Abstract
To what extent does attention modulate neural activity in early visual areas? fMRI measurements of attentional modulation in primary visual cortex (V1) show large effects, while single unit recordings show much smaller ones. This discrepancy suggests that fMRI measures of attention may be inflated, perhaps by activity related to other processes. To test whether effects measured with fMRI actually reflect attentional enhancement, we used a rapid acquisition protocol to determine their timing. Subjects were presented with two stimuli on either side of fixation and were cued to attend one and ignore the other. Attended stimuli showed a greater magnitude of response in V1, but this increase was delayed, by roughly one second in time, relative to both unattended responses and response increases due to boosting stimulus contrast. These results suggest that fMRI measurements of attention may primarily depend upon other processes that take a relatively long time to feed back to V1. Our results demonstrate the importance of using the fine timing information available in the fMRI response.
Introduction
In visual cortex, attended stimuli produce larger responses than unattended ones. There is evidence that these changes in visual cortex activity are driven by top-down signals from frontal and parietal regions (e.g. Bressler et al., 2008; Lauritzen et al., 2009). Debate remains, however, about the magnitude of effects of attention in early visual cortex. Studies using single unit recording have generally reported small effects of attention in V1 (Haenny and Schiller, 1998; Luck et al., 1997; McAdams and Reid, 2005; Moran and Desimone, 1985; Motter, 1993), while studies using fMRI have reported larger ones (e.g. Beauchamp, Cox, and DeYoe, 1997; Ghandi, Heeger, and Boynton, 1999; Somers et al., 1999). Part of this discrepancy may be explainable by an increased sensitivity of fMRI to changes in baseline neural activity that is independent of stimulus presentation (Kastner et al., 1999; Murray, 2008). It has also been suggested that attention alters the gain of the fMRI signal to a greater degree than it alters the underlying neural response (Yoshor et al., 2007).
The relative timing of neural signals can give important clues to their functional significance. If spatial attention is already focused on a location where a visual target appears, response enhancement can begin very soon after stimulus presentation. While comparison between studies is complicated by different experimental parameters, most single unit studies in V1 have found effects of attention by around 200 msec post-stimulus (Motter, 1993; Luck et al., 1997; Roelfsema et al., 2003; McAdams and Reid, 2005; Roberts et al, 2007). This timing is in line with behavioral estimates of attentional latency (Muller and Findlay, 1988; Muller and Rabbitt, 1989). Thus, for the neural enhancement measured with fMRI to be attributable to visual attention, it should begin in the approximate range of 0 to 200 msec following a stimulus.
We measured the fine timing of attentional response enhancement with fMRI. Prior work has demonstrated that fMRI has relatively good temporal precision (e.g. Menon, Luknowsky, and Gati, 1998; Miezin et al., 2000; Saad et al., 2001), though no prior study has used it to characterize neural feedback. Our results indicate that enhancement of V1 response occurs late, likely too late for it to be involved in spatial attention. Enhancement of later visual area V4 responses occurred earlier in time, demonstrating that the method had adequate sensitivity to detect shorter latencies.
Results
Participants viewed two drifting gratings, one on either side of a central fixation (Figure 1), and were cued to attend one and ignore the other. Their task was to discriminate accelerating gratings from decelerating gratings, at the cued location. The cued location remained constant for the duration of each scan, but changed between scans. Stimuli were presented at two contrast levels: 50% and 100%. We performed a rapid acquisition fMRI protocol (TR=250 ms), gathering data from visual areas V1 and V4. The rapid TR allowed data collection from only three slices, so to optimize slice placement, we used online analysis of a functional localizer scan (See Methods). The slices were positioned to overlap with regions active during the functional localizer, both in the calcarine sulcus to target V1, and along ventral visual cortex to target V4.
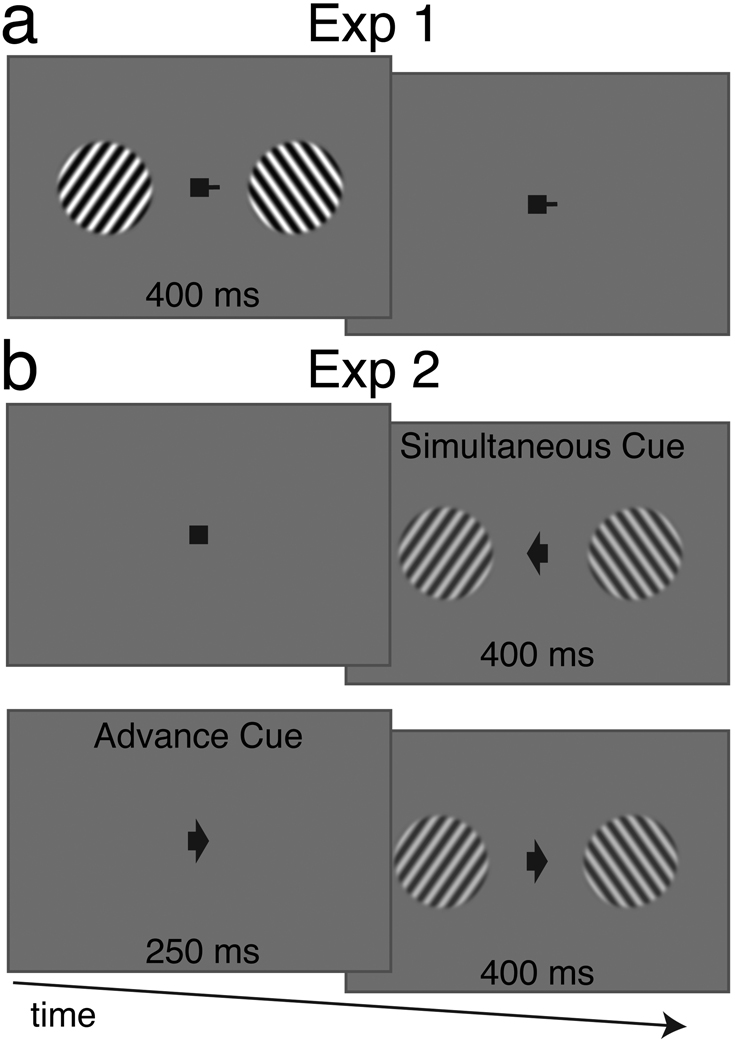
Figure 1.
Design of Experiments 1 and 2. Subjects viewed two sets of drifting gratings on either side of fixation with a duration of 400 ms. The task was to identify the motion of one grating as accelerating or decelerating. A cue at fixation indicated which of the gratings the subject should attend. In Experiment 1 (a), the cued location was constant for the entire scan. One half of trials contained gratings with 50% contrast and the other half contained trials with 100% contrast. In Experiment 2 (b), the cued location varied from trial to trial. The cue appeared either simultaneous (top) with, or 250 ms in advance of (bottom), the appearance of the gratings.
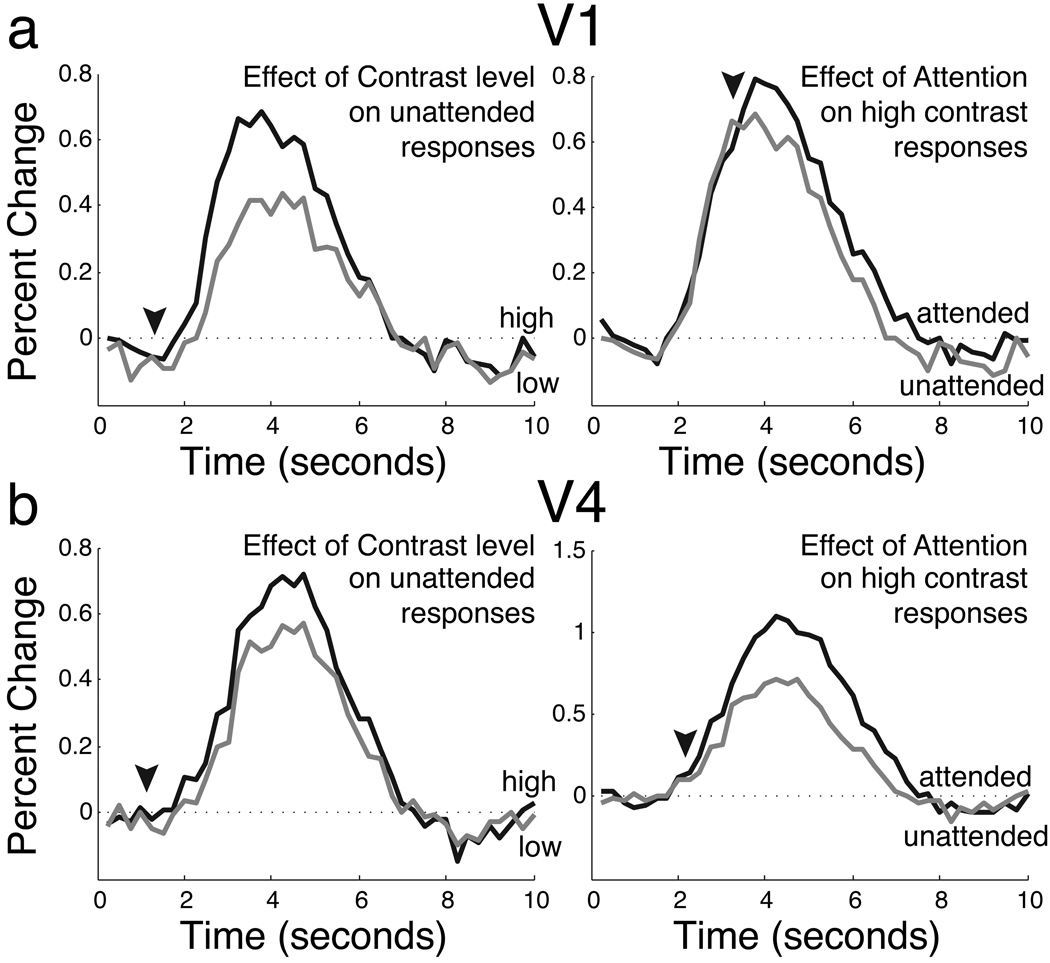
Attention increased the overall size of the neural response in both areas (V1, p<.05; V4, p<.01). Averaged event-related responses for V1 and V4 are shown in Figure 2. In addition to an overall increase in response strength, the attended responses appeared to be delayed relative to the unattended responses. Examination of the time courses reveals that the attended and unattended responses only began to separate during the rising slope of the response (Figure 2, arrowheads). The timing of enhancement due to increased stimulus contrast, on the other hand, began immediately.
Figure 2.
Experiment 1 responses in V1 (c) at two contrast levels (top) and with and without attention (bottom). Arrowheads indicate points at which the timecourses diverge. In the top panel, responses to high (black) and low (gray) contrast stimuli diverge immediately. In the bottom panel, responses to attended (black) and unattended (gray) stimuli diverge later.
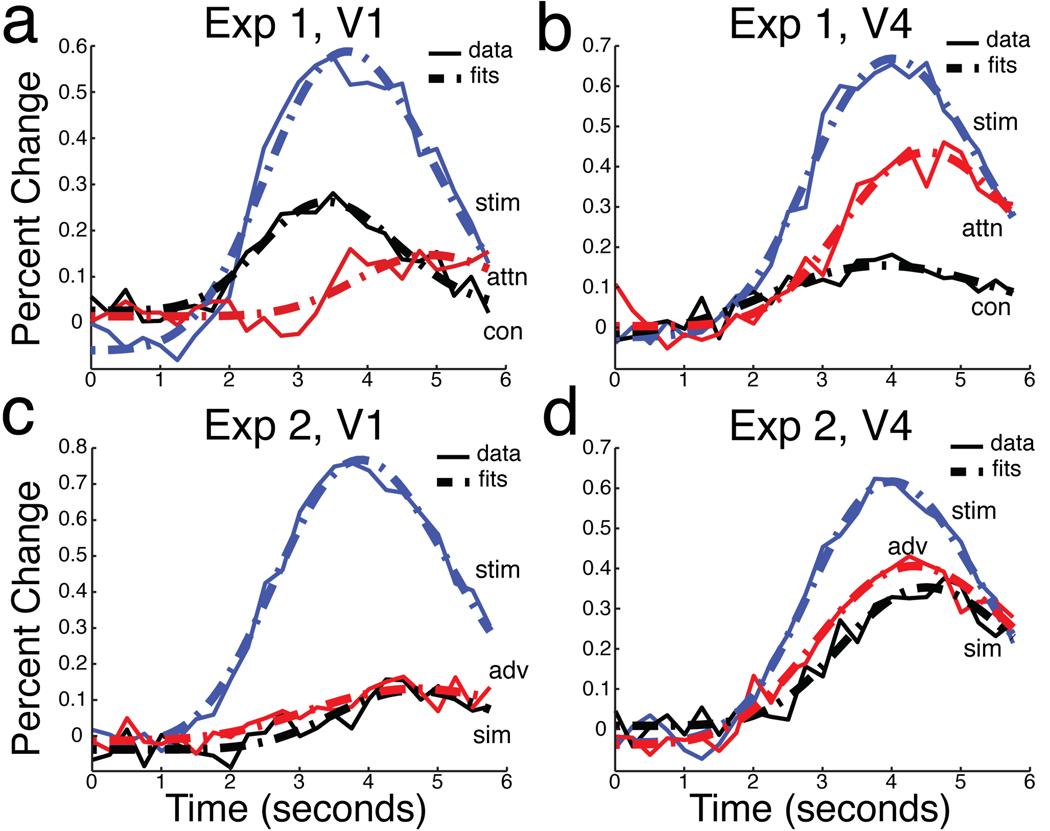
To compare the timing of stimulus-related, attention-related, and contrast increase-related effects, we created new time courses by averaging and subtracting combinations of the original event-related responses. To characterize stimulus-driven activity, we averaged responses to the high and low contrast unattended stimuli. To characterize activity due to attention, we averaged responses to unattended stimuli (high and low contrast) and subtracted them from the average response to attended stimuli. To characterize activity due to an increase in contrast, we averaged the responses to low contrast stimuli (attended and unattended) and subtracted them from the average response to high contrast stimuli. The stimulus-related, attention-related, and contrast increase-related time courses are plotted in Figure 3 for areas V1 and V4. The contrast increase-related response begins at the same time as the stimulus-related response, while the attention effect begins later in time.
Figure 3.
Response components and fits in Experiments 1 and 2. Estimated HRFs are plotted in solid lines, and the best-fitting gamma functions are plotted in broken lines. In Experiment 1 (a,b), Stimulus-driven (stim) components are plotted in blue; attention-related (attn) components are plotted in red; contrast-related (con) components are plotted in black. In Experiment 2 (c,d), Stimulus-driven (stim) components are plotted in blue; attention-related components are plotted in red for the Advance condition (adv) and in black for the Simultaneous condition (sim).
We next quantified the timing differences between conditions. Because the shapes of the contrast-related time course and the attention-related time course were different, simple cross-correlation techniques would have yielded biased results. To overcome this, we fit a model of the hemodynamic response to each time course and used the fit to estimate the latency of the response. First, we fit a gamma function to each subject’s stimulus, contrast, and attention-related time courses (Figure 3, broken traces). Then, we estimated the time point at which each of these fits crossed threshold levels of activity. We next computed a delay for the attention and contrast- related conditions by subtracting the threshold crossing time point for the stimulus-related component. Finally, we plotted these delays as a function of the threshold, which spanned from 15% to 85% of the peak level of activity.
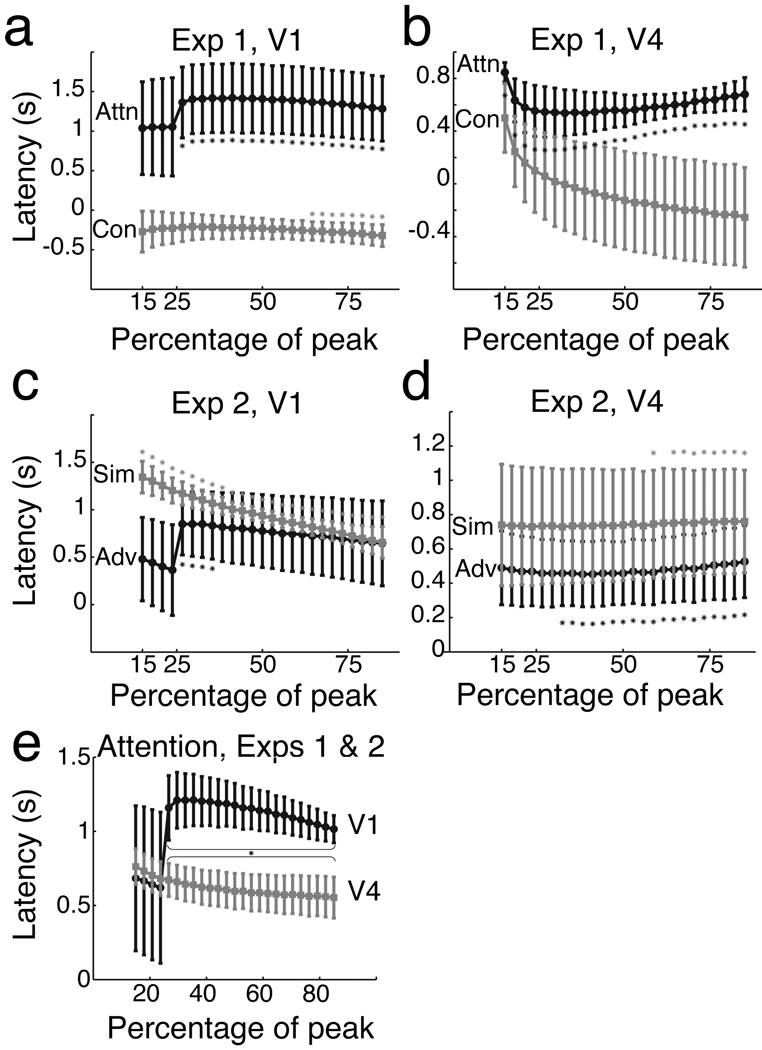
Attention-related activity in V1 was very delayed relative to stimulus-related activity. Figure 4 shows the delay as a function of threshold, and notes the many individual time points at which the relative delay is reliably above zero (p<.05). The average delay across all threshold levels was 1322±445 (s.e.m) milliseconds in V1. The contrast-related activity actually preceded the stimulus-related activity, and hence plots in Figure 4 as negative numbers. This was due to the higher contrast responses starting earlier in time than the lower contrast responses. The average delay of the contrast-related activity was −249±132 milliseconds in V1.
Figure 4.
Estimated response latencies in Experiments 1 and 2. The relative delay between the stimulus-driven activity, and the attention-related or contrast-related activity is measured between various percentages of each response. In Experiment 1 (V1, a; V4, b), the black trace shows the delay of attention-related activity and the gray trace shows the delay of contrast-related activity. In Experiment 2 (V1 c; V4 d), the black trace shows the delay of attention-related activity with the Advance cue, and the gray shows the delay with the Simultaneous cue. Errorbars indicate one standard error; asterisks denote points significantly different than zero. We combined Experiments 1 and 2 to compare the timing of attention in V1 and V4 (e); points where V1 delays were significantly longer are marked with an asterisk.
Attention-related activity was less delayed in area V4, averaging 598±100 milliseconds. This delay differed significantly from zero at most threshold levels. The contrast increase-related delay was small (−71±340 milliseconds) and unreliable in V4.
This first experiment revealed very delayed response enhancement in V1 when subjects attended one location for the duration of the scan. However, it is possible that subjects returned attention to the central fixation between trials, and shifted it to the target location only once they detected a target, instead of maintaining it there throughout the scan. In this alternative account, the delays we observed simply might be due to the time needed to shift attention across space to the peripheral location, rather than reflecting the operation of already spatially focused attention.
Experiment 2 tested this possibility by requiring subjects to shift attention to the peripheral target on every trial. A central arrow cue at the start of each trial directed the observer’s attention to one side or the other (Figure 1b), with direction randomized across trials. This forced subjects to shift their attention from the central cue to the peripheral target location. The target, which was the same as in the first experiment, appeared either at the same time as the cue (Simultaneous cue condition), or 250 milliseconds after the cue (Advance cue condition). Given that behavioral performance is improved between 50–200 milliseconds after an attention cue (Eriksen and Collins, 1969), we predicted that the Advance cue would provide sufficient time for spatial attention to have reached the target location by the time the stimulus appeared. Differences in delays between the two conditions are thus likely to be due to the time to shift spatial attention to the target location.
Figure 3(c,d) shows the results of Experiment 2. Attention increased the amplitude of response by a small but reliable amount in V1, and by a larger amount in V4 (both p<.01). As in Experiment 1, attended and unattended responses began at the same time and only separated during the rising slope of the response.
We characterized stimulus-related activity and attention-related activity as before, though this was now done separately for both cueing conditions. Stimulus-related activity was computed by averaging fMRI responses across both unattended conditions. Attention-related activity in the two cueing conditions (Simultaneous and Advance) was computed by subtracting unattended from attended responses separately for each condition. These three response components are plotted in Figure 3, panels c and d.
Long delays were maintained in V1 even when the attentional cue was given in advance. We quantified the size of the relative delays in activity using the same gamma-fitting procedure used in the previous experiment (Figure 4c,d). Both Simultaneous and Advance cue responses showed significantly positive delays at individual threshold levels in V1 (p<.05). The average delay was 702±355 ms for the Advance cue and 957±66 ms in V1 for the Simultaneous cue. This difference likely reflects the amount of time required for attention to shift locations, and its relatively small size suggests that it cannot account for the long delays we observe for attentional effects generally.
V4 also showed delayed attention-related activity in both conditions. Delays were significantly positive at many threshold levels, and the average delay was 476±195 ms for the Advance cue and 743±323 ms for the Simultaneous cue.
We next compared the attention-related delays in V1 and V4 (Figure 4e). To increase our power, we combined the attention-related activity in both experiments. First, we averaged each subject’s latency estimates in the Advance and Simultaneous cue conditions (Experiment 2). Then, because all four subjects in Experiment 2 participated in Experiment 1, we averaged each subject’s combined Experiment 2 delays with their attention delay from Experiment 1. The averaged delays for the combined experiments were 1059±190 ms in V1 and 617±117 ms in V4. Delays in V1 were reliably greater than delays in V4 (p<.01). Thus, while attention-related activity was significantly delayed in both V1 and V4, the delays were longer in V1 than they were in V4. Our ability to measure shorter delays in V4 also implies that the long delays we observed in V1 were not simply artifacts of our delay estimation methods.
The delays we observed in V1 are likely greater than the amount of time required by subjects to allocate attention, which is generally in the range of a few hundred milliseconds (Muller and Findlay, 1988; Muller and Rabbitt, 1989). To confirm this timing for our paradigm, we carried out a psychophysical experiment (Figure S1). Subjects performed the task of Experiment 2, but with 5 cue-stimulus intervals of 0, 250, 500, 750, and 1000 msec. Performance was worst when the cue-stimulus interval was 0, and improved reliably when subjects were given 250 msec to allocate attention. For both subjects, increasing delays beyond 500 msec did not reliably increase performance further, indicating that attentional allocation was generally complete by that time.
Discussion
The aim of this work was to measure the timing of the attentional enhancement of activity in human visual cortex with fMRI. We found that enhancement due to attention was delayed in both V1 and V4 beginning approximately 700–1300 ms after the stimulus-driven response in V1, and 475–750 ms after the stimulus-driven activity in V4. Advance cuing of the target location in Experiment 2 reduced, but did not eliminate, the delay in attentional enhancement. Critically, not all increases in response are delayed to relative to baseline conditions, as shown by the slightly negative delays observed for contrast increase-related responses. Similarly, the shorter delays found for V4 indicate that our method had reasonable temporal sensitivity.
Classical behavioral estimates place the beginning of attentional enhancement between 100–300 msecs after cue onset (Muller and Findlay, 1988; Muller and Rabbitt, 1989). Measures of the timing of enhancement using single unit recording place it between 0–200 msecs post-stimulus (Motter, 1993; Luck et al., 1997; Roelfsema et al., 2003; McAdams and Reid, 2005; Roberts et al, 2007, though see Buffalo et al., 2009), while EEG and MEG measurements place it within a range of 0 to 250 msecs (Noesselt et al, 2002; Boehler et al., 2008; Kelley, Gomez-Ramirez, and Foxe, 2008; Poghosyan and Ioannides, 2008). With fMRI, attention-related activity in parietal regions known to influence V1 (IPS; Bressler et al., 2008) has been shown to lead activity in V1 by approximately 500 ms (Lauritzen et al., 2009).
Our estimate of the latency of attention effects in V1 is well outside the range of previous data. In our task, performance peaked with a cue 500 milliseconds before the stimulus. Thus, it is unlikely that the long delays we observed in V1 reflected unusually slow attentional deployment in the task we used. Thus, the long delayed increase in V1 activity may be unrelated to attentional processing.
Response components that begin close to one second after stimulus-driven activity could be due to non-sensory processes that correlate with the presence of spatial attention. For example, signals in V1 have been reported due to reward (Shuler and Bear, 2006), awareness (Ress and Heeger, 2003), and task structure (Jack et al., 2006). Additionally, late effects could be due to a sub-type of attentional processing, for example scrutiny for high resolution detail, that arises late in processing (Buffalo et al., 2009). The delayed enhancement of V1 activity we observed could be due any or all of these processes. How these results relate to attention-related decreases in activity driven by feedback (Murray, et al, 2002) is an open question.
Our results do not, of course, rule out attention having early effects in V1. Attention likely influences both initial stimulus-related activity and later neural response. Our results suggest only that the later effects dominate fMRI measurements. It remains possible that other paradigms (e.g. using different tasks, pulse sequences, attentional conditions) could isolate early effects of attention with fMRI, but it is worth noting that our paradigm is similar to many used in the literature. Furthermore, our data do not address possible effects of endogenous attention caused by the appearance of the stimuli, since the simultaneous appearance, and equal contrast, of the attended and unattended gratings would lead to equal endogenous attention.
Not all prior studies have found attentional effects in V1. Single unit recordings have yielded varying amounts of attentional modulation: from strong (Motter, 1993; McAdams and Reid, 2005), to weak (Haenny and Schiller, 1988; Yoshor et al., 2007), to none at all (Moran and Desimone, 1985, although the lack of modulation in this study may have been due to methodological limitations). Similarly, scalp-recorded electrical potential (ERP) and magnetic field (ERMF) studies are divided between those that show effects in V1 (Kelly, Gomez-Ramirez, and Foxe, 2008; Poghosyan and Ioannides, 2008; Oakley and Eason, 1990), and those that do not (Heinze et al., 1994; Mangun and Hillyard, 1988; Wijers, Lange, Mulder, and Mulder, 1997). In contrast, fMRI experiments have consistently shown effects of spatial attention in V1 (e.g. Beauchamp, Cox, and DeYoe, 1997; Kastner et al., 1999; Gandhi, Heeger, and Boynton, 1999; Somers, Dale, Seiffert, and Tootell, 1999). Our results suggest that some of these fMRI effects may be due to processes other than attention, that are engaged relatively long after stimulus presentation.
An alternative explanation for prior fMRI results is that attentional effects are mainly due to stimulus-independent changes in baseline activity (Kastner et al., 1999). This hypothesis received support from a study that found little effects of attention using an event-related paradigm in which sustained baseline effects are automatically subtracted out (Murray, 2008). Our first experiment used a similar method, and nevertheless found effects of attention in V1. It is possible that differences in the task (two attended locations; spatial frequency discrimination) produced the discrepant results.
Our method for estimating the timing of neural activity relies on assumptions of linearity in the BOLD response, such that delays in the timing of neural activity are reliably preserved in the BOLD response (Boynton, Engel, Glover, and Heeger, 1996; Dale and Buckner, 1997). While previous work has demonstrated that fMRI can be used to measure temporal shifts in neural activity with reasonable precision (Menon, Luknowsky, and Gati, 1998; Miezin et al., 2000; Saad et al., 2001), further characterization of the linearity of the BOLD response may refine interpretation of these data. Given its excellent spatial resolution, the ability to use fMRI to resolve discrete neural events separated by hundreds of milliseconds in time should prove to be a useful tool to test for feedback effects in cortex.
Methods
Subjects
In Experiment 1, one left-handed and four right-handed individuals (two male, three female) participated. The subjects were between 27 and 41 years of age (mean = 31.4) and had normal or corrected to normal vision. In Experiment 2, one left-handed individual and three right-handed individuals (two male, two female) participated. The subjects were between 27 and 31 years of age (mean = 29) and had normal or corrected to normal vision. The studies were performed under a protocol approved by the UCLA Office for Protection of Research Subjects.
Stimuli and task
Subjects were instructed to fixate a square in the center of a screen while they performed a demanding two alternative, forced choice motion discrimination task (Figure 1). On each trial, drifting gratings simultaneously appeared on either side of fixation for 400 milliseconds. The subjects were cued to a location and were required to identify the motion of the cued grating as accelerating or decelerating. Whether the target grating as accelerating or decelerating was randomly assigned on each trial. Non-target gratings were independently assigned, so no task-related information was available at the uncued location.
In both experiments, subjects viewed drifting gratings that were contained within two 5.5 degree apertures, located 7.5 degrees on either side of fixation. The spatial frequency of the gratings was 1.5 cycles/degree and they were oriented at one of four possible orientations {0; π/4; π/2;3 π/4}, randomly chosen on each trial. The gratings drifted at one of three base rates (0.53, 0.56, or 0.59 degrees/second), randomly chosen on each trial and would accelerate or decelerate at one of 40 levels between 5.3 × 10−7 and 8.2 × 10−1 degrees/second2. The acceleration or deceleration level used on each trial was chosen using a 1-up, 3-down staircasing method.
While the task was the same in the two experiments, the stimuli and the cueing method varied. In Experiment 1, a short line extended from a central fixation square to the left or the right, directing the subject to attend to the grating that would appear on that side (Figure 3). For the duration of the scan, the fixation cue was static. Subjects were therefore not required to shift their attention as part of the task. However, the fixation cue changed between scans so subjects attended both locations equally during the course of the experiment. On half of the trials, the gratings were 100% contrast and on the other half of the trials the gratings were 50% contrast, but during each trial, the contrast of the two gratings was the same. The trials were 1.25 seconds long.
In Experiment 2, at the beginning of each trial, the fixation square was replaced by an arrow that faced either to the left or right side of the screen (Figure 3). The arrow cue directed the subject to attend to the drifting grating that appeared on the cued side of the screen. Additionally, there were two possible timings for the cue. During the Simultaneous condition, the cue appeared at the same time that the drifting gratings appeared. During the Advance condition, the cue appeared 250 milliseconds before the drifting gratings appeared. In both cases, the arrow cue was present for the entire duration of the grating. During other periods of the scan, the arrow was replaced by a fixation square. All gratings were 50% contrast, and the trials were 1.5 seconds long.
Experimental design
Stimuli were generated in Matlab, and presented with the Psychophysics toolbox extensions (Brainard, 1997). Using a Sharp XG P-25 projector, the stimuli were back-projected onto a screen positioned in the magnet bore. The projector was calibrated using a Photoresearch PR-650 spectral radiometer. Subjects viewed the stimuli via a mirror mounted on the head coil. Responses were collected using a magnet-compatible button box (Resonance Technologies, Inc.).
In addition to the trial types described above, the scans also contained trials without any stimuli. These null trials were used to model the baseline response. Experiment 1 had three conditions: the null condition, the low contrast condition, and the high contrast condition. Experiment 2 had five conditions: the null condition, Simultaneous cue to the left, Advance cue to the left, Simultaneous cue to the right, Advance cue to the right. The trials, including null trials, were ordered using m-sequences (Buracas and Boynton, 2002). The m-sequence length was 95 trials in Experiment 1, and 124 trials in Experiment 2. Because the BOLD response is sluggish and lasts many seconds, the responses to the last several stimuli in the m-sequence would not be recorded without extending the scan. For this reason, the first fifteen trials were appended to the end of the scan.
The first scans of each session presented contrast-reversing patterns designed to localize brain regions sensitive to the stimulus locations. The stimuli during these functional localizers consisted of flickering checkerboard patterns in the location of the experimental stimuli. Localizer scans contained five cycles of 16 seconds checkerboard then 16 seconds of gray screen, for a total length of 160 seconds.
MR data acquisition
All imaging was performed using the 3-Tesla Siemens Allegra MR scanner located at the Brain Mapping Center of the University of California, Los Angeles. Subjects participated in multiple scanning sessions. In the first scanning session, subjects viewed standard phase-encoded retinotopic stimuli to identify visual areas (Engel, Glover, and Wandell, 1997; DeYoe et al., 1996; Sereno et al., 1995). Twelve slices of fMRI data, oriented perpendicular to the Calcarine fissure, were acquired using an EPI sequence (TR = 1000 ms; TE = 45 ms; voxel size = 3.1 × 3.1 × 4 mm; gap = 5 mm). In addition, two high resolution T1-weighted anatomical scans were acquired for use in cortical unfolding of the retinotopic data.
In subsequent scanning sessions, a single twelve slice functional scan was acquired (with parameters as above) while subjects viewed a functional localizer. Immediately after the end of this scan, regions of responsive visual cortex were identified using the Siemens’ built-in analysis software. The responses during this functional scan were used to guide slice selection in a rapid MR protocol (three slices, TR = 250 ms). Slices were chosen to overlap with responsive areas near the calcarine sulcus, as well as responsive areas on or near the ventral surface of the brain. Because, brain coverage was limited, analysis was necessarily restricted to V1 and V4.
MR data analysis
We used an intensity-based linear method (Jenkinson, Bannister, Brady, and Smith, 2002) to correct for head motion during the each scan, and to register the functional scans to an in-session anatomical scan. The fMRI time series from each active voxel was converted to a percent change scores, and voxels within each restricted visual area ROI were averaged. We used linear deconvolution to estimate the responses in each visual area from each scan’s averaged time series. The design matrix for each condition contained one column per TR. The estimated time courses were the set of parameter weights that, when convolved with the design matrix, best fit the data using ordinary least squares. Responses were estimated for each ROI in all scans, and then averaged together according to condition.
To determine if there was any effect of attention on the overall magnitude of the response, the areas of the time courses were compared. The advantage of this method is that attention effects need not coincide with the peak of the unattended response. The area under each subject’s averaged attended and unattended time courses was tested, using a one-tailed, paired t-test (because of our prior hypothesis of attentional enhancement).
To estimate response timing, we fit gamma functions (Glover, 1999) to each subject’s responses. Parameters were selected that minimized the difference between the gamma function and the observed time course (RMS error). In addition to the shape and timing gamma function parameters, we included an overall scaling parameter and allowed for the fits to begin at 0±0.25% change. We fit only the first 6 seconds of the response in V1, and 9 seconds in V4, to improve the fits during the important, early portion of the response. We resampled the resulting fits to 48 samples per second, to allow our timing estimates a finer time scale.
We then used these fit gamma functions to estimate the response latencies for each condition. First, we calculated the time for each fit function to reach a target threshold, relative to its peak (accounting for any differences in starting point). Then to calculate delay, we subtracted this time to reach threshold for the stimulus-related activity from the time to reach threshold for the attention and contrast-related activity. This analysis was repeated at each of 25 thresholds evenly spaced between 15% and 85% of peak. At each threshold level, we tested whether the delays for each condition were significantly different than zero. We also averaged the delays at all the threshold levels used and tested each condition versus zero and versus the other condition. In Experiment 1, we used two-tailed t-tests; because Experiment 1 gave us strong prior hypotheses we used one-tailed t-tests in Experiment 2.
To identify visual areas, flattened cortical maps were generated from the high-resolution anatomical scan (MPRAGE) scans using SurfRelax (Larsson, 2001). Data from the retinotopy scans were projected onto the flat maps using mrVista (http://white.stanford.edu/software). Visual areas V1, V2, V3, and V4 were identified using reversals in phase-encoded polar angle retinotopy data (Engel, Glover, and Wandell, 1997; DeYoe et al., 1996; Sereno et al., 1995). Visual area ROIs were restricted to include only voxels with activity during the functional localizer scans that correlated well (>0.3) with a sinusoid at the stimulus frequency. Because of poor slice coverage for one subject, the threshold correlation was set at 0.2. Changing the threshold for this subject did not affect the overall pattern of results, but did improve the overall signal to noise ratio.
Behavioral Experiment
To measure attentional delays behaviorally, we also ran a psychophysical version of Experiment 2. The task was as in that experiment, but the temporal interval between cue and stimulus varied between five levels: 0, 250, 500, 750, and 1000 milliseconds. Three subjects performed seven blocks of 350 trials each. Independent, interleaved staircasing procedures were used for each of the five cue-stimulus intervals. For each interval, we interpolated a psychometric function relating stimulus acceleration to percent correct, and defined threshold as the acceleration that yielded 82 percent correct.
Research Highlights.
Rapid-TR fMRI can be used to measure temporal shifts in neural activity with reasonable precision
Enhancement of V1 response occurs late, likely too late for it to be involved in spatial attention
Enhancement of V4 response was less delayed than V1 response
Supplementary Material
Results of the behavioral experiment. Acceleration threshold (lower is better performance) is plotted as a function of interval between the attentional cue and stimulus onset for three subjects. All subjects show best performance with an interval of 500 msec, suggesting that attention is fully allocated to the stimulus by that time.
Acknowledgements
The authors thank James Bisley, Mark Cohen, and Zili Liu for helpful discussions. This work was funded by NIH EY11862, and for support for fMRI research at UCLA, the authors also wish to thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon Foundation, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Seth E. Bouvier, Center for Cognitive Neuroscience, University of Pennsylvania, 3810 Walnut Street, Philadelphia, PA 19104, sbouvier@sas.upenn.edu, phone: (215) 573-6156, fax: (215) 898-1982
Stephen A. Engel, Department of Psychology, University of Minnesota, 75 East River Road, Minneapolis, MN 55455
References
- Beauchamp MS, Cox RW, DeYoe EA. Graded effects of spatial and featural attention on human area MT and associated motion processing areas. J Neurophysiol. 1997;78:516–520. doi: 10.1152/jn.1997.78.1.516. [DOI] [PubMed] [Google Scholar]
- Boehler CN, Schoenfeld MA, Heinze H-J, Hopf J-M. Rapid recurrent processing gates awareness in primary visual cortex. Proc Natl Acad Sci U S A. 2008;105:8742–8747. doi: 10.1073/pnas.0801999105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boynton GM, Engel SA, Glover GM, Heeger DJ. Linear systems analysis of functional magnetic resonance imaging in human V1. J Neurosci. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–436. [PubMed] [Google Scholar]
- Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffalo EA, Fries P, Landman R, Liang H, Desimone R. A backward progression of attentional effects in the ventral stream. Proc Natl Acad Sci U S A. 2010;107:361–365. doi: 10.1073/pnas.0907658106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buracas GT, Boynton GM. Efficient design of event-related fMRI experiments using M-sequences. Neuroimage. 2002;16:801–813. doi: 10.1006/nimg.2002.1116. [DOI] [PubMed] [Google Scholar]
- Dale AM, Buckner RL. Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapping. 1997;5:329–340. doi: 10.1002/(SICI)1097-0193(1997)5:5<329::AID-HBM1>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci U S A. 1996;93:2382–2386. doi: 10.1073/pnas.93.6.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SA, Glover GH, Wandell BA. Retinotopic organization in human visual cortex and the spatial precision of functional MRI. Cereb Cortex. 1997;7:181–192. doi: 10.1093/cercor/7.2.181. [DOI] [PubMed] [Google Scholar]
- Eriksen CW, Collins JF. Temporal course of selective attention. J Exp Psychol. 1969;80:254–261. doi: 10.1037/h0027268. [DOI] [PubMed] [Google Scholar]
- Gandhi SP, Heeger DJ, Boynton GM. Spatial attention affects brain activity in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:3314–3319. doi: 10.1073/pnas.96.6.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–429. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Haenny PE, Schiller PH. State dependent activity in monkey visual cortex. I. Single cell activity in V1 and V4 on visual tasks. Exp Brain Res. 1988;69:225–244. doi: 10.1007/BF00247569. [DOI] [PubMed] [Google Scholar]
- Heinze HJ, Mangun GR, Burchert W, Hinrichs H, Scholz M, Munte TF, Gos A, Scherg M, Johannes S, Hundeshagen H, et al. Combined spatial and temporal imaging of brain activity during visual selective attention in humans. Nature. 1994;372:543–546. doi: 10.1038/372543a0. [DOI] [PubMed] [Google Scholar]
- Jack AI, Shulman GL, Snyder AZ, McAvoy M, Corbetta M. Separate modulations of human V1 associated with spatial attention and task structure. Neuron. 2006;51:135–147. doi: 10.1016/j.neuron.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annu Rev Neurosci. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kastner S, Pinsk MA, De Weerd P, Desimone R, Ungerleider LG. Increased activity in human visual cortex during directed attention in the absence of visual stimulation. Neuron. 1999;22:751–761. doi: 10.1016/s0896-6273(00)80734-5. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Gomez-Ramirez M, Foxe JJ. Spatial Attention Modulates Initial Afferent Activity in Human Primary Visual Cortex. Cereb Cortex. 2008 doi: 10.1093/cercor/bhn022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson J. Dissertation. Stockholm, Sweeden: Karolinska Institutet; 2001. Imaging vision: functional mapping of intermediate visual processes in man. [Google Scholar]
- Lauritzen TZ, D’Esposito M, Heeger DJ, Silver MA. Top-down flow of visual spatial attention signals from parietal to occipital cortex. J Vis. 2009;9 doi: 10.1167/9.13.18. 18.1-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Chelazzi L, Hillyard SA, Desimone R. Neural mechanisms of spatial selective attention in areas V1, V2, and V4 of macaque visual cortex. J Neurophysiol. 1997;77:24–42. doi: 10.1152/jn.1997.77.1.24. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Spatial gradients of visual attention: behavioral and electrophysiological evidence. Electroencephalogr Clin Neurophysiol. 1988;70:417–428. doi: 10.1016/0013-4694(88)90019-3. [DOI] [PubMed] [Google Scholar]
- McAdams CJ, Reid RC. Attention modulates the responses of simple cells in monkey primary visual cortex. J Neurosci. 2005;25:11023–11033. doi: 10.1523/JNEUROSCI.2904-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Luknowsky DC, Gati JS. Mental chronometry using latency-resolved functional MRI. Proceedings of the National Academy of Sciences. 1998;95:10902–10907. doi: 10.1073/pnas.95.18.10902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Moran J, Desimone R. Selective attention gates visual processing in the extrastriate cortex. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- Motter BC. Focal attention produces spatially selective processing in visual cortical areas V1, V2, and V4 in the presence of competing stimuli. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- Muller HJ, Findlay JM. The effect of visual attention on peripheral discrimination thresholds in single and multiple element displays. Acta Psychol (Amst) 1988;69:129–155. doi: 10.1016/0001-6918(88)90003-0. [DOI] [PubMed] [Google Scholar]
- Muller HJ, Rabbitt PM. Reflexive and voluntary orienting of visual attention: time course of activation and resistance to interruption. J Exp Psychol Hum Percept Perform. 1989;15:315–330. doi: 10.1037//0096-1523.15.2.315. [DOI] [PubMed] [Google Scholar]
- Murray SO. The effects of spatial attention in early human visual cortex are stimulus independent. Journal of Vision. 2008;8:1–11. doi: 10.1167/8.10.2. [DOI] [PubMed] [Google Scholar]
- Murray SO, Kersten D, Olshausen BA, Schrater P, Woods DL. Shape perception reduces activity in human primary visual cortex. Proc Natl Acad Sci U S A. 2002;99(23):15164–15169. doi: 10.1073/pnas.192579399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noesselt T, Hillyard SA, Woldorff MG, Schoenfeld A, Hagner T, Jancke L, Tempelmann C, Hinrichs H, Heinze HJ. Delayed striate cortical activation during spatial attention. Neuron. 2002;35:575–587. doi: 10.1016/s0896-6273(02)00781-x. [DOI] [PubMed] [Google Scholar]
- Oakley MT, Eason RG. Subcortical gating in the human visual system during spatial selective attention. Int J Psychophysiol. 1990;9:105–120. doi: 10.1016/0167-8760(90)90065-l. [DOI] [PubMed] [Google Scholar]
- Poghosyan V, Ioannides AA. Attention modulates earliest responses in the primary auditory and visual cortices. Neuron. 2008;58:802–813. doi: 10.1016/j.neuron.2008.04.013. [DOI] [PubMed] [Google Scholar]
- Ress D, Heeger DJ. Neuronal correlates of perception in early visual cortex. Nat Neurosci. 2003;6:414–420. doi: 10.1038/nn1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M, Delicato LS, Herrero J, Gieselmann MA, Thiele A. Attention alters spatial integration in macaque V1 in an eccentricity-dependent manner. Nat Neurosci. 2007;10:1483–1491. doi: 10.1038/nn1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema PR, Khayat PS, Spekreijse H. Subtask sequencing in the primary visual cortex. Proc Natl Acad Sci U S A. 2003;100:5467–5472. doi: 10.1073/pnas.0431051100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad ZS, Ropella KM, Cox RW, DeYoe EA. Analysis and use of FMRI response delays. Hum Brain Mapp. 2001;13:74–93. doi: 10.1002/hbm.1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science. 1995;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- Shuler MG, Bear MF. Reward Timing in the Primary Visual Cortex. Science. 2006;311:1606–1609. doi: 10.1126/science.1123513. [DOI] [PubMed] [Google Scholar]
- Somers DC, Dale AM, Seiffert AE, Tootell RB. Functional MRI reveals spatially specific attentional modulation in human primary visual cortex. Proc Natl Acad Sci U S A. 1999;96:1663–1668. doi: 10.1073/pnas.96.4.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijers AA, Lange JJ, Mulder G, Mulder LJ. An ERP study of visual spatial attention and letter target detection for isoluminant and nonisoluminant stimuli. Psychophysiology. 1997;34:553–565. doi: 10.1111/j.1469-8986.1997.tb01742.x. [DOI] [PubMed] [Google Scholar]
- Yoshor D, Ghose GM, Bosking WH, Sun P, Maunsell JHR. Spatial attention does not strongly modulate neuronal responses in early human visual cortex. J Neurosci. 2007;27:13205–13209. doi: 10.1523/JNEUROSCI.2944-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of the behavioral experiment. Acceleration threshold (lower is better performance) is plotted as a function of interval between the attentional cue and stimulus onset for three subjects. All subjects show best performance with an interval of 500 msec, suggesting that attention is fully allocated to the stimulus by that time.