Abstract
Background
Analysis of samplings from periodontal pockets is important in diagnosis and therapy control of periodontitis. In this study, three different sampling techniques were compared to determine if one method can yield samples suitable for reproducible and simultaneous determination of bacterial load, cytokines, neutrophil elastase, and Arg-specific gingipains. R-gingipains are an important virulence factor of Porphyromonas gingivalis, the exact concentration of which in gingival crevicular fluid (GCF) has not yet been quantified.
Methods
GCF was sampled from four sites per patient (each two sites one method) in 36 chronic periodontitis patients. One week later, the procedure was repeated with alternative methods. The variables that had been determined were: loads of Aggregatibacter actinomycetemcomitans and P. gingivalis, levels of interleukin-6 and interleukin-8, activity of neutrophil elastase and level of R-gingipains.
Results
The detected cytokine levels were higher using paper strips compared to paper points. Bacteria were found in similar loads from the paper strips and paper points. R-gingipains were detectable in high quantities only by washing of the periodontal pocket. The level of R-gingipains correlated with the load of P. gingivalis.
Conclusion
The use of paper strips is suitable for simultaneous determination of microbial and immunological parameters. Obtaining GCF by washing can be useful for special purposes. Gingipain concentration in periodontal pockets was directly determined to be up to 1.5 μM. This value indicates that most of so far identified substrates of these proteases by in vitro assays can be easily degraded in P. gingivalis infected sites.
Keywords: gingival crevicular fluid, sampling techniques, Porphyromonas gingivalis, Arg-gingipain, cytokines
In periodontal disease, gingival crevicular fluid (GCF) is an inflammatory exudate. GCF contains substances from the host, as well as from supra- and subgingival located bacteria. Host constituents include molecules from blood and periodontal tissues. Inflammatory and immune cells that have infiltrated into the periodontal tissues are found in GCF together with markers of inflammation, including enzymes, cytokines, and interleukins. Further, products of tissue breakdown can also be detected in GCF.1
Analysis of gingival crevicular fluid and subgingival microflora became more and more important in diagnosis and therapy control of periodontal diseases. The presence of large numbers of periodontopathic bacteria in GCF, such as Aggregatibacter actinomycetemcomitans and members of the so called “red complex”, including Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola indicate clinically important microbial infection.2, 3P. gingivalis is strongly associated with severe chronic periodontitis.4 Among the variety of virulence factors of that species, arginine-specific gingipains (HRgpA, RgpB) and lysine-specific gingipain (Kgp) play a major role in maintenance of inflammatory conditions in periodontitis.5 They are able to impair neutrophil function as well as to degrade the extracellular matrix and bioactive peptides such as complement factor C5, prekallikrein and kininogen.6 Furthermore, Rgp and Kgp can inactivate interleukin (IL)-67 and inhibitors of neutrophil proteases,8 just to mention a few important targets for the gingipain activity.9
In periodontitis, levels of cytokines and activities of host derived enzymes in GCF are used for characterization of inflammation and the host response to subgingival microbiota, which in the case of P. gingivalis and other periodontopathic bacteria is initiated and regulated by locally synthesized or released inflammatory mediators, including major inflammatory cytokines. Increased levels of expression and synthesis of IL-1, tumor necrosis factor α (TNFα), IL-6, and IL-8 have been detected in periodontal tissues.6 Effective host response to bacterial challenge is primarily mediated by neutrophils and characterized by an influx of neutrophils into gingival crevice.10 Elastase levels are among the highest of any proteinase activity determined in GCF during periodontal inflammation.11 It has been shown that assessment of the granulocyte elastase activity in GCF can serve as a marker of the intracrevicular granulocyte activity.12
Different techniques were described for sampling the contents of the periodontal pocket.13 Sampling of subgingival bacteria seems to be suitable with curettes or with paper points,14 while cytokines and host enzymes were usually collected with filter paper strips.15 There are considerable variations in the application of the paper strip method of collection. The methods may be broadly divided into intracrevicular and the extracrevicular techniques. The intracrevicular sampling is the most frequently used method and can be further subdivided into a superficial (entrance of the crevice) and a deep (until a minimum of resistance is felt) method.13
The washing technique seems to be a possible alternative when other sampling methods failed, e.g., we reported recently about the levels of cathelicidin LL-37 in GCF of patients with periodontitis.16 Preliminary experiments in that study demonstrated that LL-37 was detectable in GCF by using Western blot technique only if GCF was sampled with the washing technique.
The aim of this study was to identify a method which can be used for different purposes (e.g. microbiota and immunological variables). Moreover, different sampling techniques were tested to effectively detect a parameter of special interest, in this case Rgp. Because of missing data in the literature, an additional aim of this study was to investigate the level of Rgp and its correlation to P. gingivalis in gingival crevicular fluid.
MATERIAL AND METHODS
Subject recruitment
Thirty six subjects with chronic periodontitis were recruited from patients of the Department of Conservative Dentistry (Section of Periodontology), University Hospital of Jena from January 2008 till August 2008. The definition of chronic periodontitis was based on the classification system of the “International Workshop for a Classification System of Periodontal diseases and Conditions” from 1999.17 Patients with generalized chronic periodontitis were included when they demonstrated: attachment loss ≥5 mm at more than 30% of sites and an age of ≥35 years. After hygiene phase, the plaque did not exceed 35%.18 To ensure similar periodontal conditions for comparison of sampling methods, each molar per quadrant should have a site with a probing depth between 5 and 7 mm. Subjects with significant systemic disease (e.g. diabetes mellitus, cancer or coronary heart disease), antibiotic therapy within the last 6 months and pregnant or lactating females were excluded. Only non-smokers with no history of smoking were included into the study. Ethical approval was obtained from local ethics committee of the University of Jena. Written informed consent was obtained from each subject prior to participation.
Clinical assessment
Probing depths (PD) were measured with a periodontal probe# at six sites per tooth. Bleeding on probing (BoP) was calculated as the percentage of positive sites per subject.
Sample collection
Patients were randomized per lot into one of three groups. Group 1 compared paper strips versus paper points, group 2 paper strips versus washing technique, and group 3 paper points versus washing technique. GCF was sampled in each patient with two sampling techniques only on molars with a probing depth from at least 5 mm and less or equal to 7 mm. One method was performed at an upper and lower molar of the right side and the other method at corresponding molars of the left side of the oral cavity. After one week, the collection of the samples was repeated using the opposite sites.
Samples were collected in the morning, 2–3 h after breakfast. The sites to be sampled were isolated with cotton rolls and gently air-dried. Paper strips** and paper points (ISO 30)†† were gently placed for 30 seconds into the pocket until a minimum of resistance was felt. Samples were eluted at 4°C overnight into 500 μl phosphate buffered saline (PBS). After being centrifuged at 400 g for 4 min, the paper points/strips were removed; both paper points/strips and the supernatants were kept frozen at −20°C until assayed. Crevicular washes were obtained using a previously described method.19, 20 A gel loading capillary tip was carefully inserted into the crevice at a level of approximately 1 mm below the gingival margin. In each case, 5 sequential washes with 10 μl of 0.9% sodium chloride were performed using a micropipette. The washes were transferred into a microcentrifuge tube, centrifuged at 400 g for 4 min; following that, the supernatants were immediately frozen and kept at −20°C until analyzed. All samples containing blood were discarded and sampling was repeated two days after.
Microflora
The DNA was extracted using a DNA extraction system‡‡ according to recommendations of the manufacturer from the paper points/strips after elution and 5 μl of the washes. Real-time polymerase chain reaction (PCR) was carried out using a real-time rotary analyzer§§. The primer for P. gingivalis21 and A. actinomycetemcomitans22 were designed as described before. PCR amplification was carried out in a reaction volume of 20 μl consisting of 2 μl template DNA and 18 μl of reaction mixture composed of 2 μl 10 × PCR buffer, 2.75 mM MgCl2, 0.2 mM nucleotides, 0.5 μM primer each, 10−4 SybrGreen, 1 U taq polymerase.□□ Negative and positive controls were included in each batch of specimens. The positive control consisted of 2 μl genomic DNA in concentrations in a range from 102 to 107 bacteria of the reference strains, the negative control was 2 μl of sterile water, each added to 18 μl reaction mixture. The cycling conditions comprised an initial denaturation step at 95°C for 5 min, followed by 45 cycles at 95°C for 15 seconds, at 65°C (exception A. actinomycetemcomitans 62°) for 20 seconds using a touch-down for five cycles, at 72°C for 20 seconds. The sensitivity and specificity of the method was checked by well characterized bacterial strains and subgingival plaque specimens. Furthermore, the specificity of the amplification was always assayed with the use of melting curves. For quantification, the results from unknown plaque specimens were projected on the counted pure culture standard curves of the target bacteria. The numbers of bacteria were classified by using log-stages.
Neutrophil elastase
Neutrophil granulocyte elastase (NE) activity was measured with a microplate assay by using the chromogenic substrate N-Methoxysuccinyl-Ala-Ala-pro-Val-pNa.¶¶23 The assay was performed in total volume of 150 uL with 0.75 mM final substrate concentration in 50 mM Tris-HCl, pH 7.5. The rate of pNA released was recorded at 405 nm by using a microplate reader.## One unit was calculated as the amount of enzyme that hydrolyzes 1 nmol of substrate in 1 min.
Interleukin 6 and 8
The concentrations of the cytokines IL-6 and IL-8 were determined by commercially available ELISA kits*** as described in the manufacturer's instruction. The detection level of the kits was about 2 pg/ml interleukin.
Antibody used in ELISA assay
Antibodies (IgY) anti HRgpA were raised in chickens as described by Pike et aliae.24 IgY specific for the Rgp catalytic domain (identical in RgpA and RgpB) were purified by affinity chromatography using immobilized RgpB. Anti-RgpB (clone 25G8.A8.G6) mouse mAb was produced on-site in a monoclonal facility at the University of Georgia. Since caspase-like domain of RgpB is essentially identical with the catalytic domain of RgpA obtained mAb react with equal affinity with both Rgp gingipains.
Level of arginine specific gingipains (R-gingipains)
The level of the P. gingivalis protease R-gingipains in the GCF was determined by the ELISA-technique. Hundred μl from the paper GCF eluate and 10 μl from each washing GCF diluted 10-fold to 100 μl by addition of PBS were used. The wells of the microtiter plates were coated with the chicken antibody anti Rgp in the final concentration 1 μg/ml in carbonate buffer (pH 9.6) for 12 h at 4°C. After blocking with bovine serum albumin (2% in PBS; pH 7.4) for 2 h and washing 4-times with 200 μl of PBS-T (PBS-0.05% Tween 20), HRgpA in 1% BSA/PBS-T (at concentration from 1 ng/ml to 100 ng/ml to generate a standard curve), a negative control, and the GCF samples were added to the wells and a plate incubated for 12 h at 4°C. After additional washing, secondary mAb anti Rgp catalytic domain were added to each well (100 μl, the final concentration 1 μg/ml in 1% BSA/PBS-T). The microtiter plates were incubated for 2 h, washed as described above, and incubated with 100 μl of polyclonal antibody anti mouse conjugated with horse radish peroxidase (HRP)**** at the final concentration 1μg/20ml in 1% BSA/PBS-T for 2 h. After washing, substrate TMB (3,3′,5,5′,Tetramethylbenzidine 0.4g/l)††† (100 μl per well) was applied. The reaction was stopped by addition of 100 μl of 1% H2SO4 and the absorbance was read at 450 nm.††††
In vitro study
Additionally, in vitro experiments were performed to determine the recovery levels of known concentrations of P. gingivalis, A. actinomycetemcomitans, IL-6, IL-8, NE and R-gingipains from paper points. In detail, bacteria were used in a range from 104 – 107 per μl. IL-6 and IL-8‡†‡† were diluted to a final concentration of 62.5, 125 and 250 pg/μl. R-gingipains and human neutrophil elastase was tested at concentrations between 0.3 and 1.2 ng/μl and between 0.025 and 0.1 μg/μl, respectively. Suspension or dilution media contained always 10% human serum. One μl of the final solution or suspension of tested bacteria or proteins was placed directly to the paper point and paper strip. Further processing of the samples was made as described before for the clinical samples. Independent analysis was made at least in triplicates.
Statistical analysis
The clinical data were expressed as means ± standard deviation (SD). Laboratory variables are presented as median including quartiles. Groups were compared with the paired Wilcoxon-test. The correlation between tested variables was made using Spearman-testA Statistical software‡‡‡ was used for all statistical analyses.
RESULTS
Demographic and clinical data are presented in Table 1. Subjects of all groups showed similar clinical signs; no difference between the groups was found to be statistically relevant.
Table 1.
Demographical and clinical data
| Variable | Group 1 Paper strips vs. Paper points n = 12 | Group 2 Paper strips vs. Washing n = 12 | Group 3 Paper points vs. Washing n = 12 |
|---|---|---|---|
| Age (mean ± SD) (years) | 42.17 ± 5.74 | 41.00 ± 4.75 | 40.88 ± 5.69 |
| Gender (m:f) | 7 : 5 | 5 : 7 | 5 : 7 |
| Full mouth PD (mean ± SD) (mm) | 3.99 ± 0.66 | 3.92 ± 0.55 | 3.95 ± 0.61 |
| Test site PD (mean ± SD) (mm) | 5.82 ± 0.23 | 5.90 ± 0.30 | 5.88 ± 0.29 |
| BoP (mean ± SD) (%) | 78.40 ± 21.96 | 86.01 ± 15.29 | 83.12 ± 22.74 |
Comparison of the paper based methods
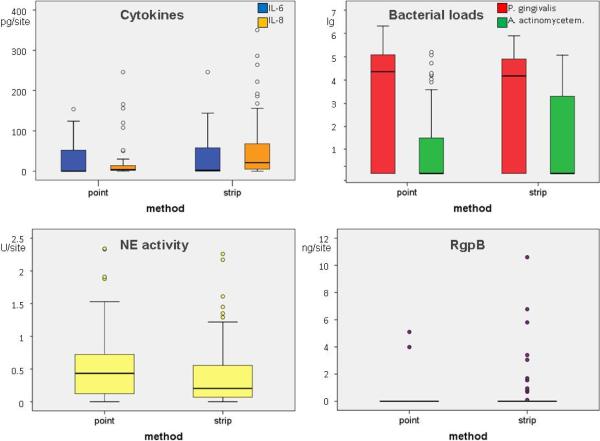
The cytokines IL-6 and IL-8 were detected more often when paper strips were used compared to paper points. The cytokine IL-6 was measured in concentrations up to 250 pg/site by means of the paper strips and 154 pg/site by means of the paper points (no significance, p=0.311). IL-8 was analyzed in significant higher levels (p=0.001) by using the paper strips (up to 350 pg/site) in relation to the paper point (246 pg/site). The levels of each cytokine measured by both methods correlated positively (IL-6: R=0.565, p<0.001; IL-8: R=0.337; p=0.019). The neutrophil elastase activity was measurable in 85% of the paper strip samples and in 98% of the paper point samples. Levels were slightly (not significant, p=0.130) higher when using paper points.
The measured bacterial loads did not differ significantly between the two sampling methods (P. gingivalis: p=0.375; A. actinomycetemcomitans: p=0.627). Two third of the samples have been found positive for P. gingivalis, contrary A. actinomycetemcomitans was detectable in 25% of the paper point samples and in 33% of the paper strip samples. R-gingipains were found only in two paper point eluates (4%) and 11 paper strip eluates (22.9%). All R-gingipains positive samples were also positive for P. gingivalis; that means Rgp was detected in 6% of the positive samples by using paper points and in 35% by using paper strips (Fig. 1, Table 2).
Figure 1.
Levels of Interleukin (IL)-6 and IL-8, activity of neutrophil elastase, bacterial loads of P. gingivalis and A. actinomycetemcomitans as well as levels of arginine specific cysteine proteases (R-gingipains derived from Porphyromonas gingivalis) in gingival crevicular fluid determined by using paper points and paper strips for sampling material
Table 2.
Comparison of sampling methods
| Variables | Result of analysis | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|---|
| Paper strips (n=48) | Paper points (n=48) | Paper strips (n=48) | Washing (n=48) | Paper points (n=48) | Washing (n=48) | ||
| IL-6 | Positive | 30 | 22 | 34 | 35 | 23 | 38 |
| ≥ median (2 pg/site) | 30 | 22 | 34 | 35 | 23 | 38 | |
| ≥ 75 quartile (32 pg/site) | 17 | 14 | 19 | 9 | 8 | 7 | |
| IL-8 | Positive | 39 | 37 | 42 | 44 | 44 | 48 |
| ≥ median (22 pg/site) | 24 | 9 | 23 | 34 | 34 | 41 | |
| ≥ 75 quartile (76 pg/site) | 11 | 5 | 10 | 18 | 18 | 18 | |
| NE activity | Positive | 41 | 47 | 48 | 22 | 47 | 46 |
| ≥ median (180 mU/site) | 25 | 33 | 27 | 12 | 24 | 21 | |
| ≥ 75 quartile (423 mU/site) | 14 | 25 | 12 | 5 | 11 | 5 | |
| A. actinomycetemcomitans | Positive | 16 | 12 | 22 | 7 | 20 | 8 |
| ≥ median (0/site) | - | - | - | - | - | - | |
| ≥ 75 quartile (1785/site) | 13 | 11 | 19 | 5 | 18 | 6 | |
| P. gingivalis | Positive | 31 | 33 | 34 | 19 | 27 | 14 |
| ≥ median (2726/site) | 30 | 32 | 32 | 17 | 23 | 10 | |
| ≥ 75 quartile (61026/site) | 15 | 16 | 17 | 6 | 15 | 3 | |
| R-gingipians | Positive | 11 | 2 | 14 | 23 | 10 | 24 |
| ≥ median (0 pg/site) | - | - | - | - | - | - | |
| ≥ 75 quartile (838 pg/site) | 8 | 2 | 13 | 20 | 7 | 22 | |
Numbers of samples given a positive result, or a result ≥ median, ≥ 75 percentile in each group
Washing as a sampling method
In general, the cytokines were detected in higher levels using the washing method compared to the paper based methods. These differences were most profound for IL-8 (significant differences compared to both paper strip (p=0.012) and paper point (p<0.001)). The NE activity was significantly lower in samples collected using the washing method compared to paper strips (p=0.001; Table 2). Bacterial organisms were found in low numbers but, nevertheless, there was a good correlation to the presence of specific bacteria collected with other sampling methods (P. gingivalis: p=0.004 compared to paper strips, p=0.001 compared to paper points; A. actinomycetemcomitans: p=0.001 compared to paper strips, p=0.002 compared to paper points; Table 2).
P. gingivalis and arginine specific cysteine proteases (R-gingipains)
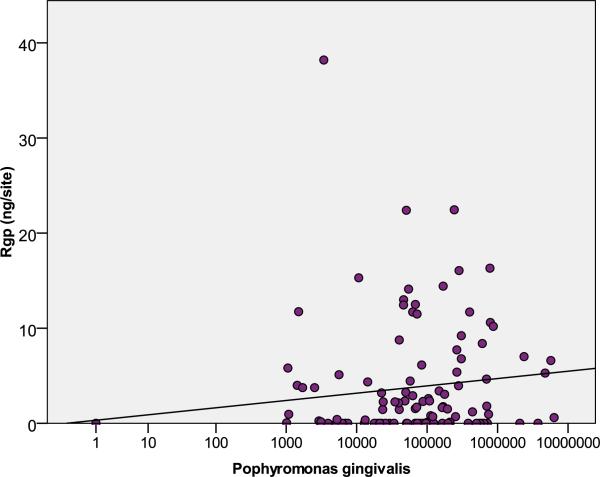
When comparing different methods of sampling, R-gingipains were detectable in 49% of GCF washes, 13% of the paper point samples and 26% of the paper strip samples. To overcome limitations of individual methods, at least partially, the higher values of each of the 144 sites obtained by the two methods were used for further analysis. Thus, 70 sites (49%) were tested positive for Rgp and 109 sites (76%) for P. gingivalis; yielding 64% overlap of the sites infected with P. gingivalis in which Rgps were found (median 1.45 ng/site in P. gingivalis positive sites, maximum 38.2 ng/site). Analysis of all sites revealed that the concentration of the Rgp gingipains correlated positively with the bacterial load of P. gingivalis (R= 0.429; p<0.001). Nevertheless, when analysis was limited to sites positive for P. gingivalis, no correlation was found (R=0.088; p=0.360) (Fig. 2) between the bacterium load and the R-gingipains level.
Figure 2.
Levels of arginine specific cysteine protease (R-gingipains derived from P. gingivalis) in gingival crevicular fluid in relation to the load of P. gingivalis
In vitro-experiments
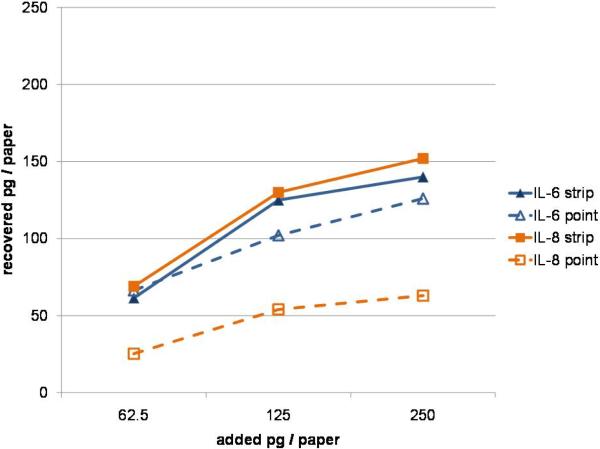
The mean recovery rate of the cytokines was higher from paper strips than from paper points. The difference was more significant for IL-8 compared to IL-6 (Table 3). Only up to 30% of applied IL-8 was eluted from paper points. Concentration-dependent effects were clearly visible. From the paper strips, the lower concentrations were nearly completely released; contrary the highest tested concentration, e.g. at 250 pg IL-8 applied only 152 pg of cytokine was recovered in solution (Fig. 3).
Table 3.
Recovery rate of cytokines, P. gingivalis, A. actinomycetemcomitans and RgpB (mean and SD).
| Variables (Range of tested concentration) | Recovery (%) Paper strips | Recovery (%) Paper points |
|---|---|---|
| Interleukin-6 (62.5 – 250 pg) | 84.75 ± 24.91 | 79.44 ± 27.98 |
| Interleukin-8 (62.5 – 250 pg) | 91.73 ± 26.98 | 36.29 ± 9.70 |
| A. actinomycetemcomitans (104 – 107) | 87.25 ± 5.80 | 83.25 ± 1.26 |
| P. gingivalis (104 – 107) | 80.75 ± 18.50 | 82.13 ± 13.26 |
| R-gingipains (0.3 – 1.2 ng) | 23.41±2.31 | 25.80 ± 4.65 |
Figure 3.
Recovered levels of cytokines applied in vitro onto paper points and paper strips
The bacterial load being still attached to papers after elution was found to be between 80.75% and 87.25%. It should be kept in mind that bacterial loads are normally counted as log stages, so deviations appear to be smaller. The recovery rate of R-gingipains from papers was low, independent of the concentration of the applied enzyme. The NE activity was measured only after elution from endodontic paper points and only when the purified elastase was applied as the highest amount (0.1 μg).
DISCUSSION
The quantity and quality of GCF samples are highly affected by the method of collection and analysis.25–27 Different approaches in sampling techniques, sampling times and data presentation seems to be critical in GCF-profile studies.28 The wide range of volumetric distribution, the site-specific nature, and the impact of distinct sampling site on volume were described as important features of GCF. A standardization of the extent of probing depth, degree of gingival inflammation and distinct sampling may improve the reliability of GCF methology.29 For this reason, only patients with comparable probing depth, good oral hygiene (plaque index < 0.35%) and low gingival inflammation (sample collection after hygiene phase) to avoid contamination of GCF samples with blood were chosen. A standardized time for collection of GCF by means of paper-based methods was applied since the clinical situation is better represented by analysis of GCF based on time of sampling than based on volume.30 This procedure allowed an instant freezing of samples to prevent proteolysis. Nevertheless, the lack of samples standardization according to protein content or collected volume can be considered as a limitation of the study.
Two paper-based sampling methods were compared: regular nitrocellulose paper point and a filter paper strip. These methods are quick and easy to use, can be applied to individual sites and are not traumatic when correctly used.13 Further, GCF was collected by an intracrevicular washing technique. This technique uses the installation and continuous re-aspiration of definite solutions, e.g. Hanks' balanced salt solution31 or PBS32 at the gingival crevice. The method is highly sensitive, but requires participation of a trained, experienced investigator to collect samples.
The GCF collection with filter paper strips is probably the most preferred sampling method.33 Several studies used this method to analyze the level of different cytokines and other biomarkers in GCF.30, 34–36 Significantly, amounts of IL-634 and IL-835 in GCF reported in these studies are comparable with our data. Filter paper strips resulted in higher IL-8 levels compared to paper points. This finding is supported by the in-vitro-analysis; the recovery rate of IL-8 was much lower from endodontic paper points in comparison to paper strips. An earlier study also reported an incomplete recovery of proteins from paper points supposedly due binding of GCF proteins to the paper.37 The difference in elution of IL-6 and IL-8 is most likely due to difference in the structure, charge distribution and hydrophobicity of these cytokines molecules.
Further, the recovery rate of the R-gingipains from both papers was only in the range of 23 to 26 percent of the predetermined concentrations. This low recovery of R-gingipains explains the low number of positive samples collected by one of the paper based methods. Intracrevicular washing was the only method detecting relevant amounts of R-gingipains. After determination of the gingipain activity in periodontal pockets by others,38 to the best of our knowledge, this is the first study measuring the level of the arginine specific protease within gingival sulcus. Taking into account the molecular mass of R-gingipains9 and the volume of GCF in periodontitis patients30 a concentration of up to 1.5 μM was found. This finding is highly significant since it allows predicting if a specific gingipains substrate will be degraded in vivo. For example, a 150-fold less concentration would be sufficient to cleave IL-67 and is more than high enough to destroy complement,39 protease inhibitors,8 bactericidal peptides40 and impair neutrophil functions9 in periodontal pockets. This underlines the importance of R-gingipains in vivo. Nevertheless, a correlation between the level of R-gingipains9 and the load of P. gingivalis was not found. The level of synthesized and released Arg-gingipains differs between strains9 and depends on environmental conditions', e.g. contact to epithelial cells.41
Contrary the superiority of the washing method in determination of gingipains, paper-based methods detected higher levels of the NE activity. Earlier studies indicated that neutrophil elastase concentrations or activities in GCF can be used to identify differences between disease activities within patients42, 43, therefore, this enzyme activity is an excellent qualitative measure of gingival inflammation.44 Elastase is one of proteolytic enzymes present in the PMN primary granules, released on activation of the PMN and capable of degrading extracellular matrix proteins of the connective tissue.45 The higher granulocyte elastase activity has previously been observed in patients with periodontitis (both aggressive and chronic) in comparison to healthy controls.46, 47 A positive correlation between the IL-8 level and the NE activity was found in GCF of periodontitis patients which was explained by the intensity of the host inflammatory response induced by the IL-8-elicited activity to activate granulocytes.30 Periodontal therapy reduced the level of IL-8, suggesting a relationship between this cytokine and periodontal status.3434
The qualitative and quantitative composition of GCF with respect to subgingival microbiota and host mediators is well known to reflect the severity of periodontal disease. Unfortunately, clinical significance the analysis is often unclear since different sampling methods are usually used to measure cytokines content and determine microflora of discrete periodontitis sites.48, 49 In this study, the same sample was used for both analyses. Surprisingly, as shown in the in-vitro-assays, more than 80% of the bacteria were still attached on the paper points or strips after overnight elution with PBS and before extraction of DNA. The pathogenic microflora was detected in nearly all patient samples with both paper-based sampling methods. Papers are easy to insert into the gingival sulcus; the low costs of endodontic paper points indicate their usage for determination of microflora only. The outcomes of paper points for microbiological diagnostics were recently compared with the sampling of subgingival biofilm with curettes. The authors concluded that paper points are suitable for microbiological diagnostics.14
In this work we found that supernatants of GCF washes are not well suited for determination of microflora. This is in contradiction to our previous studies there relevant numbers of bacteria were detectable in samples obtained by the same method. The discrepancy can be easily explained. In this study design we added a centrifugation step to remove cells and detect only soluble cytokines and NE in GCF. At 400 g bacteria alone should not sediment but they would if associated with host cells and/or tissue debris. Indeed, the analysis of supernatants and sediments from five additional GCF washing samples revealed that majority of bacteria was in pellets and only up to 10% were present in supernatants (data not shown). This result suggests that before centrifugation 5 μl of the washing sample should be retained for microbiological diagnostics.
Keeping in mind the limitations of the used method for detection of bacterial loads, it should not be surprising that more samples were positive for R-gingipains (total: 47 samples) than for P. gingivalis (total: 33 samples). The washing method is most suitable for detection of the Rgp gingipains. In comparison to paper-based sampling methods it allows for the collection of the highest amounts of the gingipains (significant difference p=0.018 washes compared to paper point).
In conclusion, the washing technique is an alternative sampling method of GCF for special purposes when sampling by paper-based methods fails. Paper points are suitable for determination of the microflora and can be recommended for daily microbiological analysis in dental practice. Paper strips are the method of choice for most of biomarkers in immunological studies; a combined determination of periodontopathic bacteria seems to be possible.
Short summary.
The use of paper strips is suitable for simultaneous determination of microbial and immunological parameters; obtaining gingival crevicular fluid by washing of the periodontal pocket might be useful for special purposes, such as determination of bacterial proteases.
Acknowledgments
We are grateful to Claudia Ranke (University Hospital Jena) for excellent assistance in performing the assays.
Source of funding statement: Most the study was institutionally founded. In addition, this study was partially supported by grants from European Community (FP7-HEALTH-2010-261460 “Gums&Joints”), Ministry of Science and Higher Education, Warsaw, Poland (project 1642/B/P01/2008/35), and the National Institutes of Health, USA (Grant DE 09761). The Faculty of Biochemistry, Biophysics and Biotechnology of the Jagiellonian University is a beneficiary of the structural funds from the European Union (grant No: POIG.02.01.00-12-064/08 – “Molecular biotechnology for health”).
Footnotes
Conflict of interest The authors declare that there are no conflicts of interest in this study.
PCP-UNC 15, Hu Friedy, Leimen, Germany
Periopaper; Oraflow Inc., Smithtown, New York, USA
Dentsply-De-Trey GmbH, Konstanz, Germany
A&A Biotechnology, Gdynia, Poland
RotorGene 2000; Corbett Research, Sydney, Australia
Fermentas Life Science, St. Leon-Rot, Germany
Sigma-Aldrich, Taufkirchen, Germany
Spectra Max 250, Soft Max Pro 4.7, Molecular Devices, Sunnyvale, USA
BioSource, Invitrogen Corporation, Carlsbad, CA, USA
KPL, Gaithersburg, Maryland, USA
SPSS 15.0, SPSS, Chicago, IL, USA
References
- 1.Lamster IB, Ahlo J. Analysis of gingival crevicular fluid as applied to the diagnosis of oral and systemic diseases. Ann N Y Acad Sci. 2007;1098:216–229. doi: 10.1196/annals.1384.027. [DOI] [PubMed] [Google Scholar]
- 2.Holt SC, Ebersole JL. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000. 2005;38:72–122. doi: 10.1111/j.1600-0757.2005.00113.x. [DOI] [PubMed] [Google Scholar]
- 3.Genco R, Kornman K, Williams R. Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol. 1996;1:926–932. doi: 10.1902/annals.1996.1.1.926. [DOI] [PubMed] [Google Scholar]
- 4.Lopez N. Occurence of Actinobacillus actinomycetemcomitans, Porphyromonas gingivalis, and Prevotella intermedia in progressive adult periodontitis. J Periodontol. 2000;71:948–954. doi: 10.1902/jop.2000.71.6.948. [DOI] [PubMed] [Google Scholar]
- 5.Potempa J, Pike RN. Corruption of innate immunity by bacterial proteases. J Innate Immun. 2009;1:70–87. doi: 10.1159/000181144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lamont R, Jenkinson H. Life below the gum line: pathogenic mechanisms of Phorphyromonas gingivalis. Microbiol Mol Biol Rev. 1998;62:1244–1263. doi: 10.1128/mmbr.62.4.1244-1263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banbula A, Bugno M, Kuster A, Heinrich PC, Travis J, Potempa J. Rapid and efficient inactivation of IL-6 gingipains, lysine- and arginine-specific proteinases from Porphyromonas gingivalis. Biochem Biophys Res Commun. 1999;261:598–602. doi: 10.1006/bbrc.1999.1075. [DOI] [PubMed] [Google Scholar]
- 8.Kantyka T, Latendorf T, Wiedow O, et al. Elafin is specifically inactivated by RgpB from Porphyromonas gingivalis by distinct proteolytic cleavage. Biol Chem. 2009;390:1313–1320. doi: 10.1515/BC.2009.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y, Nguyen K, Potempa J. Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon's knife to a meat chopper-like brutal degradation of proteins. Periodontology 2000. 2010;54:15–44. doi: 10.1111/j.1600-0757.2010.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Genco RJ. Host responses in periodontal diseases: Current concepts. J Periodontol. 1992;63:338–355. doi: 10.1902/jop.1992.63.4s.338. [DOI] [PubMed] [Google Scholar]
- 11.Cox SW, Eley BM. Cathepsin B/L-, elastase-, tryptase-, trypsin- and dipeptidyl peptidase IV-like activities in gingival crevicular fluid. A comparison of levels before and after basic periodontal treatment of chronic periodontitis patients. J Clin Periodontol. 1992;19:333–339. doi: 10.1111/j.1600-051x.1992.tb00655.x. [DOI] [PubMed] [Google Scholar]
- 12.Lamster IB. Evaluation of components of gingival crevicular fluid as diagnostic tests. Ann Periodontol. 1997;2:123–137. doi: 10.1902/annals.1997.2.1.123. [DOI] [PubMed] [Google Scholar]
- 13.Griffiths GS. Formation, collection and significance of gingival crevice fluid. Periodontol 2000. 2003;31:32–42. doi: 10.1034/j.1600-0757.2003.03103.x. [DOI] [PubMed] [Google Scholar]
- 14.Jervoe-Storm PM, AlAhdab H, Koltzscher M, Fimmers R, Jepsen S. Comparison of curet and paper point sampling of subgingival bacteria as analyzed by real-time polymerase chain reaction. J Periodontol. 2007;78:909–917. doi: 10.1902/jop.2007.060218. [DOI] [PubMed] [Google Scholar]
- 15.Wolff LF, Koller NJ, Smith QT, Mathur A, Aeppli D. Subgingival temperature: relation to gingival crevicular fluid enzymes, cytokines, and subgingival plaque micro-organisms. J Clin Periodontol. 1997;24:900–906. doi: 10.1111/j.1600-051x.1997.tb01209.x. [DOI] [PubMed] [Google Scholar]
- 16.Puklo M, Guentsch A, Hiemstra P, Eick S, Potempa J. Analysis of neutrophil-derived antimicrobial peptides in gingival crevicular fluid suggests importance of cathelicidin LL-37 in the innate immune response against periodontogenic bacteria. Oral Microbiol Immunol. 2008;23:328–335. doi: 10.1111/j.1399-302X.2008.00433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armitage GC. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 18.O'Leary T, Drake R, Naylor J. The plaque control records. J Periodontol. 1972;43:38. doi: 10.1902/jop.1972.43.1.38. [DOI] [PubMed] [Google Scholar]
- 19.Sigusch B, Klinger G, Holtz H, Süss J. In vitro phagocytosis by crevicular phagocytes in various forms of periodontitis. J Periodontol. 1992;63:496–501. doi: 10.1902/jop.1992.63.6.496. [DOI] [PubMed] [Google Scholar]
- 20.Guentsch A, Erler M, Preshaw PM, Sigusch BW, Klinger G, Glockmann E. Effect of smoking on crevicular polymorphonuclear neutrophil function in periodontally healthy subjects. J Periodontal Res. 2006;41:184–188. doi: 10.1111/j.1600-0765.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- 21.Ashimoto A, Chen C, Bakker I, Slots J. Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol. 1996;11:266–273. doi: 10.1111/j.1399-302x.1996.tb00180.x. [DOI] [PubMed] [Google Scholar]
- 22.Tran SD, Rudney JD. Improved multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans, Bacteroides forsythus, and Porphyromonas gingivalis. J Clin Microbiol. 1999;37:3504–3508. doi: 10.1128/jcm.37.11.3504-3508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakajima K, Powers J, Ashe B, Zimmerman M. Mapping the extended substrate binding site of cathepsin G and human leukocyte elastase. Studies with peptide substrates related to the alpha 1-protease inhibitor reactive site. J Biol Chem. 1979;254:4027–4032. [PubMed] [Google Scholar]
- 24.Pike R, Bagarozzi DJ, Travis J. Immunological cross-reactivity of the major allergen from perennial ryegrass (Lolium perenne), Lol p I, and the cysteine proteinase, bromelain. Int Arch Allergy Immunol. 1997;112:412–414. doi: 10.1159/000237489. [DOI] [PubMed] [Google Scholar]
- 25.Egelberg J, Attstrom R. Comparison between orifrice and intracrevicular methods of sampling gingival fluid. J Periodontal Res. 1973;8:384–388. doi: 10.1111/j.1600-0765.1973.tb00773.x. [DOI] [PubMed] [Google Scholar]
- 26.Persson GR, Page RC. Effect of sampling time and repitition on gingival crevicular fluid and aspartate aminotransferase activity. J Periodontal Res. 1990;25:236–242. doi: 10.1111/j.1600-0765.1990.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 27.Lamster IB, Harper D, Goldstein S, Celenti RS, Oshrain RL. The effect of sequential sampling on crevicular fluid volume and enzyme activity. J Clin Periodontol. 1989;16:252–258. doi: 10.1111/j.1600-051x.1989.tb01650.x. [DOI] [PubMed] [Google Scholar]
- 28.Ozkavaf A, Aras H, Huri CB, et al. Relationship between the quantity of gingival crevicular fluid and clinical periodontal status. J Oral Sci. 2000;42:231–238. doi: 10.2334/josnusd.42.231. [DOI] [PubMed] [Google Scholar]
- 29.Hatipoglu H, Yamalik N, Berberoglu A, Eratalay K. Impact of the distinct sampling area on volumetric features of gingival crevicular fluid. J Periodontol. 2007;78:705–715. doi: 10.1902/jop.2007.060331. [DOI] [PubMed] [Google Scholar]
- 30.Jin L, Soder B, Corbet EF. Interleukin-8 and granulocyte elastase in gingival crevicular fluid in relation to periodontopathogens in untreated adult periodontitis. J Periodontol. 2000;71:929–939. doi: 10.1902/jop.2000.71.6.929. [DOI] [PubMed] [Google Scholar]
- 31.Skapski H, Lehner T. A crevicular washing method for investigating immune components of crevicular fluid in man. J Periodontal Res. 1976;11:19–24. doi: 10.1111/j.1600-0765.1976.tb00046.x. [DOI] [PubMed] [Google Scholar]
- 32.Salonen JI, Paunio KU. An intracrevicular washing method for collection of crevicular contents. Scand J Dent Res. 1991;99:406–412. doi: 10.1111/j.1600-0722.1991.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 33.Ozkavaf A, Aras H, Huri CB, et al. Analysis of factors that may affect the enzymatic profile of gingival crevicular fluid: sampling technique, sequential sampling and mode of data presentation. J Oral Sci. 2001;43:41–48. doi: 10.2334/josnusd.43.41. [DOI] [PubMed] [Google Scholar]
- 34.Gamonal J, Acevedo A, Bascones A, Jorge O, Silva A. Characterization of cellular infiltrate, detection of chemokine receptor CCR5 and interleukin-8 and RANTES chemokines in adult periodontitis. J Periodontal Res. 2001;36:194–203. doi: 10.1034/j.1600-0765.2001.360309.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin SJ, Chen YL, Kuo MY, Li CL, Lu HK. Measurement of gp130 cytokines oncostatin M and IL-6 in gingival crevicular fluid of patients with chronic periodontitis. Cytokine. 2005;30:160–167. doi: 10.1016/j.cyto.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 36.Bozkurt FY, Berker E, Akkus S, Bulut S. Relationship between interleukin-6 levels in gingival crevicular fluid and periodontal status in patients with rheumatoid arthritis and adult periodontitis. J Periodontol. 2000;71:1756–1760. doi: 10.1902/jop.2000.71.11.1756. [DOI] [PubMed] [Google Scholar]
- 37.Johnson RB, Streckfus CF, Dai X, Tucci MA. Protein recovery from several paper types used to collect gingival crevicular fluid. J Periodontal Res. 1999;34:283–289. doi: 10.1111/j.1600-0765.1999.tb02255.x. [DOI] [PubMed] [Google Scholar]
- 38.Eley BM, Cox SW. Correlation between gingivain/gingipain and bacterial dipeptidyl peptidase activity in gingival crevicular fluid and periodontal attachment loss in chronic periodontitis patients. A 2-year longitudinal study. J Periodontol. 1996;67:703–716. doi: 10.1902/jop.1996.67.7.703. [DOI] [PubMed] [Google Scholar]
- 39.Popadiak K, Potempa J, Riesbeck K, Blom AM. Biphasic effect of gingipains from Porphyromonas gingivalis on the human complement system. J Immunol. 2007;178:7242–7250. doi: 10.4049/jimmunol.178.11.7242. [DOI] [PubMed] [Google Scholar]
- 40.Carlisle MD, Srikantha RN, Brogden KA. Degradation of human alpha- and beta-defensins by culture supernatants of Porphyromonas gingivalis strain 381. J Innate Immun. 2009;1:118–122. doi: 10.1159/000181015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eick S, Reissmann A, Rodel J, Schmidt KH, Pfister W. Porphyromonas gingivalis survives within KB cells and modulates inflammatory response. Oral Microbiol Immunol. 2006;21:231–237. doi: 10.1111/j.1399-302X.2006.00282.x. [DOI] [PubMed] [Google Scholar]
- 42.Binder TA, Goodson JM, Socransky SS. Gingival fluid levels of acid and alkaline phosphatase. J Periodontal Res. 1987;22:14–19. doi: 10.1111/j.1600-0765.1987.tb01534.x. [DOI] [PubMed] [Google Scholar]
- 43.Lamster IB, Oshrain RL, Fiorello LA, Celenti RS, Gordon JM. A comparison of 4 methods of data presentation for lysosomal enzyme activity in gingival crevicular fluid. J Clin Periodontol. 1988;15:347–352. doi: 10.1111/j.1600-051x.1988.tb01010.x. [DOI] [PubMed] [Google Scholar]
- 44.Herrmann JM, Gonzales JR, Boedeker RH, Vonholdt J, Meyle J. Microassay for the detection of elastase activity in the gingival crevice. J Clin Periodontol. 2001;28:31–37. doi: 10.1034/j.1600-051x.2001.280105.x. [DOI] [PubMed] [Google Scholar]
- 45.Nicu EA, Van der Velden U, Everts V, Van Winkelhoff AJ, Roos D, Loos BG. Hyper-reactive PMNs in FcgammaRIIa 131 H/H genotype periodontitis patients. J Clin Periodontol. 2007;34:938–945. doi: 10.1111/j.1600-051X.2007.01136.x. [DOI] [PubMed] [Google Scholar]
- 46.Guentsch A, Puklo M, Preshaw PM, et al. Neutrophils in chronic and aggressive periodontitis in interaction with Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Periodontal Res. 2009;44:368–377. doi: 10.1111/j.1600-0765.2008.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giannopoulou C, Demeurisse C, Cimasoni G. Elastase release from gingival crevicular and peripheral neutrophils in periodontitis and health. Arch Oral Biol. 1994;39:741–745. doi: 10.1016/0003-9969(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 48.Guentsch A, Jentsch H, Pfister W, Hoffmann T, Eick S. Moxifloxacin as an adjunctive antibiotic in the treatment of severe chronic periodontitis. J Periodontol. 2008;79:1894–1903. doi: 10.1902/jop.2008.070493. [DOI] [PubMed] [Google Scholar]
- 49.Teles R, Sakellari D, Teles F, et al. Relationships among gingival crevicular fluid biomarkers, clinical parameters of periodontal disease, and the subgingival microbiota. J Periodontol. 2010;81:89–98. doi: 10.1902/jop.2009.090397. [DOI] [PMC free article] [PubMed] [Google Scholar]