Abstract
Annual immunization with a trivalent inactivated vaccine (TIV) is considered efficacious for prevention of seasonal influenza in older adults. However, significant controversy exists in the current literature regarding the clinical effectiveness of TIV immunization in this highly heterogeneous population. Frailty is an important geriatric syndrome characterized by decreased physiologic reserve and increased vulnerability to stressors. Using a validated set of frailty criteria, we conducted a prospective observational study to evaluate TIV-induced strain-specific hemagglutination inhibition (HI) antibody titers and post-vaccination rates of influenza-like illness (ILI) and infection in frail and nonfrail older adults. The results indicate that frailty was associated with significant impairment in TIV-induced strain-specific HI titers and increased rates of ILI and laboratory-confirmed influenza infection. These findings suggest that assessing frailty status in the elderly may identify those who are less likely to respond to TIV immunization and be at higher risk for seasonal influenza and its complications.
Keywords: Frailty, Influenza immunization, Antibody response, Influenza infection, Influenza-like illness, Older adults
1. Introduction
Seasonal influenza causes significant morbidity and mortality in older adults [1–3]. A large number of studies have shown the efficacy of annual immunization with TIV (Cochrane Database Systemic Review) [4], which is the current vaccination strategy against influenza infection in this population. For example, the efficacy of TIV immunization in relatively young and healthy seniors has been demonstrated by controlled trials [5,6] and prospective observational studies [7,8]. Nichol and colleagues have shown the effectiveness of annual TIV immunization in working adults aged 50–64 years as well as in the elderly [9–12]. However, Simonsen et al. reported that increased vaccination coverage over the past 2 decades failed to reduce influenza-related mortality in older adults [13–15]. A nested case–control study, with the majority of its study population being over 70 years, demonstrated no significant benefit of TIV immunization against pneumonia secondary to influenza infection [16]. Impaired functional status has also been associated with decreased mortality benefit of TIV immunization in the elderly [17]. These studies suggest significant controversy in the current literature regarding the clinical effectiveness of TIV immunization in this highly heterogeneous population as well as the need for further evaluation of the effectiveness of TIV immunization in subsets of seniors who are older and frail.
Frailty is an important geriatric syndrome characterized by decreased physiologic reserve and increased vulnerability with multi-system dysregulation, leading to hospitalization, dependency, and early mortality in older adults [18–21]. A 5-item set of criteria has been validated in multiple studies to identify seniors who are frail and vulnerable to adverse health outcomes in the community [18,21–24]. Based on these criteria, frailty has an estimated prevalence of 7% among community-dwelling men and women 65 years and older, and up to 30% in those over 80 years [18,23]. Evidence from our group and others suggests that frail older adults manifest a heightened inflammatory state and significant dysregulation in the innate and T cell compartments that appear to be above and beyond age-related senescent immune remodeling [22,25–31]. However, potential impact of frailty on TIV-induced antibody response and its clinical effectiveness in the elderly population has not been adequately investigated.
The objective of this study was to evaluate TIV-induced strain-specific HI antibody titers as well as post-vaccination rates of influenza-like illness (ILI) and laboratory confirmed influenza infections in frail and nonfrail older adults. We hypothesized that frail older persons would have lower HI titers to TIV immunization and higher post-vaccination rates of ILI and laboratory-confirmed influenza infections than nonfrail controls. The results indicate that assessing frailty status in the elderly may identify those who are less likely to respond to TIV immunization and be at higher risk for seasonal influenza and its complications.
2. Materials and methods
2.1. Study design and participants
This is a prospective observational study of the potential influence of the frailty syndrome on strain-specific antibody response and clinical effectiveness of influenza immunizations with TIV in older adults. The study was performed during 2007–2008 influenza season. Community-dwelling older adults over 70 were recruited via collaborating physicians and community newspaper advertisement and flyers at outpatient clinics, senior centers, retirement communities, and residential areas in Baltimore, Maryland. Potential candidates who consented to participate were screened by trained clinical research coordinators according to the validated frailty criteria (see below). Information about clinical diagnosis, medication usage, and TIV immunization in the previous 5 influenza seasons was obtained by self-report and confirmed by review of medical records with participants’ permission from their primary care physician’s offices. Exclusion criteria included allergies to eggs or influenza vaccine components, acute illness such as a viral infection or acute exacerbation of chronic conditions, recent use (within past year) of immune modulating agents (glu-cocorticoid steroids, methotrexate, etc.), rheumatoid arthritis or other systemic inflammatory conditions, active malignancy or on radiation or chemotherapy, uncompensated congestive heart failure or endocrine disorders, Parkinson’s disease, dementia, or stroke with residual hemiparesis. These exclusion criteria were designed to eliminate any contraindications for vaccine and minimize confounding immune effects of existing medical conditions or medications. They also addressed the possibility that specific diseases would mimic or overshadow the frailty phenotype. These enrollment criteria (except for allergies to eggs or influenza vaccine) have been successfully applied in our previous frailty studies [25,32]. The Johns Hopkins Institutional Review Board approved the study protocol, and written informed consent was obtained from all participants.
2.2. Determination and classification of frailty
Participants were categorized as frail, prefrail, and nonfrail according to the validated and widely utilized frailty criteria [18]. This set of criteria is based on the presence or absence of five measurable characteristics: slowed motor performance (by walking speed), poor endurance and energy (by self-report of exhaustion), weakness (by grip strength), shrinking (by unintentional weight loss), and low physical activity. Older persons with three or more out of these five characteristics were defined as frail, those with one or two as prefrail, and those with none as nonfrail.
2.3. TIV immunization
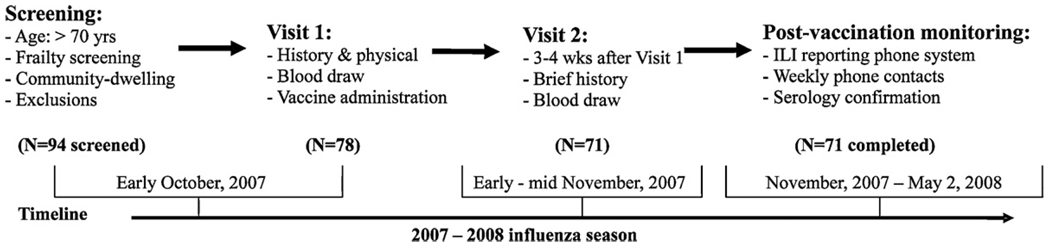
As shown in Fig. 1, study participants were recruited in early October 2007, 3–4 weeks before the peak influenza immunization in the Baltimore area (late October). After initial screening, eligible candidates with written informed consent came to the General Clinical Research Center (GCRC) at Johns Hopkins Bayview Medical Center (JHBMC), now the Johns Hopkins Institute of Clinical and Translational Research, or were seen at home if they preferred. They underwent pre-vaccination evaluation and vaccine administration (Visit 1). Commercially available standard TIV of 2007–2008 formula supplied as a 0.5mL dose in a pre-filled syringe (Fluarix, GlaxoSmithKline) containing 15mcg of hemaglutinin for each of the following 3 strains, A/Solomon Islands/3/2006 (H1N1), A/Wisconsin/67/2005 (H3N2), and B/Malaysia/2506/2004, was administered by intramuscular injection by a licensed health-care provider. During the 4th week after TIV immunization, participants returned to the GCRC at JHBMC or were seen at home for a post-vaccination evaluation (Visit 2). Serum samples were collected at each visit and stored at −80 °C for pre- and post-vaccination antibody titer measurements.
Fig. 1.
Pre-vaccination screening and blood draw, TIV immunization, as well as post-vaccination blood draw and influenza surveillance.
2.4. Influenza surveillance during post-vaccination season
Participants were instructed to report any influenza-like symptoms by calling a central phone number with recording capability 24 h a day, 7 days a week throughout the post-vaccination season. Anyone who called to report symptoms was contacted immediately by the research team to ask the participants (or their family member to help) to take a body temperature (if not already done so) and confirm the participants’ symptoms and signs. In addition, all participants were contacted weekly by phone and asked a series of questions regarding their general health status and the presence of such symptoms and signs. Clinical cases of ILI were defined by either (i) presence of fever (100 °F orally or 101 °F rectally) plus one or more of the following: cough, headache, myalgias, or sore throat [33]; or (ii) in the absence of fever, the occurrence of two or more of the following symptoms: cough, coryza, sore throat, myalgia, headache, or photophobia [34,35]. Participants whose symptoms and signs met the above diagnostic criteria for ILI were visited by the research team 3–4 weeks after the onset of ILI to obtain a post-ILI serum sample for post-ILI serology. Influenza infection was confirmed based on the serology criterion of a 4-fold or more rise in post-ILI strain-specific antibody titer. Influenza surveillance was conducted over a period of 27 weeks and was discontinued on Friday, May 2, 2008, about 4 weeks after the last influenza case of the season was reported in the Baltimore area.
2.5. Measurement of strain-specific anti-influenza antibody titers
Strain-specific anti-influenza antibody titers against hemagglutinin (HA) were measured using hemagglutination inhibition (HI) assay. Appropriate influenza reference antigens and anti-sera were obtained from the WHO Collaborating Center for Influenza, the Center for Disease Control and Prevention (Atlanta, GA). All freshly thawed serum samples were pre-treated with receptor destroying enzyme (RDE) (Denka Seiken, Tokyo, Japan) and by pre-adsorption with turkey red blood cells (RBC) (RDI Division of Fitzgerald Industries International, Concord, MA) to remove non-specific inhibitors and agglutinin, respectively. After careful titration of reference antigens against turkey RBC (0.5%) and treated reference sera, HI antibody titers were measured using V-shaped 96-well microtiter plates, according to the standard microtiter technique [36]. Paired pre- and post-vaccination serum samples as well as post-ILI serum samples (for those who had ILI) from the same subject were analyzed simultaneously for HI antibodies against each of the three vaccine strains. HI titers were recorded as the reciprocal of the highest serum dilution that produced complete inhibition of RBC agglutination.
2.6. Data analysis
Summary statistics of demographic and clinical characteristics were constructed for all study participants and distributions these characteristics were summarized across the nonfrail, prefrail, and frail groups and across the groups of participants with no ILI, ILI cases, and influenza cases. Geometric mean of HI titers (GMT) and standard deviations were presented. Comparisons between log-transformed HI titers pre- and post-immunization HI titers were analyzed by paired t-tests. Seroprotection was defined by post-immunization HI titer equal or greater than 1:40; seroconversion was defined by 4-fold or high post- over pre-immunization increase in HI titers; and rates of seroprotection and seroconversion were obtained in all participants as well as in individual study groups. GMT titer ratio was calculated as post-immunization GMT titer divided by pre-immunization GMT titer. The Jonckheere–Terpstra tests were used to determine statistical significance of stepwise increase/decrease trends in log-transformed HI titers and GMT ratios across the study groups. Fisher exact tests were performed to determine statistical significance of differences in rates of sero-protection or seroconversion between nonfrail and frail groups or between the groups of participants with no ILI and influenza cases. The Cochran–Armitage tests were used to determine statistical significance of stepwise increase/decrease trends in overall rates ILI or influenza infection during post-vaccination season across the study groups. Results from our exploratory analysis showed no significant difference in all demographic and clinical characteristics across the frailty study groups except for age. To assess the effect of frailty independent of age, linear regressions were used to model log-transformed HI titers, GMT ratios; logistic regressions were used to model post-vaccination rates of ILI and influenza infection. Intercooled Stata software, version 9 was used for model estimation and diagnostics (Stata Corporation, College Station, TX).
3. Results
3.1. Characteristics of the study participants
Of 94 persons initially screened, 78 (83%) met the eligibility criteria and enrolled into the study. Seven individuals were lost follow-up due to either moving out of the area for the winter season (n = 4), refusal of providing a post-vaccination blood specimen (n = 2), or being hospitalized after an accidental fall and subsequent death (n = 1). This yielded a final sample size of 71 (91% of the total enrolled). Table 1 summarizes major demographic and clinical characteristics of the study population and across the frail (n = 17), prefrail (n = 32), and nonfrail (n = 22) groups. The mean age of the participants was 84.5 years with a range of 72–95. The majority of the participants were female and Caucasian with education level of high school and above. Participants had an average of 3–4 chronic diseases including hypertension, other cardiovascular diseases (coronary artery disease, congestive heart failure, atrial fibrillation, and stroke), hyperlipidemia, osteoarthritis, and hypothyroidism. On average, participants took 3–4 commonly prescribed medications, such as diuretics, HMG-CoA reductase inhibitors, β-blockers, thyroid hormone supplement, and ACE-inhibitors. Consistent with previously reported prevalence of frailty in older adults over 80 years of age [18,23], 17 (24%) subjects were frail. Compared with nonfrail controls, frail participants were older (86.0±3.1 vs. 82.0±5.4, p = .01). No significant difference was observed between frail and nonfrail participants in race, sex, education, BMI, total number of medical diagnoses or specific chronic conditions, and total number of medications or usage of specific drugs. All participants had TIV immunization in each of the prior 5 influenza seasons.
Table 1.
Demographic and clinical characteristics and study variables in all study participants and across the frailty spectrum.
| Variables | All subjects (n = 71) | Nonfrail (n = 22) | Prefrail (n = 32) | Frail (n = 17) | pa |
|---|---|---|---|---|---|
| Age (years), mean (SD+) | 84.5 (4.6) | 82.0 (5.4) | 85.4 (4.1) | 86.0 (3.1) | .01 |
| Race (white), % | 91.6% | 95.5% | 90.6% | 88.4% | .14 |
| Sex (female), % | 77.5% | 81.8% | 68.8% | 88.2% | .68 |
| Education (high school or above), % | 94.4% | 100.0% | 93.7% | 88.2% | .42 |
| BMI (kg/m2), mean (SD) | 25.0 (4.2) | 25.0 (3.0) | 24.5 (3.2) | 25.9 (7.0) | .47 |
| Total # chronic diseases, mean (SD) | 3.7 (1.5) | 3.2 (1.3) | 3.8 (2.4) | 4.1 (4.5) | .43 |
| Common clinical conditions | |||||
| Hypertension, % | 67.1% | 81.0% | 59.4% | 64.7% | .29 |
| Other cardiovascular disease, % | 38.0% | 27.3% | 46.9% | 35.3% | .79 |
| Hyperlipidemia, % | 54.9% | 59.1% | 59.4% | 41.2% | .34 |
| Osteoarthritis, % | 29.6% | 22.7% | 28.1% | 41.2% | .30 |
| Hypothyroidism, % | 23.9% | 22.7% | 25.0% | 23.5% | .98 |
| Total # medications, mean (SD) | 3.3 (1.5) | 3.5 (2.4) | 3.0 (2.8) | 4.2 (5.3) | .34 |
| Commonly used medications | |||||
| Diuretics, % | 47.9% | 54.6% | 43.8% | 47.1% | .75 |
| HMG-CoA reductase inhibitor (lipid-lowering drugs), % | 43.7% | 50.0% | 43.8% | 35.3% | .52 |
| β-Blockers, % | 32.4% | 31.8% | 34.4% | 29.4% | .94 |
| Thyroid hormone supplement, % | 29.0% | 27.3% | 31.3% | 26.7% | .93 |
| ACE-inhibitors, % | 21.1% | 27.3% | 18.8% | 17.7% | .69 |
Determined using Fisher exact test for categorical variables or t test for continuous variables between frail and nonfrail participants.
3.2. TIV-induced strain-specific antibody response in all participants and across the frailty study groups
As shown in Table 2, the study population as a whole “All (n = 71)” had significantly higher post-immunization HI titers compared to pre-immunization HI titers to H1N1, H3N2, and B strains (GMT titers [Mean±geometric SD]: 308±2.1 vs. 174±2.1, p = .001; 408±2.6 vs. 279±2.2, p = .01; 85±1.8 vs. 78±1.7, p .005, respectively, paired t test), indicating active immunogenicity of the vaccine used in the study. Among the study groups, nonfrail participants had significantly higher post-immunization than pre-immunization HI titers to H1N1, H3N2, and B strains (387±2.0 vs. 201±2.0, p < .001; 497±1.9 vs. 309±1.6, p < .001; and 105±1.5 vs. 88±1.4, p = .01, respectively). Prefrail participants had significantly higher post-immunization than pre-immunization HI titers to H1N1 and H3N2 (282±2.3 vs. 157±2.2, p = .01 and 388±2.4 vs. 278±2.1, p = .01, respectively). The difference between post-immunization and pre-immunization HI titers to B strain in these participants was not statistically significant (81±1.3 vs. 78±1.6, p = .23). In contract, there was no statistically significant difference between post-immunization and pre-immunization HI titers to any of the above vaccine strains among frail participants (201±2.1 vs. 149±1.9 to H1N1, p = .43; 307±2.3 vs. 255±2.0 to H3N2, p = .17; and 67±2.1 vs. 65±2.0 to B, p = .33, respectively). In addition, post-immunization HI titers to all three vaccine strains had significant stepwise decrease from the nonfrail and prefrail to the frail participants, adjusted for age (387±2.0, 282±2.3, 201±2.1, respectively, to H1N1, p = .03; 497±1.9, 388±2.4, 307±2.3, respectively, to H3N2, p = .02; and 105±1.5, 81±1.3, 67±2.1, respectively, to B, p = .05).
Table 2.
Pre- and post-TIV immunization HI titers and seroprotection or seroversion rates to H1N1, H3N2, and B vaccine strains in all study subjects and across the nonfrail, prefrail, and frail study groups.
| HI antibody responses | All subjects (n = 71) | Nonfrail (n = 22) | Prefrail (n = 32) | Frail (n = 17) | p values |
|---|---|---|---|---|---|
| H1N1 | |||||
| Pre-immunization HI titersa | 174±2.1 | 201±2.0 | 157±2.2 | 149±1.9 | .17* |
| Post-immunization HI titersa | 308±2.1 | 387±2.0 | 282±2.3 | 201±2.1 | .03* |
| Pre- vs. post- p valuesb | .001 | <.001 | .01 | .43 | .86** |
| Seroprotection ratesc | 94% | 95% | 94% | 96% | ND |
| Seroconversion ratesd | 7% | 13% | 6% | 0 | |
| H3N2 | |||||
| Pre-immunization HI titersa | 279±2.2 | 309±1.6 | 278±2.1 | 255±2.0 | .16 |
| Post-immunization HI titersa | 408±2.6 | 497±1.9 | 388±2.4 | 307±2.3 | .02* |
| Pre- vs. post- p valuesb | .01 | .001 | .01 | .17 | .71** |
| Seroprotection ratesc | 92% | 91% | 94% | 88% | .05** |
| Seroconversion ratesd | 13% | 27% | 6% | 6% | |
| B | |||||
| Pre-immunization HI titersa | 78±1.7 | 88±1.4 | 78±1.6 | 65±2.0 | .19* |
| Post-immunization HI titersa | 85±1.8 | 105±1.5 | 81±1.3 | 67±2.1 | .05* |
| Pre- vs. post- p valuesb | .005 | .01 | .23 | .33 | .87** |
| Seroprotection ratesc | 82% | 86% | 78% | 82% | ND |
| Seroconversion ratesd | 1% | 5% | 0 | 0 |
ND: not done.
These p values derived from linear regression analysis adjusted for age.
These p values derived from Fisher exact test between nonfrail and frail groups.
Mean±geometric SD of geometric mean titers (GMT).
These p values derived from paired t tests of the pre–post immunization difference in log transformed HI titers.
Seroprotection defined by post-vaccination HI titer≥1:40.
Seroconversion defined by 4-fold or higher HI titer increase after TIV immunization or post- over pre-vaccination HI titer ratio ≥4.
Next, we examined rates of seroprotection and seroconversion. Seroprotection is conventionally defined by post-immunization HI titer equal or greater than 1:40. The rates of seroprotection were high to all three strains in the study population (94%, 92%, and 82% to H1N1, H3N2 and B strain, respectively) and they did not differ among nonfrail, prefrail and frail study groups (Table 2). Serocon-version is defined by 4-fold or higher post- over pre-immunization HI titer rise. The rates of seroconversion were low to all three strains in the study population [7% (5 participants), 13% (9), and 1% (1) to H1N1, H3N2 and B strain, respectively)]. Among the study groups, nonfrail participants had seroconversion rates of 13% (3 participants), 27% (6) and 5% (1) to H1N1, H3N2 and B strain, respectively; prefrail participants had seroconversion rates of 6%, 6%, and none, respectively; while only 6% (1) frail participants was seroconverted to H3N2 and none to H1N1 or B (Table 2). The difference in rates of seroconversion to H3N2 between nonfrail and frail groups was statistically significant (27% vs. 6%, respectively, p = .05, Fisher exact test).
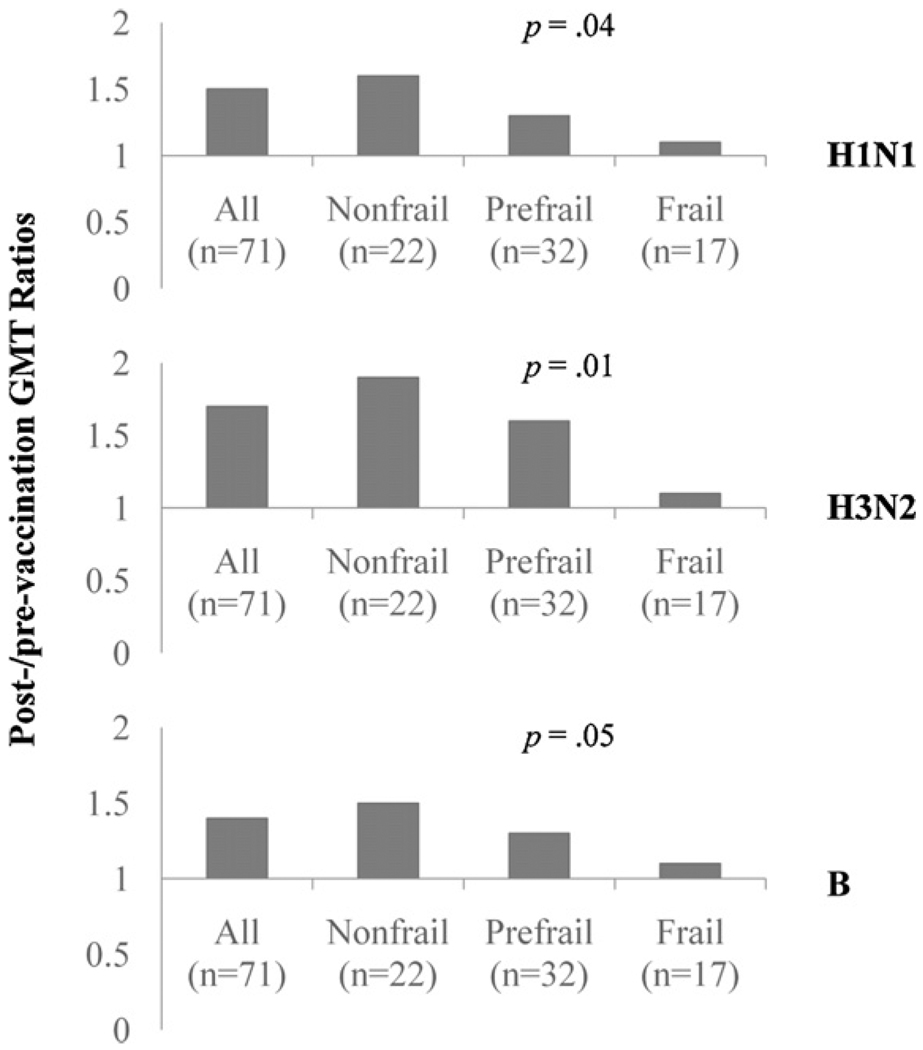
We also evaluated the GMT titer ratios for TIV-induced anti-body response in all participants and among three study groups. As shown by Fig. 2, GMT ratios in all participants were 1.5, 1.7, and 1.4 to H1N1, H3N2, and B, respectively. Among the study groups, GMT ratios to all three vaccine strains had significant stepwise decrease from the nonfrail and prefrail to the frail participants, adjusted for age (1.6, 1.3, 1.1, respectively, to H1N1, p = .04; 1.9, 1.6, 1.1, respectively, to H3N2, p = .01; and 1.5, 1.3, 1.1, respectively, to B, p = .05).
Fig. 2.
GMT ratios to H1N1, H3N2, and B strains in all study participants “All (n = 71)”, nonfrail (n = 22), prefrail (n = 32), and frail (n = 17) groups. p Values were derived from linear regression analysis for stepwise trend of decrease in nonfrail, prefrail, to frail study groups, adjusted for age.
Taken together, these results demonstrate that frailty is associated with significant impairment in antibody responses to TIV immunization among community-dwelling older adults.
3.3. Rates of influenza-like illness (ILI) and confirmed influenza infection
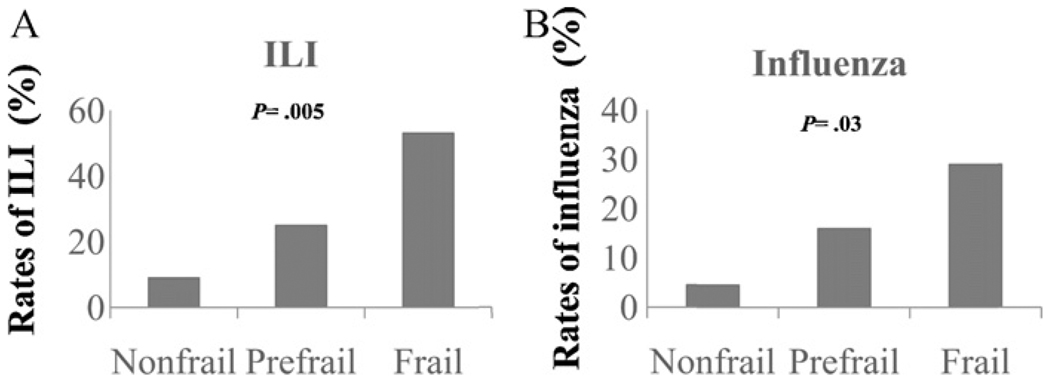
A total of 19 (26.8%) participants developed ILI during the post-vaccination season. Eleven (15.5%) participants were confirmed for influenza infection by post-ILI serology, among which seven cases were influenza A/H3N2, one case of influenza A/H1N1, and three cases of influenza B. Three cases (all influenza A/H3N2) were hospitalized for severe influenza infection and secondary pneumonia and two subsequently died (one from respiratory failure and one from cardiac arrest). As shown in Fig. 3, the rates of ILI and confirmed influenza infection had significant stepwise increase from the nonfrail and prefrail to the frail participants (9%, 25%, and 53%, respectively, p = .002 for ILI; 5%, 16%, and 29%, respectively, p = .02 for influenza infection). These trends remained statistically significant after adjusting for age (p = .005, 0.03 for ILI and influenza infection, respectively). Among three influenza A/H3N2 cases who were hospitalized influenza cases, two were frail (one met 3 and the other met 5 of the 5 frailty criteria) and subsequently died; the other one was prefrail (met 1 of the 5 frailty criteria). The remaining four influenza A/H3N2 cases include two prefrail (both met 1 of the 5 frailty criteria), one frail (met 4 of the 5 frailty criteria), and one nonfrail. The influenza A/H1N1 case was frail (met 3 of the 5 frailty criteria). One influenza B case was frail (met 4 of the 5 frailty criteria) and two were prefrail (met 2 of the 5 frailty criteria for both cases). Regarding the time course of the ILI and influenza cases, the first ILI case occurred in late November, 7 weeks after the participant received TIV administration. The subsequent 9 ILI cases occurred in December, 6 of which were confirmed influenza. The rest were reported in January and February except for one ILI case in early March. These results indicate that despite TIV immunization, frailty is associated with significantly higher rates of ILI and influenza infection during post-vaccination season.
Fig. 3.
Rates of influenza-like illness (ILI) (A) and laboratory confirmed influenza infection (B) during post-vaccination season. p Values were obtained from logistic regression analysis for stepwise trend of increase in nonfrail, prefrail, to frail study groups, adjusted for age.
3.4. Demographic characteristics and TIV-induced strain-specific antibody response among participants with no ILI, ILI cases, and serologically confirmed influenza cases
Age did not differ among participants with no ILI, ILI cases, and serologically confirmed influenza cases (mean + SD: 84.3 + 4.9 vs. 85 + 3.5 vs. 84 + 3.4, respectively, p = .61). There was no significant difference in other variables listed in Table 1 among these groups (data not shown). Table 3 summarizes pre- and post-immunization HI titers and rates of seroprotection and seroconversion across the three groups. Participants with no ILI had significantly higher post-immunization than pre-immunization HI titers to all three strains (378 + 2.3 vs. 179 + 2.2, p = .001; 468 + 2.7 vs. 289 + 2.4, p = .01; and 87 + 1.6 vs. 79 + 1.3, p = .04, respectively). ILI cases had significantly higher post-immunization than pre-immunization HI titers to H1N1 and H3N2 (274 + 2.4 vs. 151 + 2.5, p = .05 and 389 + 2.6 vs. 268 + 2.4, p = .05, respectively) and marginally higher post-immunization HI titer to B (82 + 1.5 vs. 76 + 1.3, p = .08). There was no statistically significant difference between post-immunization and pre-immunization HI titers to any vaccine strains in influenza cases (195 + 2.3 vs. 144 + 2.3 to H1N1, p = .13; 301 + 2.7 vs. 230 + 2.3 to H3N2, p = .09; and 66 + 1.4 vs. 64 + 1.5 to B, p = .41, respectively). Post-immunization HI titers to H1N1 and H3N2 had significant stepwise decrease from the participants with no ILI and ILI cases to the influenza cases (378 + 2.3, 274 + 2.4, 195 + 2.3, respectively, to H1N1, p = .05; 468 + 2.7, 389 + 2.6, 301 + 2.7, respectively, to H3N2, p = .04). Decrease in that to B strain across these three groups had marginal significance (87 + 1.6, 82 + 1.5, 66 + 1.4, respectively, p = .07). Seroprotection rates were high in all three groups and did not differ between the groups. In participants with no ILI, five (10%) were seroconverted to H1N1, eight (15%) were seroconverted to H3N2, and one (2%) was seroconverted to B strain. None of the ILI or influenza cases were seroconverted to H1N1 or B and one ILI case (5%) was seroconverted to H3N2. These results indicate significant difference in overall TIV-induced strain-specific antibody response among participants with no ILI compared to the ILI and influenza cases.
Table 3.
Pre- and post-TIV immunization HI titers and seroprotection or seroversion rates to H1N1, H3N2, and B vaccine strains in subjects without ILI and in influenza or ILI cases.
| HI antibody responses | Subjects with no ILI (n = 52) | ILI cases (n = 19) | Influenza cases (n = 11) | p values |
|---|---|---|---|---|
| H1N1 | ||||
| Pre-vaccination titersa | 179±2.2 | 151±2.5 | 144±2.3 | .17* |
| Post-vaccination titersa | 378±2.3 | 274±2.4 | 195±2.3 | .05* |
| Pre- vs. post- p valuesb | .001 | .05 | .13 | .74** |
| Seroprotection ratesc | 98% | 89% | 91% | ND |
| Seroconversion ratesd | 10% | 0 | 0 | |
| H3N2 | ||||
| Pre-vaccination titersa | 289±2.4 | 268±2.4 | 230±2.3 | .16* |
| Post-vaccination titersa | 468±2.7 | 389±2.6 | 301±2.7 | .04* |
| Pre- vs. post- p valuesb | .01 | .05 | .09 | .43** |
| Seroprotection ratesc | 96% | 84%% | 91% | ND |
| Seroconversion ratesd | 15% | 5% | 0% | |
| B | ||||
| Pre-vaccination titersa | 79±1.3 | 76±1.3 | 64±1.5 | .19* |
| Post-vaccination titersa | 87±1.5 | 82±1.5 | 66±1.4 | .07* |
| Pre- vs. post- p valuesb | .04 | .08 | .41 | .89** |
| Seroprotection ratesc | 83% | 89% | 82% | ND |
| Seroconversion ratesd | 2% | 0 | 0 |
ND: not done.
These p values derived from Jonckheere–Terpstra trend tests across participants with no ILI, ILI cases, and serologically confirmed influenza cases.
These p values derived from Fisher exact test between subjects with no ILI and influenza cases.
Mean±geometric SD of geometric mean titers (GMT).
These p values derived from paired t tests of the pre–post immunization difference in log transformed HI titers.
Seroprotection defined by post-vaccination HI titer≥1:40.
Seroconversion defined by 4-fold or higher HI titer increase after TIV immunization or post- over pre-vaccination HI titer ratio ≥4.
4. Discussion
Here we demonstrate for the first time, the significant impact of the geriatric syndrome of frailty on TIV immunization. Specifically, we have shown that frailty is associated with decreased HI titer response to TIV immunization and increased rates of post-vaccination ILI and influenza infection in community-dwelling older adults. Participants with no ILI had better antibody response to TIV immunization as indicated by significantly higher post-immunization HI titers and most seroconversions than ILI or influenza cases, suggesting a correlation of TIV-induced antibody response with protection against influenza in the study population.
Consistent with the observation of age-related decrease in TIV-induced antibody response reported in the literature (reviewed in references [7,37,38]), the overall antibody response to TIV immunization in this study was poor. The rates of seroconversion to more than one vaccine strains, which were seldom reported in previous studies, were strikingly low; only two participants (2.8%, one non-frail and one prefrail participant) seroconverted to both H1N1 and H3N2; one (nonfrail) seroconverted to both H3N2 and B strains; none had positive seroconversion to all three vaccine strains. It was noted that the pre-immunization HI titers were high, most of which were greater than 1:40. This is likely the result of prior annual vaccination and suggests that a post-immunization titer of 1:40, or seroprotection, as the threshold of immune protection against influenza may not be applicable to these elderly individuals. Nonetheless, TIV-induced antibody response appears to be correlated with clinical protection against influenza as discussed above. This is in contrast to a previous study by Gravenstein and colleagues in a veteran’s home setting where no such correlation was observed [39].
The post-vaccination rate of serologically confirmed influenza infection observed in this study is consistent with the influenza infection rate recently reported by Shahid and colleagues in 2007–2008 influenza season [40]. Three hospitalized severe influenza cases were all H3N2 infections, which is consistent with the report by Thompson et al. that H3N2 influenza infections are associated with the highest hospitalization rates in older adults [1]. We used this influenza-specific measure and post-vaccination rate of ILI to minimize confounders associated with all-cause mortality or other outcome measures not specific to seasonal influenza. Moreover, this study specifically targeted to a frail subset of community-dwelling older adults. Except for age, which was adjusted in our analyses, there was no significant difference in demographic and clinical profiles between frail and nonfrail participants. Of note, several studies evaluated TIV-induced antibody response and/or TIV efficacy in “frail” institutionalized older persons [41–43]. However, despite a specific type of disability or disabilities that require long-term functional and healthcare support, many nursing home residents may not be truly frail and can still mount a robust antibody response to TIV immunization. In fact, a quantitative review of a large number of studies has demonstrated better antibody responses to TIV immunization in nursing home residents compared to those living in the community [38]. In addition, a recent Australian study has shown that incompletely matched influenza vaccine still provided protection among institutionalized older persons [43].
This study has several limitations. First, it has a relatively small sample size. For example, the result of no positive seroconversion to H1N1 or B strains in the frail group could partly be due to this limitation. However, significant stepwise decrease in rates of antibody response and increase in rates of ILI and influenza infection were observed across the nonfrail, prefrail and frail groups. Secondly, new virus strains appeared in the circulation in early 2008, which led to significant reformulation of the 2008–2009 TIV vaccine. As such, laboratory confirmation by post-ILI serology might have underestimated the rate of influenza infection among all ILI cases, particularly those that occurred after January 1, 2008. In addition, no data is available on TIV-induced cell-mediated immunity (CMI) from this study. Other studies have shown CMI as an important part of TIV-induced immunity against influenza in older adults [8,44,45], and further investigations into potential impact of frailty on CMI are warranted. While two frail participants were hospitalized for severe influenza and secondary pneumonia and subsequently died, we did not intend to recruit frail older individuals who had terminal illness as their frailty phenotype could be driven by a single terminal illness. Recognizing these limitations and the need for further confirmation and expansion, findings from this study provide initial evidence suggesting that assessing frailty status in the elderly may identify those who are less likely to respond to TIV immunization and be at higher risk for seasonal influenza and its complications. These results also emphasize the need for more targeted and effective influenza immunization and preventive strategies for this vulnerable community-dwelling elderly population.
Acknowledgements
Dr. Sean Leng is a current recipient of the Paul Beeson Career Development Award in Aging Research, K23 AG028963. This research was also supported in part by the Intramural Research Program of the National Institute on Aging, National Institutes of Health (NIH) and by Johns Hopkins Older American Independence Center funded by National Institute on Aging, P30 AG021334.
Abbreviations
- TIV
trivalent inactivated vaccine
- ILI
influenza-like illness
- HA
hemagglutinin
- HI
hemagglutination inhibition
References
- 1.Thompson WW, Shay DK, Weintraub E, et al. Influenza-associated hospitalizations in the United States. JAMA. 2004 September;292(11):1333–1340. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007 June;25(227):5086–5096. doi: 10.1016/j.vaccine.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Thompson WW, Shay DK, Weintraub E, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. 2003 January;289(2):179–186. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 4.Rivetti D, Jefferson T, Thomas R, et al. Vaccines for preventing influenza in the elderly. Cochrane Database Syst Rev. 2006;3 doi: 10.1002/14651858.CD004876.pub2. CD004876. [DOI] [PubMed] [Google Scholar]
- 5.Govaert TM, Thijs CT, Masurel N, Sprenger MJ, Dinant GJ, Knottnerus JA. The efficacy of influenza vaccination in elderly individuals. A randomized double-blind placebo-controlled trial. JAMA. 1994 December;272(21):1661–1665. [PubMed] [Google Scholar]
- 6.Praditsuwan R, Assantachai P, Wasi C, Puthavatana P, Kositanont U. The efficacy and effectiveness of influenza vaccination among Thai elderly persons living in the community. J Med Assoc Thai. 2005;88(2):256–264. [PubMed] [Google Scholar]
- 7.Murasko DM, Bernstein ED, Gardner EM, et al. Role of humoral and cell-mediated immunity in protection from influenza disease after immunization of healthy elderly. Exp Gerontol. 2002 January;37(2–3):427–439. doi: 10.1016/s0531-5565(01)00210-8. [DOI] [PubMed] [Google Scholar]
- 8.McElhaney JE, Ewen C, Zhou X, et al. Granzyme B: correlates with protection and enhanced CTL response to influenza vaccination in older adults. Vaccine. 2009 April;27(18):2418–2425. doi: 10.1016/j.vaccine.2009.01.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nichol KL, D’Heilly SJ, Greenberg ME, Ehlinger E. Burden of influenza-like illness and effectiveness of influenza vaccination among working adults aged 50–64 years. Clin Infect Dis. 2009 February;48(13):292–298. doi: 10.1086/595842. [DOI] [PubMed] [Google Scholar]
- 10.Nichol KL, Nordin J, Mullooly J. Influence of clinical outcome and outcome period definitions on estimates of absolute clinical and economic benefits of influenza vaccination in community dwelling elderly persons. Vaccine. 2006 March;24(10):1562–1568. doi: 10.1016/j.vaccine.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007 October;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 12.Nichol KL. Challenges in evaluating influenza vaccine effectiveness and the mortality benefits controversy. Vaccine. 2009 October;27(45):6305–6311. doi: 10.1016/j.vaccine.2009.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Simonsen L, Reichert TA, Viboud C, Blackwelder WC, Taylor RJ, Miller MA. Impact of influenza vaccination on seasonal mortality in the US elderly population. Arch Intern Med. 2005 February;165(3):265–272. doi: 10.1001/archinte.165.3.265. [DOI] [PubMed] [Google Scholar]
- 14.Simonsen L, Taylor RJ, Viboud C, Miller MA, Jackson LA. Mortality benefits of influenza vaccination in elderly people: an ongoing controversy. Lancet Infect Dis. 2007 October;7(10):658–666. doi: 10.1016/S1473-3099(07)70236-0. [DOI] [PubMed] [Google Scholar]
- 15.Simonsen L, Viboud C, Taylor RJ, Miller MA, Jackson L. Influenza vaccination and mortality benefits: new insights, new opportunities. Vaccine. 2009 October;27(45):6300–6304. doi: 10.1016/j.vaccine.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 16.Jackson ML, Nelson JC, Weiss NS, Neuzil KM, Barlow W, Jackson LA. Influenza vaccination and risk of community-acquired pneumonia in immunocompetent elderly people: a population-based, nested case-control study. Lancet. 2008 August;372(9636):398–405. doi: 10.1016/S0140-6736(08)61160-5. [DOI] [PubMed] [Google Scholar]
- 17.Jackson LA, Nelson JC, Benson P, et al. Functional status is a confounder of the association of influenza vaccine and risk of all cause mortality in seniors. Int J Epidemiol. 2006 April;35(2):345–352. doi: 10.1093/ije/dyi275. [DOI] [PubMed] [Google Scholar]
- 18.Fried LP, Tangen C, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 March;56A(3):M1–M11. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 19.Fried LP, Walston J. Frailty. In: Halter JB, editor. Hazzard’s principles of geriatric medicine and gerontology. 6th ed. Anne Arbor: McGraw-Hill; 2007. [Google Scholar]
- 20.Fried LP, Hadley EC, Walston JD, et al. From bedside to bench: research agenda for frailty. Sci Aging Knowl Environ. 2005 August;2005(31):e24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 21.Fried LP, Xue QL, Cappola AR, et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: implications for etiology and treatment. J Gerontol A: Biol Sci Med Sci. 2009 October;64(10):1049–1057. doi: 10.1093/gerona/glp076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leng SX, Xue QL, Tian J, Walston JD, Fried LP. Inflammation and frailty in older women. J Am Geriatr Soc. 2007 June;55(6):864–871. doi: 10.1111/j.1532-5415.2007.01186.x. [DOI] [PubMed] [Google Scholar]
- 23.Fried LP, Ferrucci L, Darer J, Williamson JD, Anderson G. Untangling the concepts of disability, frailty, and comorbidity: implications for improved targeting and care. J Gerontol A: Biol Sci Med Sci. 2004 March;59(3):255–263. doi: 10.1093/gerona/59.3.m255. [DOI] [PubMed] [Google Scholar]
- 24.Makary MA, Segev DL, Pronovost PJ, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010 June;210(6):901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Leng S, Chaves P, Koenig K, Walston J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: a pilot study. J Am Geriatr Soc. 2002 July;50(7):1268–1271. doi: 10.1046/j.1532-5415.2002.50315.x. [DOI] [PubMed] [Google Scholar]
- 26.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical morbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002 November;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 27.Leng S, Xue Q, Tian J. Association of neutrophil and monocyte counts with fraily in community-dwelling older women. Exp Gerontol. 2009;(44):511–516. doi: 10.1016/j.exger.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Qu T, Walston JD, Yang H, et al. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech Ageing Dev. 2009 March;130(3):161–166. doi: 10.1016/j.mad.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Semba RD, Margolick JB, Leng S, Walston J, Ricks MO, Fried LP. T cell subsets and mortality in older community-dwelling women. Exp Gerontol. 2005 January;40(1–2):81–87. doi: 10.1016/j.exger.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 30.De FU, Wang GC, Fedarko NS, Walston JD, Casolaro V, Leng SX. T-lymphocytes expressing CC chemokine receptor-5 are increased in frail older adults. J Am Geriatr Soc. 2008 May;56(5):904–908. doi: 10.1111/j.1532-5415.2008.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao X, Li H, Leng SX. Inflammation and immune system alterations in frailty. Clin Geriatr Med. 2011 February;27(1):79–87. doi: 10.1016/j.cger.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leng SX, Cappola AR, Andersen RE, et al. Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res. 2004 April;16(2):153–157. doi: 10.1007/BF03324545. [DOI] [PubMed] [Google Scholar]
- 33.Bennett NM. Diagnosis of influenza. Med J Aust. 1973 June; Suppl. 22 doi: 10.5694/j.1326-5377.1973.tb111170.x. [DOI] [PubMed] [Google Scholar]
- 34.Dolin R, Reichman RC, Madore HP, Maynard R, Linton PN, Webber-Jones J. A controlled trial of amantadine and rimantadine in the prophylaxis of influenza A infection. N Engl J Med. 1982 September;307(10):580–584. doi: 10.1056/NEJM198209023071002. [DOI] [PubMed] [Google Scholar]
- 35.McElhaney JE, Hooton JW, Hooton N, Bleackley RC. Comparison of single versus booster dose of influenza vaccination on humoral and cellular immune responses in older adults. Vaccine. 2005 May;23(25):3294–3300. doi: 10.1016/j.vaccine.2005.01.080. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Center for Disease Control and Prevention 2009. 11th ed. Washington, DC: Public Health Foundation; 2009. Epidemiology and prevention of vaccine-preventable diseases. Available from: URL: http://www.cdc.gov/vaccines/pubs/pinkbook/ [Google Scholar]
- 37.Beyer WE, Palache AM, Baljet M, Masurel N. Antibody induction by influenza vaccines in the elderly: a review of the literature. Vaccine. 1989 October;7(5):385–394. doi: 10.1016/0264-410x(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 38.Goodwin K, Viboud C, Simonsen L. Antibody response to influenza vaccination in the elderly: a quantitative review. Vaccine. 2006 February;24(8):1159–1169. doi: 10.1016/j.vaccine.2005.08.105. [DOI] [PubMed] [Google Scholar]
- 39.Gravenstein S, Drinka P, Duthie EH, et al. Efficacy of an influenza hemagglutinin-diphtheria toxoid conjugate vaccine in elderly nursing home subjects during an influenza outbreak. J Am Geriatr Soc. 1994 March;42(3):245–251. doi: 10.1111/j.1532-5415.1994.tb01746.x. [DOI] [PubMed] [Google Scholar]
- 40.Shahid Z, Kleppinger A, Gentleman B, Falsey AR, McElhaney JE. Clinical and immunologic predictors of influenza illness among vaccinated older adults. Vaccine. 2010 August;28(38):6145–6151. doi: 10.1016/j.vaccine.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roos-Van Eijndhoven DG, Cools HJ, Westendorp RG, Ten Cate-Hoek AJ, Knook DL, Remarque EJ. Randomized controlled trial of seroresponses to double dose and booster influenza vaccination in frail elderly subjects. J Med Virol. 2001;63(4):293–298. doi: 10.1002/1096-9071(200104)63:4<293::aid-jmv1004>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 42.Iorio AM, Camilloni B, Basileo M, Neri M, Lepri E, Spighi M. Effects of repeated annual influenza vaccination on antibody responses against unchanged vaccine antigens in elderly frail institutionalized volunteers. Gerontology. 2007;53(6):411–418. doi: 10.1159/000110579. [DOI] [PubMed] [Google Scholar]
- 43.Dean AS, Moffatt CR, Rosewell A, et al. Incompletely matched influenza vaccine still provides protection in frail elderly. Vaccine. 2010 January;28(3):864–867. doi: 10.1016/j.vaccine.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 44.McElhaney JE, Xie D, Hager WD, et al. T cell responses are better correlates of vaccine protection in the elderly. J Immunol. 2006 May;176(10):6333–6339. doi: 10.4049/jimmunol.176.10.6333. [DOI] [PubMed] [Google Scholar]
- 45.Gijzen K, Liu WM, Visontai I, et al. Standardization and validation of assays determining cellular immune responses against influenza. Vaccine. 2010 April;28(19):3416–3422. doi: 10.1016/j.vaccine.2010.02.076. [DOI] [PubMed] [Google Scholar]