (E2), 4',6-diamidino-2-phenylindole (DAPI), and propidium iodide were from Sigma-Aldrich (St. Louis, MO). An antibody against ER-α was from Upstate-Millipore (Billerica, MA); antibodies against pS2 and poly-(ADP-ribose)-polymerase (PARP) were from Santa Cruz Biotechnology (Santa Cruz, CA); anti-p53 antibody was from Calbiochem (Gibbstown, NJ); an antibody specific for detection of S15 phosphorylated p53 was from Cell Signaling (Danvers, MA); and anti-actin antibody was from Sigma-Aldrich. Luciferase reporter assay kit was from Promega (Madison, WI). Reagents for reverse transcription-PCR (RT-PCR) were from Invitrogen-Life Technologies (Carlsbad, CA).
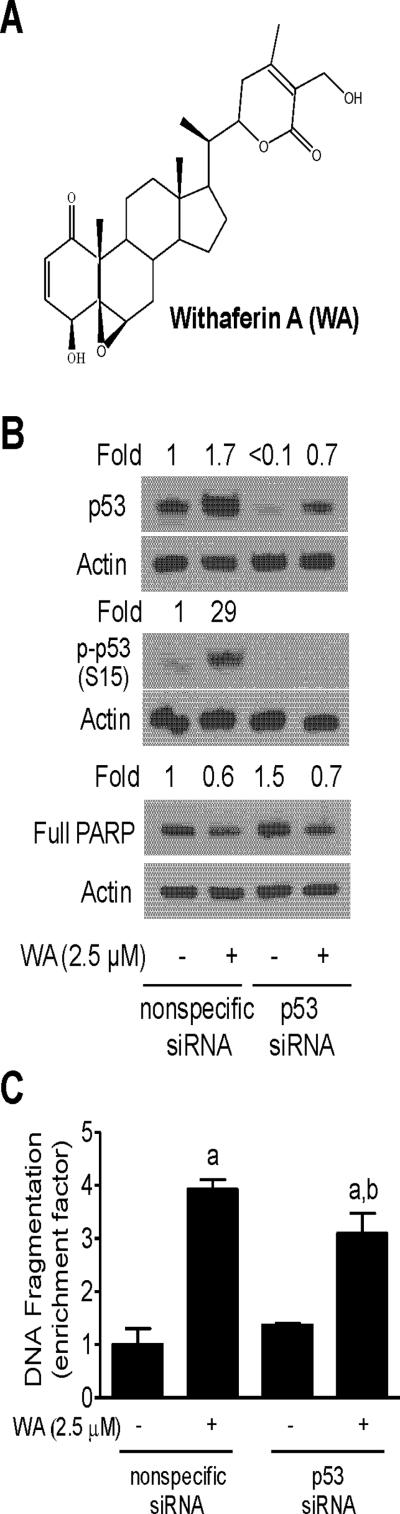
Figure 1.
p53 tumor suppressor was largely dispensable for WA-induced apoptosis. (A) Chemical structure of WA. (B) Western blotting for p53, S15 phosphorylated p53, and full PARP protein using lysates from MCF-7 cells transiently transfected with a nonspecific siRNA or a p53-targeted siRNA and treated for 24 h with DMSO (control) or 2.5 μM WA. Numbers above bands indicate change in protein levels relative to DMSO-treated-nonspecific siRNA-transfected MCF-7 cells. (C) Histone-associated DNA fragment release into the cytosol in MCF-7 cells transiently transfected with a nonspecific siRNA or a p53-targeted siRNA and treated for 24 h with DMSO (control) or 2.5 μM WA. Results shown are mean ± SD (n= 3). Significantly different (P < 0.05) (a) compared with corresponding DMSO-treated control, and (b) between WA-treated nonspecific siRNA transfected and WA-treated p53 siRNA transfected cells by one-way ANOVA followed by Bonferroni's test.
Cell Culture
MCF-7, T47D, and MDA-MB-231 human breast cancer cells were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in phenol red-free medium (MEM for MCF-7 and RPMI1640 for T47D) containing 5% charcoal/dextran-stripped fetal bovine serum (FBS) for 2 days prior to experimental procedures. The MDA-MB-231 cells stably transfected with empty pIRES vector or pIRES vector encoding for ER-α were cultured in phenol red-free RPMI1640 media containing 5% charcoal/dextran-stripped FBS (to remove endogenous steroids) and 500 μg/mL G418. The pIRES-ERα vector was a generous gift from Dr. Ben H. Park (Johns Hopkins University, Baltimore, MD). The entire coding region of ER-α was PCR-amplified using MCF7 cDNA as template and cloned into the expression vector, pIRESNEO. Cell culture media were purchased from Mediatech (Manassas, VA), whereas FBS, antibiotic mixture, and G418 were from Invitrogen-Life Technologies. Charcoal/dextran-stripped FBS was from HyClone (Rockford, IL).
Western Blotting
Cell lysate was prepared as described by us previously [35]. Lysate proteins were resolved by sodium-dodecyl sulfate polyacrylamide gel electrophoresis and wet-transferred onto polyvinylidene fluoride membrane. The membrane was blocked with 5% milk in Tris-buffered saline containing 0.05% Tween 20. The membrane was incubated by rocking at 4°C with an appropriate primary antibody for overnight. Membrane was washed three times for 15 min each with Tris-buffered saline containing 0.05% Tween 20 and then treated with an appropriate secondary antibody. Immunoreactive bands were visualized using enhanced chemiluminescence procedure. Membrane was stripped and re-probed with anti-actin antibody as a loading control.
RNA Interference of p53
The MCF-7 cells were seeded in six-well plates and transfected at 50% confluency with 100 nM of a nonspecific siRNA or 100 nM of a p53-targeted siRNA using OligofectAMINE (Invitrogen-Life Technologies) according to the manufacturer's instructions. Twenty-four hours after transfection, the cells were treated with DMSO or 2.5 μM WA for 24 h. The cells were then collected and processed for immunoblotting and determination of histone-associated DNA fragment release in cytosol, a well-accepted marker of apoptosis.
Determination of Cell Viability and Histone-Associated DNA Fragment Release in Cytosol
Cell viability was determined by trypan blue dye exclusion assay essentially as described by us previously [36]. Histone-associated DNA fragment release into the cytosol was determined using Cell Death Detection ELISA kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer's instructions.
Reverse Transcription-PCR (RT-PCR)
RT-PCR was done to determine the effect of WA treatment on mRNA levels of ER-α and ER-β. Total RNA was extracted using Invitrogen RNeasy mini kit and by following the manufacturer's instructions. Complementary DNA was synthesized from 1–2 μg of total RNA with reverse transcriptase and oligo(dT)20. PCR reaction was carried out using High Fidelity Taq Polymerase, gene-specific primers, and cDNA. PCR products were resolved by 2% agarose gel electrophoresis prestained with ethidium bromide. An UV illuminator was used to visualize the bands. PCR primers and amplification conditions were as follows: GAPDH (25 cycles): forward- 5'-TGATGACATCAAGAAGGTGGTGAAG-3', reverse- 5'-TCCTTGGAGGCCATGTGGGCCAT-3', 95°C for 30 sec, 55°C for 30 sec, 68°C for 30 sec, ER-α (32 cycles): forward- 5'-GCACCCTGAAGTCTCTGGAA-3', reverse- 5'-TGGCTAAAGTGGTGCATGAT-3', 94°C for 1 min, 55°C for 1 min, 68°C for 1 min; ER-β (35 cycles): forward- 5'- TGAAAAGGAAGGTTAGTGGGAACC-3', reverse- 5'- TGGTCAGGGACATCATCATGG-3', 94°C for 1 min, 58°C for 1 min, 68°C for 1 min.
Luciferase Assay
To assess the effect of WA treatment on ER signaling, we transfected MCF-7 cells with the ERE2e1b-luciferase reporter gene, a generous gift from Dr. Brian G. Rowan, Tulane University School of Medicine, New Orleans, LA [37] prior to treatment with WA in the absence or presence of 10 nM E2. Briefly, cells were co-transfected with 1 μg of pERE2e1bluciferase plasmid and 0.1 μg of pRL-CMV plasmid using FuGENE6 (Roche Applied Science). Twenty-four hours after transfection, the cells were treated with DMSO or WA in the absence or presence of E2 and processed for determination of luciferase activity assay using Dual-Luciferase Reporter Assay kit.
Immunofluorescence Microscopy for ER-α Expression
MCF-7 cells were plated on coverslips, allowed to attach by overnight incubation, and exposed to DMSO or 2.5 μM WA for 24 h. The cells were then fixed with 2% paraformaldehyde for 1 h at room temperature and permeabilized using 0.05% Triton X-100 for 5 min. The cells were incubated with PBS containing 0.5% bovine serum albumin, and 0.15% glycine for 1 h followed by overnight incubation with ER-α antibody at 4°C. The cells were treated with 2 μg/mL Alexa Fluor 488-conjugated secondary antibody for 1 h at room temperature. After washing with PBS, the cells were counterstained with 10 ng/mL of DAPI for 5 min at room temperature to visualize nuclear DNA. Localization of ER-α was visualized using a Leica DC300F fluorescence microscope at 100× objective lens magnification. Fluorescence images were converted to 8-bit grayscale and green fluorescence threshold was set using Image J software. ER-α-associated immunofluorescence intensity was quantified for control and WA-treated cells under identical settings.
Analysis of Cell Cycle Distribution
MDA-MB-231 cells stably transfected with empty pIRES vector or pIRES vector encoding for ER-α were treated with DMSO or 2.5 μM WA for 24 h. After treatment, cells were collected, fixed with 70% ethanol overnight at 4°C, stained with propidium iodide, and analyzed using a flow cytometer as described by us previously [38].
RESULTS
Role of p53 in WA-induced Apoptosis
p53 is a well-accepted facilitator of apoptotic cell death by different stimuli [30]. We employed RNA interference in MCF-7 cells, which express wild-type p53, to determine the possible involvement of p53 in WA-mediated apoptosis. As shown in Figure 1B, WA treatment for 24 h caused an increase in the level of total as well as S15 phosphorylated p53, which has been implicated in apoptosis induction [30]. WA-mediated induction and S15 phosphorylation of p53 were markedly suppressed in MCF-7 cells transiently transfected with a p53-targeted siRNA (Figure 1B). Moreover, RNA interference of p53 conferred modest but statistically significant protection against WA-induced apoptosis (Figure 1C). For example, 24 h exposure of nonspecific siRNA transfected MCF-7 cells to 2.5 μM WA resulted in about 3.9-fold enrichment of histone-associated DNA fragment release into the cytosol over DMSO-treated control (Figure 1C). WA-mediated enrichment of histone-associated DNA fragment release into the cytosol was about 27% greater in the MCF-7 cells transfected with the nonspecific siRNA compared with p53 silenced cells (Figure 1C). Consistent with these results, the WA-mediated decrease in protein level of full PARP was modestly attenuated by knockdown of p53 protein (Figure 1B). These results indicated that p53 protein only modestly contributed to WA-induced apoptosis, at least in the MCF-7 cells.
E2 Conferred Protection against WA-induced apoptosis
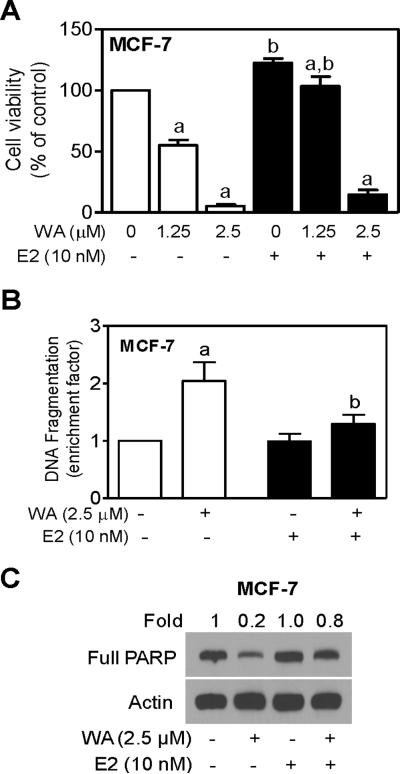
We next designed experiments to determine if growth inhibitory and proapoptotic response to WA was influenced by E2. For these studies, MCF-7 cells were cultured in phenol red-free medium supplemented with 5% charcoal/dextran stripped FBS for 2 days prior to treatments with WA and/or E2. Figure 2A shows dose-response effect of WA treatment on viability of MCF-7 cells without or with E2. Consistent with our previous observations [24], viability of MCF-7 cells was decreased in a concentration-dependent manner by WA treatment alone. Presence of E2 conferred partial but marked protection against WA-mediated growth inhibition especially at the 1.25 μM (p<0.05 compared with DMSO-treated control). Difference did not reach statistical significant at 2.5 μM WA concentration (Figure 2A). In agreement with these results, WA-induced apoptosis was significantly attenuated in the presence of E2 as judged by analysis of histone-associated DNA fragment release into the cytosol (Figure 2B) and Western blotting for full PARP protein level (Figure 2C). Together, these results indicated that growth inhibition and apoptosis induction resulting from WA exposure were markedly attenuated in the presence of E2, at least in the MCF-7 cell line.
Figure 2.
WA-induced apoptosis was attenuated in the presence of 17β-estradiol (E2). (A) Trypan blue dye exclusion assay for effects of WA and/or E2 treatments (24 h treatment) on viability of MCF-7 cells. Live cells were counted and normalized as 100% to DMSO-treated cells in the absence of E2. (B) Histone-associated DNA fragment release into the cytosol in MCF-7 cells treated for 24 h with WA and/or E2. Results shown are expressed as enrichment factor relative to DMSO-treated control cells (without WA or E2 treatment). (C) Immunodetection of full PARP protein using lysates from MCF-7 cells treated for 24 h with WA in the absence or presence of E2. Results shown are mean ± SD (n= 3). Significantly different (P < 0.05) (a) compared with DMSO-treated control and (b) between without and with E2 treatment groups by one-way ANOVA followed by Bonferroni's test.
WA Treatment Decreased ER-α Protein Expression in MCF-7 and T47D Cells
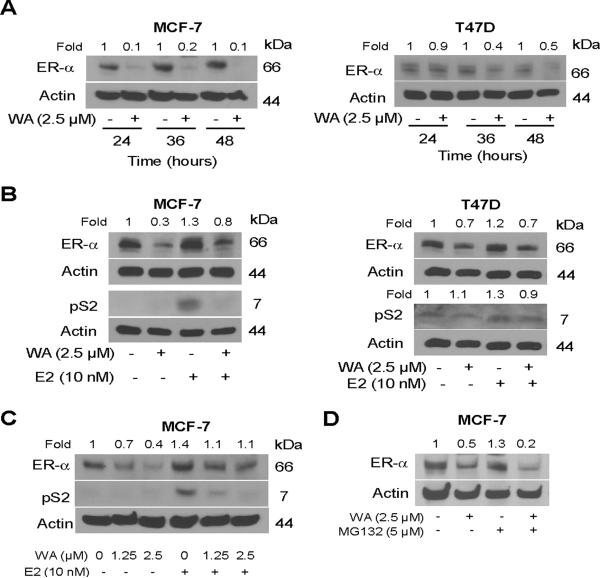
We proceeded to address the question of whether WA treatment altered ER-α protein expression using MCF-7 and T47D cells. As shown in Figure 3A, 24 h exposure of MCF-7 cells to 2.5 μM WA resulted in suppression of ER-α protein level by about 90% (varied between 50–90% in different experiments) and this effect was sustainable for up to 48 h after treatment. To the contrary, expression of ER-β protein was increased by about 20–30% by a similar WA treatment (results not shown). WA-mediated suppression of ER-α expression was relatively more pronounced in the MCF-7 cell line compared with T47D. Nevertheless, expression of ER-α protein was decreased markedly on 36–48 h treatment of T47D cells with 2.5 μM WA (Figure 3A). Expression of ER-α protein was modestly increased (by about 20–30% compared with DMSO-treated control) in the presence of E2 in both MCF-7 and T47D cells (Figure 3B). WA-mediated suppression of ER-α protein expression was partially reversible in the presence of E2 in both cell lines (Figure 3B). As expected, E2 stimulation caused an increase in protein level of ER-α target gene product pS2 (Figure 3B). E2-mediated induction of pS2 protein expression was suppressed in the presence of WA in the MCF-7 cell line (Figure 3B). Inhibition of E2-induced pS2 protein expression by WA was evident at a concentration as low as 1.25 μM, at least in the MCF-7 cell line (Figure 3C). These results showed suppression of ER-α protein expression by WA in human breast cancer cells. Extent of WA-mediated suppression of ER-α protein expression was variable between MCF-7 and T47D cells with the MCF-7 cell line being more sensitive to this effect.
Figure 3.
WA treatment downregulated protein expression of estrogen receptor-α (ER-α) in MCF-7 and T47D cells. (A) Western blot analysis for ER-α protein levels using lysates from MCF-7 (left panel) or T47D cells (right panel) treated for 24, 36, or 48 h with DMSO (control) or the indicated concentration of WA. (B) Western blot analysis for ER-α and pS2 proteins using lysates from MCF-7 (left panel) or T47D cells (right panel) treated for 24 h with 2.5 μM WA in the absence or presence of 10 nM 17β-estradiol (E2). (C) Western blot analysis for dose-response effect of WA treatment (24 h treatment without or with 10 nM E2) on ER-α and pS2 proteins in MCF-7 cells. (D) Western blot analysis for ER-α protein using lysates from MCF-7 cells treated for 24 h with 2.5 μM WA in the absence or presence of MG132. Cells were pre-treated for 2 h with MG132 and then exposed to WA in the presence of MG132. Numbers above bands denote change in protein level relative to respective DMSO-treated control.
We also considered the possibility whether WA promoted proteasomal degradation of ER-α analogous to synthetic pure anti-estrogen ICI 182,780. As shown in Figure 3D, WA-mediated decrease in ER-α protein level was sustained in the presence of proteasomal inhibitor MG132. These results indicated that WA-mediated decrease in protein level of ER-α was not due to its increased degradation.
WA SuppressedER-α mRNA and Nuclear Level of ER-α Protein in MCF-7 Cells
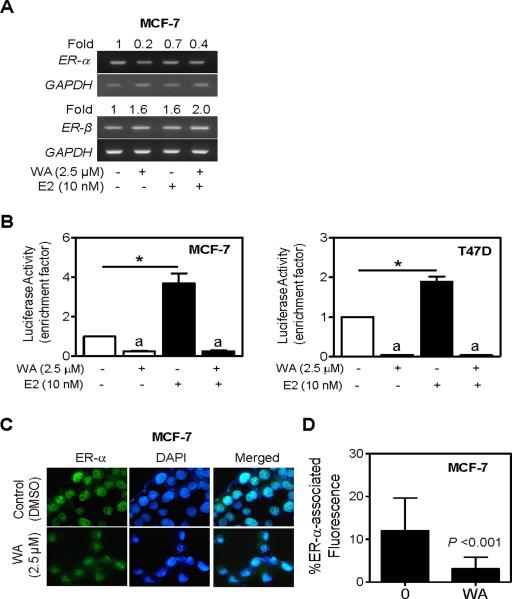
Next, we questioned whether WA-mediated decline in protein level of ER-α was due to its transcriptional repression. We addressed this question by determining the effect of WA and/or E2 treatments on level of ER-α mRNA by RT-PCR. As can be seen in Figure 4A, WA treatment caused a decrease in the level of ER-α mRNA in the MCF-7 cell line regardless of E2 stimulation. Similar to protein expression results, the MCF-7 cell line was more sensitive to WA-mediated suppression of ER-α mRNA compared with T47D cells (results not shown). In absence of E2 stimulation, WA treatment resulted in a modest increase (about 60% increase compared with DMSO-treated control) in ER-β expression (Figure 4A), which was consistent with induction of its protein level (data not shown).
Figure 4.
WA treatment suppressed ER-α mRNA expression. (A) RT-PCR for ER-α and ER-β mRNA expression in MCF-7 cells treated for 24 h with 2.5 μM WA in the absence or presence of 10 nM 17β-estradiol (E2). Numbers above bands are indicative of change in mRNA levels relative to DMSO-treated control (first lane). (B) ERE2e1b-associated luciferase reporter activity in MCF-7 (left panel) and T47D cells (right panel) treated for 24 h with 2.5 μM WA in the absence or presence of 10 nM E2. Results shown are mean ± SD (n= 3). Significantly different (P < 0.05) (a) compared with corresponding DMSO-treated control, and (*) between the indicated groups by one-way ANOVA followed by Bonferroni's test. (C) Fluorescence microscopic analysis of ER-α-associated immunofluorescence in MCF-7 cells following 24 h treatment with DMSO or 2.5 μM WA (100× objective magnification). (D) Quantitation of ER-α-associated immunofluorescence in MCF-7 cells treated for 24 h with DMSO or 2.5 μM WA. Combined results from triplicate experiments are shown (mean ± SD, n= 20 for control and n= 33 for WA). Statistical significance was determined by two-tailed t-test.
To further assess the effect of WA treatment on ER signaling, we transfected MCF-7 or T47D cells with the ERE2e1b-luciferase reporter gene prior to treatment with WA in the absence or presence of 10 nM E2. As can be seen in Figure 4B, basal ERE2e1b-associated luciferase activity was inhibited by about 75–96% in the presence of 2.5 μM WA in both cell lines. The ERE2e1b-associated luciferase activity was increased by 3.7-fold and 1.9-fold in the presence of 10 nM E2 in MCF-7 and T47D cells, respectively, in absence of WA treatment. The E2-stimulated ERE2e1b-luciferase activity was also inhibited significantly upon WA exposure in both MCF-7 and T47D cells (Figure 4B).
Mechanism underlying ER-α activation is quite complex and involves a series of events, including its translocation to the nucleus [39]. We used the MCF-7 cell line to determine the effect of WA treatment on nuclear level of ER-α. In DMSO-treated control MCF-7 cells, ER-α staining was predominant in the nucleus (Figure C). Quantitation of ER-α-associated immunofluorescence revealed about 74% decrease in MCF-7 cells treated for 24 h with 2.5 μM WA in comparison with DMSO-treated control cells (P<0.001 by two-tailed t-test) (Figure 4D).
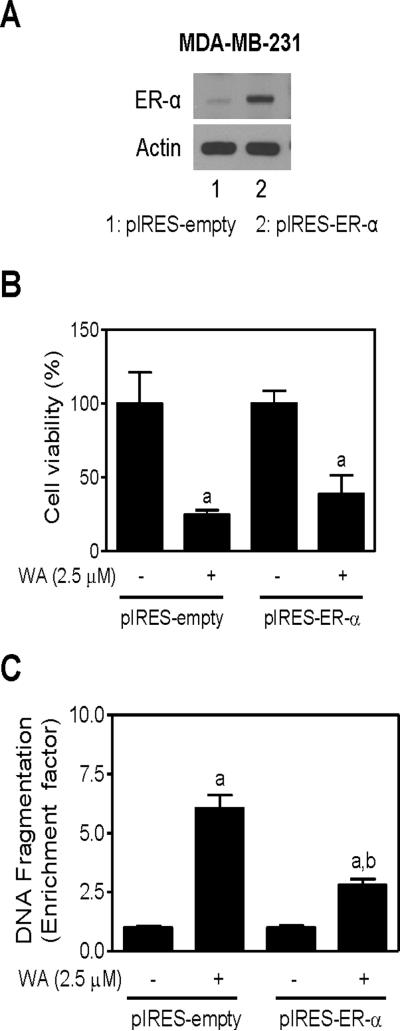
Ectopic Expression of ER-α Protected WA-induced Apoptosis in MDA-MB-231 Cells
To determine the impact of ER-α expression on proapoptotic response to WA, we utilized MDA-MB-231 cells stably overexpressing ER-α (hereafter abbreviated as pIRES-ER-α). As shown in Figure 5A, the ER-α protein was overexpressed in pIRES-ER-α compared with empty vector transfected control cells (pIRES-empty). The pIRES-empty cells were slightly more sensitive to cell killing by WA compared with pIRES-ER-α cells but the difference did not reach statistical significance (Figure 5B). Enrichment of histone-associated DNA fragment release into the cytosol resulting from 24 h exposure to 2.5 μM WA was significantly greater in pIRES-empty cells (6.0-fold enrichment over DMSO-treated pIRES-empty cells) than in ER-α overexpressing MDA-MB-231 cells (2.8-fold enrichment over DMSO-treated pIRES-ER-α cells) (Figure 5C). Similar response was observed in the presence of E2 (results not shown).
Figure 5.
Overexpression of ER-α in MDA-MB-231 cell line conferred protection against WA-induced apoptosis. (A) Western blotting for ER-α using lysates from MDA-MB-231 cells stably transfected with empty pIRES vector (pIRES-empty; lane 1) and pIRES vector encoding for ER-α (pIRES-ER-α; lane 2). Effects of WA treatment on (B) cell viability as determined by trypan blue dye exclusion assay and (C) apoptosis induction as determined by quantification of histone-associated DNA fragment release into the cytosol in pIRES-empty and pIRES-ER-α cells after 24 h treatment with DMSO or 2.5 μM WA. For cell viability data, results are normalized (100%) to DMSO-treated control for each cell line. Results shown are mean ± SD (n= 3). Significantly different (P < 0.05) compared with (a) respective DMSO-treated control for each cell line and (b) between WA-treated pIRES-empty cells and WA-treated pIRES-ER-α cells by one-way ANOVA followed by Bonferroni's test.
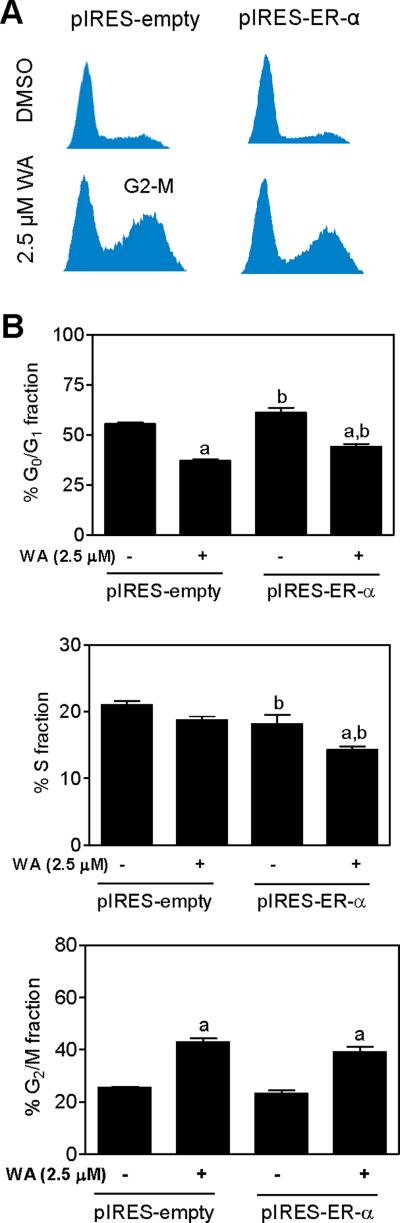
Effect of ER-α Overexpression on WA-induced Cell Cycle Arrest in MDA-MB-231 Cells
We have shown previously that WA-treated MCF-7 and MDA-MB-231 cells are arrested in G2/M phase of the cell cycle [25]. We designed experiments to test whether cell cycle arrest from WA exposure was influenced by ER-α status. Figure 6A depicts flow histograms obtained from pIRES-empty and pIRES-ER-α cells following 24 h treatment with DMSO or 2.5 μM WA. WA-mediated G2/M phase cell cycle arrest was observed regardless of ER-α status (Figure 6B) or E2 stimulation (results not shown). These results demonstrated ability of WA to cause G2/M phase cell cycle arrest irrespective of ER-α activation.
Figure 6.
ER-α was largely dispensable for WA-induced G2/M phase cell cycle arrest. (A) Representative flow histograms depicting cell cycle distribution in MDA-MB-231 cells stably transfected with empty pIRES vector (pIRES-empty) or pIRES vector encoding for ER-α (pIRES-ER-α) and treated for 24 h with DMSO or 2.5 μM WA. (B) Cell cycle distribution in pIRES-empty and pIRES-ER-α cells after 24 h treatment with DMSO or 2.5 μM WA. Results shown are mean ± SD (n= 3). Significantly different (P < 0.05) compared with (a) respective DMSO-treated control for each cell line and (b) between WA-treated pIRES-empty cells and WA-treated pIRES-ER-α cells by one-way ANOVA followed by Bonferroni's test.
DISCUSSION
Primary objective of the present study was to identify mechanistic molecular variables influencing growth inhibitory and proapoptotic response to WA, which is a highly promising natural product with in vitro and in vivo anticancer activity against human breast cancer cells [24,25]. Our initial inquiry focused on possible involvement of p53 in regulation of WA-induced apoptotic cell death. This was a logical research direction because the p53 tumor suppressor is a known regulator of apoptosis by different stimuli [30,40]. We found that WA treatment robustly increased not only protein level of p53 but also its phosphorylation at S15. However, RNA interference of p53 only moderately attenuates WA-induced apoptosis. Lack of a strong effect of p53 RNA interference should be viewed as a therapeutic advantage for WA because loss of function mutation of p53 is quite common in human cancers. Mechanism by which WA treatment stabilizes p53 or increases its phosphorylation at S15 is not known. p53 protein has short half-life and normally maintained at a low level in unstressed cells due to interaction of p53 with MDM2 [30]. Expression of p53 is also negatively regulated by several other proteins independently of MDM2, including c-Jun N-terminal kinase, TAF1, Aurora kinase etc [30]. Possibility that WA affects interaction of p53 with these proteins leading to its stabilization can't be ruled out. Similarly, S15 phosphorylation of p53 is mediated a variety of kinases (e.g., ataxia telangiectasia mutated, ataxia telangiectasia and Rad3 related, and casein kinase 1 to name a few) [30]. Precise mechanism underlying WA-mediated increase in S15 phosphorylation of p53 remains to be determined. However, this question is not important in the context of the present study because of modest protection conferred by p53 knockdown on WA-induced apoptosis.
It is well-established that estrogens promote growth of breast cancer in humans and long term exposure to estrogens is a known risk factor for this malignancy [41]. Promotional effect of estrogens in breast cancer is mediated by ER-α, which belongs to the nuclear receptor superfamily [39,42]. The ER-α primarily functions as a ligand-activated transcription factor, which upon estrogenic stimulation, binds to the promoter region of target genes (e.g., pS2, c-Myc etc.) to regulate their expression [43,44]. Detectable ER-α expression and functional ER-α-mediated gene transcription are observed in about 65% of human breast cancers [42]. A number of approaches, including selective estrogen receptor modulators that compete for binding to ER (e.g., tamoxifen and raloxifene) and reduction of estrogen levels through inhibition of aromatase are in clinical practice [31,32]. The present study indicates that WA functions as an anti-estrogen. This conclusion is based on the following observations: (a) E2 confers significant protection against WA-induced apoptosis, and (b) protein expression of ER-α is decreased markedly in breast cancer cells, which is not a cell line-specific response. Mechanism for WA-mediated downregulation of ER-α protein expression involves its transcriptional repression. In this regard, WA behaves differently than the pure synthetic anti-estrogen ICI 182,780, which triggers degradation of ER-α protein through a proteasome-dependent pathway [45]. WA-mediated decline in ER-α protein level is not relieved by inhibition of the proteasome using MG132 (Figure 3D). Thus, it is reasonable to conclude that WA treatment decreases protein level of ER-α by causing its transcriptional repression. In this regard, WA resembles aromatic isothiocyanates, which are highly promising cancer chemopreventive constituents of cruciferous vegetables [46].
Besides promotion of breast cancer cell proliferation, estrogens increase their survival by upregulating Bcl-2, a known anti-apoptotic protein, as well as by downregulation of proapoptotic factors [47–49]. Protection against WA-induced apoptosis in the presence of E2 observed in the present study is likely attributable to some of these alterations. Even though further studies are needed to systematically explore these possibilities, WA treatment causes downregulation of Bcl-2 protein and induction of proapoptotic proteins in MCF-7 cells [24]. It is also possible that WA treatment inhibits activation of Akt, a kinase known to increase transcriptional activity of ER-α through its phosphorylation [50]. Further work is needed to test possibility as well.
We have shown previously that WA-treated MDA-MB-231 and MCF-7 cells are arrested in G2/M phase of the cell cycle [25]. Cell cycle arrest resulting from WA exposure is irreversible in both cell types and probably contributes to its growth suppressive effect because a fraction of cells arrested in G2/M phase of the cell cycle after WA treatment are driven to apoptosis [25]. In this study, we show that the cell cycle arrest by WA is not affected by E2 stimulation or ER-α overexpression.
In conclusion, the present study demonstrates, for the first time, that natural product WA downregulates ER-α expression in human breast cancer cells, and this effect is due to suppression of ER-α mRNA level. Based on these results, WA can be classified as an anti-estrogen. We also conclude that p53 tumor suppressor is largely dispensable for proapoptotic effect of WA, at least in MCF-7 cells, which is a therapeutic advantage because mutation of p53 is common in human cancers.
ACKNOWLEDGMENTS
This investigation was supported by the USPHS grant 1 RO1 CA142604-01 (to SVS) awarded by the National Cancer Institute.
Abbreviations
- WA
Withaferin A
- ER
estrogen receptor
- E2
17β-estradiol
- DAPI
4',6-diamidino-2-phenylindole
- PARP
poly-(ADP-ribose)-polymerase
- reverse transcription-PCR
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
- DMSO
dimethyl sulfoxide
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Jemal A, Ward E, Thun MJ. Temporal trends in breast cancer mortality by state and race. Cancer Causes Control. 2008;19:537–545. doi: 10.1007/s10552-008-9113-1. [DOI] [PubMed] [Google Scholar]
- 3.Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15:36–47. doi: 10.1093/oxfordjournals.epirev.a036115. [DOI] [PubMed] [Google Scholar]
- 4.Hulka BS, Stark AT. Breast cancer: cause and prevention. Lancet. 1995;346:883–887. doi: 10.1016/s0140-6736(95)92713-1. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL, Bernstein L. Epidemiology and prevention of breast cancer. Annu Rev Public Health. 1996;17:47–67. doi: 10.1146/annurev.pu.17.050196.000403. [DOI] [PubMed] [Google Scholar]
- 6.Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981–2002. J Nat Prod. 2003;66:1022–1037. doi: 10.1021/np030096l. [DOI] [PubMed] [Google Scholar]
- 7.Stan SD, Kar S, Stoner GD, Singh SV. Bioactive food components and cancer risk reduction. J Cell Biochem. 2008;104:339–356. doi: 10.1002/jcb.21623. 2008. [DOI] [PubMed] [Google Scholar]
- 8.Agarwal R, Diwanay S, Patki P, Patwardhan B. Studies on immunomodulatory activity of Withania somnifera (Ashwagandha) extract in experimental immune inflammation. J Ethnopharmacol. 1999;67:27–35. doi: 10.1016/s0378-8741(99)00065-3. [DOI] [PubMed] [Google Scholar]
- 9.Gupta SK, Mohanty I, Talwar KK, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 10.Ahmad M, Saleem S, Ahmad AS, et al. Neuroprotective effects of Withania somnifera on 6-hydroxydopamine induced Parkisonism in rats. Hum Exp Toxicol. 2005;24:137–147. doi: 10.1191/0960327105ht509oa. [DOI] [PubMed] [Google Scholar]
- 11.Owais M, Sharad KS, Shehbaz A, Saleemuddin M. Antibacterial efficacy of Withania somnifera (Ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomed. 2005;12:229–235. doi: 10.1016/j.phymed.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Rasool M, Varalakshmi P. Immunomodulatory role of Withania somnifera root powder on experimental induced inflammation: an in vivo and in vitro study. Vascul Pharmacol. 2006;44:406–410. doi: 10.1016/j.vph.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 13.Singh D, Aggarwal A, Maurya R, Naik S. Withania somnifera inhibits NF-kB and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother Res. 2007;21:905–13. doi: 10.1002/ptr.2180. [DOI] [PubMed] [Google Scholar]
- 14.Devi PU, Kamath R, Rao BS. Radiosensitization of a mouse melanoma by withaferin A: in vivo studies. Indian J Exp Biol. 2000;38:432–437. [PubMed] [Google Scholar]
- 15.Devi PU, Sharad AC, Solomon FE. In vivo growth inhibitory and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma. Cancer Lett. 1995;95:189–193. doi: 10.1016/0304-3835(95)03892-z. [DOI] [PubMed] [Google Scholar]
- 16.Devi PU, Sharada AC, Solomon FE, Kamath MS. In vivo growth inhibitory effect of Withania somnifera (Ashwagandha) on a transplantable mouse tumor, sarcoma 180. Indian J Exp Biol. 1992;30:169–172. [PubMed] [Google Scholar]
- 17.Ichikawa H, Takada Y, Shishodia S, Jayaprakasam B, Nair MG, Aggarwal BB. Withanolides potentiate apoptosis, inhibit invasion, and abolish osteoclastogenesis through suppression of nuclear factor-kappaB (NF-kappaB) activation and NF-kappaB-regulated gene expression. Mol Cancer Ther. 2006;5:1434–1445. doi: 10.1158/1535-7163.MCT-06-0096. [DOI] [PubMed] [Google Scholar]
- 18.Kaileh M, Vanden Berghe W, Heyerick A, et al. Withaferin A strongly elicits IkappaB kinase beta hyperphosphorylation concomitant with potent inhibition of its kinase activity. J. Biol. Chem. 2007;282:4253–4264. doi: 10.1074/jbc.M606728200. [DOI] [PubMed] [Google Scholar]
- 19.Mohan R, Hammers HJ, Bargagna-Mohan P, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 20.Falsey RR, Marron MT, Gunaherath GM, et al. Actin microfilament aggregation induced by withaferin A is mediated by annexin II. Nat Chem Biol. 2006;1:33–38. doi: 10.1038/nchembio755. [DOI] [PubMed] [Google Scholar]
- 21.Yang H, Shi G, Dou QP. The tumor proteasome is a primary target for the natural anticancer compound withaferin A isolated from “Indian winter cherry”. Mol Pharmacol. 2007;71:426–437. doi: 10.1124/mol.106.030015. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan S, Ranga RS, Burikhanov R, Han SS, Chendil D. Par-4-dependent apoptosis by the dietary compound withaferin A in prostate cancer cells. Cancer Res. 2007;67:246–253. doi: 10.1158/0008-5472.CAN-06-2430. [DOI] [PubMed] [Google Scholar]
- 23.Malik F, Kumar A, Bhushan S, et al. Reactive oxygen species generation and mitochondrial dysfunction in the apoptotic cell death of human myeloid leukemia HL-60 cells by a dietary compound withaferin A with concomitant protection by N-acetyl cysteine. Apoptosis. 2007;12:2115–2133. doi: 10.1007/s10495-007-0129-x. [DOI] [PubMed] [Google Scholar]
- 24.Stan SD, Hahm ER, Warin R, Singh SV. Withaferin A causes FOXO3a- and Bim-dependent apoptosis and inhibits growth of human breast cancer cells in vivo. Cancer Res. 2008;68:7661–7669. doi: 10.1158/0008-5472.CAN-08-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stan SD, Zeng Y, Singh SV. Ayurvedic medicine constituent withaferin A causes G2 and M phase cell cycle arrest in human breast cancer cells. Nutr Cancer. 2008;60(S1):51–60. doi: 10.1080/01635580802381477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oh JH, Lee TJ, Kim SH, et al. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis. 2008;13:1494–1504. doi: 10.1007/s10495-008-0273-y. [DOI] [PubMed] [Google Scholar]
- 27.Mandal C, Dutta A, Malick A, et al. Withaferin A induces apoptosis by activating p38 mitogen-activated protein kinase signaling cascade in leukemic cells of lymphoid and myeloid origin through mitochondrial death cascade. Apoptosis. 2008;13:1450–1464. doi: 10.1007/s10495-008-0271-0. [DOI] [PubMed] [Google Scholar]
- 28.Yu Y, Hamza A, Zhang T, et al. Withaferin A targets heat shock protein 90 in pancreatic cancer cells. Biochem Pharmacol. 2010;79:542–551. doi: 10.1016/j.bcp.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Hahm ER, Singh SV. Withaferin A inhibits activation of signal transducer and activator of transcription 3 in human breast cancer cells. Carcinogenesis. 2010;31:1991–1998. doi: 10.1093/carcin/bgq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bode AM, Dong Z. Post-translational modification of p53 in tumorigenesis. Nat Rev Cancer. 2004;4:793–805. doi: 10.1038/nrc1455. [DOI] [PubMed] [Google Scholar]
- 31.Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 32.Land SR, Wickerham DL, Costantino JP, et al. Patient-reported symptoms and quality of life during treatment with tamoxifen or raloxifene for breast cancer prevention: the NSABP Study of Tamoxifen and Raloxifene (STAR) P-2 trial. JAMA. 2006;295:2742–2751. doi: 10.1001/jama.295.23.joc60075. [DOI] [PubMed] [Google Scholar]
- 33.Lippman ME, Allegra JC, Thompson EB, et al. The relation between estrogen receptors and response rate to cytotoxic chemotherapy in metastatic breast cancer. N Engl J Med. 1978;298:1223–8. doi: 10.1056/NEJM197806012982203. [DOI] [PubMed] [Google Scholar]
- 34.Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor α mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67:5337–44. doi: 10.1158/0008-5472.CAN-06-4582. [DOI] [PubMed] [Google Scholar]
- 35.Xiao D, Srivastava SK, Lew KL, et al. Allyl isothiocyanate, a constituent of cruciferous vegetables, inhibits proliferation of human prostate cancer cells by causing G2/M arrest and inducing apoptosis. Carcinogenesis. 2003;24:891–7. doi: 10.1093/carcin/bgg023. [DOI] [PubMed] [Google Scholar]
- 36.Xiao D, Choi S, Johnson DE, et al. Diallyl trisulfide-induced apoptosis in human prostate cancer cells involves c-Jun N-terminal kinase and extracellular-signal regulated kinase-mediated phosphorylation of Bcl-2. Oncogene. 2004;23:5594–606. doi: 10.1038/sj.onc.1207747. [DOI] [PubMed] [Google Scholar]
- 37.Shah YM, Kaul A, Dong Y, Ip C, Rowan BG. Attenuation of estrogen receptor α (ERα) signaling by selenium in breast cancer cells via downregulation of ERα gene expression. Breast Cancer Res Treat. 2005;92:239–250. doi: 10.1007/s10549-005-3203-5. [DOI] [PubMed] [Google Scholar]
- 38.Singh SV, Herman-Antosiewicz A, Singh AV, et al. Sulforaphane-induced G2/M phase cell cycle arrest involves checkpoint kinase 2-mediated phosphorylation of Cdc25C. J Biol Chem. 2004;279:25813–25822. doi: 10.1074/jbc.M313538200. [DOI] [PubMed] [Google Scholar]
- 39.Rosen EM, Fan S. Inhibition of estrogen receptor signaling. Breast Cancer Online. 2005:8. [Google Scholar]
- 40.Vousden KH, Lu X. Live or let die: the cell's response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 41.Endogenous Hormones and Breast Cancer Collaborative Group Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94:606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 42.Spears M, Bartlett J. The potential role of estrogen receptors and the SRC family as targets for the treatment of breast cancer. Expert Opin Ther Targets. 2009;13:665–674. doi: 10.1517/14728220902911509. [DOI] [PubMed] [Google Scholar]
- 43.Evans RM. The steroid and thyroid hormone receptor superfamily. Science. 1988;240:889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 45.Preisler-Mashek MT, Solodin N, Stark BL, Tyriver MK, Alarid ET. Ligand-specific regulation of proteasome-mediated proteolysis of estrogen receptor-α. Am J Physiol Endocrinol Metab. 2002;282:E891–E898. doi: 10.1152/ajpendo.00353.2001. [DOI] [PubMed] [Google Scholar]
- 46.Kang L, Ding L, Wang ZY. Isothiocyanates repress estrogen receptor α expression in breast cancer cells. Oncol Rep. 2009;21:185–192. [PMC free article] [PubMed] [Google Scholar]
- 47.Leung BS, Potter AH. Mode of estrogen action on cell proliferative kinetics in CAMA-1 cells. I. Effect of serum and estrogen. Cancer Invest. 1987;5:187–194. doi: 10.3109/07357908709011735. [DOI] [PubMed] [Google Scholar]
- 48.Perillo B, Sasso A, Abbondanza C, Palumbo G. 17beta-estradiol inhibits apoptosis in MCF-7 cells, inducing bcl-2 expression via two estrogen-responsive elements present in the coding sequence. Mol Cell Biol. 2000;20:2890–2901. doi: 10.1128/mcb.20.8.2890-2901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Frasor J, Danes JM, Komm B, Chang KC, Lyttle CR, Katzenellenbogen BS. Profiling of estrogen up- and down-regulated gene expression in human breast cancer cells: insights into gene networks and pathways underlying estrogenic control of proliferation and cell phenotype. Endocrinol. 2003;144:4562–4574. doi: 10.1210/en.2003-0567. [DOI] [PubMed] [Google Scholar]
- 50.Sun M, Paciga JE, Feldman RI, et al. Phosphatidylinositol-3-OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res. 2001;61:5985–5991. [PubMed] [Google Scholar]