Abstract
Objective
Clinical and preclinical evidence suggest a hyperactive glutamatergic system in clinical depression. Recently, the metabotropic glutamate receptor 5 (mGluR5) has been proposed as an attractive target for discovery of novel therapeutic approaches against depression. The goal of this study was to compare mGluR5 binding (PET study) and mGluR5 protein expression (postmortem study) between subjects with major depressive disorder and healthy controls.
Method
Images of mGluR5 receptor binding were acquired using PET and [11C]ABP688 that binds to an allosteric site with high specificity in 11 unmedicated subjects with major depression and 11 matched healthy controls; the amount of mGluR5 protein was investigated using Western blot method in brain samples of 15 depressed subjects and 15 matched controls (postmortem study).
Results
The PET study revealed decreased regional mGluR5 binding in the prefrontal cortex, the cingulate cortex, the insula, the thalamus and the hippocampus of the depressed individuals (uncorrected p<0.001). Severity of depression correlated negatively with mGluR5 binding in the hippocampus (cluster-level corrected p=0.029). The postmortem study showed reduced mGluR5 protein expression in the prefrontal cortex (Brodmann's area 10) in depression (p<0.014), while prefrontal mGluR1 protein expression was unchanged.
Conclusions
The reductions in mGluR5 binding found in the depressed sample are compatible with reduced protein expression in postmortem tissue. Thus, both studies suggest that basal or compensatory changes in excitatory neurotransmission play roles in the pathophysiology of major depression.
Introduction
Almost all established antidepressants target the monoamine systems (1). However, full and partial resistance to these drugs and their delayed onset of action suggest that dysfunctions of monoaminergic neurotransmitter systems found in major depressive disorder represent the downstream effects of other, more primary abnormalities. Several lines of evidence suggest a glutamatergic dysfunction as a primary abnormality in depression: A single dose of glutamate N-methyl-D-aspartate (NMDA) receptor antagonist ketamine produced rapid and large antidepressant effects in patients with treatment-resistant depression (2). Inhibitors of glutamate release (e.g., lamotrigine, riluzole) have antidepressant properties (3). Abnormal glutamate levels were found in depressed subjects as determined by magnetic resonance spectroscopy (4). Finally, there is evidence for abnormal NMDA signaling in postmortem tissue preparations (5).
Metabotropic glutamate receptors are known to regulate glutamate neurotransmission and interact with monoamine neurotransmitters that are involved in the neurobiology of depression (6). The metabotropic glutamate receptor 5 (mGluR5) has been proposed as an attractive target for modulating glutamatergic neurotransmission (7) because it is not only present at postsynaptic neurons but also on glia cells where it appears to modulate the stimulation of extrasynaptic NMDA receptors (8). In rats, prolonged blockade of mGlu5 receptors exerts a strong anxiolytic- and antidepressant-like effect (9), and mGluR5 knockout mice express an antidepressant-like phenotype (10).
This is the first study to assess mGluR5 binding and protein expression in depressed subjects. We applied PET with the radiolabeled mGluR5 antagonist 3-(6-methyl-pyridin-2-ylethynyl)-cyclohex-2-enone-O-11C-methyl-oxime ([11C]ABP688) (11), which binds to an allosteric site of the mGluR5 with high selectivity. To support the results from the PET study, a second study was performed to investigate mGluR5 protein levels in the postmortem prefrontal cortex. Next, in order to evaluate the specificity of potential mGluR5 abnormalities in depression, amounts of postmortem mGluR1 protein were investigated in the same cohort of depressed subjects.
Subjects and Methods
PET Study
Subjects
Subjects who met DSM-IV criteria for a current major depressive episode and for major depressive disorder (n=11; 5 females; mean±SD age= 40.8±13.9 years, range: 22 to 59 years; Beck Depression Inventory (BDI) total score 25.3±8.0, range: 13 to 35; Beck Anxiety Inventory (BAI) total score 18.6±10.5, range: 6 to 37) were included, as well as healthy controls (n=11; 5 females; ages 40.6±14.2 years, range 23 to 62 years; BDI total score 1.0±1.4, range: 0 to 4; BAI total score 0.6±0.8, range: 0 to 2) were included. Additional clinical characteristics of the depressed sample are included in the supplementary information (Table S1). The subjects were recruited through advertisement in local newspapers and posters at Zurich University Hospital, and evaluated during screening visits in the outpatient psychiatry clinic of the Zurich University Hospital. Psychiatric diagnoses were established both with an unstructured clinical interview by a psychiatrist and with a structured interview using the Structured Clinical Interview for the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (12,13). The clinical evaluation also included physical examination, and in some cases electrocardiography, and laboratory tests, including liver and kidney function tests, hematology profile, thyroid function tests, urine analysis, and toxicology (drug screen). Exclusion criteria included current medical or neurological disorders, lifetime history of psychosis, manic episode, substance dependence, autism, posttraumatic stress disorder, eating disorders, and exposure to psychotropic medications within 4 weeks of scanning (8 weeks for fluoxetine), regular cigarette smoking, and pregnancy. Magnetic resonance imaging (MRI) was performed on each subject and assessed by a radiologist to exclude any brain structural pathology. Subjects were entered into the study after a full explanation of the purpose of the study and the study procedures, and after written consent had been obtained as approved by the local ethical committee (Kantonale Ethikkommission Zürich).
Positron Emission Tomography
We applied a bolus/infusion protocol (14), which we had previously evaluated for PET with [11C]ABP688 (15). The incentive to use the bolus/infusion protocol was that an equilibrium between tracer in tissue and blood can be achieved (14,16). Prior to scanning, catheters were placed in the right antecubital vein for tracer injection. Subjects were scanned in a single session using PET with [11C]ABP688 in 3-dimensional mode in a whole body scanner (Discovery VCT, GE Healthcare, Milwaukee) with an axial field of view of 14.6 cm and an in-plane resolution of 7 mm. For attenuation correction, a low dose CT was acquired before tracer injection. Using a previously evaluated equilibrium protocol (15,17), a total of 500–650 MBq in a 50 ml solution was administered with an infusion pump. Half the activity was given as a bolus over 2 minutes and half was subsequently infused over 58 minutes. Simultaneously to the start of the bolus injection, we initiated a series of 20 scans. Ten frames of 60s were followed by 10 frames of 300s leading to a total duration of 60 minutes per acquisition. Transaxial images were reconstructed to a 128×128 matrix with 35 slices of 2.34×2.34×3.27mm voxel size using filtered backprojection. In each subject, it was verified that equilibrium was reached by analysing the tissue time-activity curve in a cingular (high receptor density) and a cerebellar (low receptor density) region. As shown in the supplementary information (Figure S1), the activity reached an equilibrium at 30 minutes. The ratio of tissue activity divided by the cerebellar activity at equilibrium (CT/Ccerat 45–60 minutes) was chosen as a measure for receptor density divided by affinity. At equilibrium CT/Cceris equal to the ratio of the distribution volumes of the tracer in target and reference tissue, (CT/Ccer)eq=VT/VND, where VND is the distribution volume of the non-displaceable compartment (18), in our case the cerebellum. VT/VND is referred to as distribution volume ratio (DVR). One can furthermore demonstrate that DVR equals BPT + 1, where BPT is the binding potential in the target tissue, which is often used as measure for receptor density divided by affinity (14).
Statistical Analysis
All calculations were performed using a dedicated PMOD software version 3 (PMOD Technologies, www.pmod.com) and SPM99 (Wellcome Department of Imaging Neuroscience, University College London, London, UK). The DVR images were then transformed to a common space, the Montreal Neurological Institute [MNI] template, using PMOD, and subsequently transferred to SPM99. The PET images were then filtered using a 15-mm gaussian smoothing kernel to compensate for misalignment error arising during spatial normalization and individual anatomical variation.
Based on the postmortem study, we had the a priori hypothesis that mGluR5 binding was reduced in the right prefrontal cortex. To qualitatively compare mGluR5 binding between the PET study and the postmortem study (see below and Figure 4), we extracted PET data from a predefined region-of-interest of 32.4 ccm (4051 voxels) corresponding to the location of the postmortem tissue sample (anatomical borders of the region in Talairach coordinates: x[+10 to +30], y[+60 to +70], z[0, +20]) and calculated the mean value for this region for each study participant. In an additional exploratory analysis using SPM99, the [11C]ABP688 DVR whole brain images were compared between groups in a voxel-wise analysis using a two-sample t-test model. In separate analyses in the depression group, we calculated correlations between DVR images and BDI and BAI scores. Volumes with significant differences in DVR (Table 1) were transferred to PMOD for data extraction and display. Extracted mean values of the volumes of interest were used to estimate the magnitude of group differences (percent change) and to correlate individual DVR values with symptom dimension scores (BAI, BDI).
Figure 4.
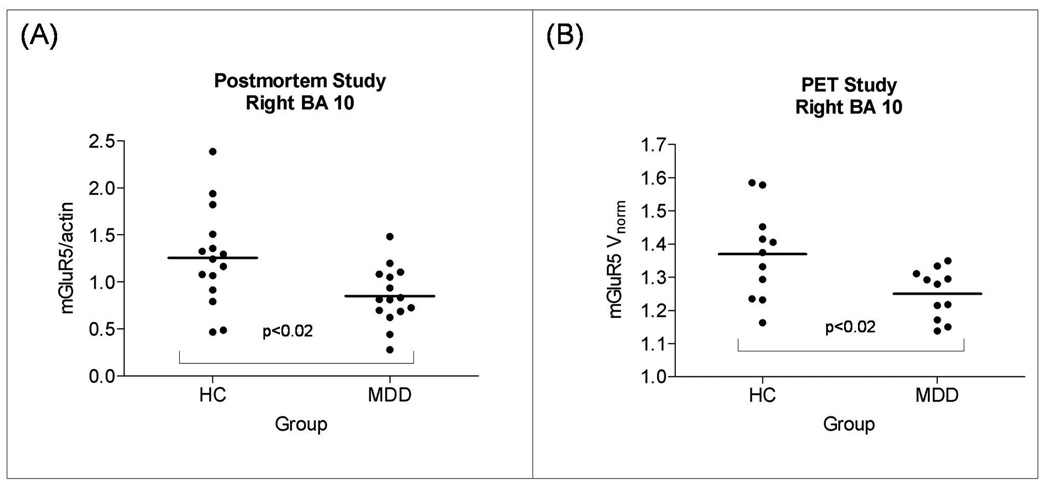
Column scatter graphs displaying mGluR5 protein levels found in the postmortem study (A), and mGluR5 binding found in the PET study (B). Both measures were derived from the identical location within the right frontal polar cortex (Brodmann's area 10; see method section). In A, the mGluR5 protein levels were normalized to actin. In B, mGluR5 DVR. Unpaired t-tests were used to compare subjects with major depressive disorder (MDD) with healthy controls (HC). In A, group means (SDs) of the mGluR5/actin ratio were 1.25 (0.52) in HC and 0.85 (0.30) in major depression. In B, group means (SDs) mGluR5 DVR were 1.37 (0.14) in HC and 1.25 (0.08) in major depression.
Table 1.
Brain regions with lower [11C]ABP688 DVR in subjects with major depressive disorder than in healthy controls (N=22)
| Brain Regions | x y z coordinatesb |
t-values | % reduction in depression |
|---|---|---|---|
| R inferior parietal lobe | 42, −47, 23 | 3.7 | 13.2 |
| R mesencephalon / thalamus | 14, −24, −12 | 3.6a | 9.6 |
| R middle / inferior temporal gyrus | 46, −45, −4 | 3.4 | 11.9 |
| L inferior parietal lobe | −38, −31, 33 | 3.3 | 11.7 |
| R anterior insula | 30, 13, 18 | 3.2 | 9.6 |
| L middle / inferior temporal gyrus | −48, −50, 3 | 3.2 | 15.2 |
| R precentral gyrus | 20, −25, 75 | 3.0 | 22.6 |
| R anterior insula / inferior prefrontal gyrus | 34, 33, −2 | 2.9 | 9.6 |
| R lateral prefrontal cortex (Brodmann's area 46) | 38, 47, 5 | 2.9 | 15.2 |
Regions were derived from contrast analyses comparing [11C]ABP688 DVR between controls and patients. All results were significant at puncorrected<0.005.
Cluster-level, corrected p value = 0.057 after applying the cluster test for multiple testing
Coordinates correspond to the stereotaxic array of Talairach and Tournoux (1988), and denote mm from the origin (anterior commissure) with positive × indicating right of midline, positive y indicating anterior, and positive z indicating dorsal to a plane containing both the anterior and posterior commissures.
Postmortem Study
Subjects
Postmortem brain samples were collected at autopsy from a total of 30 subjects at the Cuyahoga County Coroner's Office, Cleveland, OH. Informed written consent was obtained from the legal next-of-kin who were interviewed. Retrospective psychiatric assessments were conducted in accordance with an approved Institutional Review Board protocol from University Hospitals of Cleveland and the University of Mississippi Medical Center. There was no evidence of a neurological disorder in any of the subjects. A trained interviewer administered the Schedule for Affective Disorders and Schizophrenia lifetime version (SADS-L (19)) to knowledgeable next-of-kin of 10 of the depressed subjects and ten of the control subjects. The SCID was administered to next-of-kin of the five remaining depressed and control subjects (13). Axis I psychopathology was assessed and consensus diagnosis was reached in conference using information from the interview and medical records. Responses from the 10 depressed subjects evaluated with the SADS-L were also recorded in the SCID. All 15 of the depressed subjects were experiencing a depressive episode within the last two weeks of life. The average duration of depression was 8.8 ± 10.6 years. Control subjects had no Axis I psychiatric diagnosis at the time of death except for nicotine dependence. Blood and urine samples from all subjects were examined by the coroner's office for psychotropic medications and substances of abuse, including ethanol. Fifteen depressed subjects and 15 controls were as closely as possible matched for age, gender, postmortem interval, brain pH, smoking, and history of alcohol abuse (more details are included in the supplementary information, Tables S2 and S3).
Immunoblotting
The study was carried out on blocks of tissue which were dissected from the frontal pole of the right hemisphere consisting of Brodmann's area 10. Tissue from the left hemisphere was not available. Frozen blocks were cut into 50 µm-thick sections and samples containing all six cortical layers of the gray matter of area 10 were collected and stored in −80° C until assayed. For the study of cerebellum, several sections of the right cerebellar hemisphere were collected from psychiatrically healthy controls and stored until assayed. Western blot experiments were performed as previously described (5,20) except that in the current study tissue samples were not heated (at 95°C for 8 min) before being subjected to gel electrophoresis. mGluR5 was labeled using anti-mGluR5 rabbit polyclonal antibody (1:1000; Millipore, Temecula, CA, USA; no AB5675), mGluR1 was detected using anti-mGluR1 rabbit polyclonal antibody (1:500; Millipore; no. 07-617), and secondary anti-rabbit antibody (1:3000; Amersham Biosciences, no. NA934). Actin was used as a control for transfer and loading, and was detected on each blot using an anti-actin antibody (Millipore; no MAB1501). To minimize inter-blot variability and to aid in quantifying blots, each gel was loaded with 3 concentrations of a cortical tissue standard (dissected from a psychiatrically healthy subject) consisting of 10, 20, and 40 µg of total protein. The same cortical tissue standard was used for all experimental gels.
Data analysis
Immunoreactive bands were analyzed using MCID Elite 7.0 (Imaging Research, St. Catherines, ON, Canada). Linear regression was used to plot a standard curve for each gel, from which relative optical density values of samples were converted to cortical standard protein units for each experimental sample for each gel. To control for accuracy of tissue loading and efficiency of transfer, data were normalized to actin detected on the same blots. The final data are expressed in cortical standard protein units and presented as ratio of mGluR/actin. The data were analyzed statistically using a two-tailed unpaired t-test, and linear regression analysis was performed to test for potential associations between age, pH, postmortem interval, time in freezer, and duration of depression and mGluR protein level. A p value <0.05 was considered significant.
Results
PET Study
The major depression sample included four subjects who were naïve to psychotropic drugs and six had been medication-free for a mean of 15.3±13.2 months (range: 4–96). Three subjects had a comorbid anxiety disorder. The mean [11C]ABP688 dose administered did not differ significantly between the depressed and control groups (600±49 and 565±186 MBq, respectively).
In an a priori defined region-of-interest in Brodmann's area 10 (prefrontal cortex) corresponding to the tissue sample of the postmortem study, we found a reduction of mGluR5 DVR by 8.8% (p=0.018; Figure 4). Table 1 and Figure 1 display the results of the analysis comparing [11C]ABP688 DVR between depressed subjects and controls. The depressed subjects had lower DVR than controls in a region including the right mesencephalon and the right thalamus (cluster-level corrected p value=0.057), the right inferior parietal lobe, the right inferior temporal gyrus, the left inferior parietal lobe, the right anterior insula, the left middle temporal gyrus, the bilateral precentral gyrus, the bilateral lateral prefrontal cortex, the posterior cingulate cortex, the bilateral medial orbital cortex, the left frontal polar cortex, and the left hippocampus. There was no region that showed higher [11C]ABP688 DVR in controls than in depressed subjects at puncorrected<0.01.
Figure 1.
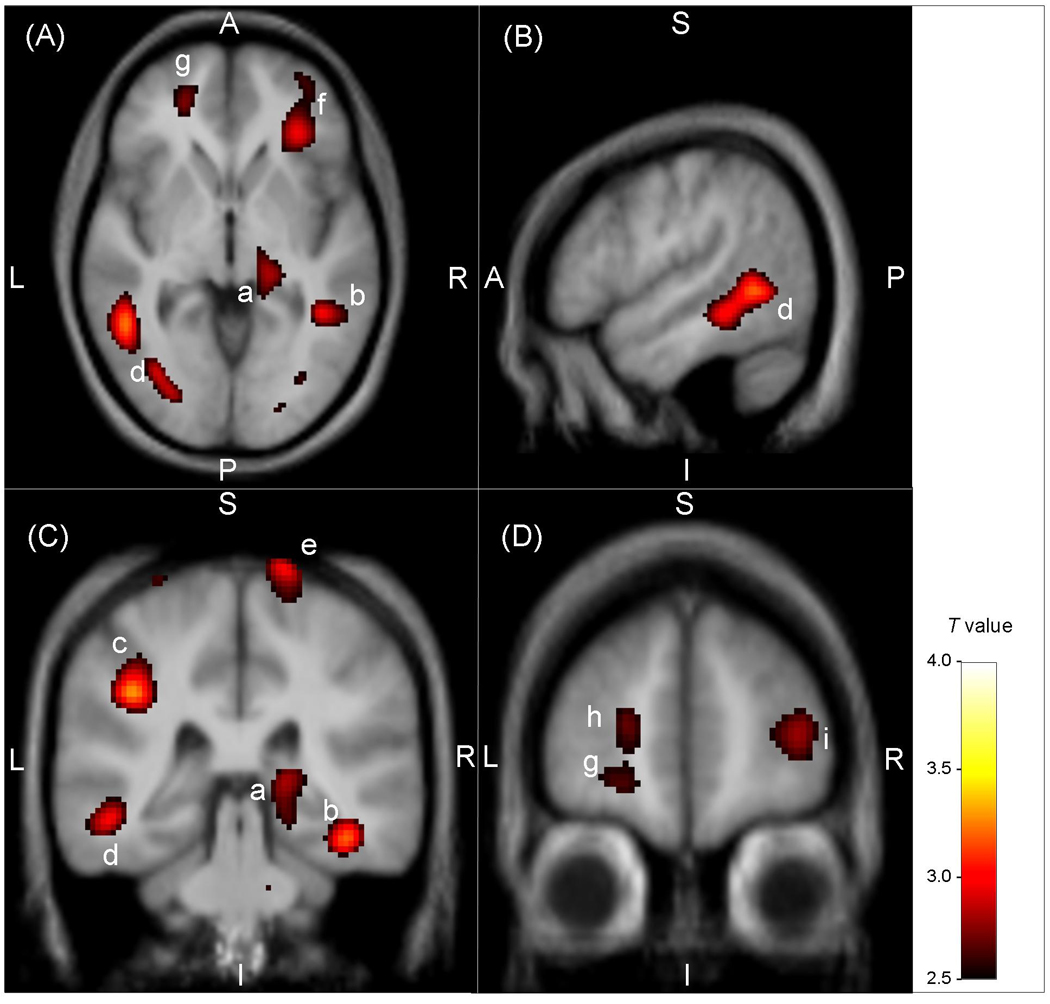
Statistical parametric map illustrating brain regions where the metabotrophic glutamate receptor subtype 5 (mGluR5) density was decreased in subjects with major depressive disorder versus healthy controls. The voxel T values correspond to p < 0.01 for the contrast of DVR between groups and are displayed on axial (A, z: −2), sagittal (B, x: −52) and coronal (C, y: −33; D, y: 51) sections of a spatially normalized and averaged MRI from the study sample. The regions are labelled with the following letters ordered by decreasing t values (Table 1): (a) right mesencephalon / thalamus, (b) right middle / inferior temporal gyrus, (c) left inferior parietal lobe, (d) left middle / inferior temporal gyrus, (e) right precentral gyrus, (f) right anterior insula / inferior prefrontal gyrus, (g) left medial orbital cortex (Brodmann's area 11), (h) left frontal polar cortex (Brodmann's area 10), (i) right lateral prefrontal cortex (Brodmann's area 46). The color bar indicates the voxel T value. Abbreviations: A, anterior; P, posterior; S, superior; I, inferior; L, left; R, right.
In the regions listed in Table 1 having reduced mGluR5 binding in depression versus controls, we found significant negative correlations between depressive symptoms as assessed by the Beck Depression Inventory (BDI) and mGluR5 binding in the depression sample (N=11) in all regions (p ranging from 0.04 to 0.0001). To identify correlations between illness severity and mGluR5 binding across the whole brain, we conducted a secondary voxelwise analysis on [11C]ABP688 DVR in the eleven subjects with major depressive disorder. Depressive symptoms as assessed by the BDI correlated negatively with mGluR5 binding in the bilateral hippocampus (right: cluster-level corrected p=0.029, see Figure 2; left: puncorrected<0.001). Anxiety symptoms as assessed with the Beck Anxiety Inventory (BAI) correlated negatively with mGluR5 binding in the bilateral thalamus, the bilateral orbital frontal cortex, the right frontal polar cortex and the left mid-cingulate cortex (all correlations at puncorrected<0.001). More details on these correlational analyses are included in the supplementary information (Table S4).
Figure 2.
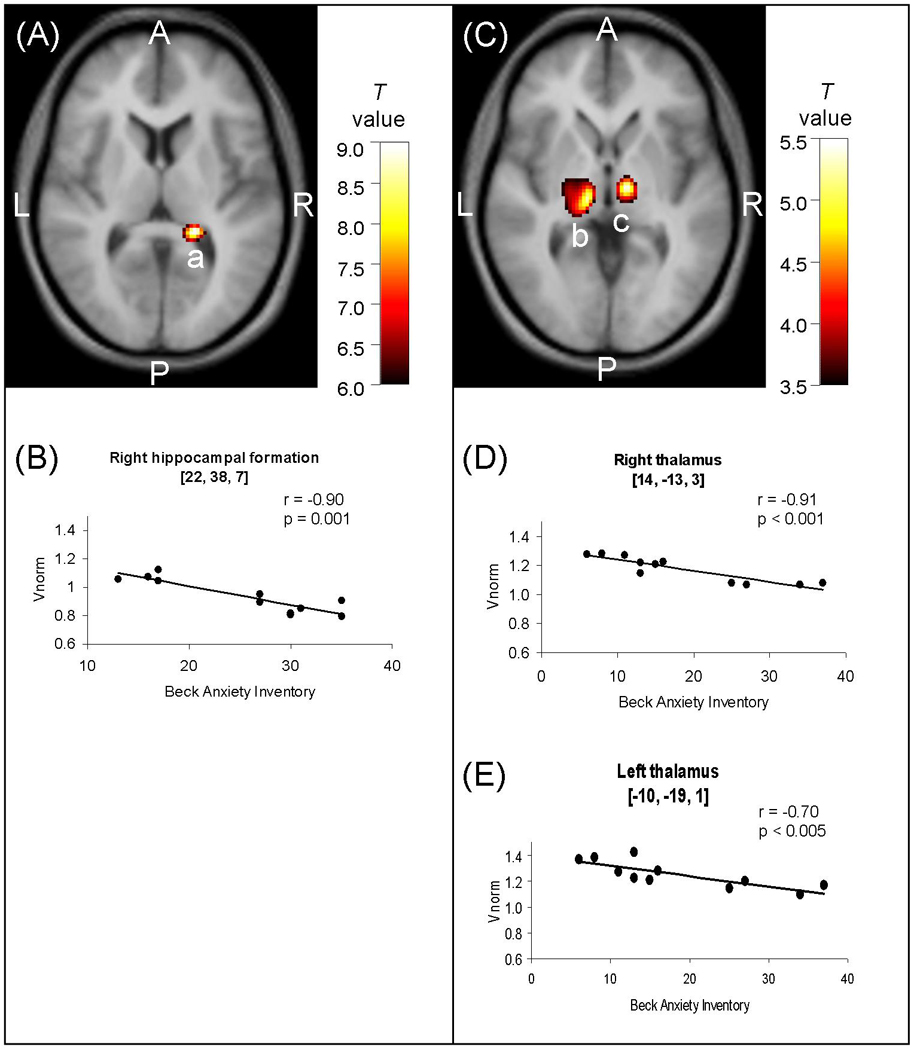
Image sections (A, C) obtained with Statistical Parametric Mapping software (SPM99; Wellcome Department of Imaging Neuroscience, London, England) and graphs (B, D, E) illustrating correlations between mGluR5 DVR and symptom severity as assessed by the Beck Depression Inventory (BDI; A, B) and the Beck Anxiety Inventory (BAI; C, D, E) in the depressed sample (N=11). In A and C, correlation maps are displayed on axial sections of a spatially normalized and averaged MRI from the study sample; in A (z=7), voxel t values correspond to p<0.0001; in C (z=−4), voxel t values correspond to p<0.003; the color bars indicate the voxel T value; a, right hippocampal formation; b, left thalamus; c, right thalamus. In B, D and E, symptoms scores are plotted against mGluR5 DVR. Correlation coefficients and p values in the right hippocampal formation (B), the right thalamus (D) and the left thalamus (E) were determined using Pearson correlation. The regression lines were determined using linear regression. Abbreviations: A, anterior; P, posterior; L, left; R, right.
Sex-based subsamples were too small to assess sex-differences within diagnostic groups. Across the whole sample (depressed subjects plus controls), no significant sex difference in mGluR5 binding was evident in the regions-of-interest defined over the areas where the groups had differed most in the initial voxel-wise analysis (p>0.08). There was also no significant correlation between mGluR5 binding and age (p>0.20) in these brain regions, neither in depressed subjects (p>0.15) nor in controls (p>0.12). In the depressed group, there was no correlation between illness duration and mGluR5 binding in any of the regions that showed reduced mGluR5 binding (p>0.137). Subjects with a history of antidepressant use (N=7) had lower mGluR5 binding in the left precentral gyrus than subjects who were drug-naïve (N=4; p=0.003), while there was no difference in mGluR5 binding between these two subgroups with major depression in all other regions-of-interest. Figure S3 in the supplementary information indicates drug status and presence of comorbid anxiety disorders for each of the 11 major depression subjects in the right prefrontal cortex.
Postmortem Study
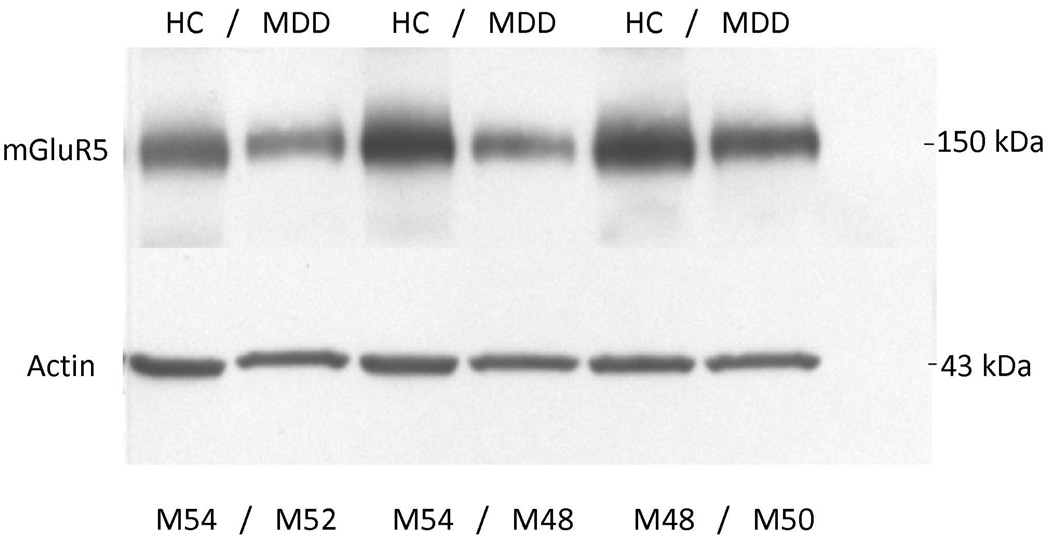
Western blot analyses of the prefrontal cortex from 15 depressed and 15 matched control subjects consistently revealed mGluR5 as a band corresponding to the molecular mass of 150 kDa. Figure 3 shows a representative immunoblot of mGluR5 and actin from three male depressed subjects and three male controls used in the analysis. The average mGluR5/actin ratio from subjects with major depression (0.85 ± 0.30) was significantly lower (−32 %) than that of matched controls (1.25±0.52, t=2.61, df=28; p=0.014; Figure 4). As cerebellum radioactivity concentration was used to perform normalization of the PET data, we investigated the level of mGluR5 protein in the cerebellum. We did not detect mGluR5 immunoreactivity in the cerebellum from control subjects using Western blot method (supplementary information, Figure S3).
Figure 3.
mGluR5 immunoreactivity in the right prefrontal cortex (Brodmann's area 10) from six male subjects used in the analysis. The bottom panel shows immunoreactive actin detected on the same blot. Each well was loaded with 15 µg of total protein. HC, healthy control; MDD, major depressive disorder; M, male.
Amounts of mGluR1 protein (150 kDa) were analyzed in the prefrontal cortex from 13 depressed and 13 matched control subjects. The same subjects (plus two additional control and two additional major depressives) were used in the mGluR5 analysis (see Table S3). The average mGluR1/actin ratio from depressed subjects (0.98±0.31) was unchanged compared to controls (1.07±0.19, t=0.95, df=24; p=0.35).
Linear regression analysis showed no significant correlations between the amount of mGluR5 or mGluR1immunoreactivity and age, postmortem interval, brain pH, time in freezer, or duration of depression.
Discussion
The present study is the first to analyze mGluR5 binding and protein expression in subjects with major depressive disorder and controls using imaging and postmortem approaches. In the PET study, we found decreased mGluR5 binding in depressed subjects versus controls, in multiple areas of the frontal, temporal and parietal cortices, in the right thalamus, bilateral insula, left hippocampus, left posterior cingulate cortex and precentral gyrus. In major depression subjects, the severity of depressive symptoms correlated negatively with mGluR5 binding in the right and left hippocampus, and anxiety symptoms correlated negatively with mGluR5 binding in the right and left thalamus, the bilateral orbital frontal cortex, the right frontal polar cortex and the left mid-cingulate cortex. In line with the PET study results, the postmortem examination revealed reduced mGluR5 protein levels in the right frontal polar cortex in depressed subjects compared to healthy controls. The postmortem study also revealed that the reduction in mGluR5 protein was specific given that we did not detect a difference in mGluR1 immunoreactivity between diagnostic groups.
Abnormalities in the glutamate receptor system have been previously observed in postmortem brain tissue from major depressives and suicide victims (6). We have reported brain region specific abnormalities in the NMDA receptor complex in depression (5,20). These studies coincide with clinical reports demonstrating potent antidepressant activity of ketamine, a NMDA receptor antagonist (2). In addition, several studies have demonstrated that selective mGluR5 antagonists have antidepressant-like effects in animals (21), and mGluR5 knockout mice showed decreased immobility in the forced swim test, which has been interpreted as antidepressant-like phenotype (10). It has been postulated that antidepressant properties of mGluR5 antagonists may involve inhibition of NMDA receptor-mediated neurotransmission and/or induction of brain-derived neurotrophic factor gene expression in the hippocampus (22).
In our PET study, the regional reductions of DVR indicate reduced binding of [11C]ABP688, which may reflect reduced mGluR5 density, a change in the affinity of the binding site or increased concentration of an unknown endogenous ligand. A reduction in mGluR5 density is consistent with our postmortem study showing reduced mGluR5 protein level in the prefrontal cortex in major depression. Thus, reduced binding to mGluR5 in depression most likely reflects reduced density of functional receptors due to decreased total protein concentration.
At the present time, we can only speculate about mechanisms that explain the widespread decreases in [11C]ABP688 binding to mGluR5 in depression in the parieto-temporo-frontal regions including the insula and orbitofrontal cortex in depression. Reduced mGluR5 binding may represent a biological trait associated with increased risk of depression, possibly due to genetic factors. Alternatively, the mGluR5 receptor binding may be reduced because receptor expression was down-regulated, possibly through the influence of repeated stress, increased glutamate activity, and/or hormonal changes (e.g., glucocorticoids) in depression. The negative correlation between mGluR5 and depression severity in the hippocampus, and between mGluR5 and anxiety in the ventrolateral thalamus suggest that reduced mGluR5 binding reflects a primary pathogenic marker or an ineffective compensatory change.
Neuroimaging, neuropathological and lesion analysis studies have provided consistent evidence that neuronal networks involving the medial and orbital prefrontal cortex and related mesiotemporal and striato-pallido-thalamic structures, as well as cortical areas, play a major role in the pathogenesis of depression (23). Neuroanatomical experiments in monkeys have shown that the orbital cortex is associated with sensory association areas in the inferior temporal cortex and somato-sensory areas associated with the insula (24). Reduced mGluR5 binding in this orbital prefrontal network may relate to impairments in the coding of affective characteristics of stimuli in depression (23). The other extended cortical network associated with depression is connected with the medial prefrontal cortex and includes regions where we also found reduced mGluR5 binding: the frontal polar cortex, posterior cingulate cortex, hippocampal formation and mesencephalic structures (25,26). Since this “visceromotor” network is involved in visceral reactions to emotional stimuli (23), reduced mGluR5 binding in this network may relate to emotional dysregulation and vegetative symptoms in depression.
Several limitations of our methods also merit comment. Our experiment’s cross-sectional design could not distinguish whether abnormalities in mGluR5 receptor binding reflected a biological vulnerability for depression or were a consequence of the illness (27). Another possible limitation is the confounding effect of medication. In the PET study, subjects were unmedicated within at least four weeks before scanning; nevertheless, we found an association between a history of antidepressant use and reduced mGluR5 binding in the left precentral gyrus suggesting that drug effects cannot be fully excluded. In the postmortem study, depressed subjects were medication-free at the time of death (based on clean postmortem toxicology screening), however, this does not exclude that antidepressants had a long-term effect on mGluR5 protein expression.
To obviate the need for the potentially painful arterial cannulation in depressed subjects, we used the bolus-infusion technique and normalized the PET images using the cerebellar radioactivity concentration. The use of the cerebellum as a reference region was based on the convincing in vivo and in vitro evidence showing that mGluR5 level is extremely low in the cerebellum relative to the brain regions that are thought to be involved in depression (31). However, some previous studies using the mGluR5 radioligand [18F]FPEB questioned the use of the cerebellum as a reference region because of the relatively high specific cerebellar binding particularly in rhesus brain tissue (28,29), but also, to a weaker extend, in human brain tissue as measured in a single subject using a relatively non-specific in vitro screen (“no-wash” assay) (29). In contrast, a recent study demonstrated that quantification of mGluR5 receptor with 18F-FPEB with non-invasive modeling using cerebellum as reference region may be feasible (30). In support of this, recent in vitro and in vivo studies suggested negligible binding in the cerebellum when using ABP688 and validated the use the cerebellum as a reference region (31,32). In addition, the postmortem study did not identify mGluR5 protein expression (supplementary information, Figure S3), and we are not aware of any other study showing detectable expression of mGluR5 protein in the cerebellum using Western blotting. Previous studies on cerebellar mRNA expression have demonstrated presence (33) and absence (34) of mGluR5 mRNA, or weak mRNA expression exclusively in Bergmann glia (35). Taken together, all studies on cerebellar mGluR5 concentration using other types of measures than in one contradictory single-subject study (29) found negligible mGluR5 expression in the cerebellum, suggesting that the cerebellum can be used as a reference region.
In conclusion, the data reported herein demonstrate reduced binding and protein expression of mGluR5 in major depressive disorder. The findings suggest that neurotransmission at mGluR5 is reduced in depression possibly as a result of basal or compensatory changes in the glutamate system activity. Implications of these findings are that mGluR5 receptor expression might be well-suited as a biomarker for depression, and a target for the discovery of novel antidepressant medications. These findings in living subjects, corroborated in postmortem tissue, encourage future studies designed to investigate genetic and environmental influences on mGluR5 and interactions among mGluR5, ionotropic glutamate receptors and monoaminergic receptor systems in mood and anxiety disorders.
Supplementary Material
Figure S1: Typical tissue time-activity curves after injecting half the activity as a bolus and the other half as a constant infusion until 60 minutes. The activity reaches a steady state at 30 minutes. At equilibrium, the ratio of the tracer concentration in tissue and blood is directly representing the total distribution volume of the tracer, which itself is directly related to receptor density (Blasberg et al., 1989; Carson et al, 1993).
Figure S2: Column scatter graphs displaying mGluR5 DVR in the 11 depressed subjects found in the PET study within the right frontal polar cortex (Brodmann's area 10; see method section). Gray shading indicates that the data was derived from subjects suffering from a comorbid anxiety disorder. Triangles indicate that data were derived from subjects who were drug-naïve at the time of the study.
Figure S3: mGluR5 immunoreactivity detected in prefrontal cortex (lanes 1 and 2) and in cerebellum (lanes 3 and 4) from two psychiatrically healthy controls. Each well was loaded with 15µg of total protein. The bottom panel shows immunoreactive actin detected on the same blot as a control for protein loading.
Acknowledgements
The authors would like to thank the PET team of Zurich University for the support in the data acquisition. The PET study was supported by Novartis Pharma AG, OPO Foundation, Zurich, Switzerland; Olga Mayenfisch Foundation, Zurich, Switzerland; Vontobel Foundation, Zurich, Switzerland; Hartmann Muller Foundation, Zurich, Switzerland. The PET data were analyzed at Zurich University Hospital by collaborators (Alfred Buck, Valerie Treyer, Alexandra Deschwanden, Gregor Hasler) who were independent of Novartis Pharma AG.
The postmortem study was supported by grant from the IDeA Program of the National Center of Research Resources (RR17701), and NARSAD (BK). We gratefully acknowledge the assistance of Drs James C Overholser, George Jurjus, Herbert Y Meltzer, Lisa Konick, Lesa Dieter and Nicole Herbst in the establishment of retrospective psychiatric diagnoses and in tissue collection in the postmortem study. We acknowledge the invaluable contributions made by the families consenting to donate brain tissue and be interviewed. The excellent assistance of the Cuyahoga County Coroner’s Office, Cleveland, OH, USA, is gratefully acknowledged.
Footnotes
Location of work: PET study: Zurich, Switzerland; postmortem study: Jackson, MS, USA
Conflict of Interest
Alexandra Deschwanden, Beata Karolewicz, Anteneh M. Feyissa, Valerie Treyer, Simon M. Ametamey, Anass Johayem, Craig A. Stockmeier, Alfred Buck and Gregor Hasler do not have a conflict of interest regarding the content of this article. Yves Auberson and Judit Sovago work for Novartis Pharma AG (Basel, Switzerland) that is developing and testing drugs targeting the mGlu5 receptor.
References
- 1.Mann JJ. The medical management of depression. N Engl J Med. 2005;353(17):1819–1834. doi: 10.1056/NEJMra050730. [DOI] [PubMed] [Google Scholar]
- 2.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 3.Kendell SF, Krystal JH, Sanacora G. GABA and glutamate systems as therapeutic targets in depression and mood disorders. Expert Opin Ther Targets. 2005;9(1):153–168. doi: 10.1517/14728222.9.1.153. [DOI] [PubMed] [Google Scholar]
- 4.Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200. doi: 10.1001/archpsyc.64.2.193. [DOI] [PubMed] [Google Scholar]
- 5.Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(1):70–75. doi: 10.1016/j.pnpbp.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashimoto K. Emerging role of glutamate in the pathophysiology of major depressive disorder. Brain Res Rev. 2009 doi: 10.1016/j.brainresrev.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 7.Sanacora G, Zarate CA, Krystal JH, Manji HK. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat Rev Drug Discov. 2008;7(5):426–437. doi: 10.1038/nrd2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Ascenzo M, Fellin T, Terunuma M, Revilla-Sanchez R, Meaney DF, Auberson YP, Moss SJ, Haydon PG. mGluR5 stimulates gliotransmission in the nucleus accumbens. Proc Natl Acad Sci U S A. 2007;104(6):1995–2000. doi: 10.1073/pnas.0609408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pilc A, Klodzinska A, Branski P, Nowak G, Palucha A, Szewczyk B, Tatarczynska E, Chojnacka-Wojcik E, Wieronska JM. Multiple MPEP administrations evoke anxiolytic- and antidepressant-like effects in rats. Neuropharmacology. 2002;43(2):181–187. doi: 10.1016/s0028-3908(02)00082-5. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Need AB, Baez M, Witkin JM. Metabotropic glutamate 5 receptor antagonism is associated with antidepressant-like effects in mice. J Pharmacol Exp Ther. 2006;319(1):254–259. doi: 10.1124/jpet.106.103143. [DOI] [PubMed] [Google Scholar]
- 11.Ametamey SM, Kessler LJ, Honer M, Wyss MT, Buck A, Hintermann S, Auberson YP, Gasparini F, Schubiger PA. Radiosynthesis and preclinical evaluation of 11C-ABP688 as a probe for imaging the metabotropic glutamate receptor subtype 5. J Nucl Med. 2006;47(4):698–705. [PubMed] [Google Scholar]
- 12.First MB, Spitzer RL, Gibbon M, Williams JBW. Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2001. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. [Google Scholar]
- 13.First MB, Gibbon M, Spitzer RL, Williams JBW. Nonpatient Edition (SCID-I/NP) New York: New York State Psychiatric Institute; 1996. Structured clinical interview for DSM-IV Axis I Disorders. [Google Scholar]
- 14.Carson RE, Channing MA, Blasberg RG, Dunn BB, Cohen RM, Rice KC, Herscovitch P. Comparison of bolus and infusion methods for receptor quantitation: application to [18F]cyclofoxy and positron emission tomography. J Cereb Blood Flow Metab. 1993;13(1):24–42. doi: 10.1038/jcbfm.1993.6. [DOI] [PubMed] [Google Scholar]
- 15.Burger C, Deschwanden A, Ametamey S, Johayem A, Mancosu B, Wyss M, Hasler G, Buck A. Validation of a Bolus/Infusion Protocol for 11C-ABP688, a PET Tracer for mGluR5. Nucl Med Biol. 2010 doi: 10.1016/j.nucmedbio.2010.04.107. in press. [DOI] [PubMed] [Google Scholar]
- 16.Blasberg RG, Carson RE, Kawai R, Patlak CG, Sawada Y, Channing M, Chelliah M, Herscovitch P. Strategies for the study of the opaite receptor in brain: application to the opiate antagonist cyclofoxy. J Cereb Blood Flow Metab. 1989;9:S732. [Google Scholar]
- 17.Treyer V, Streffer J, Wyss MT, Bettio A, Ametamey SM, Fischer U, Schmidt M, Gasparini F, Hock C, Buck A. Evaluation of the metabotropic glutamate receptor subtype 5 using PET and 11C-ABP688: assessment of methods. J Nucl Med. 2007;48(7):1207–1215. doi: 10.2967/jnumed.107.039578. [DOI] [PubMed] [Google Scholar]
- 18.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, Holden J, Houle S, Huang SC, Ichise M, Iida H, Ito H, Kimura Y, Koeppe RA, Knudsen GM, Knuuti J, Lammertsma AA, Laruelle M, Logan J, Maguire RP, Mintun MA, Morris ED, Parsey R, Price JC, Slifstein M, Sossi V, Suhara T, Votaw JR, Wong DF, Carson RE. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 19.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35(7):837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 20.Karolewicz B, Szebeni K, Gilmore T, Maciag D, Stockmeier CA, Ordway GA. Elevated levels of NR2A and PSD-95 in the lateral amygdala in depression. Int J Neuropsychopharmacol. 2009;12(2):143–153. doi: 10.1017/S1461145708008985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palucha A, Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol Ther. 2007;115(1):116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 22.Legutko B, Szewczyk B, Pomierny-Chamiolo L, Nowak G, Pilc A. Effect of MPEP treatment on brain-derived neurotrophic factor gene expression. Pharmacol Rep. 2006;58(3):427–430. [PubMed] [Google Scholar]
- 23.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213(1–2):93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saleem KS, Kondo H, Price JL. Complementary circuits connecting the orbital and medial prefrontal networks with the temporal, insular, and opercular cortex in the macaque monkey. J Comp Neurol. 2008;506(4):659–693. doi: 10.1002/cne.21577. [DOI] [PubMed] [Google Scholar]
- 25.Drevets WC. Orbitofrontal cortex function and structure in depression. Ann N Y Acad Sci. 2007;1121:499–527. doi: 10.1196/annals.1401.029. [DOI] [PubMed] [Google Scholar]
- 26.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003;460(3):425–449. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 27.Hasler G, Drevets WC, Manji HK, Charney DS. Discovering endophenotypes for major depression. Neuropsychopharmacology. 2004;29(10):1765–1781. doi: 10.1038/sj.npp.1300506. [DOI] [PubMed] [Google Scholar]
- 28.Hamill TG, Krause S, Ryan C, Bonnefous C, Govek S, Seiders TJ, Cosford ND, Roppe J, Kamenecka T, Patel S, Gibson RE, Sanabria S, Riffel K, Eng W, King C, Yang X, Green MD, O'Malley SS, Hargreaves R, Burns HD. Synthesis, characterization, and first successful monkey imaging studies of metabotropic glutamate receptor subtype 5 (mGluR5) PET radiotracers. Synapse. 2005;56(4):205–216. doi: 10.1002/syn.20147. [DOI] [PubMed] [Google Scholar]
- 29.Patel S, Hamill TG, Connolly B, Jagoda E, Li W, Gibson RE. Species differences in mGluR5 binding sites in mammalian central nervous system determined using in vitro binding with [18F]F-PEB. Nucl Med Biol. 2007;34(8):1009–1017. doi: 10.1016/j.nucmedbio.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 30.Barret O, Tamagnan G, Batis J, Jennings D, Zubal G, Russell D, Marek K, Seibyl J. Quantitation of glutamate mGluR5 receptor with 18F-FPEB PET in humans. J Nucl Med. 2010;51 Supplement 2:215. [Google Scholar]
- 31.Elmenhorst D, Minuzzi L, Aliaga A, Rowley J, Massarweh G, Diksic M, Bauer A, Rosa-Neto P. In vivo and in vitro validation of reference tissue models for the mGluR(5) ligand [(11)C]ABP688. J Cereb Blood Flow Metab. 2010;30(8):1538–1549. doi: 10.1038/jcbfm.2010.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ametamey SM, Treyer V, Streffer J, Wyss MT, Schmidt M, Blagoev M, Hintermann S, Auberson Y, Gasparini F, Fischer UC, Buck A. Human PET studies of metabotropic glutamate receptor subtype 5 with 11C-ABP688. J Nucl Med. 2007;48(2):247–252. [PubMed] [Google Scholar]
- 33.Malherbe P, Kew JN, Richards JG, Knoflach F, Kratzeisen C, Zenner MT, Faull RL, Kemp JA, Mutel V. Identification and characterization of a novel splice variant of the metabotropic glutamate receptor 5 gene in human hippocampus and cerebellum. Brain Res Mol Brain Res. 2002;109(1–2):168–178. doi: 10.1016/s0169-328x(02)00557-0. [DOI] [PubMed] [Google Scholar]
- 34.Daggett LP, Sacaan AI, Akong M, Rao SP, Hess SD, Liaw C, Urrutia A, Jachec C, Ellis SB, Dreessen J, et al. Molecular and functional characterization of recombinant human metabotropic glutamate receptor subtype 5. Neuropharmacology. 1995;34(8):871–886. doi: 10.1016/0028-3908(95)00085-k. [DOI] [PubMed] [Google Scholar]
- 35.Berthele A, Platzer S, Laurie DJ, Weis S, Sommer B, Zieglgansberger W, Conrad B, Tolle TR. Expression of metabotropic glutamate receptor subtype mRNA (mGluR1-8) in human cerebellum. Neuroreport. 1999;10(18):3861–3867. doi: 10.1097/00001756-199912160-00026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Typical tissue time-activity curves after injecting half the activity as a bolus and the other half as a constant infusion until 60 minutes. The activity reaches a steady state at 30 minutes. At equilibrium, the ratio of the tracer concentration in tissue and blood is directly representing the total distribution volume of the tracer, which itself is directly related to receptor density (Blasberg et al., 1989; Carson et al, 1993).
Figure S2: Column scatter graphs displaying mGluR5 DVR in the 11 depressed subjects found in the PET study within the right frontal polar cortex (Brodmann's area 10; see method section). Gray shading indicates that the data was derived from subjects suffering from a comorbid anxiety disorder. Triangles indicate that data were derived from subjects who were drug-naïve at the time of the study.
Figure S3: mGluR5 immunoreactivity detected in prefrontal cortex (lanes 1 and 2) and in cerebellum (lanes 3 and 4) from two psychiatrically healthy controls. Each well was loaded with 15µg of total protein. The bottom panel shows immunoreactive actin detected on the same blot as a control for protein loading.