Abstract
The organisation of the cerebral cortex into distinct modules may be described along several dimensions, most importantly, structure, connectivity and function. Identification of cortical modules by differences in whole-brain connectivity profiles derived from diffusion tensor imaging or resting state correlations have already been shown. These approaches, however, carry no task-related information. Hence, inference on the functional relevance of the ensuing parcellation remains tentative. Here, we demonstrate, that Meta-Analytic Connectivity Modelling (MACM) allows the delineation of cortical modules based on their whole-brain co-activation pattern across databased neuroimaging results. Using a model free approach, two regions of the medial pre-motor cortex, SMA and pre-SMA were differentiated solely based on their functional connectivity. Assessing the behavioural domain and paradigm class meta-data of the experiments associated the clusters derived from the co-activation based parcellation moreover allows the identification of their functional characteristics. The ensuing hypotheses about functional differentiation and distinct functional connectivity between pre-SMA and SMA were then explicitly tested and confirmed in independent datasets using functional and resting state fMRI. Co-activation based parcellation thus provides a new perspective for identifying modules of functional connectivity and linking them to functional properties, hereby generating new and subsequently testable hypotheses about the organization of cortical modules.
Keywords: database, fMRI, areas, connectivity, action, SMA
Introduction
In this paper, we propose a set of neuroinformatic tools to investigate a given seed region’s structural-connectional and functional properties. This method relies on data-driven algorithms capitalizing on the host of task-dependent imaging data and meta-information archived in the BrainMap database. The feasibility of our approach and its potential in generating testable hypotheses is demonstrated here by an exemplary seed region in the medial premotor cortex. Evidence from primate research indicated that microstructure and connectivity of the cortex are the main determinants of its functional segregation (Luppino et al., 1991; Matelli et al., 1991). Early histological investigations into the (micro-) structural heterogeneity of the cerebral cortex have resulted in several detailed, though partially incongruent, anatomical maps (Brodmann, 1909; Vogt and Vogt, 1919). Although histological examination allows topographical delineation of cortical modules, mere microstructure is poorly informative in the absence of a strong a priori hypothesis when attempting to deduce functional roles of those modules.
The neuroimaging era has now enabled the precise localisation of functional responses across the whole brain and led to a wealth of information on the neural correlates of various processes. In the context of differentiating cortical modules, however, fMRI has predominantly a confirmatory role. That is, using appropriate experimental designs, fMRI is an extremely powerful tool for testing hypotheses about, e.g., a functional differentiation between two regions (Reddy and Kanwisher, 2006) or a dichotomy between the neural correlates of two processes (Charron and Koechlin, 2010). While many hypotheses derived in particular from primate work (Bremmer et al., 2001) and lesion mapping studies (Riecker et al., 2005) could be explicitly tested using this approach, neuroimaging is intrinsically less well suited to delineate the organization of a particular brain region. Whereas fMRI and positron emission tomography (PET) are compelling approaches for testing hypotheses about a functional differentiation between cortical modules, their potential for delineating them - given a particular brain region - is limited.
Apart from fMRI allowing powerful functional mapping, several neuroimaging-based methods for assessing human brain connectivity, i.e., interactions between different brain regions, have evolved over the recent years. Among those, effective connectivity analyses, such as dynamic causal modelling (Friston et al., 2003) or structural equation modelling (Buchel and Friston, 1997), allow the investigation of task-dependent influences among cortical areas (Grefkes et al., 2008). As an alternate approach, fMRI time-series signals measured during task or resting state may be correlated between different cortical regions to infer their functional connectivity (Hampson et al., 2002; Ramnani et al., 2004). Probably even more than functional activation studies, however, these methods are primarily confirmative.
In contrast to those largely hypothesis-driven methods to study connectivity, approaches for widely data-driven connectivity analyses have recently emerged. This has first been demonstrated for the analysis of anatomical connectivity using diffusion tensor imaging (DTI, Johansen-Berg et al., 2004). The key idea behind connectivity-based parcellation is to first analyse the connectivity of each individual voxel in a particular seed region of interest to the rest of the brain separately. By comparing the anatomical connectivity profiles of the individual seed voxels with each other, these can then be grouped into distinct clusters of homogeneous connectivity (Anwander et al., 2007; Johansen-Berg et al., 2004; Klein et al., 2007). Apart from DTI, functional resting state MRI emerged as another highly suitable cornerstone for the parcellation of grey matter. Those signal fluctuations likely conveying meaningful functional relationships between brain regions regions is illustrated by the fact that they were reported to widely correspond with both task-state networks (Biswal et al., 1995; Smith et al., 2009) and structural connectivity (Greicius et al., 2009; Hagmann et al., 2008). Approaches investigating cortical sub-specialization capitalized on this type of inter-regional connectivity, such as for the successful parcellation of the premotor cortex (Kim et al., 2010), insular cortex (Cauda et al., 2011), and thalamus (Zhang et al., 2008). Taken together, DTI and resting-state correlations thus allow the delineation of cortical modules based on their connectivity pattern without a need for prior knowledge. Neither modality, however, carries any task-dependent information in order to form hypotheses on which tasks may selectively activate them or modulate their connectivity.
A task-dependent approach to connectivity-based parcellation that addresses this dilemma is meta-analytic connectivity modelling (MACM). MACM is based on assessing the brain-wise co-activation patterns of a seed region across a large number of databased neuroimaging results (Laird et al., 2009a). Importantly and in contrast to the aforementioned approaches, the experiments underlying the difference in co-activation pattern may then be described behaviourally, linking them to functional properties of the ensuing parcellation. This unique advantage of MACM thus allows formulation of hypotheses for subsequent targeted experiments on functional activation properties and inter-regional connections.
Here we demonstrate that MACM can be used reliably to identify cortical modules in a data-driven fashion based on co-activation patterns across the brain by applying it to a seed volume of interest (VOI) in the medial premotor cortex. First, we identified for each voxel of the seed VOI those experiments in the BrainMap database that reported activation at that particular location. By performing an Activation Likelihood Estimation (ALE) meta-analysis over these experiments, we derived the brain wide co-activation pattern for each particular seed voxel. Individual seed voxels were then clustered into distinct groups based on similarities and differences in these co-activation maps. Differences in co-activation pattern of the ensuing clusters were tested by directly contrasting the regional MACM patterns, yielding hypotheses about differential connectivity between them. Finally, behavioural domain and paradigms class meta-data of experiments associated with the ensuing clusters. This allowed to characterise the functional properties of these connectivity defined cortical modules.
Materials and Methods
The potential of co-activation based cortical parcellation is demonstrated in the medial premotor cortex, i.e., the region of (pre-) SMA. A volume of interest (VOI) was defined by merging two activation sites from a neuroimaging study of speeded motor responses (Jakobs et al., 2009). The posterior activation was consistently observed during left, right and bilateral responses (supp. figure 1), the anterior showed increased activation when subjects responded to (randomly) bilateral as compared to unilateral stimuli. Here, both clusters were combined into a single volume of interest (VOI). We then assessed whether the two original regions could be recovered in a model-free analysis from this merged, i.e., single, VOI based on similarities between co-activation patterns of the individual seed voxels across neuroimaging experiments.
Meta-analytic connectivity mapping
Co-activation based parcellation was performed using the BrainMap database (Laird et al., 2009a) (www.brainmap.org). From that database, only those experiments were considered, that reported stereotaxic coordinates from normal mapping studies (no interventions, no group comparison) in healthy subjects using either fMRI or PET. These inclusion criteria yielded (at the time of analysis) approximately 6200 functional neuroimaging experiments. Note that we considered all eligible BrainMap experiments because any pre-selection of taxonomic categories would constitute a fairly strong a priori hypothesis about how brain networks are organized. In fact, it remains elusive how well psychological constructs, such as emotion and cognition, map on the human brain (Laird et al., 2009a; Poldrack, 2006). To enable a reliable mapping of the co-activation pattern for each individual voxel of the seed region -in spite of the variable and usually low number of foci located precisely at any particular voxel- we proceeded as follows. Importantly, this procedure was performed for each individual seed voxel, i.e., each voxel within the seed region in the medial premotor cortex. First, we identified for the currently considered seed voxel the 50 experiments in BrainMap that reported activation closest to it. This was done by computing for each databased experiment the distance between the current seed voxel and the nearest activation of that particular experiment. The 50 closest experiments were then considered to be associated with the currently assessed seed voxel and selected for further analysis. To evaluate a potential influence of this criterion, analysis was repeated using the closest 25, 100 or 250 experiments. It has to be considered, however, that the density of activation foci may not necessarily be stationary across the seed region. The outlined approach thus has the advantage of using the same number of experiments for each seed voxel but may introduce slight differences between them with respect to the distance of the furthest experiment that was included. Therefore, we repeated the analysis using all experiments reporting foci within a 4 or 6 mm radius around a particular seed voxel. This latter approach has the advantage that for each seed voxel the distance to an activation in the further associated experiment is identical across all individual seed voxels but has the drawback, that the number of experiments may vary between seed voxels.
The brain wide co-activation pattern for each individual seed voxel was then computed by a meta-analysis over the experiments that were associated with that particular voxel by the procedure outlined above. That is, experiments were defined by activation at or close to a particular seed voxel, and quantitative meta-analysis over all foci reported in these experiments was performed to assess how likely any other voxel throughout the brain co-activates with that seed-voxel.
Meta-analysis was performed using the revised version (Eickhoff et al., 2009) of the activation likelihood estimation (ALE) approach. The key idea behind ALE is to treat the foci reported in the associated experiments not as single points, but as centres for 3D Gaussian probability distributions that reflect the spatial uncertainty associated with neuroimaging results. For each experiment, the probability distributions of all reported foci are then combined into a modelled activation (MA) map for that particular experiment. The voxel-wise union of these MA (modelled activation) - values for all experiments associated with a particular seed voxel then yielded an ALE score for each voxel of the brain that describes the co-activation probability of that particular location with the current seed voxel (Fig. 1). No threshold was applied to retain the complete pattern of co-activation likelihood. The ALE scores of all voxel within the grey matter (based on 10% probability according to the ICBM [International Consortium on Brain Mapping] tissue probability maps) were then recorded before moving to the next voxel of the seed region.
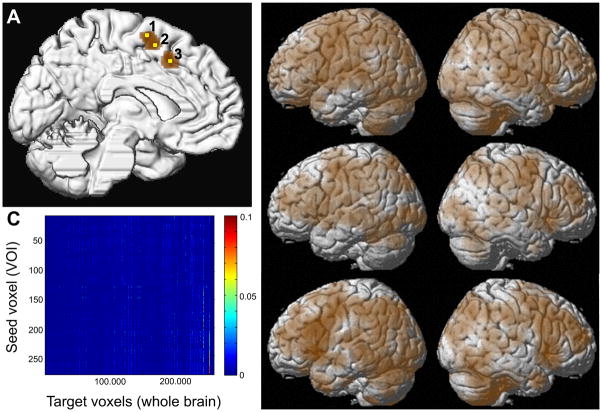
Figure 1.
(A) Location of the seed VOI (brown) and the three exemplary voxels for which co-activation maps are illustrated, displayed on a surface rendering of the MNI single subject template. The yellow colored exemplary seed voxel 1 is located at −4/−6/+68, seed voxel 2 is located at −2/0/+60, and seed voxel 3 at −6/+12/+48 (all coordinates in MNI space).
(B) Brain-wide co-activation maps of three voxels indicated by the yellow numbers in panel A as revealed by meta-analytical connectivity modelling using ALE meta-analysis on the brain-wide foci reported in those 50 experiments in BrainMap that featured the closest activation peaks to the respective seed voxels.
(C) Co-activation matrix summarising the co-activation likelihood (ALE values) of all seed voxels to the rest of the grey matter. The grey matter mask is based on at least 10% probability according to the ICBM (International Consortium on Brain Mapping). This matrix containing the brain wide co-activation pattern of each individual seed voxel served as the basis for co-activation based parcellation of the medial premotor seed region.
Cortical parcellation based on co-activation patterns
The brain-wide co-activation profiles for all seed voxels were subsequently combined into a NS × NB co-activation matrix, where NS is the number of seed voxels (283) and NB the number of target voxels (~260,000 voxels located within the grey matter) at 2 × 2 ×2 mm3 resolution. Sets of voxels that feature similar brain-wide co-activation profiles were identified by hierarchical cluster analysis (Eickhoff et al., 2007; Timm, 2002). In this approach, each voxel initially forms an individual cluster, and a hierarchy is then built by progressively merging the least dissimilar cluster to derive successively larger sets. We used Euclidean distance between the brain-wide co-activation profiles (ALE scores of all target voxels) as similarity measure and Ward linkage criterion for cluster merging (Timm, 2002). To evaluate potential influences of these parameters, analyses were repeated for all combinations of the different filter criteria for assigning experiments to a particular seed voxel (cf. above), distance measures (Euclidean, correlation, cosine) and linkage algorithm (weighted, average, single, complete). Finally, we also assessed seed voxel clustering using the spectral reordering approach that has previously been employed for connectivity-based parcellation using DTI and resting-state data (Johansen-Berg et al., 2004; Kim et al., 2010). This approach involves first to compute the cross-correlation matrix of the whole brain co-activation profiles obtained for the individual seed voxels. The matrix is then reordered to minimize the cross-correlation values off the diagonal, hereby forcing closely correlated voxels close to each other. Clusters are then identified in the reordered matrix as sets of seed voxels whose connectivity patterns were strongly correlated with each other and weakly with the rest of the matrix.
Characterisation of the derived clusters: Co-activations
Following the co-activation based parcellation of the seed region into separate clusters, MACM was performed on each of the ensuing clusters in order to characterise their co-activation profiles. In this context, “clusters” refers to sets of voxels within the seed region that were identified by the co-activation based parcellation outlined above as having similar co-activation patterns to each other but distinct to the rest of the seed voxels. The co-activation profiles of the different clusters were obtained by first identifying all experiments in the BrainMap database that featured at least one focus of activation in a particular cluster derived from the co-activation based hierarchical cluster analysis. Then, an ALE meta-analysis was performed on these experiments as described above. In contrast to the MACM analyses underlying the co-activation based parcellation, in which the ALE maps were not thresholded to retain the complete pattern of co-activation likelihood, statistical inference was now sought.
To establish which regions were significantly co-activated with one of the clusters identified by the above described co-activation parcellation of the seed region, ALE scores for the MACM analysis of this cluster were compared to a null-distribution that reflects a random spatial association between experiments, but regards the within-experiment distribution of foci as fixed (Eickhoff et al., 2009). This random-effects inference assesses above-chance convergence between experiments, not clustering of foci within a particular experiment. The observed ALE scores from the actual meta analysis of experiments activating within a particular cluster were then tested against the ALE scores obtained under this null-distribution yielding a p-value based on the proportion of equal or higher random values. The resulting non-parametric p-values were then thresholded at a family-wise error (FWE) corrected threshold of p < 0.05.
Regions that co-activated with both clusters delineated in the medial premotor cortex by the co-activation based parcellation were identified using a minimum-statistic conjunction by computing the intersection of the thresholded ALE-maps (Caspers et al., 2010; Kurth et al., 2010). Differences in co-activation patterns not only describe the features that have driven the differentiation of the seed region, but also represent hypotheses about functional connections that may inform subsequent models of effective and functional connectivity. They were assessed by first performing MACM separately on the experiments associated with either cluster and computing the voxel-wise difference between the ensuing ALE maps. All experiments contributing to either analysis were then pooled and randomly divided into two groups of the same size as the two original sets of experiments. That is, if 100 experiments in BrainMap featured activation in cluster A and 75 featured an activation in cluster B, the resulting pool of (175) experiments would be randomly divided into a group of 100 and a group of 75 experiments. ALE-scores for these two randomly assembled groups were calculated and the difference between these ALE-scores was recorded for each voxel in the brain. Repeating this process 10,000 times then yielded a null-distribution for the differences in ALE-scores between the MACM analyses of the two clusters. The observed difference in ALE scores was then tested against this null-distribution yielding a p-value for the difference at each voxel based on the proportion of equal or higher random differences. The resulting non-parametric p-values were thresholded at p < 0.001 and inclusively masked by the respective main effects, i.e., the significant effects in the MACM for the particular cluster, to focus inference on regions reliably co-activating with that cluster.
Notably, there is still no established method to correct ALE difference maps for multiple comparisons. Nevertheless, permutation of the experiments’ associations with either cluster served as a statistical tool to estimate the magnitude of the difference. Additionally, we opted for a conventional conservative threshold of p < 0.001 to account for intra-laboratory idiosyncrasies, inter-subject variability, as well as the limited spatial resolution of fMRI and PET. The conjunction of these aspects allowed for focussing inference on regions reliably co-activating with one of the clusters.
Characterisation of the derived clusters: Function
Functional characterisation of the co-activation based clusters is another crucial aspect, as it provides a first link between the derived parcellation and the putatively corresponding functional differentiation. Moreover, these characterisations may also provide hypotheses that may inform explicitly targeted further experiments that may then confirm a differential response between the defined regions. The functional characterisation of the clusters derived from co-activation based parcellation of the medial frontal seed region was based on the BrainMap metadata that describes the included specific mental process isolated by the statistical contrast of each included experiment. It is important to appreciate that we ran MACM without any taxonomic constraints to delineate genuine brain networks that might be implicated in diverse brain functions regardless of actual experiment tasks. Only after that, nodes of the thus derived “untapped” brain networks were functionally characterized because this order of steps permits taxonomic profiling of computationally-derived brain networks, both in a bottom-up fashion. Behavioural domains (BD) include the main categories of cognition, action, perception, emotion, interoception, as well as their related subcategories. The respective paradigm classes (PC) classify the specific task employed (a complete list of BDs and PCs can be found in the supplementary material and at http://brainmap.org/scribe/). We analyzed the behavioural domain and paradigm class metadata associated with each identified cluster to determine the frequency of domain “hits” relative to its likelihood across the entire database. In particular, functional roles of the derived clusters were identified by significant over-representation of BDs and PCs in the experiments activating the respective cluster relative to the BrainMap database using a binomial test (p<0.05, corrected for multiple comparisons using Bonferroni’s method (Laird et al., 2009b).
Results
Meta-analytic connectivity mapping
Individual co-activation maps for each voxel within the seed VOI were computed by ALE meta-analysis over those experiments in BrainMap that featured the closest activation foci to that respective seed voxel. Following analysis of all seed voxels, the ALE values at all voxels in the rest of the brain were then combined into a functional co-activation matrix, reflecting how likely each seed voxel co-activated with any other voxel in the brain (Fig. 1).
Cortical parcellation based on co-activation patterns
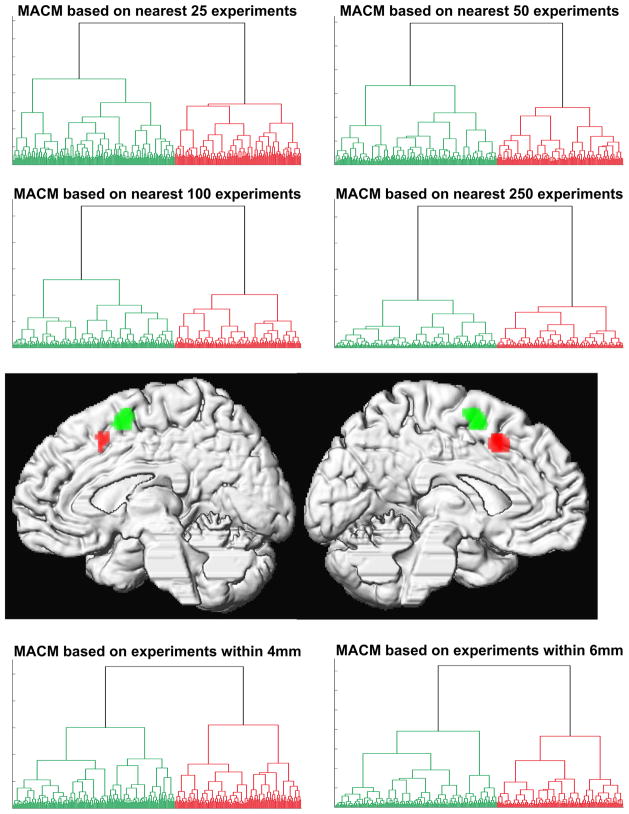
Hierarchical cluster analysis performed on this matrix, treating individual seed voxels as observations and the brain-wide co-activation (ALE scores) as response variables, revealed a separation of the seed region into two co-activation based clusters that corresponded exactly to the two activation clusters obtained from different contrasts of the original fMRI study (Jakobs et al., 2009). Importantly, this parcellation was stable across all explored analysis parameters. Each combination of different filter criteria for assigning experiments to a particular seed voxel, distance measures for quantifying dissimilarity between co-activation profiles and linkage algorithms to assemble clusters yielded exactly the same parcellation with no single voxel changing attribution (Fig. 2, supp. figures 2–3). Finally, the same parcellation was also confirmed by spectral reordering of the cross-correlation matrix of co-activation profiles (supp. figure 4). Without a priori constraints imposed on the analysis, cluster formation driven by dissimilarity in co-activation patterns thus recovered the two original regions from the combined VOI in a highly robust manner.
Figure 2.
Hierarchical cluster analysis of the co-activation profile matrix (cf. Fig. 1C) revealed a highly reliable separation of the seed voxels into two distinct clusters independent of filter criterion and cluster parameters (cf. supp. figures 2–4). Projecting the voxels back onto their brain location revealed that these clusters were spatially continuous and corresponded to an anterior and posterior cluster in the medial premotor cortex (cf. supp. figure 1)
Please note that we employed a number of different parameter combinations as an acid test for the robustness of the obtained co-activation based parcellations. In fact, finer grained parcellation into more than two clusters appeared to result in “hyper-parcellation” already with little reduction of the absolute distance value. Moreover, separation of the seed region into more than two clusters demonstrated to be highly dependent on the employed parameter combination. Both these observations speak for less distinct functional-connectional properties of the ensuing sets of voxels when more than two voxel groups were predicted. Furthermore, the reordered correlation matrix clearly indicated a parcellation into two clusters of extremely high within-cluster and very low between-cluster correlation. That is, across all the different employed approaches, a parcellation into two clusters emerged as clearly the most robust choice that was least susceptible to methodological variations (SFig. 2–4).
Characterisation of the derived clusters: co-activations
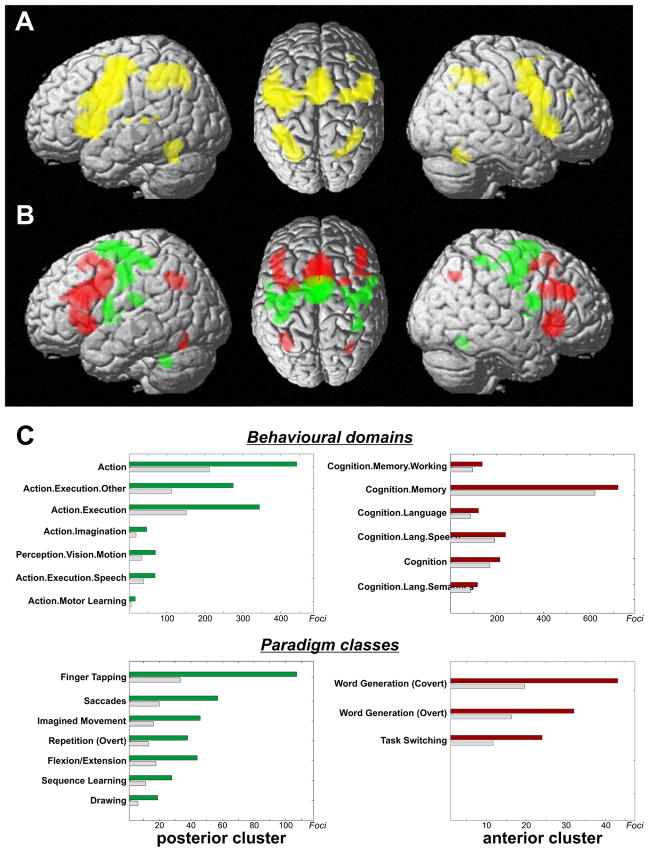
Task-based co-activations of each cluster were delineated by performing an ALE meta-analysis across all experiments featuring at least one activation in that region (Fig. 3). Conjunction analysis revealed an overlap between the thresholded (p<0.05, FWE corrected) co-activation maps of both clusters in dorsal and ventral lateral premotor cortex, BA 44, primary motor and somatosensory cortices, anterior insula, basal ganglia (particularly putamen), thalamus, superior cerebellum as well as intraparietal sulcus and adjacent inferior parietal lobule (Fig. 3A). Contrasting the co-activation maps for the posterior and anterior cluster showed the clear distinction in functional connectivity pattern underlying the parcellation of the seed VOI (Fig. 3B). The posterior cluster showed higher co-activation probabilities in dorsal premotor cortex, primary motor and somatosensory cortices, cerebellum and basal ganglia (putamen). In contrast, the anterior cluster showed significantly higher co-activation probabilities in ventral premotor cortex, middle frontal gyrus and BA 44, anterior insula and intraparietal sulcus/inferior parietal cortex. The stable distinction of the two clusters in the above analyses thus seems to originate from different co-activation likelihood of the posterior and anterior seed region with areas implicated in sensory-motor functions and cognitive control, respectively.
Figure 3.
(A) Conjunction analysis over the MACM maps for the two main clusters indicates that several fronto-parietal regions show significant co-activation with both medial premotor regions.
(B) Contrasting the MACM maps revealed that the anterior cluster showed significantly higher co-activation probabilities with ventral premotor, inferior frontal and posterior parietal cortices. The posterior cluster showed significantly higher co-activation probabilities with dorsal premotor cortex, primary sensory-motor cortices, cerebellum and basal ganglia. It should be noted that, at the given threshold, many brain regions appear both in the conjunction as well as the contrast analysis. This indicates voxels, which show functional connectivity with both clusters, which, however, was significantly stronger for one of them. That is, the MACM maps of both clusters differ mainly quantitatively, i.e., connectivity likelihood between cluster and target voxels.
(C) Functional characterisation by behavioural domain and paradigm class metadata. The red/green bars denote the number of foci for that particular behavioural domain/paradigm class within the anterior/posterior cluster. The grey bars represent the number of foci that would be expected to hit the particular cluster if all foci with the respective behavioural domain or paradigm class were randomly distributed throughout the cerebral cortex. That is, the grey bars denote the by-chance frequency of that particular label given the size of the cluster. This analysis indicated that the posterior cluster was strongly related to motor functions whereas the anterior cluster showed lower specificity but was activated predominantly by more cognitive processes, such as language, working memory, and task switching.
Characterisation of the derived clusters: Function
Functional characterisation using the meta-data of the experiments in BrainMap confirmed these results. All behavioural domains (BDs) and paradigm classes (PCs) that were significantly overrepresented in experiments activating the posterior cluster were related to motor functions (execution, imagery and learning, overt speech and saccades). The only exception was a significant overrepresentation of visual motion experiments that may be attributable to the high prevalence of (reflexive) eye movements in these tasks (Fig. 3C). In spite of the similar number of experiments, conspicuously fewer PCs were overrepresented in the anterior VOI indicating lower specificity to particular cognitive processes (based on the current taxonomy). Strikingly, none of the overrepresented BDs and PCs related to motor behaviour. Rather this region was primarily activated in experiments assessing “higher” cognitive processes, such as working memory, language and task switching.
Differentiation at the subsequent level of linkage
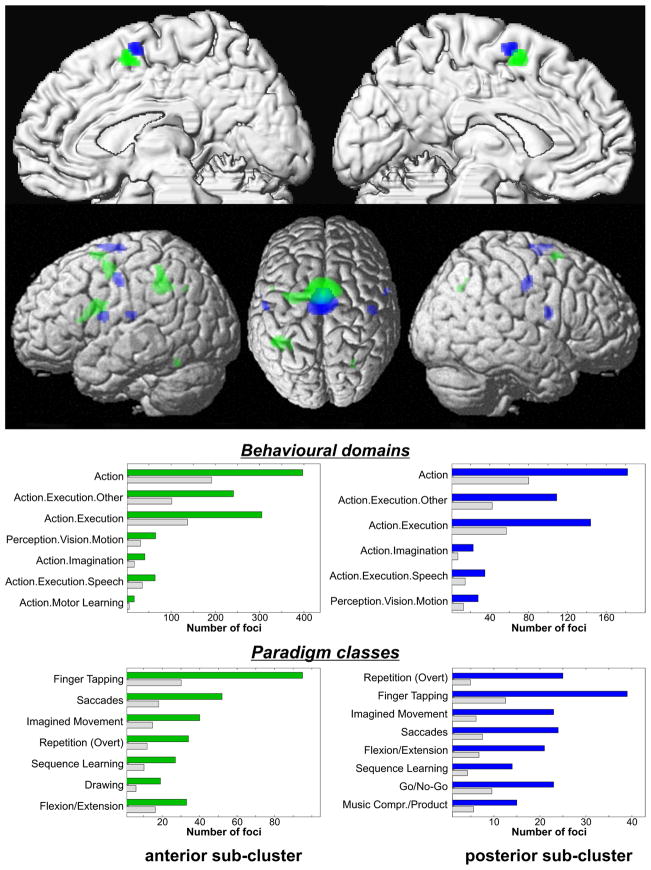
At the next lower linkage, two sets of voxels within the posterior cluster were separated, albeit cluster attribution was less stable than for the main clusters (supp. figure 5). When plotting the location of the unanimously defined voxels, the sub-clusters corresponded to the anterior respectively posterior portion of the caudal cluster in the two-cluster solution. The posterior sub-cluster showed higher probability for co-activation with primary motor and somatosensory cortex, as well as with inferior frontal cortex just posterior to BA 44 (Fig. 4). The anterior sub-cluster, in contrast, co-activated more strongly with the premotor cortex, left BA 44 and the parietal lobe. Mirroring these smaller differences in co-activation pattern, associated BDs and PCs were highly similar and varied mainly in relative position (Fig. 4). In summary, the first two levels of linkage thus indicate that the more anterior a voxel is located in the seed region, the more likely it is to co-activate with pre-frontal and parietal regions rather than the primary sensory-motor cortices.
Figure 4.
MACM maps and functional characterisation of the two sub-clusters jointed at the second-to-last linkage. As indicated by the top panel, the posterior sub-cluster showed higher probability for co-activation with primary sensory-motor and inferior frontal cortices, the anterior one co-activated stronger with premotor cortex, left BA 44 and the inferior parietal lobule. In the lower panel, the assessment of BD and PC profiles for experiments activating either region indicated that overrepresented BDs and PCs were very similar. Again, the blue/light green bars denote the number of foci for that particular behavioural domain/paradigm class within the two sub-clusters. The grey bars represent the number of foci that would be expected to hit the particular sub-cluster if all foci with the respective label were randomly distributed.
Testing hypotheses on the function of the co-activation based clusters
Two main hypotheses on functional differentiation and connectivity were derived from the performed meta-analytical modelling. Behavioural domain analyses indicated that the two main clusters derived from the hierarchical cluster analysis of co-activation profiles would be differentially activated by motor and working memory tasks. This hypothesised functional differentiation was tested in an fMRI experiment involving a working memory (counting E’s among a series of visually presented letters) and a motor task (alternating tapping with the left and right index finger at a self-paced frequence) in 56 subjects (cf. supplementary methods). These task-based fMRI data were acquired as part of an ongoing project. As suggested by the functional characterisation via the BrainMap meta-data, the anterior cluster was significantly stronger activated by the working memory task while the posterior one was significantly stronger activated by the motor paradigm (Fig. 5). Moreover, the whole-brain activation patterns (voxel-level FWE corrected p< 0.05) closely mirrored the networks obtained from the MACM analysis. This analysis thus fully confirmed the hypothesis about the functional differentiation between the co-activation based clusters.
Figure 5.
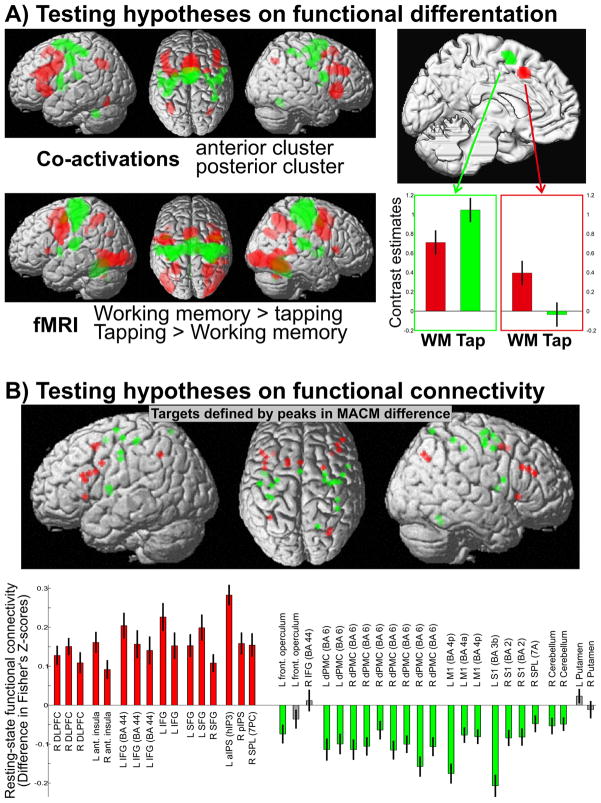
(A) Testing the hypothesis derived from the behavioural domain analysis that the anterior cluster should be more involved in working memory, the posterior in action. The top left panel shows the differential co-activation map of the anterior (red) and posterior (green) cluster. The lower left panel shows the results of the fMRI analysis for “working memory > finger tapping” (red) and “finger tapping > working memory” (green) and illustrates the close correspondence with the hypothesis from the MACM analysis. The right panel shows the activation (contrast estimates and 95% confidence intervals) of the two medial premotor clusters by the two tasks and demonstrates the distinction in functional activation between them as hypothesised.
(B) Testing the hypotheses on functional connectivity derived from the differential MACM analysis. The upper panel shows the location of the 39 peaks from the difference analysis between the co-activation maps for which a differential (resting state) functional connectivity was hypothesised. Low frequency resting state correlations between the two medial premotor clusters and these 39 locations were calculated, transformed into Fisher’s z-scores and compared for higher connectivity with either cluster. The lower panel summarises the results of this analysis (cf. details in supplementary figures 6–9). This bar diagram provides the mean and standard error of the Fisher z-transformed correlation coefficients with the two clusters. Coloured bars indicate a significantly (p < 0.05, Bonferroni-corrected for multiple comparisons) higher correlation with the anterior (red) or posterior (green) cluster, grey bars non-significant differences. All regions which showed significantly higher co-activation likelihoods with the anterior cluster also showed significantly higher resting-state connectivity with it. For the posterior cluster this was true for all but four regions, again confirming the hypotheses derived from the MACM analysis.
Testing hypotheses on the connectivity of the co-activation based clusters
By significant differences in co-activation likelihood, MACM indicated differential connectivity of the anterior and posterior cluster with inferior frontal, prefrontal and parietal as well as sensory-motor and dorsal premotor cortices, respectively. This hypothesis was explicitly tested by performing resting-state functional connectivity analysis in 62 subjects (cf. supplementary methods). In particular, we first identified all local maxima (peaks) in the MACM difference map. For each of these peaks we then computed the low-frequency resting state correlation with the two medial premotor seed regions. We tested the hypothesis that those peaks that showed significantly higher co-activation likelihood with the anterior than the posterior cluster would also show significantly stronger resting state connectivity and vice versa. Statistical analysis confirmed that all those regions that showed significantly higher co-activation likelihoods with the anterior cluster also showed significantly stronger (Bonferroni-corrected p < 0.05) resting state connectivity with that cluster. In turn, all four regions (bilateral putamen, left IFG/frontal operculum) showing higher co-activation probabilities with the posterior cluster also featured significantly higher resting state correlation with it. The hypotheses derived from the MACM analysis about differential connectivity of the two co-activation defined clusters thus again received convincing support from an independent dataset.
Discussion
Here, we outlined how co-activation patterns across neuroimaging experiments may be used to identify cortical modules in a model-free manner. Using this approach, we showed a highly robust distinction in task-based functional connectivity between two medial premotor regions that could be linked to differences in functional properties. Whereas an anterior cluster was associated with cognitive functions and co-activated with pre-frontal and parietal cortices, the posterior one was activated by action-related tasks and co-activated with (pre-) motor areas.
Methods for connectivity-based parcellation
We applied hierarchical cluster analysis (Eickhoff et al., 2007; Timm, 2002) to combine the individual seed voxels into larger regions, showing that the two main clusters remained completely stable, with no single voxel changing attribution. In contrast, the first connectivity-based parcellation applied a spectral reordering algorithm to the connectivity cross-correlation matrix (Johansen-Berg et al., 2004), identifying clusters in the reordered matrix as sets of seed voxels whose connectivity patterns were strongly correlated with each other and weakly with the rest of the matrix. Subsequent studies also employed k-means clustering to identify sets of seed voxels with similar connectivity (Kim et al., 2010; Klein et al., 2007; Tomassini et al., 2007). While all of these approaches yielded the same parcellation of our dataset, hierarchical cluster analysis has the major advantage of allowing parcellation of the seed regions at various levels of linkage. That is, there is no need to specify a priori the number of clusters to be identified. Instead, an organisational hierarchy is generated that should, in theory, allow a multi-layered delineation of cortical fields from individual modules to larger regions.
Organisation of the medial pre-motor cortex
The pre-SMA is conceptualised to be more strongly involved than the SMA in more complex, “cognitive” aspects of motor behaviour, such as motor selection or inhibition (Picard and Strick, 1996; Rizzolatti and Luppino, 2001; Vogt et al., 2007). Supporting this view, connectivity tracing in non-human primates revealed that the pre-SMA receives afferences from the inferior parietal lobule (Luppino et al., 1993) and the prefrontal cortex. Functional characterisation and co-activation pattern of the anterior cluster relate very well to these pre-SMA properties. Invasive tracing, moreover, provided no evidence for direct connections towards the primary motor cortex from pre-SMA but only from SMA proper (Luppino et al., 1993; Rizzolatti and Wolpert, 2005). Based on these observations and functional activation studies, the SMA has been implicated in executive aspects of motor control, e.g. movement initiation (Chouinard and Paus, 2006; Cunnington et al., 2002; Eickhoff et al., 2008a; Picard and Strick, 1996). This is well reflected in the recruitment of the posterior cluster by movement execution and its co-activation with lateral premotor, primary motor, and somatosensory cortices. Together these comparisons allow to confidently relate the anterior field to pre-SMA and the posterior one to SMA proper (Picard and Strick, 1996; Rizzolatti and Wolpert, 2005).
Aspects of brain connectivity
How does our approach compare to previous connectivity-based parcellation approaches and what could be the specific contribution of MACM to exploring brain connectivity? To answer these questions, the three major concepts of brain connectivity, anatomical, effective and functional connectivity, will be briefly reviewed.
Anatomical connectivity is predominantly assessed based on diffusion tensor imaging (DTI). On these, tractography algorithms may be employed to derive information about the most likely fibre tract direction from particular seed or the course and strength of pathways connecting two different regions of the cortex (Behrens et al., 2003; Ramnani et al., 2004). Nevertheless, it still has to be emphasized that diffusion-based tractography does not provide information about anatomical connectivity sensu stricto, i.e. axonal connectivity as revealed by tracer studies in macaques (Pons and Kaas, 1985), but can merely assess the presence and strength of macroanatomical fibre bundles between regions. Moreover, DTI does not allow to directly infer the nature of functional interactions using the respective pathways.
Effective connectivity is defined as the “influence a neural system has on another”, i.e., (context-dependent) interactions among different nodes in neural networks (Friston, 2002). In the widely used Dynamic Causal Modelling (DCM) and Structural Equation Modelling (SEM) approaches, this is assessed by taking an explicit network perspective and modelling the interactions between regions as a function of the experimental context (Buchel and Friston, 1997; Stephan et al., 2007). The derived model parameters then describe effective connectivity. Other approaches such as psychophysiological interactions or Granger causality mapping are used to identify regions, showing a context-dependent change in their coupling with a particular seed region (Friston et al., 1997; Goebel et al., 2003) under the assumptions of the employed model.
Functional connectivity, finally, is a heterogeneous concept, attributable to its definition as the “temporal correlation of spatially distant neurophysiological events” (Friston et al., 1996). It thus summarises any analysis that assesses correlations among brain signals without the explicit modelling of the underlying networks (as in effective connectivity), ranging from spike train correlations (Nuding et al., 2009) to EEG coherence (Gross et al., 2001) and various fMRI based methods. The latter correlate regional BOLD signal changes in order to quantify the degree of functional connectivity between them or identify regions that correlate with a particular seed (Hampson et al., 2002; Ramnani et al., 2004; Xiong et al., 1999). Such analyses may be performed on experimentally perturbed time-courses as an approach to task-based connectivity analysis without the a priori assumptions of effective connectivity models. However, they gained particular popularity in the context of resting state analyses. While the underlying physiology of correlations in the absence of a structured task remains somewhat elusive, it seems plausible that “resting” state, as a mixture of various cognitive processes, may likewise sample the repertoire of operations brain networks can perform (Buckner and Vincent, 2007; Smith et al., 2009). Finally, MACM may also be regarded as an approach to functional connectivity analysis. In line with the original definition of functional connectivity and the tradition of spike coincidence analyses, MACM assesses correlation of activation (across experiments) between brain regions. In contrast to functional connectivity analyses on fMRI time-series, however, not changes in the voxel-specific BOLD signal over scans but rather occurrences of activation across many different experiments represent the units of observation in MACM analyses.
Comparison to anatomical and resting state connectivity
The observed co-activation patterns are in good agreement with the (pre-) SMA connectivity as delineated by DTI tractography (Johansen-Berg et al., 2004). This concordance is noteworthy as DTI and co-activation represent unrelated techniques performed on independent samples. Together with previous computational modelling (Honey et al., 2007), this supports the notion of a good congruency between anatomical and functional connectivity. Combining MACM and DTI analyses on the same seed region may thus provide an indication of the anatomical connections by which functional networks are implemented and vice versa. In spite of the congruency, however, some differences are noteworthy. e.g., the putative pre-SMA cluster showed dense anatomical connections to the medial parietal cortex (Johansen-Berg et al., 2004), which were not mirrored by co-activation data. Several reasons might explain the observed discrepancy. First, the mentioned authors assessed the anatomical connectivity between pre-SMA and medial parietal cortex by means of diffusion tensor imaging (DTI), while we employed MACM. These two approaches examining brain connectivity are subject to different sources of measurement errors and noise that may vary systematically or haphazardly. Second, there are major conceptual differences between anatomical connectivity, a task-independent property of the brain, and functional connectivity, a task-dependent property of the brain. Third, this might also entail an underestimation of those functional connections, as revealed by MACM that are challenging to effectuate under the constraints of scanner-compliant task designs.
Correlation of resting state fMRI fluctuations in a medial premotor seed VOI with the rest of the brain also showed a differentiation into two regions (Kim et al., 2010) that closely match those from the DTI parcellation and the current results. This congruency strongly supports the correspondence of the brain’s functional architecture during rest and activation revealed by independent component analysis (Smith et al., 2009) and demonstrated that it also extends to seed-based analyses and connectivity based parcellations. The present analysis now shows how both approaches may be combined, in particular, how co-activation patterns across many neuroimaging experiments may be used to derive hypotheses that can subsequently be tested in independent resting-state datasets. This combination may provide a very important route for further research, as it would combine the strength of either method. Resting state analysis is readily performed even in clinical populations and may yield information about subject or group specific functional connectivity patterns but is dependent upon a motivated choice of the assessed regions and often difficult to associate with functional characteristics. MACM, on the other hand, allows the delineation of robustly co-activated networks across many different experimental designs and yields statistically testable associations with functional domains but is unsuited to derive information about a particular population of interest or even connectivity in individual subjects. It becomes evident that combining the large scale approach of MACM with the possibility of subject-level resting state functional connectivity analysis may offset the drawbacks and combine the strengths of each approach. In particular, MACM may be used to build hypotheses about the differential connectivity patterns of cortical modules and link them to particular cognitive, sensory or motor functions, as demonstrated here for the medial premotor cortex. Resting state functional connectivity analysis informed by the MACM results may then be used to test this hypothesis and further characterise it, e.g., by relating the degree of functional connectivity to neuropsychological or clinical measures obtainable in single subjects. Likewise, functional connectivity analyses on time series obtained for tasks informed by the MACM results may be used to test for the functional dissociations implicated by the co-activation patterns.
Contribution of MACM as a hypothesis generation approach for neuroimaging
Importantly, the functional nature of clusters identified by DTI or resting-state connectivity could only be qualitatively interpreted by experts. In comparison, the probably most distinct advantage of co-activation analysis is the availability of functional meta-data on the experiments associated with a particular cluster (Laird et al., 2009b). Using this information, functional characteristics of identified cortical modules may be delineated by statistical assessment of the functional domains or paradigms that are associated with the observed co-activation patterns. MACM may hence provide the crucial link between connectivity-based parcellation and functional properties. It should be appreciated that connectivity-based parcellation not only based on BrainMap but also based on DTI and resting state data can be further functionally characterized using MACM. In other words, task-relatedness of a cluster can be explored using BrainMap regardless of whether that cluster has been derived from task-dependent (fMRI, BrainMap), task-independent (resting state data) or structural (DTI) imaging information. In this context, the probably most important role that MACM may fulfil is the generation of hypotheses for targeted neuroimaging experiments. Today’s functional neuroimaging has a multitude of methods and approaches for testing hypotheses about the location of a particular cognitive function or about a potential dissociation of the neural substrates of different processes. However, neuroimaging is less well suited to delineate cortical modules and create new hypotheses about the functional differentiation between them. That is, if the tasks that differentiate between the different modules within a particular region are known, fMRI may be used to specifically test this dissociation. If they are unknown, experiments cannot be specifically designed to reveal a functional distinction. Here, MACM may be in a unique position in that it allows summarizing the findings of thousands of previous neuroimaging findings in a statistically rigorous fashion. Hereby, it can identify those functional domains and paradigms that are associated with a particular cortical module or area and generate hypotheses about how two neighboring regions, e.g., defined by their co-activation patterns, may be differentially activated. In the present case, this is exemplified by a proof of principle analysis showing that the hypothesis on the functional dissociation between both clusters derived from the co-activation based parcellation could indeed be confirmed in an independent fMRI study.
Conclusions and outlook
Following earlier reports that cortical modules may be defined by differences in anatomical connectivity (Johansen-Berg et al., 2004; Klein et al., 2007; Tomassini et al., 2007) or resting state correlations (Kim et al., 2010) we demonstrated that SMA and pre-SMA may be distinguished by a model-free analysis of co-activation patterns across activation studies. Analysis of the behavioural domain and paradigm class meta-data (Laird et al., 2009a) moreover allowed the delineation of functional characteristics for the ensuing cortical modules. Using this approach, co-activation maps and functional characterisation via neuroimaging databases provide valuable tools for generating hypotheses that may be explicitly tested in targeted neuroimaging experiments as demonstrated in this report. A challenging task for future research will be to compare and integrate cortical maps from different connectivity-based approaches. DTI, resting state analyses, and MACM each focus on a different aspect of connectivity, and we therefore predict that in spite of their good convergence, each capture different features of cortical organisation (Eickhoff et al., 2010; Honey et al., 2007; Ramnani et al., 2004). In addition, a necessary step towards understanding cortical organisation is to relate connectivity-defined modules and functional differentiations to microstructural maps of the human cerebral cortex (Amunts et al., 2007; Eickhoff et al., 2005; Eickhoff et al., 2008b; Zilles et al., 2002). It remains to be tested if changes in connectivity profiles coincide with the histologically defined borders, or if they represent two largely independent principles of cortical organisation, whose intersection constitutes the fundamental modules of functional specialisation.
Supplementary Material
Supplementary Figure 1: Co-activation based parcellation was demonstrated in an exemplary analysis of the medial premotor cortex, i.e., the location of the (pre-) SMA. The volume of interest (VOI) in this region was defined by combining two regions of activation observed in a recent neuroimaging study on speeded motor responses. The activations displayed in green were defined by consistent activation for left, right and bilateral responses to fixed or randomly jittered stimuli. The activations depicted in red correspond to increased activation during responses to (randomly) bilateral as compared to unilateral stimuli. It can be seen, that in the region of the medial premotor cortex the former engages a more posterior region (supposedly SMA), the latter a more anterior one (pre-SMA). This observed functional differentiation within medial premotor cortex provided the basis for the performed connectivity-based parcellation analysis, aiming at recovering the distinction between these two regions in a model-free analysis on their co-activation patterns across thousands of neuroimaging experiments.
Supplementary Figure 2,3: To evaluate any influence of the algorithmic parameters on the co-activation based parcellation of the medial premotor cortex, the hierarchical cluster analysis was repeated for all combinations of different distance measures (Euclidean, correlation, cosine distance) and linkage algorithms (weighted, average, single, complete). Results are shown here for analyses based on performing MACM using the closest 50 (Supplementary figure 2) and 100 (Supplementary figure 3) experiments, respectively, which feature the closest focus of activation. Hierarchical cluster analysis of the co-activation matrices revealed a highly reliable separation of the seed voxels into two clusters, as each analysis revealed exactly the same parcellation of the VOI. Independently of all employed parameters, the two main clusters always aligned perfectly with the SMA and pre-SMA activations from the fMRI study that were combined into the seed VOI.
Supplementary Figure 4: For each of the various filter criteria used to assign experiments to seed voxels for MACM (cf. fig. 2, supp. Fig. 2–3), a connectivity similarity matrix was defined by cross-correlation of co-activation profiles between individual voxels of the seed VOI (left). The arrangement of the seed voxels was then permuted based on a spectral reordering algorithm for envelope reduction in sparse matrices, forcing large values toward the diagonal (right). Break points between clusters in that reordered matrix then represent locations where co-activation patterns change. These analyses confirmed the parcellation derived from the hierarchical cluster analyses. Again, each individual analysis indicated again exactly the same parcellation of the seed region into two main clusters with no voxel changing cluster-attribution.
Supplementary Figure 5: The second-highest linkage consistently merged two sets of voxels within the posterior cluster. While this was the case independently of the filter criterion used to define the experiments attributed to each voxel, the clustering did not precisely overlap between the different analyses. In particular, the number of voxels attributed to the smaller of the two sub-clusters varied between 46 and 75. When plotting the location of the unanimously defined voxels belonging to either of the two sub-clusters in all analyses, it was apparent that these corresponded to the anterior respectively posterior portion of the posterior set obtained from the two-cluster solution.
Supplementary Figures 6–9: Details of the resting state functional connectivity analysis for all peaks obtained in the MACM contrast. Low frequency resting state correlations between the two clusters obtained by the connectivity-based parcellation on one hand and the 39 peaks derived from the difference analysis between the co-activation maps on the other were calculated in 62 subjects, transformed into Fisher’s z-scores and compared. The left panel if each image shows the location of the peak on the MNI single subject and its coordinates in MNI space. The colour of the crosshair indicates whether a particular location showed significantly higher co-activation likelihood with the anterior (red) or the posterior (green) cluster. The left (red) and right (green) bars indicate the mean correlations and standard error (across subjects) of the Fisher’s z scores for resting state correlations with the anterior and posterior cluster, respectively. The lower right panel shows significance as assessed by a paired T-test. The colour of the bar indicates whether the anterior (red) or the posterior (green) cluster showed a higher functional connectivity with that region. Significance (p < 0.05, Bonferroni-corrected for multiple comparisons) is indicated by asterisks.
Acknowledgments
This work was partly funded by the Human Brain Project (R01-MH074457-01A1; S.B.E., A.R.L., P.T.F), the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model; K.Z., S.B.E.), the DFG (IRTG 1328, S.B.E.) and the Helmholtz Alliance for Mental Health in an Aging Society (HelMA; K.Z.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Amunts K, Schleicher A, Zilles K. Cytoarchitecture of the cerebral cortex--more than localization. Neuroimage. 2007;37:1061–1065. doi: 10.1016/j.neuroimage.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Anwander A, Tittgemeyer M, von Cramon DY, Friederici AD, Knosche TR. Connectivity-Based Parcellation of Broca’s Area. Cerebral Cortex. 2007;17:816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, Matthews PM, Brady JM, Smith SM. Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med. 2003;50:1077–1088. doi: 10.1002/mrm.10609. [DOI] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, Zafiris O, Kubischik M, Hoffmann K, Zilles K, Fink GR. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29:287–296. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Großhirnrinde. Barth; Leipzig: 1909. [Google Scholar]
- Buchel C, Friston KJ. Modulation of connectivity in visual pathways by attention: cortical interactions evaluated with structural equation modelling and fMRI. Cerebral Cortex. 1997;7:768–778. doi: 10.1093/cercor/7.8.768. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–1096. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F, D’Agata F, Sacco K, Duca S, Geminiani G, Vercelli A. Functional connectivity of the insula in the resting brain. Neuroimage. 2011;55:8–23. doi: 10.1016/j.neuroimage.2010.11.049. [DOI] [PubMed] [Google Scholar]
- Charron S, Koechlin E. Divided representation of concurrent goals in the human frontal lobes. Science. 2010;328:360–363. doi: 10.1126/science.1183614. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. The primary motor and premotor areas of the human cerebral cortex. Neuroscientist. 2006;12:143–152. doi: 10.1177/1073858405284255. [DOI] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally- triggered movement: a study of event-related fMRI. Neuroimage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Dafotakis M, Grefkes C, Shah NJ, Zilles K, Piza-Katzer H. Central adaptation following heterotopic hand replantation probed by fMRI and effective connectivity analysis. Exp Neurol. 2008a;212:132–144. doi: 10.1016/j.expneurol.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, Behrens TE. Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. J Neurosci. 2010 doi: 10.1523/JNEUROSCI.5664-09.2010. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-Based Activation Likelihood Estimation Meta-Analysis of Neuroimaging Data: A Random-Effects Approach Based on Empirical Estimates of Spatial Uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Rottschy C, Kujovic M, Palomero-Gallagher N, Zilles B. Organisational principles of human visual cortex revealed by receptor mapping. Cerebral Cortex. 2008b;18:2637–2645. doi: 10.1093/cercor/bhn024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Rottschy C, Zilles K. Laminar distribution and co-distribution of neurotransmitter receptors in early human visual cortex. Brain Struct Funct. 2007;212:255–267. doi: 10.1007/s00429-007-0156-y. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Friston K. Beyond phrenology: what can neuroimaging tell us about distributed circuitry? Annu Rev Neurosci. 2002;25:221–250. doi: 10.1146/annurev.neuro.25.112701.142846. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Fletcher P, Liddle PF, Frackowiak RS. Functional topography: multidimensional scaling and functional connectivity in the brain. Cerebral Cortex. 1996;6:156–164. doi: 10.1093/cercor/6.2.156. [DOI] [PubMed] [Google Scholar]
- Goebel R, Roebroeck A, Kim DS, Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magn Reson Imaging. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cerebral Cortex. 2009;19:72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc Natl Acad Sci USA. 2001;98:694–699. doi: 10.1073/pnas.98.2.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen V, Sporns O. Mapping the structural core of human cerebral cortex. Plos Biology. 2008;6:1479–1493. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson M, Peterson BS, Skudlarski P, Gatenby JC, Gore JC. Detection of functional connectivity using temporal correlations in MR images. Human Brain Mapping. 2002;15:247–262. doi: 10.1002/hbm.10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Kotter R, Breakspear M, Sporns O. Network structure of cerebral cortex shapes functional connectivity at multiple time scales. Proc Natl Acad Sci USA. 2007;104:10240–10245. doi: 10.1073/pnas.0701519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobs O, Wang LE, Dafotakis M, Grefkes C, Zilles K, Eickhoff SB. Effects of timing and movement uncertainty implicate the temporo-parietal junction in the prediction of forthcoming motor actions. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TE, Robson MD, Drobnjak I, Rushworth MF, Brady JM, Smith SM, Higham DJ, Matthews PM. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proc Natl Acad Sci USA. 2004;101:13335–13340. doi: 10.1073/pnas.0403743101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Lee JM, Jo HJ, Kim SH, Lee JH, Kim ST, Seo SW, Cox RW, Na DL, Kim SI, Saad ZS. Defining functional SMA and pre-SMA subregions in human MFC using resting state fMRI: functional connectivity-based parcellation method. Neuroimage. 2010;49:2375–2386. doi: 10.1016/j.neuroimage.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein JC, Behrens TE, Robson MD, Mackay CE, Higham DJ, Johansen-Berg H. Connectivity-based parcellation of human cortex using diffusion MRI: Establishing reproducibility, validity and observer independence in BA 44/45 and SMA/pre-SMA. Neuroimage. 2007;34:204–211. doi: 10.1016/j.neuroimage.2006.08.022. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by Meta-Analysis. Brain Struct Funct. 2010 doi: 10.1007/s00429-010-0255-z. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Kurth F, Fox PM, Uecker AM, Turner JA, Robinson JL, Lancaster JL, Fox PT. ALE Meta-Analysis Workflows Via the Brainmap Database: Progress Towards A Probabilistic Functional Brain Atlas. Front Neuroinformatics. 2009a;3:23. doi: 10.3389/neuro.11.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird AR, Eickhoff SB, Li K, Robin DA, Glahn DC, Fox PT. Investigating the Functional Heterogeneity of the Default Mode Network Using Coordinate-Based Meta-Analytic Modeling. J Neurosci. 2009b;29:14496–14505. doi: 10.1523/JNEUROSCI.4004-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda R, Rizzolatti G. Corticocortical connections of area F3 (SMA-proper) and area F6 (pre-SMA) in the macaque monkey. J Comp Neurol. 1993;338:114–140. doi: 10.1002/cne.903380109. [DOI] [PubMed] [Google Scholar]
- Luppino G, Matelli M, Camarda RM, Gallese V, Rizzolatti G. Multiple representations of body movements in mesial area 6 and the adjacent cingulate cortex: an intracortical microstimulation study in the macaque monkey. J Comp Neurol. 1991;311:463–482. doi: 10.1002/cne.903110403. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G, Rizzolatti G. Architecture of superior and mesial area 6 and the adjacent cingulate cortex in the macaque monkey. J Comp Neurol. 1991;311:445–462. doi: 10.1002/cne.903110402. [DOI] [PubMed] [Google Scholar]
- Nuding SC, Segers LS, Baekey DM, Dick TE, Solomon IC, Shannon R, Morris KF, Lindsey BG. Pontine-ventral respiratory column interactions through raphe circuits detected using multi-array spike train recordings. Journal of Neurophysiology. 2009;101:2943–2960. doi: 10.1152/jn.91305.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard N, Strick PL. Motor areas of the medialwall: a reviewof their location and functional activation. Cerebral Cortex. 1996;6:342–353. doi: 10.1093/cercor/6.3.342. [DOI] [PubMed] [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Pons TP, Kaas JH. Connections of area 2 of somatosensory cortex with the anterior pulvinar and subdivisions of the ventroposterior complex in macaque monkeys. J Comp Neurol. 1985;240:16–36. doi: 10.1002/cne.902400103. [DOI] [PubMed] [Google Scholar]
- Ramnani N, Behrens TE, Penny W, Matthews PM. New Approaches for Exploring Anatomical and Functional Connectivity in the Human Brain. Biol Psychiatry. 2004;56:613–619. doi: 10.1016/j.biopsych.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Reddy L, Kanwisher N. Coding of visual objects in the ventral stream. Current Opinion in Neurobiology. 2006;16:408–414. doi: 10.1016/j.conb.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Riecker A, Mathiak K, Wildgruber D, Erb M, Hertrich I, Grodd W, Ackermann H. fMRI reveals two distinct cerebral networks subserving speech motor control. Neurology. 2005;64:700–706. doi: 10.1212/01.WNL.0000152156.90779.89. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Luppino G. The cortical motor system. Neuron. 2001;31:889–901. doi: 10.1016/s0896-6273(01)00423-8. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Wolpert DM. Motor systems. Current Opinion in Neurobiology. 2005;15:623–625. doi: 10.1016/j.conb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan KE, Harrison LM, Kiebel SJ, David O, Penny WD, Friston KJ. Dynamic causal models of neural system dynamics:current state and future extensions. J Biosci. 2007;32:129–144. doi: 10.1007/s12038-007-0012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timm NH. Applied Multivariate Analysis. Springer; New York: 2002. [Google Scholar]
- Tomassini V, Jbabdi S, Klein JC, Behrens TE, Pozzilli C, Matthews PM, Rushworth MF, Johansen-Berg H. Diffusion-weighted imaging tractography-based parcellation of the human lateral premotor cortex identifies dorsal and ventral subregions with anatomical and functional specializations. Journal of Neuroscience. 2007;27:10259–10269. doi: 10.1523/JNEUROSCI.2144-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt C, Vogt O. Allgemeinere Ergebnisse unserer Hirnforschung. Journal für Psychologie und Neurologie. 1919;25:279–461. [Google Scholar]
- Vogt S, Buccino G, Wohlschlager AM, Canessa N, Shah NJ, Zilles K, Eickhoff SB, Freund HJ, Rizzolatti G, Fink GR. Prefrontal involvement in imitation learning of hand actions: Effects of practice and expertise. Neuroimage. 2007;37:1371–1383. doi: 10.1016/j.neuroimage.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Xiong J, Parsons LM, Gao JH, Fox PT. Interregional connectivity to primary motor cortex revealed using MRI resting state images. Human Brain Mapping. 1999;8:151–156. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<151::AID-HBM13>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DY, Snyder AZ, Fox MD, Sansbury MW, Shimony JS, Raichle ME. Intrinsic functional relations between human cerebral cortex and thalamus. Journal of Neurophysiology. 2008;100:1740–1748. doi: 10.1152/jn.90463.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Palomero-Gallagher N, Amunts K. Quantitative analysis of cyto- and receptor architecture of the human brain. In: Mazziotta J, Toga A, editors. Brain Mapping, the methods. Elsevier; 2002. pp. 573–602. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1: Co-activation based parcellation was demonstrated in an exemplary analysis of the medial premotor cortex, i.e., the location of the (pre-) SMA. The volume of interest (VOI) in this region was defined by combining two regions of activation observed in a recent neuroimaging study on speeded motor responses. The activations displayed in green were defined by consistent activation for left, right and bilateral responses to fixed or randomly jittered stimuli. The activations depicted in red correspond to increased activation during responses to (randomly) bilateral as compared to unilateral stimuli. It can be seen, that in the region of the medial premotor cortex the former engages a more posterior region (supposedly SMA), the latter a more anterior one (pre-SMA). This observed functional differentiation within medial premotor cortex provided the basis for the performed connectivity-based parcellation analysis, aiming at recovering the distinction between these two regions in a model-free analysis on their co-activation patterns across thousands of neuroimaging experiments.
Supplementary Figure 2,3: To evaluate any influence of the algorithmic parameters on the co-activation based parcellation of the medial premotor cortex, the hierarchical cluster analysis was repeated for all combinations of different distance measures (Euclidean, correlation, cosine distance) and linkage algorithms (weighted, average, single, complete). Results are shown here for analyses based on performing MACM using the closest 50 (Supplementary figure 2) and 100 (Supplementary figure 3) experiments, respectively, which feature the closest focus of activation. Hierarchical cluster analysis of the co-activation matrices revealed a highly reliable separation of the seed voxels into two clusters, as each analysis revealed exactly the same parcellation of the VOI. Independently of all employed parameters, the two main clusters always aligned perfectly with the SMA and pre-SMA activations from the fMRI study that were combined into the seed VOI.
Supplementary Figure 4: For each of the various filter criteria used to assign experiments to seed voxels for MACM (cf. fig. 2, supp. Fig. 2–3), a connectivity similarity matrix was defined by cross-correlation of co-activation profiles between individual voxels of the seed VOI (left). The arrangement of the seed voxels was then permuted based on a spectral reordering algorithm for envelope reduction in sparse matrices, forcing large values toward the diagonal (right). Break points between clusters in that reordered matrix then represent locations where co-activation patterns change. These analyses confirmed the parcellation derived from the hierarchical cluster analyses. Again, each individual analysis indicated again exactly the same parcellation of the seed region into two main clusters with no voxel changing cluster-attribution.
Supplementary Figure 5: The second-highest linkage consistently merged two sets of voxels within the posterior cluster. While this was the case independently of the filter criterion used to define the experiments attributed to each voxel, the clustering did not precisely overlap between the different analyses. In particular, the number of voxels attributed to the smaller of the two sub-clusters varied between 46 and 75. When plotting the location of the unanimously defined voxels belonging to either of the two sub-clusters in all analyses, it was apparent that these corresponded to the anterior respectively posterior portion of the posterior set obtained from the two-cluster solution.
Supplementary Figures 6–9: Details of the resting state functional connectivity analysis for all peaks obtained in the MACM contrast. Low frequency resting state correlations between the two clusters obtained by the connectivity-based parcellation on one hand and the 39 peaks derived from the difference analysis between the co-activation maps on the other were calculated in 62 subjects, transformed into Fisher’s z-scores and compared. The left panel if each image shows the location of the peak on the MNI single subject and its coordinates in MNI space. The colour of the crosshair indicates whether a particular location showed significantly higher co-activation likelihood with the anterior (red) or the posterior (green) cluster. The left (red) and right (green) bars indicate the mean correlations and standard error (across subjects) of the Fisher’s z scores for resting state correlations with the anterior and posterior cluster, respectively. The lower right panel shows significance as assessed by a paired T-test. The colour of the bar indicates whether the anterior (red) or the posterior (green) cluster showed a higher functional connectivity with that region. Significance (p < 0.05, Bonferroni-corrected for multiple comparisons) is indicated by asterisks.