Abstract
Background and Purpose
Prior studies show that black patients have carotid endarterectomy less frequently than white patients. Diagnostic imaging is necessary to determine whether a patient is a candidate for the operation. We determined whether there were differences in the use of diagnostic carotid imaging and the frequency of carotid endarterectomy between elderly black and white ischemic stroke patients.
Materials and Methods
Medicare fee-for-service beneficiaries with discharge diagnoses of ischemic stroke (International Classification of Diseases 9th revision: 433, 434, 436) were randomly selected for inclusion in the National Stroke Project 1998-1999, 2000-2001. Receipt of at least one type of carotid imaging study was compared for black and white patients. Binomial logistic regression models were used to evaluate the associations between race and receipt of carotid imaging and carotid endarterectomy with adjustment for demographics, degree of carotid artery stenosis, and other clinical covariates.
Findings
Among 19,639 stroke patients (1,974 black; 17,655 white), 69.6% received at least one diagnostic carotid imaging test (blacks, 68.4%; whites, 69.7%; p=0.233). After risk-adjustment, blacks were less likely to receive carotid imaging (adjusted OR, 0.87; 95%CI, 0.78-0.97). There was no relationship between race and the receipt of carotid endarterectomy after adjustment for degree of carotid stenosis and other covariates (adjusted OR, 1.14; 95%CI, 0.66-1.96).
Conclusions
Black ischemic stroke patients were less likely to receive diagnostic carotid imaging than white patients, although the difference was small, and only significant after risk-adjustment. There was no difference in the proportion having carotid endarterectomy after adjustment for degree of carotid artery stenosis and other clinical factors.
Introduction
Carotid endarterectomy (CEA) is effective in preventing stroke in selected patients with moderate or severe stenosis in whom the operation can be performed safely (1, 2). Some reports indicate that black patients receive CEA less frequently than white patients, but many of these studies do not account for possible differences in the degree of carotid stenosis (3-13). Black patients generally have less severe extracranial carotid atherosclerosis compared with whites (14-16), which may in part explain the observed racial differences in the receipt of CEA (17). Diagnostic imaging of the carotid artery is necessary to determine whether a patient is a candidate for the operation (15, 18), and differences in the performance of this imaging by race may also be an important issue related to observed differences in the receipt of CEA. A study carried out in patients receiving care at Veterans Administration (VA) hospitals, in which there is no economic barrier to diagnostic testing or healthcare procedures among qualified veterans, found no white/nonwhite differences in the use of screening carotid ultrasound or CEA(18). A second VA study also reported no difference in black and white CEA rates after adjusting for the degree of carotid stenosis (19). Whether there are racial differences in the rates of diagnostic carotid imaging and of CEA after adjustment for differences in clinical characteristics or the degree of carotid artery stenosis in non-VA healthcare settings is uncertain.
We determined whether there was variation in the receipt of diagnostic carotid imaging among elderly black and white fee-for-service Medicare beneficiaries hospitalized with a primary discharge diagnosis of ischemic stroke. Among patients with moderate or severe stenosis who had no evidence of carotid occlusion or atrial fibrillation, we also assessed whether the receipt of CEA differed for black and white patients, adjusting for demographic and clinical characteristics, including the maximum reported degree of extracranial carotid artery stenosis.
Materials and Methods
Patients were randomly selected for the Medicare Health Care Quality Improvement Program’s National Stroke Project, an initiative authorized by the Health Care Financing Administration (now the Centers for Medicare & Medicaid Services), which has been described elsewhere (20, 21). The program was designed to improve health care quality for Medicare beneficiaries hospitalized with stroke or transient ischemic attack (TIA). A systematic random sample of up to 750 hospitalized, fee-for service Medicare beneficiaries with a primary discharge diagnosis of stroke were identified from each of the 50 states, the District of Columbia, and Puerto Rico during each of two time periods (April 1, 1998-March 31,1999, and April 1, 2000-March 31, 2001). The current analysis includes patients with a primary diagnosis of ischemic stroke (ICD-9-CM discharge codes 433.×1, 434.×1 and 436) with confirmatory documentation in the patient’s medical record. Patients were included in the analysis if they were classified as of either black or white race, and of non-Hispanic ethnicity based on chart abstracted information. Patients were excluded if they were admitted from non-acute care settings, were transferred from other acute care settings, were younger than 65 years of age, had terminal cancer, or had an illness with a life expectancy of less than 6 months. Because of variability in diagnostic criteria, patients with a diagnosis of TIA were also excluded.
In addition, those with a documented TIA less than 6 months before the index hospitalization were excluded as it was assumed that they would have received diagnostic carotid imaging prior to the index stroke hospitalization. Patients who received carotid imaging but for whom test results were not recorded in the medical record were excluded from the primary analyses. Analyses examining the receipt of CEA were restricted to patients with moderate or severe stenosis without carotid occlusion or atrial fibrillation (1, 22).
Data were obtained from medical record review by two clinical data abstraction centers using computerized abstraction tools. Patient age, sex, race (black, white), and medical history (prior stroke or TIA, atrial fibrillation, congestive heart failure, ischemic heart disease, myocardial infarction, diabetes and hypertension) were recorded. Stroke severity was measured as the number of deficits (sensory, motor, speech and/or visual) present upon arrival, and then dichotomized (≥2 deficits vs. <2 deficits) (23).
Study outcomes included the receipt of any carotid imaging test during the index hospitalization and the receipt of CEA among the subset of patients with moderate or severe carotid stenosis. The receipt of any imaging was defined as a documented test result for catheter angiography, magnetic resonance angiography (MRA), or duplex ultrasound; CT angiography was not routinely performed during the period of data collection. Patients in whom a study was planned but not done during the hospitalization were considered to not have had the test in the primary analysis, but were included in secondary analyses. The degree of carotid stenosis was categorized as mild (0-49%), moderate (50-69%), severe (70-99%), or occluded (100%) (1, 2). When multiple tests were performed with discordant results, the degree of stenosis was assigned based first on catheter angiography, then MRA, and finally duplex ultrasound. As stenosis in the symptomatic artery was not specifically recorded in the database, the degree of carotid artery stenosis for this analysis was based on the greatest reported degree of stenosis. The receipt of carotid endarterectomy was identified by physician documentation in the medical record during the index hospitalization. Patients for whom CEA was planned, but not performed during the index hospitalization, were considered in secondary analyses.
Bivariate analyses were used to compare the characteristics of black and white patients, as well as to identify patient characteristics associated with the receipt of carotid imaging and CEA among patients with moderate or severe carotid stenosis. The significance of differences for categorical variables was determined using the X2 test. To account for multiple comparisons, p-values were adjusted using the Benjamini-Hochberg False Discovery Rate (FDR) (24), in which the expected proportion of errors among the rejected null hypotheses is controlled. Binomial logistic regression analyses were used to calculate the unadjusted odds ratios and 95% confidence intervals for the association of black vs. white race with each outcome. Model covariates included race (black, white), sex, age, prior stroke or TIA, heart disease, myocardial infarction, heart failure, hypertension, diabetes, stroke severity, period of data collection, and degree of carotid stenosis (for models evaluating the receipt of CEA). Candidate variables were selected for inclusion in the multivariable models based on their significance in bivariate comparisons. Separate analyses that assessed the receipt of CEA for patients with moderate or severe stenosis were performed. Secondary binomial logistic regression analyses included both documented and planned carotid imaging tests and CEAs. All analyses were conducted using SAS Version 9.1 (SAS Institute Inc, Cary, NC).
Results
A total of 19,639 elderly ischemic stroke patients were included in the analyses; 10% (n= 1,974) were identified as black, 57% were women, and the mean age was 78.2 ± 7.3 years. Black patients were more likely to be women, to be younger, and to have a history of stroke, diabetes, and/or hypertension. White patients were more likely to have prior TIA, atrial fibrillation, heart disease and/or myocardial infarction than black patients (Table 1).
Table 1.
Demographic and Clinical Characteristics of Elderly Black and White Patients with Ischemic Stroke
| Black | White | ||
|---|---|---|---|
| Characteristic | (N=1974) | (N=17665) | p-value |
| Female | 63.3 | 56.7 | <0.0001 |
| Age | <0.0001 | ||
| ≥85 | 17.6 | 21.6 | |
| 75-84 | 39.3 | 46.3 | |
| 65-74 | 43.1 | 32.1 | |
| Prior Stroke | 57.6 | 49.2 | <0.0001 |
| Prior TIA | 9.3 | 15.5 | <0.0001 |
| Atrial Fibrillation | 10.6 | 19.9 | <0.0001 |
| Heart Failure | 17.7 | 16.0 | 0.049 |
| Heart Disease | 48.5 | 52.8 | 0.0004 |
| Myocardial Infarction | 29.8 | 32.6 | 0.013 |
| Diabetes | 44.5 | 27.9 | <0.0001 |
| Hypertension | 91.0 | 78.2 | <0.0001 |
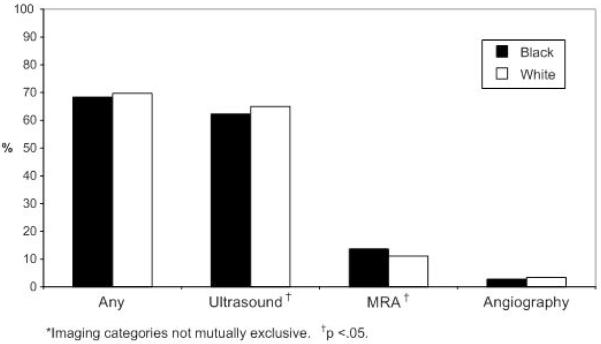
Overall, 69.6% of patients received at least one diagnostic carotid imaging test. Duplex ultrasounds were performed in 64.7%, MRA in 11.5%, and catheter angiography in 3.4% of patients. In unadjusted analyses, there were no significant differences in the proportion of black and white patients who received diagnostic carotid imaging (68.4% of black patients compared with 69.7% of white patients, p=0.233; Figure 1). In risk-adjusted analyses, black race was associated with lower receipt of carotid imaging (OR, 0.87; 95% CI, 0.78 to 0.97; Table 2). Additional covariates associated with lower rates of imaging include female sex, advanced age, prior stroke or TIA, atrial fibrillation, and heart failure. In secondary analyses, 196 patients who did not receive any diagnostic carotid imaging during the index hospitalization but had planned imaging procedure(s) after hospital discharge were combined with patients who received imaging during the hospitalization. The inclusion of these patients did not appreciably alter the association between race and receipt of imaging tests (adjusted OR, 0.85; 95% CI, 0.77 to 0.95).
Figure 1.
Proportion of Black and White Patients who Received Carotid Imaging*
Table 2.
Risk-Adjusted Analyses Predicting Receipt of Any Carotid Imaging
| Adjusted Model* |
||
|---|---|---|
| Characteristic | OR | 95% CI |
| Black (vs. White) | 0.87 | (0.78, 0.97) |
| Sex (Female vs. Male) | 0.93 | (0.87, 0.99) |
| Age | ||
| ≥85 (vs. 65-74) | 0.46 | (0.42, 0.50) |
| 75-84 (vs. 65-74) | 0.76 | (0.71, 0.82) |
| Prior Stroke | 0.64 | (0.60, 0.69) |
| Prior TIA | 0.82 | (0.75, 0.89) |
| Atrial Fibrillation | 0.73 | (0.67, 0.79) |
| Heart Failure | 0.73 | (0.67, 0.80) |
| Heart Disease | 1.09 | (1.00, 1.19) |
| Myocardial Infarction | 1.00 | (0.91, 1.09) |
| Diabetes | 0.95 | (0.88, 1.02) |
| Hypertension | 1.33 | (1.23, 1.43) |
Adjusted for covariates in table, stroke severity and wave of data collection.
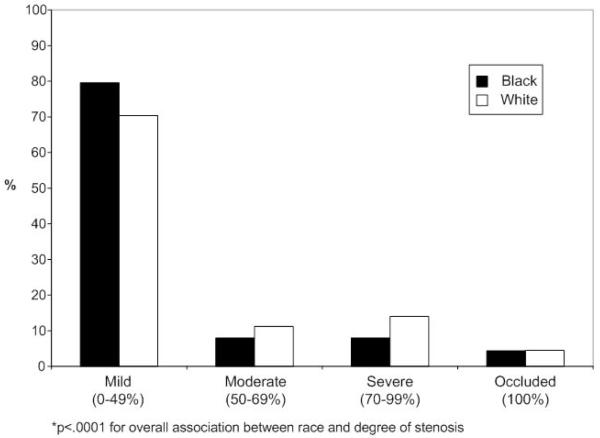
Among the 13,671 patients who had carotid imaging during the hospitalization, black patients were more likely to have mild stenosis as compared with white patients, and were less likely to have moderate or severe stenosis (Figure 2). The analyses evaluating the receipt of CEA were limited to 2,768 patients with moderate or severe carotid stenosis without carotid occlusion and no evidence of atrial fibrillation. Black patients were more likely to be women, to be younger, to have prior stroke, heart failure, diabetes, and/or hypertension, and to have moderate stenosis (Table 3). Of the group eligible for CEA as defined for this analysis, 8.6% of blacks and 9.1% of whites received carotid endarterectomy. In risk-adjusted analyses, there was no association between race and receipt of CEA after controlling for clinical covariates (OR, 1.14; 95% CI, 0.66 to 1.96; Table 4). Similar patterns were found for analyses stratified by the degree of carotid stenosis. Secondary analyses that included patients who had CEA planned after the index hospitalization also showed no difference in the receipt of CEA by race (OR 0.93; 95%CI, 0.58 to 1.49).
Figure 2.
Overall Degree of Stenosis for Black and White Patients*
Table 3.
Demographic and Clinical Characteristics of Patients with Moderate or Severe Carotid Stenosis
| Black | White | ||
|---|---|---|---|
| (N=198) | (N=2570) | p-value | |
| Female | 58.1 | 49.1 | 0.050 |
| Age | 0.158 | ||
| ≥85 | 14.1 | 16.7 | |
| 75-84 | 41.9 | 46.6 | |
| 65-74 | 43.9 | 36.7 | |
| Prior Stroke | 54.6 | 47.6 | 0.097 |
| Prior TIA | 9.6 | 14.9 | 0.097 |
| Heart Failure | 17.2 | 12.4 | 0.097 |
| Heart Disease | 55.1 | 57.9 | 0.490 |
| Myocardial Infarction | 33.8 | 36.2 | 0.507 |
| Diabetes | 48.5 | 31.5 | <0.0001 |
| Hypertension | 91.9 | 81.4 | 0.001 |
| Moderate Stenosis | 50.0 | 43.5 | 0.109 |
Table 4.
Risk-Adjusted Analyses Predicting Receipt of Carotid Endarterectomy
| Adjusted Model* |
||
|---|---|---|
| Characteristic | OR | 95% CI |
| Black (vs. White) | 1.14 | (0.66, 1.96) |
| Sex (Female vs. Male) | 0.94 | (0.71, 1.24) |
| Age | ||
| ≥85 (vs. 65-74) | 0.32 | (0.18, 0.56) |
| 75-84 (vs. 65-74) | 1.06 | (0.80, 1.41) |
| Prior Stroke | 0.88 | (0.67, 1.17) |
| Prior TIA | 1.29 | (0.90, 1.85) |
| Heart Failure | 0.79 | (0.51, 1.22) |
| Heart Disease | 1.45 | (1.03, 2.05) |
| Myocardial Infarction | 0.78 | (0.55, 1.10) |
| Diabetes | 0.84 | (0.62, 1.13) |
| Hypertension | 0.98 | (0.68, 1.42) |
Adjusted for covariates in table, stroke severity, wave of data collection, and degree of stenosis.
Discussion
In this elderly ischemic stroke population, black patients had a lower likelihood of receiving diagnostic carotid imaging than whites after risk adjustment for demographic and clinical variables. The difference, however, was small, with 68.4% of black patients and 69.7% of white patients receiving imaging, and was only significant after adjustment for age, sex, and other clinical factors. Among those receiving imaging, black patients were more likely to have mild stenosis that white patients. In analyses restricted to patients with moderate or severe stenosis, there was no difference in the receipt of CEA between black and white patients even after risk adjustment for clinical covariates.
Results of studies assessing racial differences in the receipt of diagnostic tests for ischemic stroke in both federal and non-federal health care settings have varied. Several found no difference (18, 25) whereas others reported lower rates for black patients than for white patients (3, 4, 9, 15, 26). For example, a single-state stroke registry reported that blacks were equally likely to have carotid imaging as compared to whites (25). In contrast, a study conducted in a 4-hospital urban area found that blacks had fewer inpatient tests than whites, although the adjusted association combined carotid imaging with other types of diagnostic evaluations such as CT/MRI scan, electrocardiogram and serum cholesterol measurement (26). Black patients had a lower rate of noninvasive carotid imaging than white patients in a Medicare sample of TIA patients(4), and in a more general sample of elderly Medicare fee-for-service beneficiaries (3). Our study found no difference in unadjusted comparisons, but a modest association with race after adjustment for demographic and clinical characteristics, suggesting that these factors may contribute to differences in the receipt of diagnostic testing. Variation in study population characteristics and risk adjustment for patient demographic and clinical factors may contribute to inconsistencies across studies. Additional research evaluating racial differences in the receipt of carotid diagnostic imaging in non-hospitalized patients, including those who present with TIAs, stroke symptoms, or individuals with stroke risk factors who are asymptomatic is needed.
Our finding that black patients had less severe extracranial carotid stenosis compared to whites is consistent with most (14-16), but not all prior studies (27). Direct comparisons with these studies are difficult due to variation in the study design. Study populations included patients who were younger than 65 years of age (14-16, 27), whose degree of stenosis was not categorized according to current clinical guidelines (14, 16), and who were referred for carotid imaging for reasons other than ischemic stroke such as TIA (excluded in the present analysis) (14-16, 27), asymptomatic carotid bruit (14, 16), preoperative screening(16) and syncope (14, 16). Despite these methodological differences, white patients tended to have high-grade carotid artery stenosis more frequently than patients of other racial groups in most study populations. It is possible that physicians aware of this difference may be using other clinical information not measured in our analysis to identify persons less likely to have high grade carotid artery stenosis, possibly contributing to the lower rate of testing in black patients.
A patient’s perceived risk of adverse outcomes, perceived barriers to care and cultural beliefs may affect his/her likelihood to delay or refuse recommended care (17), and may contribute to the difference in the adjusted rates of diagnostic imaging found in the present analysis. Blacks are more likely to be averse to carotid endarterectomy (28, 29), as well other invasive procedures (30-32). Patients who are not interested in receiving a procedure may not receive a preoperative diagnostic test, as the results would not otherwise affect their care (18).
Racial differences in CEA rates have been documented using Medicare administrative claims data (3-7), as well as in other national data (8, 9) and statewide (10-13) hospital discharge information. These studies found greater utilization of CEA among white as compared with black patients (white:black rate ratios ranging from 2.2 to more than 4) (5-8, 10-13); however, clinical characteristics that may confound the association between black and white race and receipt of the operation, such as the degree of stenosis, were not assessed. The lack of information related to the degree of stenosis in these studies may, in part, explain the discrepancy between their results and that of the present analysis.
The absence of a relationship between black vs. white race and the performance of CEA found in our analysis is consistent with studies conducted in VA populations that also accounted for the degree of stenosis, either through adjustment for CEA appropriateness, inclusion of a stenosis variable, or both (19, 33). Oddone et al. found that the appropriateness of CEA was the strongest predictor of receipt of the procedure among patients with at least moderate stenosis (19). Further analysis of the patients deemed to be appropriate candidates for the operation also found no association between black vs. white race and the likelihood of receipt of CEA (33).
This study has limitations. Clinical information, such as stroke location and its relationship to carotid stenosis, whether the carotid artery with the greatest degree of stenosis was symptomatic or asymptomatic, ischemic stroke subtype, contraindications for surgery (34), information about clinical decision making, and patient preferences was not available. Although studies have shown that black patients have higher perioperative stroke rates than whites (35, 36), information on CEA complication rates were not available. The assessment of stroke severity was based on the cumulative number of deficits, a novel measure used in the NSP study; however, prior analyses using this assessment found a positive association between the cumulative number of deficits and adverse outcomes, including in-hospital mortality or discharge to a skilled nursing facility suggesting the scale has predictive validity (23). Results from this study reflect elderly fee-for-service Medicare beneficiaries and may not be generalizable to younger populations (however, the majority of CEA procedures are performed in patients 65 years of age and older (37)). Additional testing may have been conducted prior to the index hospitalization or after discharge, resulting in the scheduling of CEA procedures prior to the index event. We minimized this inception cohort bias by excluding patients with TIAs prior to the index event and by including planned testing and post-discharge procedures in secondary analyses. We also excluded TIA events because the diagnosis of these events can be imprecise, even among stroke subspecialists (38). The impact of other unmeasured variables, including cognitive status, socioeconomic factors, other medical conditions not reflected in the patient’s medical records and variation in surgical risk, could not be assessed. Data were collected in years 1998-2001; however, other studies found that racial differences in the receipt of secondary therapies, if anything, have narrowed over time (39), and there is no reason to suspect that racial differences would have emerged over the intervening years.
This analysis revealed no significant differences in the receipt of carotid endarterectomy among elderly black and white patients with recent stroke. There was, however, a small difference in the receipt of carotid imaging, which may be explained by any of several unmeasured factors including patient preferences. Additional research is needed to better understand the reasons for this difference.
Acknowledgments
The data upon which this publication is based were provided by the Centers for Medicare & Medicaid Services, an agency of the U.S. Department of Health and Human Services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
This project was supported by grant number 5 R03 HS013940 from the Agency for Healthcare Research and Quality and grant number 5 R03 AG022075 from the National Institute of Aging. Dr. Goldstein is also supported by the Veterans Administration and an American Stroke Association-Bugher Foundation Center for Stroke Prevention Research Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosures: None
References
- 1.Biller J, Feinberg WM, Castaldo JE, et al. Guidelines for carotid endarterectomy: A statement for healthcare professionals from a special writing group of the stroke council, american heart association. Circulation. 1998;97:501–509. doi: 10.1161/01.cir.97.5.501. [DOI] [PubMed] [Google Scholar]
- 2.Chaturvedi S, Bruno A, Feasby T, et al. Carotid endarterectomy--an evidence-based review: Report of the therapeutics and technology assessment subcommittee of the american academy of neurology. Neurology. 2005;65:794–801. doi: 10.1212/01.wnl.0000176036.07558.82. [DOI] [PubMed] [Google Scholar]
- 3.Escarce JJ, Epstein KR, Colby DC, et al. Racial differences in the elderly’s use of medical procedures and diagnostic tests. American journal of public health. 1993;83:948–954. doi: 10.2105/ajph.83.7.948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell JB, Ballard DJ, Matchar DB, et al. Racial variation in treatment for transient ischemic attacks: Impact of participation by neurologists. Health Services Research. 2000;34:1413–1428. [PMC free article] [PubMed] [Google Scholar]
- 5.Escarce JJ, McGuire TG. Changes in racial differences in use of medical procedures and diagnostic tests among elderly persons: 1986-1997. American journal of public health. 2004;94:1795–1799. doi: 10.2105/ajph.94.10.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillum RF. Carotid endarterectomy in older women and men in the united states: Trends in ethnic disparities. J Natl Med Assoc. 2005;97:957–962. [PMC free article] [PubMed] [Google Scholar]
- 7.Jha AK, Fisher ES, Li Z, et al. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353:683–691. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- 8.Gillum RF. Epidemiology of carotid endarterectomy and cerebral arteriography in the united states. Stroke. 1995;26:1724–1728. doi: 10.1161/01.str.26.9.1724. [DOI] [PubMed] [Google Scholar]
- 9.Oddone EZ, Horner RD, Monger ME, et al. Racial variations in the rates of carotid angiography and endarterectomy in patients with stroke and transient ischemic attack. Arch Intern Med. 1993;153:2781–2786. [PubMed] [Google Scholar]
- 10.Giacomini MK. Gender and ethnic differences in hospital-based procedure utilization in california. Arch Intern Med. 1996;156:1217–1224. [PubMed] [Google Scholar]
- 11.Kennedy BS, Fortmann SP, Stafford RS. Elective and isolated carotid endarterectomy: Health disparities in utilization and outcomes, but not readmission. J Natl Med Assoc. 2007;99:480–488. [PMC free article] [PubMed] [Google Scholar]
- 12.Maxwell JG, Rutherford EJ, Covington D, et al. Infrequency of blacks among patients having carotid endarterectomy. Stroke. 1989;20:22–26. doi: 10.1161/01.str.20.1.22. [DOI] [PubMed] [Google Scholar]
- 13.Mort EA, Weissman JS, Epstein AM. Physician discretion and racial variation in the use of surgical procedures. Arch Intern Med. 1994;154:761–767. [PubMed] [Google Scholar]
- 14.Gil-Peralta A, Alter M, Lai SM, et al. Duplex doppler and spectral flow analysis of racial differences in cerebrovascular atherosclerosis. Stroke. 1990;21:740–744. doi: 10.1161/01.str.21.5.740. [DOI] [PubMed] [Google Scholar]
- 15.Oddone EZ, Horner RD, Sloane R, et al. Race, presenting signs and symptoms, use of carotid artery imaging, and appropriateness of carotid endarterectomy. Stroke. 1999;30:1350–1356. doi: 10.1161/01.str.30.7.1350. [DOI] [PubMed] [Google Scholar]
- 16.Wang MY, Mimran R, Mohit A, et al. Carotid stenosis in a multiethnic population. J Stroke Cerebrovasc Dis. 2000;9:64–69. doi: 10.1053/jscd.2000.0090064. [DOI] [PubMed] [Google Scholar]
- 17.Horner RD, Oddone EZ, Matchar DB. Theories explaining racial differences in the utilization of diagnostic and therapeutic procedures for cerebrovascular disease. The Milbank Quarterly. 1995;73:443–462. [PubMed] [Google Scholar]
- 18.Goldstein LB, Matchar DB, Hoff-Lindquist J, et al. Veterans administration acute stroke (vast) study: Lack of race/ethnic-based differences in utilization of stroke-related procedures or services. Stroke. 2003;34:999–1004. doi: 10.1161/01.STR.0000063364.88309.27. [DOI] [PubMed] [Google Scholar]
- 19.Oddone EZ, Horner RD, Johnston DC, et al. Carotid endarterectomy and race: Do clinical indications and patient preferences account for differences? Stroke. 2002;33:2936–2943. doi: 10.1161/01.str.0000043672.42831.eb. [DOI] [PubMed] [Google Scholar]
- 20.Jencks SF, Cuerdon T, Burwen DR, et al. Quality of medical care delivered to medicare beneficiaries: A profile at state and national levels. JAMA. 2000;284:1670–1676. doi: 10.1001/jama.284.13.1670. [DOI] [PubMed] [Google Scholar]
- 21.Jencks SF, Huff ED, Cuerdon T. Change in the quality of care delivered to medicare beneficiaries, 1998-1999 to 2000-2001. JAMA. 2003;289:305–312. doi: 10.1001/jama.289.3.305. [DOI] [PubMed] [Google Scholar]
- 22.Wolf PA, Clagett GP, Easton JD, et al. Preventing ischemic stroke in patients with prior stroke and transient ischemic attack: A statement for healthcare professionals from the stroke council of the american heart association. Stroke. 1999;30:1991–1994. doi: 10.1161/01.str.30.9.1991. [DOI] [PubMed] [Google Scholar]
- 23.Dallas MI, Rone-Adams S, Echternach JL, et al. Dependence in prestroke mobility predicts adverse outcomes among patients with acute ischemic stroke. Stroke. 2008;39:2298–2303. doi: 10.1161/STROKEAHA.107.506329. [DOI] [PubMed] [Google Scholar]
- 24.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57:289–300. [Google Scholar]
- 25.Jacobs BS, Birbeck G, Mullard AJ, et al. Quality of hospital care in african american and white patients with ischemic stroke and tia. Neurology. 2006;66:809–814. doi: 10.1212/01.wnl.0000203335.45804.72. [DOI] [PubMed] [Google Scholar]
- 26.Tuhrim S, Cooperman A, Rojas M, et al. The association of race and sex with the underuse of stroke prevention measures. J Stroke Cerebrovasc Dis. 2008;17:226–234. doi: 10.1016/j.jstrokecerebrovasdis.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Ryu JE, Murros K, Espeland MA, et al. Extracranial carotid atherosclerosis in black and white patients with transient ischemic attacks. Stroke. 1989;20:1133–1137. doi: 10.1161/01.str.20.9.1133. [DOI] [PubMed] [Google Scholar]
- 28.Oddone EZ, Horner RD, Diers T, et al. Understanding racial variation in the use of carotid endarterectomy: The role of aversion to surgery. J Natl Med Assoc. 1998;90:25–33. [PMC free article] [PubMed] [Google Scholar]
- 29.Bosworth HB, Stechuchak KM, Grambow SC, et al. Patient risk perceptions for carotid endarterectomy: Which patients are strongly averse to surgery? J Vasc Surg. 2004;40:86–91. doi: 10.1016/j.jvs.2004.03.040. [DOI] [PubMed] [Google Scholar]
- 30.Gordon HS, Paterniti DA, Wray NP. Race and patient refusal of invasive cardiac procedures. J Gen Intern Med. 2004;19:962–966. doi: 10.1111/j.1525-1497.2004.30131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heidenreich PA, Shlipak MG, Geppert J, et al. Racial and sex differences in refusal of coronary angiography. Am J Med. 2002;113:200–207. doi: 10.1016/s0002-9343(02)01221-4. [DOI] [PubMed] [Google Scholar]
- 32.Rathore SS, Masoudi FA, Wang Y, et al. Socioeconomic status, treatment, and outcomes among elderly patients hospitalized with heart failure: Findings from the national heart failure project. Am Heart J. 2006;152:371–378. doi: 10.1016/j.ahj.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horner RD, Oddone EZ, Stechuchak KM, et al. Who doesn’t receive carotid endarterectomy when appropriate? J Vasc Surg. 2004;39:162–168. doi: 10.1016/j.jvs.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Halm EA, Chassin MR, Tuhrim S, et al. Revisiting the appropriateness of carotid endarterectomy. Stroke. 2003;34:1464–1471. doi: 10.1161/01.STR.0000072514.79745.7D. [DOI] [PubMed] [Google Scholar]
- 35.Chaturvedi S, Madhavan R, Santhakumar S, et al. Higher risk factor burden and worse outcomes in urban carotid endarterectomy patients. Stroke. 2008;39:2966–2968. doi: 10.1161/STROKEAHA.108.516062. [DOI] [PubMed] [Google Scholar]
- 36.Matsen SL, Chang DC, Perler BA, et al. Trends in the in-hospital stroke rate following carotid endarterectomy in california and maryland. J Vasc Surg. 2006;44:488–495. doi: 10.1016/j.jvs.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 37.Halm EA, Tuhrim S, Wang JJ, et al. Risk factors for perioperative death and stroke after carotid endarterectomy: Results of the new york carotid artery surgery study. Stroke. 2009;40:221–229. doi: 10.1161/STROKEAHA.108.524785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Castle J, Mlynash M, Lee K, et al. Agreement regarding diagnosis of transient ischemic attack fairly low among stroke-trained neurologists. Stroke. 2010;41:1367–1370. doi: 10.1161/STROKEAHA.109.577650. [DOI] [PubMed] [Google Scholar]
- 39.Schwamm LH, Reeves MJ, Pan W, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]