Abstract
Lung cancer is the most common cause of cancer mortality in male and female patients in the US. Although it is clear that tobacco smoking is a major cause of lung cancer, about half of all women with lung cancer worldwide are never-smokers. Despite a declining smoking population, the incidence of non-small cell lung cancer (NSCLC), the predominant form of lung cancer, has reached epidemic proportions particularly in women. Emerging data suggest that factors other than tobacco, namely endogenous and exogenous female sex hormones, have a role in stimulating NSCLC progression. Aromatase, a key enzyme for estrogen biosynthesis, is expressed in NSCLC. Clinical data show that women with high levels of tumor aromatase (and high intratumoral estrogen) have worse survival than those with low aromatase. The present and previous studies also reveal significant expression and activity of estrogen receptors (ERα, ERβ) in both extranuclear and nuclear sites in most NSCLC. We now report further on the expression of progesterone receptor (PR) transcripts and protein in NSCLC. PR transcripts were significantly lower in cancerous as compared to non-malignant tissue. Using immunohistochemistry, expression of PR was observed in the nucleus and/or extranuclear compartments in the majority of human tumor specimens examined. Combinations of estrogen and progestins administered in vitro cooperate in promoting tumor secretion of vascular endothelial growth factor and, consequently, support tumor-associated angiogenesis. Further, dual treatment with estradiol and progestin increased the numbers of putative tumor stem/progenitor cells. Thus, ER- and/or PR-targeted therapies may offer new approaches to manage NSCLC.
Keywords: Progesterone, Estrogen, Steroid hormone receptor, Non-small cell lung cancer, VEGF, Progenitor cells, Cancer stem cells, Angiogenesis
1. Introduction
Lung cancer is the most common cause of cancer mortality in female and male patients in the US. In contrast to that in men, lung cancer mortality among women has reached epidemic proportions, increasing 600% since 1950 and accounting for 25% of all female cancer deaths [1]. About 1.75 times more women in the United States are expected to die from lung cancer than from breast cancer in 2010 [2]. Non-small cell lung cancer (NSCLC) is the predominant type of lung cancer and includes major histologic types such as adenocarcinoma, squamous carcinoma and large cell carcinoma. Of these, adenocarcinoma is the most common lung cancer in current series. Survival rates from NSCLC are unacceptably low (about 15% five-year survival), and new approaches to treat and prevent this disease are urgently needed. Although there is clear evidence that tobacco smoking is a major cause of lung cancer, about 53% of all women with lung cancer worldwide are never-smokers [3]. Despite a decline of the smoking population, the incidence of NSCLC, particularly adenocarcinoma subtypes, is rapidly increasing [1,2]. Adenocarcinoma represents three-fourths of primary lung cancers in women and is also the most frequent histologic type of NSCLC in nonsmokers and young people [2,3]. Such data suggest that etiologic factors other than tobacco may also have a role in development and progression of lung cancer.
Emerging evidence shows that female sex hormones, especially endogenous and exogenous estrogens (E), are key contributors to NSCLC progression in women [4–12]. Aromatase, a cytochrome P-450 enzyme that mediates the final, rate-limiting step in estrogen synthesis, catalyzing conversion of androstenedione and testosterone to estrone and estradiol, respectively, is expressed in both primary and metastatic NSCLC. Clinical data now show that women with high levels of lung tumor aromatase expression and activity (and consequently high intratumoral estrogen levels) have significantly worse survival than those with low levels of this enzyme [4,5,8]. In both male and female patients, about 73% of NSCLCs show higher levels of intratumoral estradiol in cancer tissue than in paired non-neoplastic lung tissue. Such results confirm that estradiol is locally produced in NSCLC by aromatase.
Despite earlier conflicting data on the presence of estrogen receptors (ER) in lung, numerous studies now confirm significant expression and activity of ERα and ERβ in most NSCLC, particularly in adenocarcinoma [4,6–12]. In the epithelium of the lung, ERβ is abundant while ERα tends to be relatively lower. Further, levels of ERβ and ERα mRNA expression are noted to be increased in lung cancer as compared to normal epithelia [13,14]. ERα and ERβ proteins occur in nuclei and extranuclear sites. In lung, as in the breast, extranuclear ERs derive from the same transcript as nuclear ER [7,15–17]. Nuclear and extranuclear ER appear to act in concert to promote cell growth [15,18]. In normal lung, ERs are involved in important physiologic functions, including alveolar formation in development, activation of alveolar regeneration and maintenance of pulmonary diffusion capacity in the adult [19–21]. In lung malignancy, estrogens stimulate rapid cellular effects on MAPK and AKT kinases and phosphorylation of steroid receptor coactivators (SRC-3/AIB1) that appear to correlate with later stimulation of NSCLC cell proliferation, angiogenesis and tumor progression, and these actions in lung tumors are inhibited by the pure antiestrogen fulvestrant and by aromatase inhibitors [4–8,22–27]. Estrogen-induced transcription is mediated by ERα and ERβ in cell nuclei and is augmented by protein-protein interactions of ER forms with other transcription factors or extranuclear complexes (MAPK and AKT kinases) that, in turn, modulate ERα and ERβ and downstream gene transcription [15]. Recent work offers additional proof that estradiol significantly increases NSCLC proliferation in the presence of either ERα or ERβ [26,27].
Several decades ago, the Coronary Drug Project identified men who had a previous myocardial infarction and randomly assigned them, as part of a multi-component clinical trial, to conjugated equine estrogen at 2.5 mg/day or to placebo, anticipating a reduction in future cardiac events in the estrogen treatment arm. However, this intervention was stopped for primary endpoint futility when increased lung cancer mortality was observed in the estrogen therapy group [28]. Data from more recent randomized, prospective trials suggest that hormone replacement therapy with estrogen plus progestins increases both the incidence of and the mortality from lung cancer in postmenopausal women [29,30], although conflicting data based on earlier retrospective studies are reported [31]. Of special note, similar prospective trials suggest that hormone replacement therapy with estrogen alone is not sufficient to enhance mortality from lung cancer in women [32]. The reason for this apparent contradiction is not known and requires further investigation of the role of progestins in lung cancer progression.
Effects of progestins are mediated by progesterone receptor (PR), and reported PR expression in lung tumors is variable, with some studies reporting a high expression frequency [39–63%; 25,33–35] and others showing little or no expression [36–38]. Low PR expression is a prognostic factor for poor clinical outcome in some studies of NSCLC [33,35] but other survival studies with PR showed no correlation with outcome [25]. Progesterone supplementation has also been shown to inhibit the growth of PR-positive lung tumors in mice [33]. In contrast, treatment of mice with an antiprogestin (mifepristone; RU-486) reduced the progression of spontaneous lung tumors [39]. In embryonic lung cells, combined treatment with estrogen and progesterone increased expression of vascular endothelial growth factor (VEGF) mRNA and protein (40). Pretreatment with antiestrogen ICI 182,780 and antiprogestin RU-486 completely abolished the sex steroid-induced effects. Thus, estrogen and progestins appear to cooperate in promoting expression of VEGF in primary embryonic lung cells and are also involved in regulating expression of key molecules for prenatal lung development and postnatal lung function [40]. Support for this notion also comes from recent reports showing that progestins and estrogen can promote the expansion of stem/progenitor cells in mammary tissues [41,42].
Cancer progression is also dependent on the development of a rich vascular network, a process regulated by a number of potent growth factors, particularly VEGF [43,44]. It has been clearly established that VEGF is produced by many tumor cells, including NSCLC cells [43–46], and the VEGF content of malignant cells has been shown to correlate with the prognosis of patients with lung cancer [45,46]. It is also reported that VEGF produced by tumor cells is essential for the expansion of lung cancer, largely by increasing proliferation of endothelial cells from neighboring blood vessels through interactions with VEGF receptors present on these cells. Only limited information is currently available on the roles of estrogens and progestins in regulating angiogenic growth factors and tumor-associated angiogenesis in human lung cancer [7,19,47]. Although much emphasis has been placed on the role of estrogens in NSCLC progression, this work reports further on the expression of PR and the potential activity of progestins in this malignancy.
2. Experimental
2.1. Cell culture and reagents
Human NSCLC cells (A549, H23, H1975, H3255, HCC827) were obtained from the American Type Culture Collection (Manassas, VA). Lung cancer cell lines were routinely maintained in RPMI 1640 media with 10% fetal bovine serum (FBS; Invitrogen/Life Technologies) and antibiotic-antimycotic solution (Mediatech). For steroid-free conditions, medium was changed 48 h before studies to phenol-red free RPMI 1640 with 0.1% dextran-coated, charcoal-treated (DCC)-FBS [48,49]. Lung tumor cells were characterized previously for ER (ERα, ERβ) expression using ligand binding, immunoassay and immunofluorescence methods [6,7,27]. Human umbilical vein endothelial cells (HUVEC; Clonetics/BioWhittaker) at passages 4–6 were grown on attachment factor-coated tissue culture flasks in MEM containing 10 ng/mL basic-FGF and 15% FBS [50–53]. All cultures were maintained in a humidified incubator at 37°C under 5% CO2, 95% air and free of Mycoplasma and pathogenic murine viruses. Recombinant human VEGF-165 (rhVEGF), anti-VEGF antibody, IgG antibody control and a Quantikine VEGF ELISA kit were acquired from R&D Systems, Inc. (Minneapolis, MN). Progesterone, medroxyprogesterone acetate (MPA), RU-486 and estradiol-17β were purchased from Sigma-Aldrich Corp. (St. Louis, MO).
2.2. VEGF secretion
Secretion of VEGF, a primary proangiogenic factor, was quantitated in the extracellular media of NSCLC cells by ELISA assays using established methods as before [50–52]. NSCLC cells were grown in maintenance medium containing 10% FBS in 100-mm tissue culture dishes and allowed to reach 60–70% confluence. Cells were washed twice with PBS, and the medium was changed to phenol red-free, serum-free medium and incubated for 24 h. The serum-free medium was replaced, and the cells were treated with or without 10 nM progesterone or MPA for 18 h. Conditioned medium was collected for determination of VEGF. VEGF was quantitated using a Quantikine kit according to the manufacturer’s protocol. VEGF values were calculated by plotting absorbance at 450 and 540 nm and comparing unknown values to standards.
2.3. Cell proliferation assays
Proliferation of NSCLC cells was quantitated as before [6,7,48]. To determine effects of progesterone, cells were cultured in phenol red-free, steroid-free conditions for 48 h, then treated in triplicate with vehicle or increasing concentrations of progesterone alone (0.1 nM- 1000 nM), mifepristone alone (RU-486; 0.1 nM- 1000 nM) or progesterone with a fixed dose of progesterone receptor antagonist mifepristone (1 µM). After 72 h, cells were counted using the colorimetric assay CellTiter 96 Aqueous (Promega) to determine the number of viable cells. Correlation of cell numbers with colorimetric assay data were verified in preliminary experiments.
To assess paracrine effects of progesterone- and MPA-induced VEGF on endothelial cell proliferation, NSCLC cells were first grown in medium containing 10% FBS in 100-mm tissue culture dishes and allowed to reach approximately 70–80% confluence [52,53]. Cells were then washed twice with PBS and placed in serum-free, phenol red-free medium overnight. The medium was then changed, and the cells were treated with 10 nM progesterone or MPA for 24 h. Conditioned medium was collected, filtered through a 0.2-µm pore size membrane, and stored at −80 C. HUVEC cells were seeded at 5 × 103 cells/well in culture medium with FBS into a 96-well plate overnight as described above. For HUVEC cells, the medium was replaced with phenol red-free medium containing 0.5% DCC-FBS for 12 h, after which the medium was removed, and conditioned medium was added for 48 h with and without the anti- VEGF antibody or IgG control as in earlier studies [53]. To neutralize the VEGF effect in hormone-treated conditioned medium or the rhVEGF before addition to the cells, aliquots (100 µl containing 100 ng/ml rhVEGF or conditioned medium) were incubated with anti-VEGF antibody (2µg/ml) or a control IgG (2µg/ml) at 37 C for 1 h and then placed over the cells.
2.4. Gel electrophoresis and Western blot
Cultured NSCLC cells treated with or without progestins or vehicle controls for 30 and 240 min were harvested and lysed. Total cell proteins were resolved by 7.5% SDS-PAGE, transferred to polyvinylidene difluoride membranes (90 V for 2 h), and probed with monoclonal antibodies directed against human progesterone receptor or α-actin or GADPH loading controls (Sigma).
2.5. Aldefluor assay and CD133 labeling using flow cytometry
Aldehyde dehydrogenase (ALDH) activity and CD133 expression were analyzed by established double labeling methods [54–56] using the Aldefluor Assay Kit (Stem Cell Technologies) and CD133/1 antibody (Miltenyi) according to the manufacturer’s instructions. Briefly, cells were suspended in assay buffer and incubated with the ALDH substrate Aldefluor or with Aldefluor and the ALDH inhibitor diethylaminobenzaldehyde DEAB (negative control) for 30 min at 37C. Cells were subsequently washed and labeled with Allophycocyanin (APC)-conjugated CD133/1 for 30 min on ice. Cells were washed with assay buffer twice and analyzed using FACSCalibur (BD Bioscience) and CellQuest software (BD Bioscience). Data were analyzed using FlowJo (Tree Star Inc.). Experiments were performed in triplicate.
2.6. Tumor sphere assays in vitro and tumorigenic potential in vivo
For tumor sphere experiments in vitro, single cell suspensions of NSCLC cells were plated on 1% agarose-coated plates at a density of 1×105 and grown for 7–10 days. Subsequent cultures after dissociation of primary spheres were plated on ultralow attachment plates at a density of 5×103 to 1×104. Tumor sphere cultures were grown in a serum-free basal medium as previously described [57,58].
To compare tumorigenic properties of A549 cell subsets in vivo, CD133+/ALDH+ cells and CD133−/ALDH− cell subsets were harvested and implanted subcutaneously in 7–8 week old NOD/SCID mice primed with subcutaneous estradiol-17β (1.7 mg/biodegradable pellet; Innovative Research) [7,27]. Tumor cells were injected (in 200 µl PBS) subcutaneously at concentrations of 200 or 20,000 cells per mouse (3 mice/group) using established methods [55,58]. Tumors were measured twice weekly. Incidence of tumor xenograft formation was scored 10 weeks after injection [7,27]. Experiments were carried out in accordance with guidelines provided by our institutional Animal Care and Use Committee.
2.7.Patient material
Paraffin-embedded, formalin-fixed human lung tissue was obtained from the Department of Pathology and Laboratory Medicine within the David Geffen School of Medicine at UCLA under appropriate Institutional Review Board (IRB) and Health Insurance Portability and Accountability Act (HIPPA) regulations and approval.
2.8. Immunohistochemistry
PR, ERα and ERβ were detected in lung tissue samples using a standard two-step indirect immunohistochemistry (IHC) protocol similar to those previously described [5,59–61]. Antibodies used included anti-human mouse monoclonal progesterone receptor antibody (IgG1) clone 1A6 (catalogue MAB429) from Millipore (Billerica, MA), anti-human ERα antibody HC-20 (Santa Cruz Biotechnology) and anti-human ERβ1 antibody PPG5/10 (ABDSerotec). Briefly, combined sodium citrate (pH 6.0) and incubation in a pressure cooker (5 min, 125° C) was used for antigen retrieval. Slides were incubated for 1 hr (ERα) or overnight at 4° C (PR and ERβ) with primary antibodies used at a the following dilutions: PR 1:20, ERα 1:200 and ERβ 1:50. A two-step polymer-HRP method (Dako; Carpinteria, CA) was used for detection. No staining was observed for negative controls, which included either breast or lung samples incubated with a non-immune primary antibody. Expression was quantified by a pathologist (M.A.) and checked by a second pathologist (V.M.) for cellular frequency of staining (0–100%) as well as staining intensity (0–3) for nuclear or for extranuclear/cytoplasmic staining as before [5].
2.7. Gene expression analyses
A publicly available lung cancer gene expression data set was used for gene expression analysis [62]. This data was available through the Gene Expression Omnibus (GEO) website (accession number GSE11969), and was chosen because gene expression for non-malignant lung tissue was included. The data was generated on an Agilent Homo sapiens 21.6K custom array, with 21619 probes [62]. Samples were pre-processed using a protocol implemented into the R function Sample Network. Briefly, the sample network function evaluates array quality by considering the inter-array correlation (IAC) between samples. The IAC is the Pearson correlation coefficient between a given pair of arrays across all probe sets. The Sample Network R function defines a sample dissimilarity measure as 1 minus the inter-array correlation. Arrays with low inter-array correlation are automatically removed since they are likely to represent outliers. Hierarchical clustering did not reveal a batch effect in the data. Mean expression for progesterone receptor was determined for defined non-malignant and malignant human lung tissues [62].
2.8. Statistics
The Mann-Whitney U test was used for two-group comparisons. The Kruskal-Wallis and Spearman rank correlation tests were used for multi-group comparisons [5,63]. For preclinical laboratory studies, statistical analysis was carried out using ANOVA or student’s t-test as appropriate, with values reported as the mean ± SEM. A P-value < 0.05 was considered significant.
3. Results
3.1. Nuclear and extranuclear expression of ER in archival human NSCLC specimens from the clinic
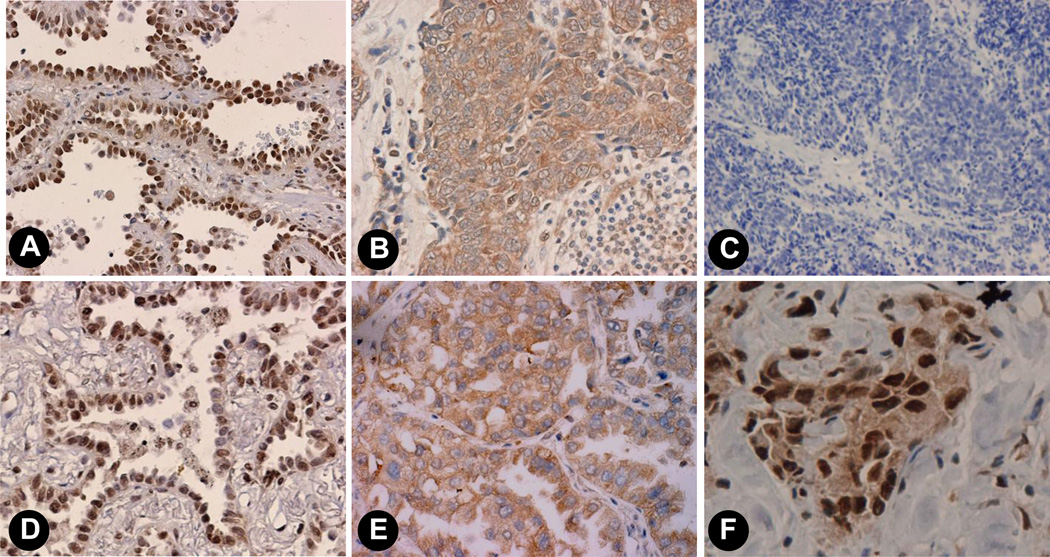
We previously demonstrated the presence of ERα and ERβ in most human NSCLC by standard IHC methods using archival formalin-fixed, paraffin-embedded human tumor specimens (6,7,27). Fig. 1 shows representative examples of the immunoreactivity observed for nuclear (Fig.1A) and extranuclear (Fig.1B) ERα as well as nuclear (Fig. 1D) and extranuclear (Fig. 1E) staining patterns for ERβ. Further, both nuclear and extranuclear ERβ staining occur concurrently in an adenocarcinoma specimen (Fig.1F). As before, no specific staining was observed in controls in the absence of primary antibodies (Fig. 1C).
Fig. 1. Immunohistochemical (IHC) detection of nuclear and extranuclear ERα and ERβ proteins in archival human lung adenocarcinomas.
Formalin-fixed paraffin-embedded tumors were processed for IHC using anti-ERα Ab HC-20 (Santa Cruz) and anti-ERβ1 Ab PPG5/10 (ABDSerotec). Panels A and B show nuclear and extranuclear staining for ERα and panels D and E show nuclear and extranuclear staining for ERβ. Panel C shows no staining in a control specimen with no addition of primary antibody. Panel F is an example of adenocarcinoma that shows both specific nuclear and extranuclear staining for ERβ.
3.2. Expression of progesterone receptor transcripts in malignant and non-malignant lung tissue
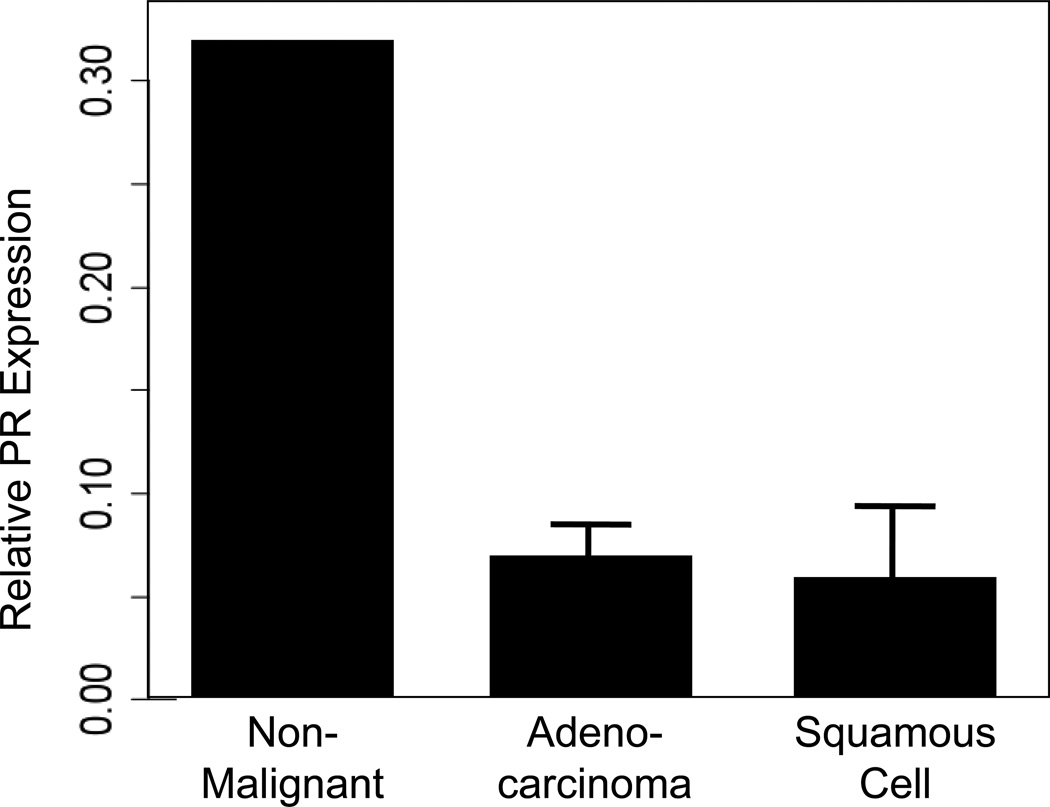
We first considered PR transcript expression in malignant as compared with non-malignant human lung tissue samples. To do this, we conducted a meta-analysis of publically-available gene expression data. These data were derived using an Agilent Homo sapiens 21.6K custom array as described [62]. Expression data were processed to remove artifactual outliers and to determine batch effect parameters which might skew data. At the transcript level, non-malignant (normal) lung tissue demonstrated a greater than 3-fold higher expression of PR as compared to either adenocarcinoma or squamous cell carcinoma (Fig. 2). Among the 149 lung tumor samples, there were 78 at stage I, 26 at stage II and 45 at stage III. There was no correlation of PR transcript expression with the clinical stage for individuals with adenocarcinomas (P=0.705), squamous cell carcinomas (P=0.519), nor all patients combined (P=0.402).
Fig. 2. Mean mRNA expression levels for PR.
Data were downloaded from GEO and processed as described in the Experimental section. Bar plots showing mean PR expression in non-malignant lung tissue (n=5) compared to adenocarcinoma (n=90) and squamous cell carcinoma (n=35). Expression levels in non-malignant lung were significantly higher than adenocarcinoma (Kruskal-Wallis P=0.0037) and squamous cell carcinoma (Kruskal-Wallis P=0.0051).
3.3. Progesterone receptor protein expression in human lung tissue
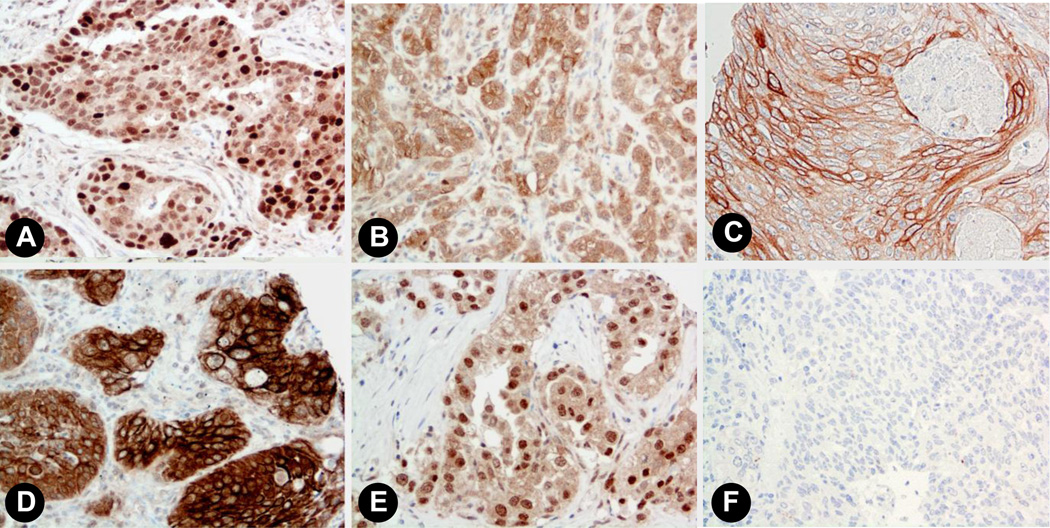
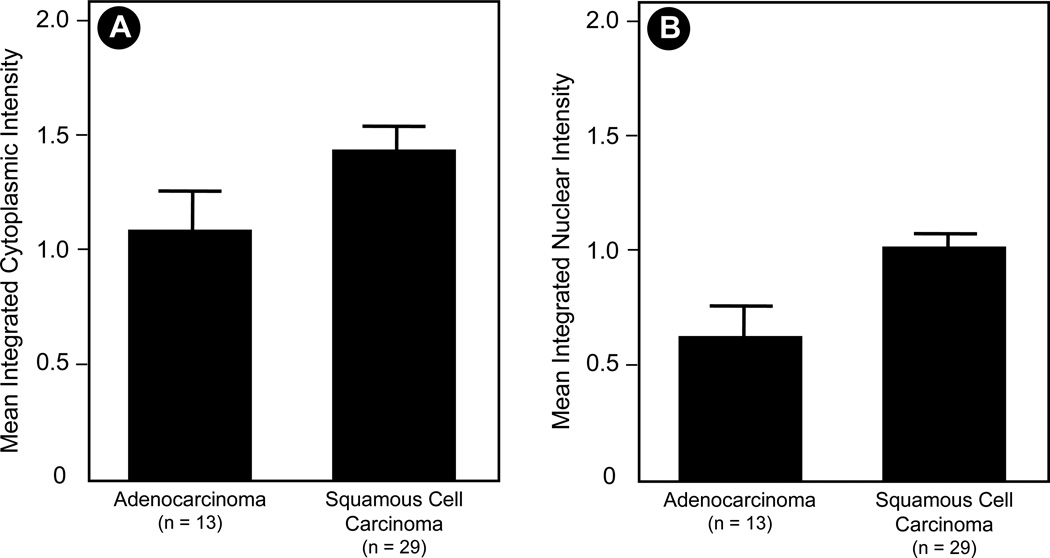
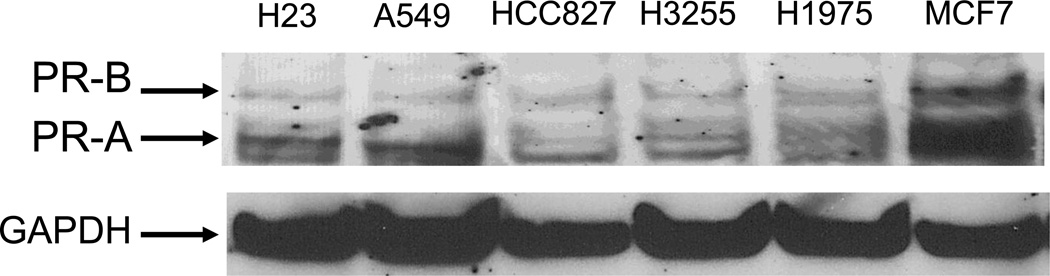
We further examined the protein expression of PR in human lung tissues. Non-small cell lung cancer samples (n=42) were stained for PR using standard IHC protocols as outlined in the Experimental section. We observed a diverse array of staining patterns ranging from both nuclear and extranuclear expression, only nuclear or only extranuclear staining, to below the level of detection of staining in either cellular compartment. Representative images are shown in Figure 3. We quantified the expression level of PR in NSCLC samples from 42 individuals. Results for both nuclear and extranuclear staining patterns are shown in Figure 4. To date, we have observed no correlation between either nuclear or extranuclear PR staining and clinical stage (Spearman correlation coefficient=0.12, P=0.45 for extranuclear staining; and Spearman correlation coefficient=0.052, P=0.75 for nuclear staining). Significant expression of PR was also found in a series of human NSCLC cell lines using immunoassay by Western blot (see Fig. 5).
Fig. 3. Progesterone receptor expression in human lung tissues.
Representative images showing staining patterns for progesterone receptor in normal lung and NSCLC (20x magnification). A) Breast cancer positive control. B) Adenocarcinoma showing relatively stronger extranuclear as compared to nuclear staining. C) Squamous cell carcinoma showing strong membrane staining; D) Squamous carcinoma with some of the tumor cells showing both intense membrane and cytoplasmic staining. E) Adenocarcinoma showing considerably stronger nuclear staining relative to extranuclear staining. F) Non-immune antibody-incubated negative controls showed no staining.
Fig. 4. PR protein expression in NSCLC tissues.
Human lung tissue samples were stained for PR protein expression as described in the Experimental Section. Columns are mean integrated PR expression; bars are SEM from adenocarcinoma and squamous carcinoma NSCLC specimens (42 individuals). Mean integrated (A) cytoplasmic/extranuclear (P < 0.001) and (B) nuclear (P = 0.103) expression of PR are compared for adenocarcinoma and squamous carcinoma.
Fig. 5. Expression of progesterone receptor (PR) in human NSCLC cell lines.
Significant immunoreactivity for PR-A and PR-B was detected in NSCLC cancer cell lines and in control human breast cancer cell line MCF-7. Western blots were performed as described in methods. GAPDH is shown as a loading control.
3.4. Progesterone and RU-486 effects on NSCLC cell proliferation in vitro
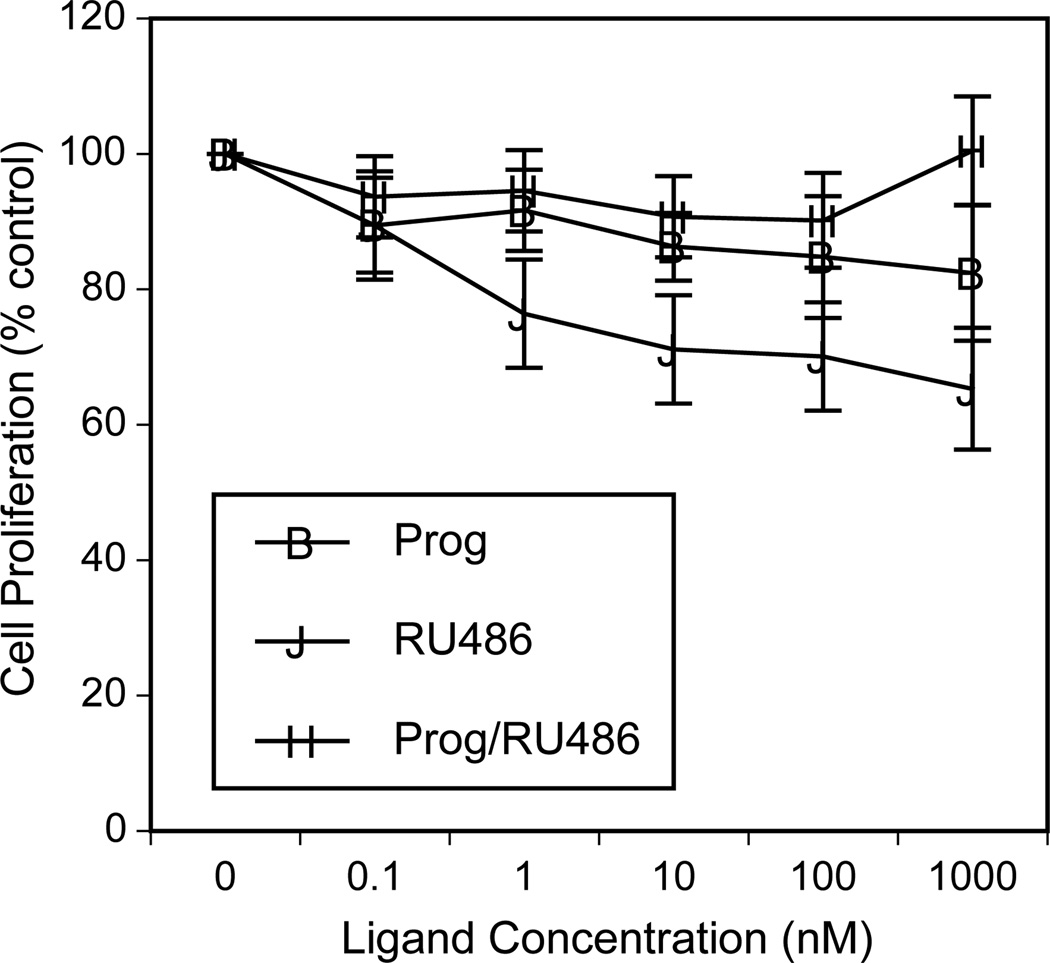
One previous report shows that progesterone alone inhibits the proliferation of NSCLC cells [33] while another study suggests that progesterone promotes proliferation [64]. To further assess effects of progestins on NSCLC proliferation, we used the H23 adenocarcinoma cell line that expresses progesterone receptors (Fig. 5). Progesterone treatment for 72 hr elicited a minimal inhibition of cell proliferation that was significantly different from controls only at the 1 µM dose (P<0.05; see Fig. 6). This inhibitory effect of the high dose of progesterone was reversed by the progesterone receptor antagonist RU-486. Of note, RU-486 administered as a single agent elicited a significant inhibition of cell proliferation over a dose range of 0.1–1000 nM (P<0.05).
Fig. 6. Effects of progesterone and RU-486 on NSCLC cell proliferation.
Treatment of H23 lung adenocarcinoma cells with increasing concentrations of progesterone alone (Prog; 0.1–1000 nM), RU-486 alone (0.1–1000 nM) or progesterone (0.1–1000 nM) combined with RU-486 (at a fixed dose of 1 µM)(Prog/RU486) was assessed in vitro. Progesterone alone elicited minimal inhibition of cell proliferation that was significantly different from controls only at 1 µM (P<0.05). This effect was blocked when cells were treated with progesterone in the presence of RU-486 at 1 µM. RU-486 alone elicited significant inhibition of cell proliferation over a dose range of 0.1–1000 nM (P<0.05). Similar results were found with A549 cells treated as above (data not shown).
3.5. Progestins and estrogen stimulate VEGF secretion by NSCLC cells
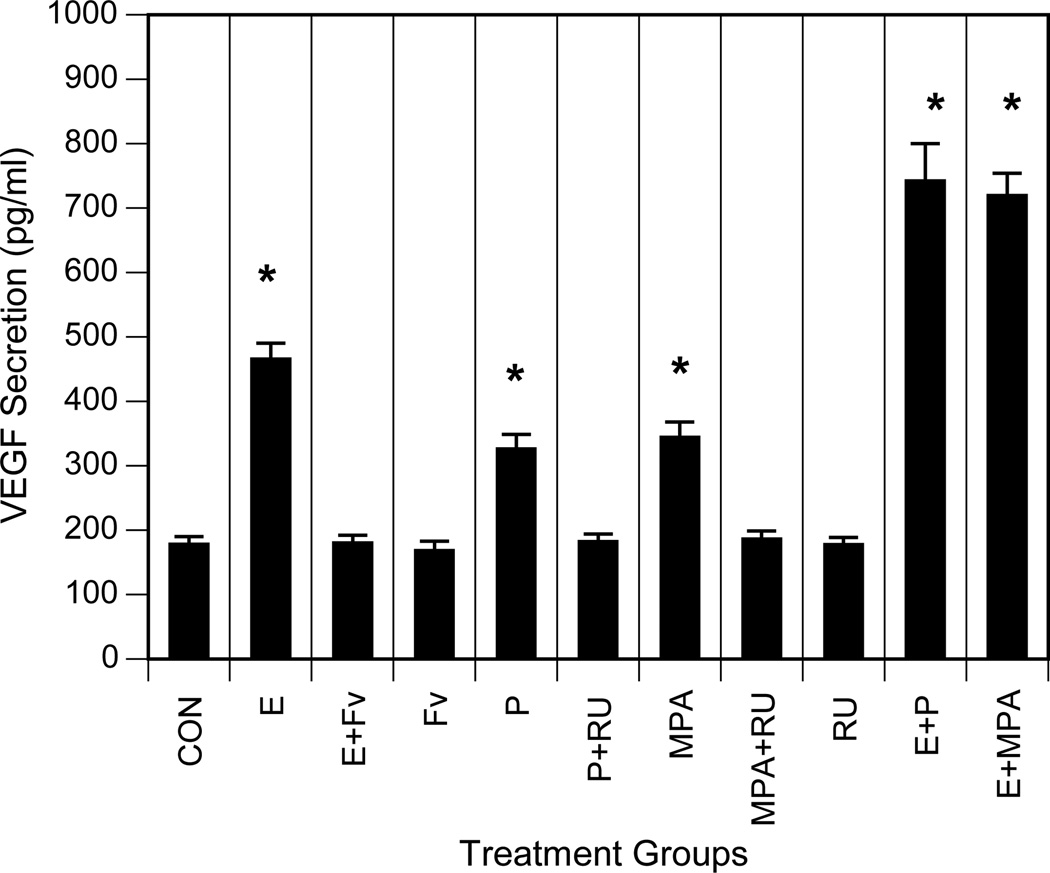
Progestins are known to increase VEGF production and angiogenesis in mammary tissues [53]. Moreover, combined treatment with estrogen and progesterone is reported to increase VEGF transcripts and protein levels in embryonic lung cells and fibroblasts [40]. Recent clinical hormone replacement therapy (HRT) trials also showed that treatment with progestins combined with estrogens increases the incidence and mortality for lung cancer in postmenopausal women [29,30]. However, the role of progestins in lung cancer biology remains to be fully elucidated. To assess the effect of progestins and estrogens on VEGF secretion by NSCLC cells, we treated A549 cells in vitro with progesterone, estradiol-17β or combinations of these agents and then performed ELISA assays to detect VEGF in extracellular media. MPA, a synthetic progestin commonly used in postmenopausal HRT and oral contraception, was also included in these studies to compare with the action of the natural steroid. Figure 7 shows that estradiol as well as progesterone and MPA induce VEGF secretion in NSCLC cells under serum-free conditions. In addition, the antiestrogen fulvestrant blocked estrogen-dependent VEGF, while the antiprogestin RU-486 suppressed progestin-dependent VEGF, indicating that these responses were receptor-dependent.
Fig. 7. Progestins and estrogens stimulate VEGF secretion by NSCLC cells.
A549 cells were maintained in medium with 10% FBS as described in Experimental. Cells were then washed with PBS and incubated in serum-free media overnight. The medium was replaced, and incubation was continued for an additional 18 h without ligand (CON; control) or with 10 nM estradiol-17β (E), 1µM ICI 182,780 (FV; fulvestrant), estradiol and ICI 182,780 (E+Fv), 10 nM progesterone (P), 10 nM MPA, 1 µM RU-48610 (RU), progesterone and RU-486 (P+RU), MPA and RU-486 (MPA+RU) or estradiol and progesterone (E+P) or estradiol and MPA (E+MPA), as indicated. Conditioned media were collected, and VEGF was measured by ELISA (expressed as picograms of VEGF per ml). Values represent the mean ± SEM from three determinations. *, VEGF induction values significantly different from controls at P<0.001.
3.6. VEGF-rich conditioned medium from NSCLC cells treated with progesterone promotes endothelial cell proliferation
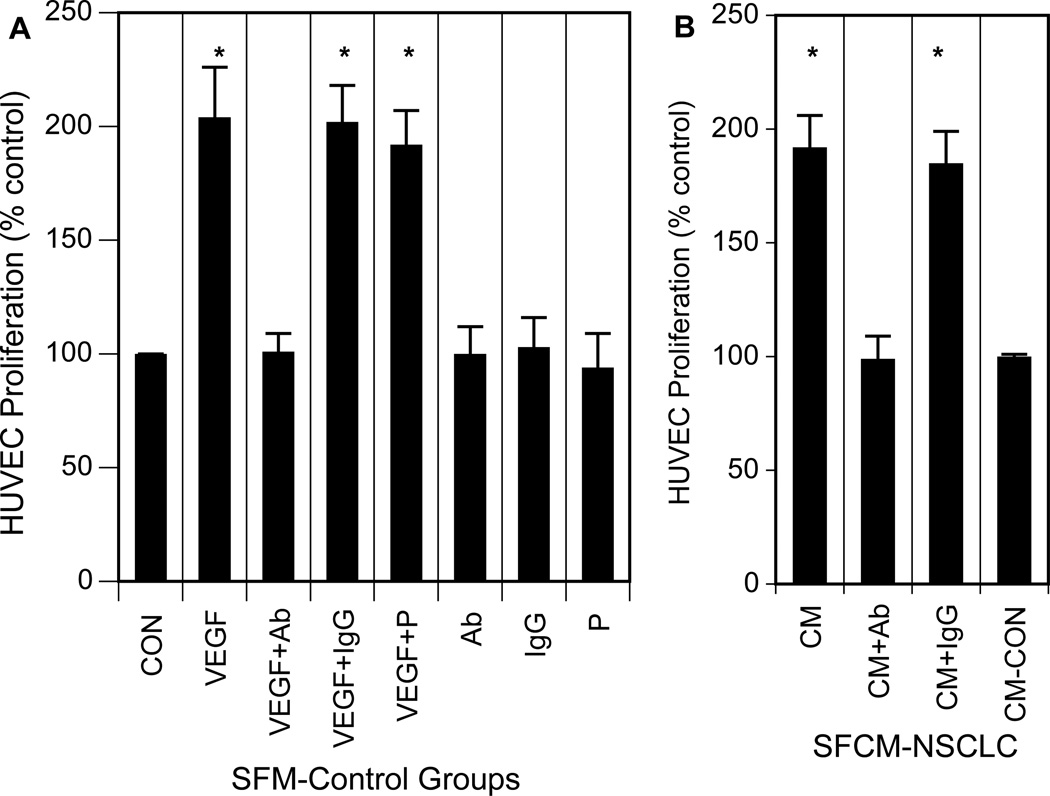
In the next series of experiments, we determined whether VEGF within conditioned medium collected from progestin-treated tumor cells was angiogenically-active and able to increase proliferation of vascular endothelial cells (HUVECs) [53]. NSCLC cells were treated with 10 nM progesterone, and the conditioned medium was collected after 24 h. HUVECs were exposed to the serum-free conditioned medium or to serum-free medium alone for 24 h. Cell proliferation was detected by measurement of cell numbers. As shown in Fig. 8, exposure of HUVECs to conditioned medium collected from progesterone-treated NSCLC cells or to VEGF significantly increased proliferation. The proliferative response of HUVECs was abolished in the presence of anti-VEGF antibody, but not in the presence of nonimmune IgG, suggesting that progestin-induced VEGF was the major factor to induce cell proliferation in vascular endothelial cells. VEGF was used to confirm that the population of HUVECs used in our test system contained an intact VEGF-dependent signal transduction pathway that leads to the proliferation of HUVECs, as also reported by others [43,44,53]. These results suggest that VEGF produced by tumor cells was sufficient to cause a proliferative response of endothelial cells either by itself or in combination with certain tumor cell- or endothelial cell-derived factors for which VEGF was the predominant partner. When nonconditioned serum-free medium was added to HUVECs as a control, and these cells were then exposed to progesterone, proliferation was equal to that in controls, indicating that prior conditioning of the medium with NSCLC cells was essential for subsequent HUVEC proliferation. An additional control experiment shows that no significant HUVEC proliferation occurred with progesterone administered directly in serum-free medium (Fig. 8). These results indicate that lung tumor cells can produce angiogenically-active VEGF in response to progestins, which can subsequently increase the proliferation of human endothelial cells.
Fig. 8. Paracrine effects of progesterone-induced VEGF on HUVEC proliferation.
Conditioned medium was prepared from NSCLC cells (A549) treated with progesterone (P) as described in Experimental. A) HUVECs were seeded overnight and then incubated 12 h. The medium was then aspirated and replaced with serum-free medium (SFM) alone as a control, VEGF (100 ng/ml), VEGF antibody (Ab), IgG control, progesterone (P; 10 nM) or combinations of these agents and the incubation was continued for an additional 24 h. Only VEGF treatment significantly effectively promoted HUVEC proliferation (*P<0.001). All results are shown as % control, with proliferation in SFM alone as control. B) Alternatively, HUVECs were treated with conditioned medium prepared from progesterone-treated NSCLC cells (SFCM-NSCLC) alone (CM) or with VEGF antibody (CM+Ab) or control IgG (CM+IgG). HUVEC proliferation was later assessed by cell counts and expressed as percent control. Control cells were cultivated in conditioned medium from NSCLC cells not treated with progesterone (CON-CM). Conditioned medium from progesterone-treated NSCLC cells significantly increased HUVEC proliferation (*P<0.001), and this effect was blocked by simultaneous exposure to VEGF antibody (*P<0.001) but not to control IgG. Values represent the mean ± SEM from three different experiments.
3.7. Estradiol and progestins promote an increase in the number of tumor stem/progenitor cells
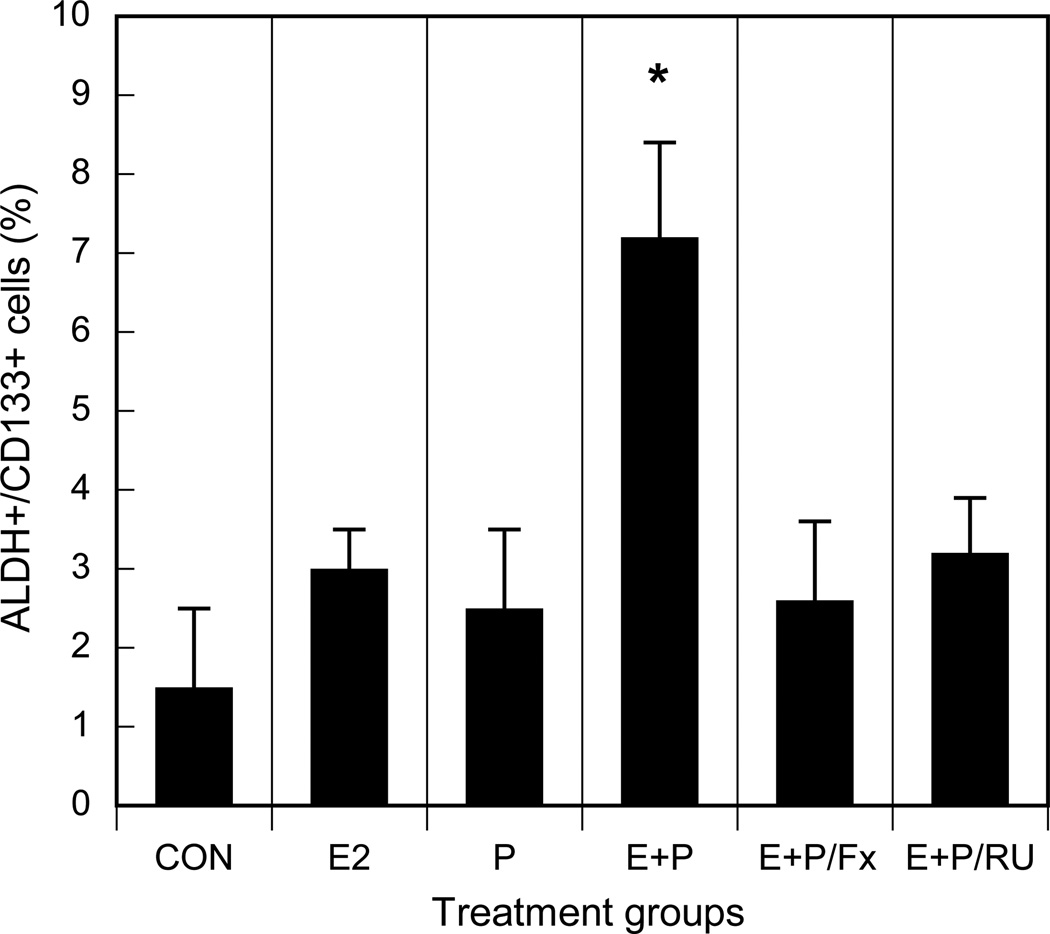
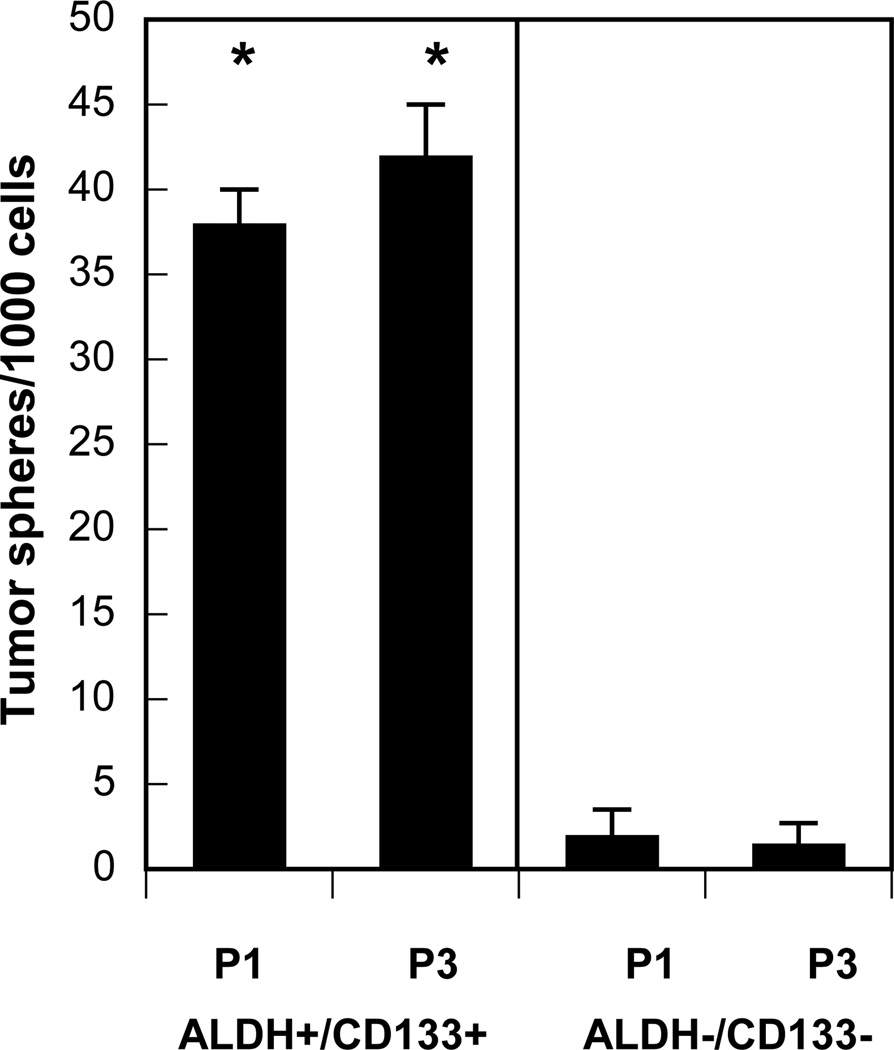
Recently, substantial evidence has accumulated to support the role of a small subset of self-renewing cells that sustain malignant growth [54,55,57]. This subpopulation is termed cancer-initiating cells or cancer stem/progenitor cells (CSPC) for their high capacity for self-renewal and superior levels of malignancy. Cancer stem cells are identified and isolated in several malignancies including lung cancer [54,55,57,58,60]. These CSPC express tissue-specific cell surface markers, such as CD44+/CD24low in breast [54,55,57] and CD133+ in brain, prostate, pancreas and lung cancers [cf.58]. CD133 (prominin-1), a 5-transmembrane glycoprotein, was recently reported as an important biomarker to identify subsets of human lung CSPC [58,65,66]. Further, isolated lung cancer cells with high aldehyde dehydrogenase-1 (ALDH) activity display in vitro and in vivo features of cancer stem cells and express surface marker CD133 [67]. Based on these data, we assessed effects of treatment with estrogen and progestins on the expansion of putative CSPC in NSCLC. We identified CD133+/ALDH+ tumor stem/progenitor cells from human lung cancer cells in vitro using Aldefluor assay (to isolate ALDH+ cells) in combination with labeled anti-CD133 antibodies followed by fluorescence-activated cell sorting analysis. Treatment with either estrogen or progesterone alone elicited relatively small increments in the numbers of CSPC subsets, but estrogen combined with progesterone stimulated a several-fold increase in the numbers of CSPC (P<0.001) (Fig. 9). This latter effect of combined therapy was blocked by use of antiestrogens. We then cultivated isolated subpopulations of tumor cells following dual therapy with estrogen plus progesterone and determined that these CD133+/ALDH+ subsets were able to grow as tumor spheres and maintain self-renewal capacity, properties indicative of CSPC. In contrast, control CD133−/ALDH− subsets were not capable of significant tumor sphere formation (Fig. 10). To confirm the tumorigenic potential of these CD133+/ALDH+ cells as compared with CD133−/ALDH− cells, we implanted the tumor cell subsets subcutaneously in immunosuppressed mice by established methods [55,58]. Tumor growth occurred in all 3 of 3 mice inoculated subcutaneously with either 200 or 20,000 CD133+/ALDH+ cells. In contrast, no tumor growth was detected with inoculation of 200 CD133−/ALDH− tumor cells, but 1 of 3 mice developed tumors with injection of 20,000 CD133−/ALDH− cells. Thus, these findings suggest that CD133+/ALDH+ tumor cells also show a greater tumorigenic capability then CD133−/ALDH− cell subsets in vivo.
Fig. 9. Estrogen and progestin increase the proportion of cancer stem/progenitor cell subsets as determined by assay of specific biomarkers, ALDH and CD133.
NSCLC cells (A549) were treated in vitro with control (CON), 10 nM estradiol-17β (E2), 10 nM progesterone (P), E2 and P (E+P) or E2 and P in the presence of either 1 µM fulvestrant (E+P/Fx) or 1 µM RU-486 (E+P/RU). Thereafter, aldehyde dehydrogenase (ALDH) activity and CD133 expression were analyzed by established double labeling methods using an Aldefluor Assay Kit and CD133 antibody, respectively, followed by FACS sorting [54–56]. The numbers of ALDH+/CD133+ lung tumor cells, representing a putative CSPC subset, was expressed as a percent of total lung tumor cells for each treatment group. Dual therapy with estrogen and progestin increases the proportion of cancer stem/progenitor cell subsets as determined by assay of specific biomarkers, ALDH and CD133 (*P<0.001). Experiments were performed in triplicate.
Fig. 10. ALDH+/CD133+ tumor cell subpopulations elicited by treatment with estrogen and progesterone form tumor spheres and exhibit self-renewal properties.
For tumor sphere experiments, single cell suspensions of NSCLC cells (A549) were plated on 1% agarose-coated plates at a density of 1×105 and grown for 7–10 days. Aldefluor-positive (ALDH+) and CD133-positive (CD133+) cells isolated after treatment with estrogen and progesterone (see Fig. 9) formed significantly greater numbers and sizes of tumor spheres as compared to Aldefluor-negative (ALDH−) and CD133-negative (CD133−) controls (P<0.001). Subsequent cultures after dissociation of primary spheres were plated on ultralow attachment plates at a density of 5×103 to 1×104 (see P1, P3, 1st and 3rd passages, respectively). Tumor sphere cultures were grown in a serum-free basal medium as previously described [57,58]. Among ALDH+/CD133+ cells isolated after control treatment in the absence of estradiol and progesterone (see Fig. 9), the number of tumor spheres formed was only 11±4 (n=3) per 1000 cells after 7–10 days; this result was significantly different than that found with ALDH+/CD133+ cells isolated after treatment with estradiol plus progesterone (P<0.01;not shown). *, values significantly different from controls at P<0.001.
4. Discussion
The clinical outcome for patients with lung cancer has not changed significantly for over two decades. New approaches to therapy are urgently needed, and these should be based on new understanding of fundamental growth-promoting pathways in the lung and possible sex differences in the natural history and therapeutic responses of NSCLC [1–12]. The incidence of NSCLC, the predominant form of lung cancer, has now reached epidemic proportions particularly in women [1]. Emerging data suggest that female sex hormones have a role in lung cancer development and progression. Endogenous and exogenous estrogens appear to be key contributors in stimulating NSCLC growth and progression. Aromatase, a key enzyme for estrogen biosynthesis, is expressed and active in NSCLC [5,6,8]. The present and previous studies also reveal significant expression and activity of ERα and ERβ in both extranuclear and nuclear sites in most NSCLC. We now report on the occurrence of PR transcripts and protein in NSCLC. Using immunohistochemistry, expression of PR protein was observed in the nucleus and/or extranuclear compartment in the majority of human tumor specimens and NSCLC cell lines examined. These findings are consistent with independent reports on the expression of PR protein in NSCLC [25,33,35]. Although some initial studies indicate that tumor PR levels correlate with clinical outcome [33,35], others suggest no apparent correlation between tumor PR and clinicopathologic characteristics [25]. Further investigation of the potential interplay between ER, PR and growth factor signaling pathways may be required to fully understand their contribution to clinical outcome [35].
Previous work confirms that estrogen signaling in human lung interacts with other signaling pathways, such as EGFR, and contributes to tumor growth and clinical outcome [4,7]. Additional studies indicate that overexpression of VEGF and EGF family ligands and their receptors also correlate with clinical outcome for NSCLC patients [45,46]. New findings show that pulmonary vascularity is altered by sex steroids, particularly estradiol, with a greater number of lung microvessels in ovariectomized female mice receiving estradiol as compared to placebo [19,47]. Similarly, cancer progression is dependent on development of a rich vascular network that supplies vital nutrients to the growing tumor (43,44,52,53). Angiogenesis, the process of new blood vessel formation, is regulated by a number of potent growth factors, one of the most effective of which is VEGF [43,44,53]. It is well-established that VEGF is produced by many tumor cells, including lung cancer cells [45,46], and VEGF produced by tumor cells is essential for malignant expansion, presumably by increasing proliferation of endothelial cells from neighboring blood vessels through interactions with VEGF receptors present on these cells. Our findings further demonstrate that combinations of estradiol and progestins administered in vitro cooperate in promoting NSCLC cell secretion of VEGF and, consequently, enhancing vascular endothelial cell proliferation, an event important for tumor-associated angiogenesis. Independent experiments suggest that progestins do not directly enhance the proliferation of vascular endothelial cells [68]. The results of recent clinical trials indicate that hormone replacement therapy with estrogens and progestins is associated with higher risk of lung cancer in postmenopausal women than treatment with estrogens alone or placebo [29,30,32]. This observation is consistent with the current studies showing that progestins as well as estrogens [7] stimulate the secretion of VEGF by NSCLC cells which in turn stimulates local angiogenesis. These observations suggest that tumor-induced VEGF secretion may function in a paracrine manner to sustain tumor growth. Although endogenous progesterone levels are very low in postmenopausal women and men, in situ production of progesterone is reported in NSCLC specimens [33]. Further, it is possible that progesterone receptor, as in breast [69,70], may also signal through ligand-independent mechanisms due to phosphorylation by kinases in the lung [33,35]. Thus, it will be important to determine if PR activation by ligand-independent pathways can induce VEGF secretion from lung tumor cells to promote angiogenesis.
In view of recent evidence suggesting that sex steroids may influence the numbers of stem/progenitor cells in adult mammary tissues [41,42], we investigated the role of estradiol and progestins in promoting expansion of stem/progenitor cells in NSCLC. Dual treatment with estrogen and progestin in vitro increased the numbers of putative tumor stem/progenitor cells as evidenced by enrichment of CSPC markers and the formation of tumor spheres with self-renewal capability in vitro and enhanced tumorigenicity in vivo. It is reported that the number of tumor spheres (composed of tumor stem/progenitor cell populations) generated upon serial passage provides an indirect measure of cancer stem cell self-renewal [55,57,58]. Independent experiments have also shown that ALDH-positive and CD133-positive lung tumor cell subsets [58,65–67] can generate tumors in vivo that recapitulate the heterogeneity of the parental cancer cells and maintain tumor progression. Immunohistochemical analysis of clinical specimens from lung cancer patients and controls further show that high tumor expression of ALDH and CD133 is correlated with a poor prognosis for patients with lung cancer. Although stem cells in other tissues, such as breast, are reported to lack expression of sex steroid receptors [41,42,55], estrogen and progesterone may impact the function of stem/progenitor cells indirectly by mediating the supply of regulatory factors from neighboring cells (such as Wnt or receptor activator of NF-κB ligand [RANKL] signaling) or by regulating the activity of more differentiated progenitor cells [41,42]. One recent report also shows that vascular endothelial cell-secreted factors can enhance the survival and self-renewal of neighboring head and neck cancer stem/progenitor cells [71]. Of note, lung cancer stem/progenitor cells are also reported to produce substantially higher levels of VEGF than that of bulk tumor cells [58]. Thus, treatments aimed to modulate the activity of ERα, ERβ and/or PR may modulate tumor progression by diverse molecular and cellular pathways and thereby offer new approaches to treat non-small cell lung cancer. Further studies are needed to pursue these alternatives for specific targets of sex steroid action in NSCLC.
Although progestins alone may not directly induce the proliferation of bulk NSCLC cells, the current and earlier findings suggest that these steroids regulate the activity of other tissue factors (such as VEGF, EGF) that indirectly determine the final proliferative or antiproliferative state. Similar actions of progesterone have been reported in breast cancers [72]. Further studies will also be required to understand the significance of extranuclear and nuclear expression patterns of steroid receptors in malignant cells. In endometrial cancers, the PR-A isoform is localized in the nucleus, and PR-B is largely cytoplasmic in the absence of ligand [73]. Similar investigations in lung cancer remain to be done [35]. The distribution of steroid receptors in different subcellular compartments may well modulate signaling by genomic, nongenomic and/or ligand-independent pathways that contribute to tumor progression [15,74].
As in studies of nuclear receptor expression in breast cancer [75,76], a standardized and validated approach to the immunohistochemical detection and interpretation of ERα, ERβ, PR and possibly GPR30 expression is needed for potential screening of human lung tumor specimens in the clinic [35,77]. A systematic examination of hormonal biomarkers could potentially be prognostic and may be useful to identify patients with NSCLC who might benefit from the administration of antihormone therapeutics.
Highlight.
-
➢
PR, ERα and ERβ are expressed in both extranuclear and nuclear sites in NSCLC.
-
➢
PR transcripts were lower in cancerous as compared to non-malignant tissue.
-
➢
Estrogen and progestins promote tumor VEGF secretion and angiogenesis.
-
➢
Estrogen and progestins stimulate putative tumor stem/progenitor cell expansion.
-
➢
ER- and/or PR-targeted therapies may offer new approaches to manage NSCLC.
Acknowledgements
Support for this work was provided by the Early Detection Research Network NCI CA86366 (L. Goodglick; D. Chia), the National Lung Cancer Partnership (L. Goodglick; R. Pietras), the Specialized Programs of Research Excellence in Lung Cancer grant P50-CA90388, NCI (R. Pietras; L. Goodglick), the DOD Lung Cancer Research Program (R. Pietras; D. Marquez-Garban) and the Stiles Program in Oncology (in vitro studies only). We are grateful to patients who volunteered to provide clinical specimens for this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
None of the authors had a conflict of interest.
References
- 1.Patel JD, Bach PB, Kris MG. Lung cancer in US women: a contemporary epidemic. JAMA. 2004;291:1763–1768. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS ONE. 2010;5:e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Subramanian J, Govindan R. Lung cancer in never smokers: A review. J Clin Oncol. 2007;25:561–570. doi: 10.1200/JCO.2006.06.8015. [DOI] [PubMed] [Google Scholar]
- 4.Stabile LP, Davis AL, Gubish CT, Hopkins TM, Luketich JD, Christie N, Finkelstein S, Siegfried JM. Human non-small cell lung tumors and cells derived from normal lung express both estrogen receptor alpha and beta and show biological responses to estrogen. Cancer Res. 2002;62:2141–2150. [PubMed] [Google Scholar]
- 5.Mah V, Seligson D, Li A, Marquez DIW, Elshimali Y, Fishbein MC, Chia D, Pietras R, Goodglick L. Aromatase expression predicts survival in women with early stage non-small cell lung cancer. Cancer Research. 2007;67:10484–10490. doi: 10.1158/0008-5472.CAN-07-2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinberg OK, Marquez-Garban DC, Fishbein MC, Goodglick L, Garban HJ, Dubinett SM, Pietras RJ. Aromatase inhibitors in human lung cancer therapy. Cancer Res. 2005;65:11287–11291. doi: 10.1158/0008-5472.CAN-05-2737. [DOI] [PubMed] [Google Scholar]
- 7.Pietras RJ, Marquez D, Chen H, Tsai E, Weinberg O, Fishbein M. Estrogen and growth factor receptor interactions in human breast and non-small cell lung cancer cells. Steroids. 2005;70:372–381. doi: 10.1016/j.steroids.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 8.Niikawa H, Suzuki T, Miki Y, Suzuki S, Nagasaki S, Akahira J, Honma S, Evans D, Hayashi S, Kondo T, Sasano H. Intratumoral estrogens and estrogen receptors in human non-small cell lung carcinoma. Clin Cancer Res. 2008;14:4417–4426. doi: 10.1158/1078-0432.CCR-07-1950. [DOI] [PubMed] [Google Scholar]
- 9.Mollerup S, Jorgensen K, Berge G, Haugen A. Expression of estrogen receptors alpha and beta in human lung tissue and cell lines. Lung Cancer. 2002;37:153–159. doi: 10.1016/s0169-5002(02)00039-9. [DOI] [PubMed] [Google Scholar]
- 10.Taioli E, Wynder EL. Endocrine factors and adenocarcinoma of the lung in women. J Natl Cancer Inst. 1994;86:869–870. doi: 10.1093/jnci/86.11.869. [DOI] [PubMed] [Google Scholar]
- 11.Beattie CW, Hansen NW, Thomas PA. Steroid receptors in human lung cancer. Cancer Res. 1985;45:4206–4214. (1985) [PubMed] [Google Scholar]
- 12.Ganti AK, Sahmoun AE, Panwalkar AW, Tendulkar KK, Potti A. Hormone replacement therapy is associated with decreased survival in women with lung cancer. J Clin Oncol. 2006;24:59–63. doi: 10.1200/JCO.2005.02.9827. [DOI] [PubMed] [Google Scholar]
- 13.Hershberger PA, Vasquez AC, Kanterewicz B, Land S, Siegfried JM, Nichols M. Regulation of endogenous gene expression in human non-small cell lung cancer cells by estrogen receptor ligands. Cancer Res. 2005;65:1598–1605. doi: 10.1158/0008-5472.CAN-04-2694. [DOI] [PubMed] [Google Scholar]
- 14.Kerr A, 2nd, Eliason JF, Wittliff JL. Steroid receptor and growth factor receptor expression in human nonsmall cell lung cancers using cells procured by laser-capture microdissection. Adv Exp Med Biol. 2008;617:377–384. doi: 10.1007/978-0-387-69080-3_36. [DOI] [PubMed] [Google Scholar]
- 15.Pietras RJ, Marquez-Garban DC. Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res. 2007;13:4672–4676. doi: 10.1158/1078-0432.CCR-07-1373. [DOI] [PubMed] [Google Scholar]
- 16.Norfleet AM, Clarke CH, Gametchu B, Watson CS. Antibodies to the estrogen receptor-alpha modulate rapid prolactin release from rat pituitary tumor cells through plasma membrane estrogen receptors. Faseb J. 2000;14:157–165. doi: 10.1096/fasebj.14.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 18.Gruber CJ, Tschugguel W, Schneeberger C, Huber JC. Production and actions of estrogens. N Engl J Med. 2002;346:340–352. doi: 10.1056/NEJMra000471. [DOI] [PubMed] [Google Scholar]
- 19.Massaro D, Clerch LB, Massaro GD. Estrogen receptor-alpha regulates pulmonary alveolar loss and regeneration in female mice: morphometric and gene expression studies. Am J Physiol Lung Cell Mol Physiol. 2007;293:L222–L228. doi: 10.1152/ajplung.00384.2006. [DOI] [PubMed] [Google Scholar]
- 20.Morani A, Warner M, Gustafsson J-A. Biological functions and clinical implications of oestrogen receptors alfa and beta in epithelial tissues. J Intern Med. 2008;264:128–142. doi: 10.1111/j.1365-2796.2008.01976.x. (2008) [DOI] [PubMed] [Google Scholar]
- 21.Chotirmall SH, Greene CM, Oglesby IK, Thomas W, O’Neill SJ, Harvey BJ, McElvaney NG. 17beta-estradiol inhibits IL-8 in cystic fibrosis by up-regulating secretory leucoprotease inhibitor. Am J Respir Crit Care Med. 2010;182(1):62–72. doi: 10.1164/rccm.201001-0053OC. [DOI] [PubMed] [Google Scholar]
- 22.Fasco MJ, Hurteau GJ, Spivack SD. Gender-dependent expression of alpha and beta estrogen receptors in human nontumor and tumor lung tissue. Mol Cell Endocrinol. 2002;188:125–140. doi: 10.1016/s0303-7207(01)00750-x. [DOI] [PubMed] [Google Scholar]
- 23.Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 24.Kawai H, Ishii A, Washiya K, Konno T, Kon H, Yamaya C, Ono I, Ogawa J. Combined overexpression of EGFR and estrogen receptor alpha correlates with a poor outcome in lung cancer. Anticancer Res. 2005;25:4693–4698. [PubMed] [Google Scholar]
- 25.Raso M, Behrens C, Herynk M, Liu S, Prudkin L, Ozburn N, Woods D, Tang X, Mehran R, Moran C, Lee J, Wistuba I. Immunohistochemical expression of estrogen and progesterone receptors identifies a subset of NSCLCs and correlates with EGFR mutation. Clin Cancer Res. 2009;15:5359–5368. doi: 10.1158/1078-0432.CCR-09-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hershberger P, Stabile L, Kanterewicz B, Rothstein M, Gubish C, Land S, Shuai Y, Siegfried J, Nichols M. Estrogen receptor beta subtype-specific ligands increase transcription, p44/p42 mitogen activated protein kinase (MAPK) activation and growth in human non-small cell lung cancer cells. J Steroid Biochem Mol Biol. 2009;116:102–109. doi: 10.1016/j.jsbmb.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Márquez-Garbán D, Chen HW, Fishbein M, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–143. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Coronary Drug Project Research Group. The Coronary Drug Project: findings leading to discontinuation of the 2.5 mg/day estrogen group. JAMA. 1973;226:652–657. [PubMed] [Google Scholar]
- 29.Chlebowski RT, Schwartz A, Wakelee H, Anderson G, Stefanick M, Manson J, Rodabough R, Chien J, Wactawski-Wende J, Gass M, Kotchen J, Johnson K, O’Sullivan M, Ockene J, Chen C, Hubbell F the Women’s Health Initiative Investigators. Oestrogen plus progestin and lung cancer in postmenopausal women (Women’s Health Initiative trial): a post-hoc analysis of a randomised controlled trial. Lancet. 2009;374:1243–1251. doi: 10.1016/S0140-6736(09)61526-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Slatore C, Chien J, Au D, Satia J, White E. Lung cancer and hormone replacement therapy: association in the vitamins and lifestyle study. J Clin Oncol. 2010;28:1540–1556. doi: 10.1200/JCO.2009.25.9739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schabath MB, Wu X, Vassilopoulou-Sellin R, et al. Hormone replacement therapy and lung cancer risk: A case-control analysis. Clin Cancer Res. 2004;10:113–123. doi: 10.1158/1078-0432.ccr-0911-3. [DOI] [PubMed] [Google Scholar]
- 32.Chlebowski RT, Anderson GL, Manson JE, Schwartz AG, Wakelee H, Gass M, Rodabough R, Johnson K, Wactawski-Wende J, Kotchen J, Ockene J, O’Sullivan M, Hubbell F, Chien J, Chen C, Stefanick ML. Lung Cancer Among Postmenopausal Women Treated With Estrogen Alone in the Women’s Health Initiative Randomized Trial. J Natl Cancer Inst. 2010;102:1413–1421. doi: 10.1093/jnci/djq285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishibashi H, Suzuki T, Suzuki S, et al. Progesterone receptor in non-small cell lung cancer-a potent prognostic factor and possible target for endocrine therapy. Cancer Res. 2005;65:6450–6458. doi: 10.1158/0008-5472.CAN-04-3087. [DOI] [PubMed] [Google Scholar]
- 34.Su JM, Hsu HK, Chang H, et al. Expression of estrogen and progesterone receptors in non-small cell lung cancer: immunohistochemical study. Anticancer Res. 1996;16:3803–3806. [PubMed] [Google Scholar]
- 35.Stabile LP, Dacic S, Land SR, Lenzner D, Dhir R, Aquafondata M, Landreneau R, Grandis J, Siegfried JM. Combined analysis of estrogen receptor beta-1 and progesterone receptor expression identifies lung cancer patients with poor outcome. Clin Cancer Res. 2011;17(1):154–164. doi: 10.1158/1078-0432.CCR-10-0992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer. 2008;59:88–94. doi: 10.1016/j.lungcan.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Di Nunno L, Larsson LG, Rinehart JJ, Beissner RS. Estrogen and progesterone receptors in non–small cell lung cancer in 248 consecutive patients who underwent surgical resection. Arch Pathol Lab Med. 2000;124:1467–1470. doi: 10.5858/2000-124-1467-EAPRIN. [DOI] [PubMed] [Google Scholar]
- 38.Abe K, Miki Y, Ono K, et al. Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol. 2010;41:190–198. doi: 10.1016/j.humpath.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Check JH, Sansoucie L, Chern J, Dix E. Mifepristone treatment improves length and quality of survival of mice with spontaneous lung cancer. Anticancer Res. 2010;30(1):119–122. [PubMed] [Google Scholar]
- 40.Trotter A, Kipp M, Schrader RM, Beyer C. Combined application of 17β-estradiol and progesterone enhance vascular endothelial growth factor and surfactant protein expression in cultured embryonic lung cells of mice. International J Pediatrics. 2009:1–8. doi: 10.1155/2009/170491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joshi P, Jackson HW, Beristain AG, Di Grappa MA, Mote PA, Clarke CL, Stingl J, Waterhouse P, Khokha R. Progesterone induces adult mammary stem cell expansion. Nature. 2010;465:803–807. doi: 10.1038/nature09091. [DOI] [PubMed] [Google Scholar]
- 42.Asselin-Labat M-L, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER, Yasuda H, Smyth GK, Martin TJ, Lindeman GJ, Jane E, Visvader JE. Control of mammary stem cell function by steroid hormone signaling. Nature. 2010;465:798–802. doi: 10.1038/nature09027. [DOI] [PubMed] [Google Scholar]
- 43.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 44.Ferrara N. Role of vascular endothelial growth factor in physiologic and pathologic angiogenesis: therapeutic implications. Semin Oncol. 2002;29:10–14. doi: 10.1053/sonc.2002.37264. [DOI] [PubMed] [Google Scholar]
- 45.Zhan P, Wang J, Lv X, Wnag Q, Qui L, Lin X, Yu L, Song Y. Prognostic value of vascular endothelial growth factor expression in patients with lung cancer: a systematic review with meta-analysis. J Thorac Oncol. 2009;4:1094–1103. doi: 10.1097/JTO.0b013e3181a97e31. [DOI] [PubMed] [Google Scholar]
- 46.Reck M, Crino L. Advances in anti-VEGF and anti-EGFR therapy for advanced non-small cell lung cancer. Lung Cancer. 2009;63:1–9. doi: 10.1016/j.lungcan.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 47.Farha S, Asosingh K, Laskowski D, Licina L, Sekiguchi H, Losordo D, Dweik R, Wiedemann H, Erzurum S. Pulmonary gas transfer related to markers of angiogenesis during the menstrual cycle. J Appl Physiol. 2007;103:1789–1795. doi: 10.1152/japplphysiol.00614.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marquez DC, Chen HW, Curran EM, Welshons WV, Pietras RJ. Estrogen receptors in membrane lipid rafts and signal transduction in breast cancer. Mol Cell Endocrinol. 2006;246:91–100. doi: 10.1016/j.mce.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 49.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor alpha to the plasma membrane. Proc Natl Acad Sci U S A. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li D, Williams JI, Pietras RJ. Squalamine and cisplatin block angiogenesis and growth of human ovarian cancer cells with or without HER-2 gene overexpression. Oncogene. 2002;21:2805–2814. doi: 10.1038/sj.onc.1205410. [DOI] [PubMed] [Google Scholar]
- 51.Petit A, Rak J, Hung M, Rockwell P, Goldstein N, Fendly B, Kerbel R. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. (1997) [PMC free article] [PubMed] [Google Scholar]
- 52.Pietras RJ. Interactions between estrogen and growth factor receptors in human breast cancers and the tumor-associated vasculature. Breast J. 2003;9:361–373. doi: 10.1046/j.1524-4741.2003.09510.x. [DOI] [PubMed] [Google Scholar]
- 53.Liang Y, Hyder SM. Proliferation of endothelial and tumor epithelial cells by progestin-induced vascular endothelial growth factor from human breast cancer cells: paracrine and autocrine effects. Endocrinology. 2005;146:3632–3641. doi: 10.1210/en.2005-0103. [DOI] [PubMed] [Google Scholar]
- 54.Ginestier C, Hur M, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer C, Liu S, Schott A, Hayes D, Birnbaum D, Wicha M, Dontu G. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. (2007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Korkaya H, Paulson A, Iovino F, Wicha M. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene. 2008;27:6120–6130. doi: 10.1038/onc.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stuelten CH, Mertins SD, Busch J, Gowens M, Scudiero D, Burkett M, Hite K, Alley M, Hollingshead M, Shoemaker RH, Niederhuber JE. Complex display of putative tumor stem cell markers in the NCI60 tumor cell line panel. Stem Cells. 2010;28:649–660. doi: 10.1002/stem.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dontu G, Abdallah W, Foley J, Jackson K, Clarke M, Kawamura M, Wicha M. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 2003;17:1253–1270. doi: 10.1101/gad.1061803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Levina V, Marrangoni AM, DeMarco R, Gorelik E, Lokshin AE. Drug-selected human lung cancer stem cells: cytokine network, tumorigenic and metastatic properties. PLoS ONE. 2008;3(8):e3077. doi: 10.1371/journal.pone.0003077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mumenthaler S, Yoon N, Li A, Mah V, Chang G, Nooraie F, et al. Tissue Microarrays: Construction and Utilization For Biomarker Studies. In: Hayat MA, editor. Methods of Cancer Diagnosis, Therapy, and Prognosis. Springer Netherlands; 2008. pp. 217–234. [Google Scholar]
- 60.Ooi AT, Mah V, Nickerson DW, Gilbert JL, Ha VL, Hegab AE, et al. Presence of a putative tumor-initiating progenitor cell population predicts poor prognosis in smokers with non-small cell lung cancer. Cancer Research. 2010;70:6639–6648. doi: 10.1158/0008-5472.CAN-10-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yanagawa J, Walser TC, Zhu LX, Hong L, Fishbein MC, Mah V, et al. Snail promotes CXCR2 ligand-dependent tumor progression in non-small cell lung carcinoma. Clin Cancer Res. 2009;15:6820–6829. doi: 10.1158/1078-0432.CCR-09-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takeuchi T, Tomida S, Yatabe Y, Kosaka T, Osada H, Yanagisawa K, et al. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J Clin Oncol. 2006;24:1679–1688. doi: 10.1200/JCO.2005.03.8224. [DOI] [PubMed] [Google Scholar]
- 63.Yoon NK, Maresh EL, Elshimali Y, Li A, Horvath S, Seligson DB, et al. Elevated MED28 expression predicts poor outcome in women with breast cancer. BMC Cancer. 2010;10:335. doi: 10.1186/1471-2407-10-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishizawa Y, Yamasaki M, Katayama H, Amakata Y, Fushiki S, Nishizawa Y. Establishment of a progesterone-sensitive cell line from human lung cancer. Oncol Rep. 2007;18(3):685–690. [PubMed] [Google Scholar]
- 65.Tirino V, Camerlingo R, Franco R, Malanga D, La Rocca A, Viglietto G, Rocco G, Pirozzi G. The role of CD133 in the identification and characterisation of tumour-initiating cells in non-small-cell lung cancer. Europ J Cardiothoracic Surg. 2009;36:446–453. doi: 10.1016/j.ejcts.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 66.Chen Y-C, Hsu H-S, Chen Y-W, Tsai T-H, How C-K, et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived CD133-positive cells. PLoS ONE. 3(7):e2637. doi: 10.1371/journal.pone.0002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, Wang H, Liu Z, Su Y, Stass SA, Katz RL. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol Cancer Res. 2009;7(3):330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keck C, Herchenbach D, Pfisterer J, Breckwoldt M. Effects of 17beta-estradiol and progesterone on interleukin-6 production and proliferation of human umbilical vein endothelial cells. Exp Clin Endocrinol Diabetes. 1998;106(4):334–339. doi: 10.1055/s-0029-1211994. [DOI] [PubMed] [Google Scholar]
- 69.Lange CA, Shen T, Horwitz KB. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteosome. Proc Natl Acad Sci USA. 2000;97:1032–1037. doi: 10.1073/pnas.97.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boonyaratanakornkit V, Bi Y, Rudd M, Edwards DP. The role and mechanism of progesterone receptor activation of extranuclear signaling pathways in regulating gene transcription and cell cycle progression. Steroids. 2008;73:922–928. doi: 10.1016/j.steroids.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 71.Krishnamurthy S, Dong Z, Vodopyanov D, Imai A, Helman JI, Prince ME, Wicha MS, Nör JE. Endothelial cell-initiated signaling promotes the survival and self-renewal of cancer stem cells. Cancer Res. 2010;70(23):9969–9978. doi: 10.1158/0008-5472.CAN-10-1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Groshong S, Owen G, Grimison B, Schauer I, Todd M, Langan T, Sclafani R, Lange C, Horwitz K. Biphasic regulation of breast cancer cell growth by progesterone: Role of the cyclin-dependent kinase inhibitors, p21 and p27Kip1. Mol Endocrinol. 1997;11:1593–1607. doi: 10.1210/mend.11.11.0006. [DOI] [PubMed] [Google Scholar]
- 73.Leslie K, Stein M-P, Kumar NS, Dai D, Stephens J, Wandinger-Ness A, Glueck DH. Progesterone receptor isoform identification and subcellular localization in endometrial cancer. Gynecol Oncol. 2005;96:32–41. doi: 10.1016/j.ygyno.2004.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhang G, Liu X, Farkas AM, Parwani AV, Lathrop KL, Lenzner D, Land SR, Srinivas H. Estrogen receptor beta functions through nongenomic mechanisms in lung cancer cells. Mol Endocrinol. 2009;23(2):146–156. doi: 10.1210/me.2008-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hammond MEH, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28(16):2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Allred DC, Carlson RW, Berry DA, Burstein H, Edge S, Goldstein L, Gown A, Hammond ME, Iglehart J, Moench S, Pierce L, Ravdin P, Schnitt S, Wolff AC. NCCN Task Force Report: estrogen receptor and progesterone receptor testing in breast cancer by immunohistochemistry. J Natl Comprehensive Cancer Network. 2009;7 suppl 6:s1–s22. doi: 10.6004/jnccn.2009.0079. [DOI] [PubMed] [Google Scholar]
- 77.Filardo EJ, Quinn JA, Sabo E. Association of the membrane estrogen receptor, GPR30, with breast tumor metastasis and transactivation of the epidermal growth factor receptor. Steroids. 2008;73(9–10):870–873. doi: 10.1016/j.steroids.2007.12.025. [DOI] [PubMed] [Google Scholar]