Abstract
The closely related δ and ε isoforms of the serine/threonine protein kinase casein kinase 1 (Csnk1) have been implicated in the generation of psychostimulant-induced behaviors. Here we show that Csnk1δ/ε produces its effects on behavior by acting on the Darpp-32-PP1 signaling pathway to regulate AMPA receptor phosphorylation in the nucleus accumbens (NAcc). Inhibiting Csnk1δ/ε in the NAcc with the selective inhibitor PF-670462 blocks amphetamine induced locomotion and its ability to increase phosphorylation of Darpp-32 at S137 and T34, decrease PP1 activity and increase phosphorylation of the AMPA receptor subunit at S845. Consistent with these findings, preventing GluR1 phosphorylation with the alanine mutant GluR1(S845A) reduces glutamate-evoked currents in cultured medium spiny neurons and blocks the locomotor activity produced by NAcc amphetamine. Thus, Csnk1 enables the locomotor and likely the incentive motivational effects of amphetamine by regulating Darrp-32-PP1-GlurR1(S845) signaling in the NAcc. As such, Csnk1 may be a critical target for intervention in the treatment of drug use disorders.
Keywords: AMPA glutamate receptors, amphetamine, casein kinase 1, Darpp-32, dopamine, GluR1
Introduction
Casein kinase 1 (Csnk1) is a serine/threonine protein kinase that phosphorylates a wide array of substrates, is involved in the regulation of diverse cellular processes, and is richly expressed in multiple tissues including brain (Knippschild et al. 2005). Recently, the closely related isoforms, Csnk1δ and Csnk1ε, both abundant in striatal forebrain regions (Löhler et al. 2009; Utz et al. 2010), were implicated in the generation of psychostimulant-induced locomotor behaviors. Mice genetically modified to overexpress Csnk1δ in forebrain show dopamine-dependent alterations in basal and amphetamine-induced locomotion (Zhou et al. 2010) while mice selectively bred for high sensitivity to methamphetamine-induced locomotion (Kamens et al. 2005) show a quantitative trait loci for increased Csnk1ε expression with a close to 10-fold increase in Csnk1ε gene expression in the nucleus accumbens (NAcc) (Palmer et al. 2005). A single nucleotide polymorphism in the human CSNK1ε gene has also been associated with increased sensitivity to the subjective effects of amphetamine (Veenstra-VanderWeele et al. 2006). These findings indicate that Csnk1 can regulate dopamine signaling and psychostimulant-induced behaviors in vivo but little is known about the mechanisms by which it produces these effects.
Midbrain dopamine and cortical glutamate projections converge onto medium spiny output neurons in the NAcc where they interact to mediate the behavioral and incentive motivational effects of amphetamine and other psychostimulants (Wise and Bozarth, 1987; Meredith et al. 1993; Sesack et al. 2003; Pierce and Kalivas, 1997). Different dopamine and glutamate receptor initiated signaling pathways in these neurons, including those using calcium-calmodulin-dependent protein kinase II (Anderson et al. 2008) and dopamine and cAMP-regulated phosphoprotein 32 (Darpp-32) (Greengard, 2001; Fernandez et al. 2006), have been proposed to bridge the contribution of these two neurotransmitters. The nature and extent of Darpp-32 activity is regulated by a number of kinases and protein phosphatases (Fernandez et al. 2006). In the rat, Csnk1 phosphorylates Darpp-32 at S137 to decrease the rate of pDarpp-32(T34) dephosphorylation by calcineurin (Desdouits et al. 1995a,b). Phosphorylation of Darpp-32 at T34 by PKA inhibits protein phosphatase 1 (PP1) activity (Hemmings et al. 1984), promoting the continued phosphorylation of a number of target proteins including the GluR1 subunit of the AMPA receptor at S845 (Snyder et al. 2000). Phosphorylation of GluR1 at S845 potentiates neuronal excitability by facilitating trafficking and synaptic insertion of GluR1-containing AMPA receptors (Ehlers, 2000; Esteban et al. 2003; Mangiavacchi and Wolf, 2004; Sun et al. 2005; Oh et al. 2006) and increasing peak current and channel open probability (Roche et al. 1996; Banke et al. 2000). As psychostimulant-induced locomotion requires NAcc AMPA receptor activation (Wolf, 1998), we hypothesized that Csnk1 can enable the behavioral effects of drugs like amphetamine by enhancing the ability of Darpp-32 to inhibit PP1 activity and thus increase AMPA receptor phosphorylation at S845.
Materials and Methods
Subjects
Male Sprague-Dawley rats weighing 250-275 g on arrival were obtained from Harlan Sprague-Dawley (Madison, WI) and housed individually in a reverse cycle room (12-h light/12-h dark) with food and water freely available. Rats were given 4-5 days to acclimate and subsequently tested during the dark period of the light cycle. For experiments involving intra-NAcc infusions, rats were anesthetized with a mix of ketamine (100 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.), placed in a stereotaxic apparatus with the incisor bar positioned 5.0mm above the interaural line (Pellegrino et al. 1979), and chronically implanted with bilateral guide cannulae (22 gauge, Plastics One, Roanoke, VA) aimed at the NAcc core (A/P, +3.4mm; L, ± 1.5mm; DV, -7.5mm from bregma and skull). Cannulae were angled at 10° to the vertical and positioned either 1 mm (for biochemistry and behavioral testing with amphetamine and PF) or 4 mm (for Lentiviral infection and subsequent testing with amphetamine) above the final injection site. Guide cannulae were secured with dental acrylic anchored to stainless steel skull screws. After surgery, 28 gauge obturators were placed into the guide cannulae (extending 1 mm for guide cannulae positioned 1 mm and flush for guide cannulae positioned 4 mm above the final injection site) and rats returned to their home cage for a 7 day recovery period. All surgical procedures were conducted using aseptic techniques according to approved Institutional Animal Care and Use Committee and Institutional Biosafety Committee protocols.
NAcc microinjections
In the behavioral and biochemistry experiments testing the effects of PF, microinjections were made in the freely moving rat in a volume of 0.5μl/side over 30 seconds through 28 gauge injection cannulae extending 1 mm beyond the guide cannula tips. In the experiment testing the effect of NAcc GluR1(S845A) expression, amphetamine microinjections were made using the same guide cannulae used to deliver the Lentiviral vectors. Thus, injection cannulae extended 4 mm beyond the guide cannula tips in this case. Injection cannulae were connected via polyethylene tubing (PE20) to Hamilton syringes and left in place for 1 minute after the injection to allow for diffusion. Microinjection cannulae were then removed, the obturators replaced, and rats returned either to the activity boxes or their home cages.
Locomotor testing
Rats were placed individually in locomotor monitoring chambers for 30-60 minutes, administered their respective NAcc infusion, and returned to the locomotor chambers for 60-minutes. In the experiments testing the effects of PF, PF (1.0, 5.0, or 10 μg/side) was microinjected into the NAcc either alone or in cocktail with amphetamine (2.5 μg/side). In each of three experiments, saline, amphetamine, and amphetamine+PF microinjections were made in counterbalanced order with at least three days between injections. In a fourth experiment, rats in different groups received microinjections into the NAcc of saline or PF alone (1.0, 5.0, or 10 μg/side) again in counterbalanced order. In the experiment testing the effect of NAcc GluR1(S845A) expression, Lenti-GFP and Lenti-GluR1(S845A) infected rats were administered amphetamine (2.5 μg/side) into the NAcc 3 weeks following infection. Locomotor activity was measured using a bank of 12 activity monitoring boxes. Each box (22 × 43 × 33 cm) was constructed of opaque plastic (rear and two side walls), a plexiglas front-hinged door, and a tubular stainless steel ceiling and floor. Two photocell beams, positioned 2.5 cm above the floor and spaced evenly along the longitudinal axis of each box, estimated horizontal locomotion. Separate interruptions of photocell beams were detected and recorded via an electrical interface by a computer situated in an adjacent room using locally developed software.
NAcc signaling
Rats in different groups were infused intracranially with saline (0.5μl/side), amphetamine (2.5μg/side), PF (10μg/side) or amphetamine+PF into the NAcc and sacrificed 20 minutes later. Brains were rapidly removed for subsequent assessment of protein levels and PP1 activity. For immunoblotting, brains were flash-frozen on dry ice, 2mm thick sections obtained with a brain matrix, and 2mm diameter punches of the NAcc taken around the injection cannula tips. Tissue punches were homogenized in ice-cold lysis buffer (50mM Tris, 150mM NaCl, 1mM EDTA, 1% NP-40 ,1% Sodium deoxycholate, 0.1% SDS) containing protease and phosphatase inhibitor cocktails (1 and 2; Sigma-Aldrich, St. Louis, MO). A total of 20 μg of protein was loaded per lane and separated by 10% SDS-PAGE. Following transfer, membranes were incubated in blocking solution (5% milk in Tris-buffered saline containing Tween 20), incubated for 16 hours at 4°C in primary antibody for Darpp32 (1:1000; Cell Signaling Technologies, Beverly, MA), pDarpp32(T34; 1:500, Millipore, Billerica, MA), pDarpp32(S137; 1:5000; generously provided by Dr. Paul Greengard, Rockefeller University, New York, NY), GluR1(1:1000; Millipore), pGluR1 (S831; 1:500, Millipore), pGluR1(S845; 1:500, Millipore), Csnk1δ/ε (1:5000; BD Transduction Laboratories, Franklin Lake, NJ), CopGFP (1:1000; Evrogen, Moscow, Russia), β-actin (1:2000; Sigma-Aldrich), or Tubulin (1:10,000; Santa Cruz) and washed in TBS-T. Membranes were then incubated in a HRP-conjugated anti-rabbit or anti-mouse IgG and visualized using the ECL detection system (ECL Advanced, GE Healthcare). Blots were digitally imaged using the GeneSnap Bio Imaging System and quantified using Genetool software (Syngene, MD, USA). PP1 activity was assayed using the Protein Serine/Threonine Phosphatase (PSP) assay system (BioLabs) by measuring the release of inorganic phosphate from labeled protein. Myelin basic protein (MyBP) labeled with Γ-32P was used as a substrate. Fresh NAcc tissue was homogenized in lysis buffer (50mM Tris pH7.4, 150mM NaCl, 0.1mM EDTA, 0.1mM EGTA, 1mM MnCl2, 5mM DTT, 5% glycerol) containing a protease inhibitor cocktail but without phosphatase inhibitors. 10μg of tissue lysate was added to 30 μl of assay buffer containing PP2A and PP2B inhibitors, 2nM okadaic acid, and 2μM FK-506. After 5 minutes of preincubation at 30°C, the reaction was started by adding 10μl of substrate. Following 10 minutes of incubation at 30°C, the reaction was terminated by adding 200μl of cold 20% trichoroacetic acid. After centrifugation at 12000g for 5 min, radioactivity was counted in 200μl of supernatant by an LS6500 multi-purpose scintillation counter (Beckman, CA, USA). Data were expressed as pmol/min/μg protein.
Construct assembly and Lentiviral vectors
cDNAs for rat GluR1 (GenBank accession No. X17184) were constructed in a pcDNA1/Amp vector at the EcoRI and BamHI sites (Invitrogen) to obtain a pcDNA1/GluR1 plasmid. Mutagenesis was performed using the Quickchange II XL site-Directed mutagenesis kit. The following primers were used to produce the GluR1 S845A mutants. Forward primer: 5'-G ACC CTC CCC CGG AAC GCT GGG GCA GGA GCC AGC -3'. Reverse primer: 5'- GCT GGC TCC TGC CCC AGC GTT CCG GGG GAG GGT C-3'. The full length of the GluR1(S845A) cDNA was subcloned into a pCDH1-CMV-MCS-EF1-copGFP vector (System Bioscience). Recombinant Lentivirus was packaged in HEK TN cells using a pPACKH Lentivector Packaging Kit as described by the manufacturer (System Bioscience). The Lentivirus was purified and concentrated by PEG-8000. The average titer of the recombinant viral stocks was 1×106 infectious units/ml. The following recombinant Lentiviral vectors were used: Lenti-GluR1(S845A) and Lenti-GFP as a control. The viability of the Lentiviral vectors was confirmed in HEK cells infected either with Lenti-GFP, Lenti-GluR1, or Lenti-GluR1(S845A). GFP positive cells were observed in all cases 48 hours post-infection but GluR1 transgene expression was increased only in the Lenti-GluR1 and Lenti-GluR1(S845A) infected HEK cells (see Fig. S1 in Supplementary Information).
For the behavioral experiment, rats were transferred to a biosafety level 2 facility following one week of recovery from implant surgery and administered bilateral NAcc microinjections of the viral vectors in a fume hood (Loweth et al. 2010). Microinjections were made in freely moving rats in a volume of 2.0 μl/side at a rate of 0.1μl/30 sec through 28 gauge cannulae extending 4 mm beyond the guide cannulae tips. Microinjection cannulae loaded with Lentivirus were connected via polyethelyne tubing (PE20) to Hamilton syringes and left in place for 5 minutes after the injection to allow for diffusion. Rats were returned to the housing room 72 hours later.
For the electrophysiology experiments, dissociated cultures of medium spiny neurons were prepared from E18 Sprague-Dawley rats. Under isoflurane anesthesia, embryos were removed and the NAcc separated from diencephalon, dissected free of meninges, and diced. Tissues were digested in 0.03% (wt/vol) trypsin. After dissociation by trituration, cells were counted, suspended in culture medium consisting of Neurobasal supplemented with 2% B-27 (both from Gibco-Invitrogen Corp), 0.5 mm GlutaMax I, 10% deactivated fetal bovine serum, and 1% penicillin/Streptomycin. Cells were plated on poly l-lysine-coated 25-mm cover slips at a density of 8 × 104 cells/cm2. Neurons were either left uninfected or infected with Lenti-GFP or Lenti-GluR1(S845A) at day 1 of culture. Recordings were performed 10-18 days following infection. Cells in these three groups were also assayed for evoked pGluR1(S845) following 15 minutes incubation with the PKA activating D1 dopamine receptor agonist SKF81297 (10 μM).
Electrophysiology
Cultured medium spiny neurons were plated on coverslips, placed in an experimental chamber, and visualized using an inverted microscope under phase-contrast illumination (Nikon, Tokyo, Japan). All experiments were performed at room temperature (~20°C). The chamber was flushed with bath solution for 5 min before recording began. During experiments, solution flow was ~0.5 ml/min. Bath solution contained (in mM/l) 140 NaCl, 1 MgCl2, 5 KCl, 10 Glucose, 10 Hepes, 2 CaCL2*2H2O at pH=7.4. Patch electrodes were pulled from borosilicate glass capillary tubing (TW150-4, World Precision Instruments Inc., Sarasota, FL) on a micropipette puller (Sutter P-97, Sutter Instrument Co., Novato, CA). They had a resistance of 2-4 M when filled with solution containing (in mM/l) 135 CsCl, 1MgCl2, 10 Hepes, 10 EGTA, 3.6 Mg-ATP, 0.1 Na-GTP, and 14 Na-Creatinephosphate. Whole-cell voltage-clamp was performed using an Axopatch 200B, 16-bit data Digidata 1332A acquisition system driven by Clampex 8.2 (Axon Instruments, Foster City, CA). Cells were held at -60 mV, and gap-free recordings were acquired for 2 min each. Multiple recordings were possible in most cells. Puffs of 0.5mM Na-glutamate dissolved in bath solution were applied for 50ms with a Picospritzer II (General Valve Corp., Fairfield, NJ). The tip of the infusion pipette was placed within 80-100 μm of the recorded cell. Current traces were filtered at 1 kHz and digitized at 2 kHz. For each cell, a representative glutamate response was chosen for group analysis and background activity was analyzed from 30 s of recording prior to glutamate application.
Histology and Immunofluorescence
After the completion of the behavioral experiments testing the effects of NAcc PF, rats were deeply anesthetized and perfused transcardially with 0.9% saline and 10% formalin. Brains were removed and stored in the formalin solution for at least 24 hours. 40-μm sections were then prepared, mounted on gelatin-coated slides, and stained with cresyl violet to identify rats with injection cannula tips located bilaterally in the NAcc core. Only rats with both cannula tips located in the NAcc core were retained for statistical analyses. Four rats failed to meet this criterion.
After completion of the behavioral experiment testing the effects of NAcc GluR1(S845A), rats were perfused with 0.9 % saline and 4% paraformaldehyde and brains transferred to a 25% sucrose solution. To detect GFP fluorescence 40 μm coronal sections were prepared, mounted on gelatin-coated slides with Fluoromount-G (southern Biotech), and analyzed using a fluorescence microscope. Injection cannula tips located in the NAcc core were identified and the distribution pattern of GFP positive cells around the cannula tips assessed. Entire brains (from midbrain to prefrontal cortex) were also examined for GFP positive cells to determine the extent of anterograde and retrograde transport of the virus from the NAcc core microinjection site. GFP positive cells were observed only in close proximity to the injection cannula tips in the NAcc and were not detected in any other brain region. Again, only rats with both cannula tips located in the NAcc core were retained for statistical analysis. Five rats failed to meet this criterion.
Drugs
PF-670462 (4-(3-cyclohexyl-5-(4-fluoro-phenyl)-3H-imidazol-4-yl) pyrimidin-2-ylamine) was generously provided by Pfizer (Groton, CT). S(+)-amphetamine sulfate was purchased from Sigma-Aldrich (St. Louis, MO). Both drugs were dissolved in physiological saline. SKF81297 was purchased from Tocris (Ellisville, MO) and dissolved in DMSO. Doses refer to the weight of the salt.
Data analyses
The PF-amphetamine locomotor and electrophysiology peak current data were analyzed with one way ANOVA. The immunoblot data were normalized to β-actin or tubulin and ratios analyzed with two way between ANOVA with amphetamine and PF as the factors. The GluR1(S845A) expression pGluR1(S845) and GluR1 protein data were analyzed with two way between ANOVA with infection and drug challenge as the factors. The NAcc GluR1(S845A) expression locomotor data were analyzed with between-within ANOVA with infection as the between factor and time as the within factor. In all cases, post hoc comparisons after ANOVA were made with the Scheffé test.
Results
Inhibiting Csnk1 blocks NAcc amphetamine-induced locomotion
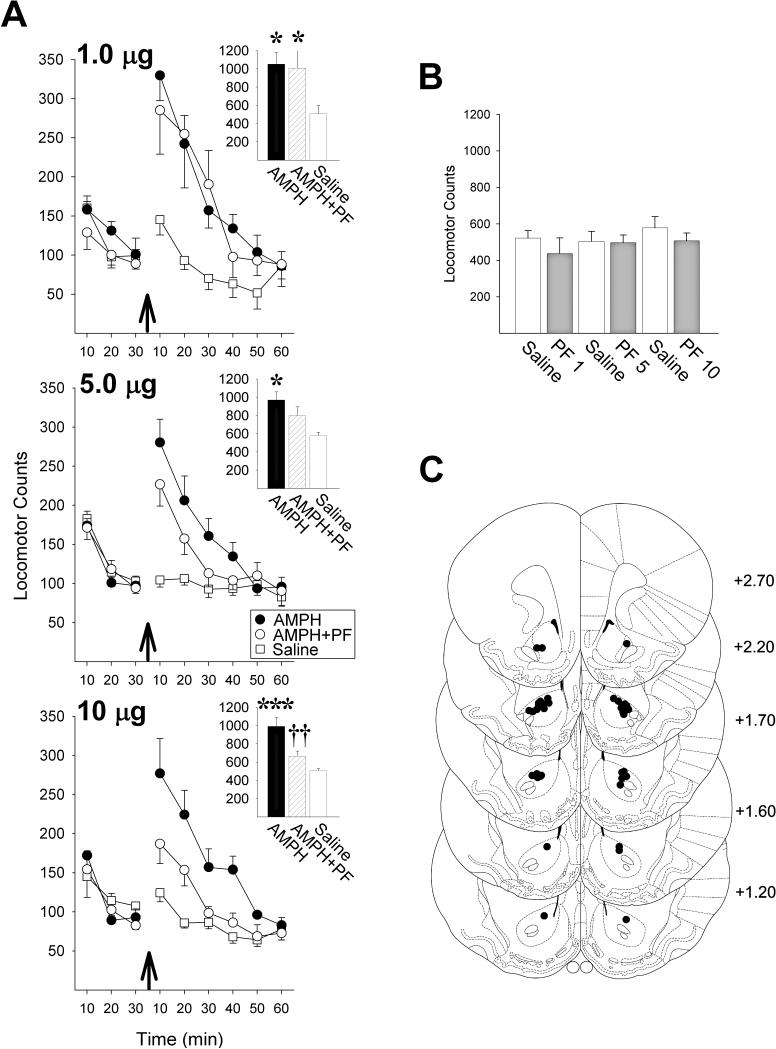
Using the selective Csnk1δ/ε inhibitor, PF-670462 (PF) (Badura et al. 2007; Walton et al. 2009), we first assessed whether inhibiting Csnk1 in the NAcc core could prevent the increased locomotion normally observed in rats when amphetamine is infused bilaterally into this site. In each of three experiments, NAcc amphetamine alone (2.5μg/side), produced a close to 2-fold increase in locomotion compared to saline (Fig. 1A; 1.0 PF: F2,10=8.84, P<0.01; 5.0 PF: F2,14=6.01, P<0.05; 10.0 PF: F2,10=20.17, P<0.001; revealed by analyses of variance, ANOVA). Consistent with results previously reported for methamphetamine in mice (Bryant et al. 2009), co-infusing PF with amphetamine into the NAcc dose-dependently reduced its ability to increase locomotion, with no effect observed at the lowest dose of PF (1.0 μg/side) and a significant attenuation observed at the highest (10.0 μg/side; P<0.01, revealed by post-hoc Scheffé comparisons). None of the doses of PF tested produced an effect on locomotion that differed from saline when infused by themselves into the NAcc (Fig. 1B).
Figure 1.
The Csnk1δ/ε inhibitor PF-670462 (PF) dose-dependently blocks NAcc amphetamine (AMPH) induced locomotion. (A) Locomotor activity counts before and after NAcc AMPH (arrows) in three experiments each testing the indicated dose of PF (n/group=6-8). Insets show 60-minute total locomotor counts following AMPH (*, P<0.05, ***, P<0.001, vs Saline; ††, P<0.01, vs AMPH; Scheffé). (B) 60-minute total locomotor counts following NAcc saline or PF alone (n/group=6-7). (C) Injection cannula placements in the NAcc core. The numbers to the right indicate millimeters from bregma (Paxinos and Watson, 1997). Data are shown as mean±SEM.
Inhibiting Csnk1 blocks NAcc amphetamine-induced alterations in pDarpp-32
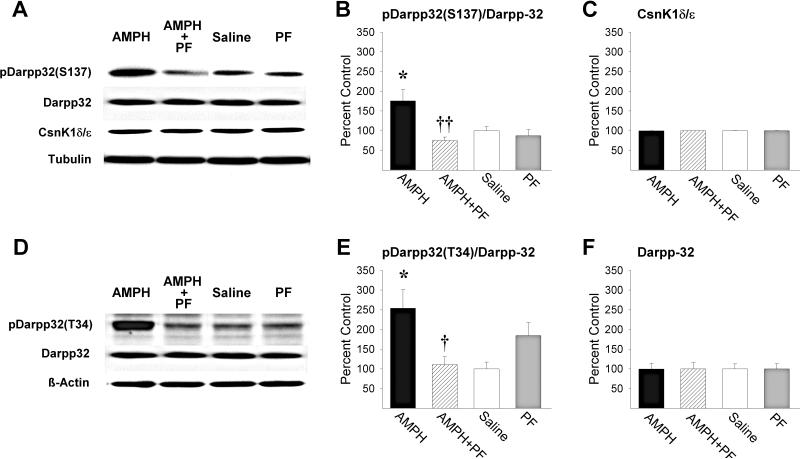
To confirm the Csnk1 inhibiting effects of PF, tissue punches were obtained in the NAcc of separate rats 20-min following the local infusion of amphetamine, amphetamine+PF, saline, or PF alone and subjected to Western blot analyses. As reported previously following systemic injections in mice (Svenningsson et al. 2003), NAcc amphetamine (2.5μg/side) significantly increased pDarpp-32(S137) relative to saline controls (Fig. 2B; P<0.05, by post-hoc Scheffé comparison following ANOVA showing a significant effect of PF, F1,20=10.15, P<0.01, and a significant amphetamine-PF interaction, F1,20=6.12, P<0.05). PF at the behaviorally effective dose (10.0 μg/side) completely blocked this effect when infused with amphetamine into the NAcc (P<0.01). As expected (Svenningsson et al. 2003), NAcc amphetamine also robustly increased PKA phosphorylation at T34 of Darpp-32 (Fig. 2E; P<0.05, by post-hoc Scheffé comparison following ANOVA showing a significant amphetamine-PF interaction, F1,20=13.01, P<0.01). Again, this effect was completely blocked by PF (P<0.05). This dose of PF produced no significant effects on pDarpp-32 relative to saline when administered alone. In addition, neither NAcc amphetamine nor PF altered Csnk1δ/ε or Darpp-32 protein levels relative to saline (Fig. 2C,F).
Figure 2.
PF blocks NAcc AMPH induced phosphorylation of Darpp-32. Representative immunoblots of (A) pDarpp-32(S137), Darpp-32 and Csnk1δ/ε and (D) pDarpp-32(T34) in the NAcc of rats in the four treatment groups. (B) pDarpp-32(S137)/Darpp-32 ratios (*, P<0.05, vs Saline; ††, P<0.01, vs AMPH; Scheffé), (C) Csnk1δ/ε protein levels, (E) pDarpp-32(T34)/Darpp-32 ratios (*, P<0.05, vs Saline; †, P<0.05, vs AMPH; Scheffé), and (F) Darpp-32 protein levels obtained in the NAcc for the four treatment groups (n/group=6). Data are shown as mean±SEM.
Inhibiting Csnk1 blocks NAcc amphetamine-induced inhibition of PP1 and phosphorylation of GluR1(S845)
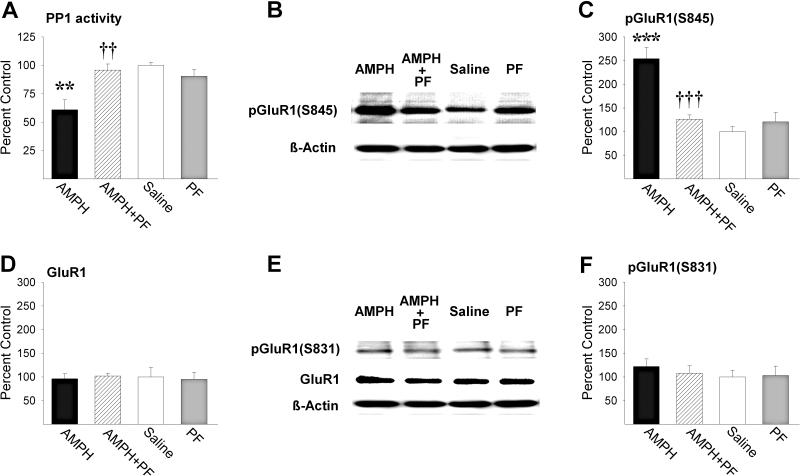
The NAcc amphetamine induced increase in pDarpp-32(T34) led to a significant decrease in PP1 activity relative to saline (Fig. 3A; P<0.01, by post-hoc Scheffé comparison following ANOVA showing significant effects of amphetamine, F1,20=7.73, P<0.05, PF, F1,20=4.42, P<0.05, and a significant amphetamine-PF interaction, F1,20=13.54, P<0.01). PF (10.0 μg/side) blocked this effect when infused with amphetamine into the NAcc (P<0.01). As predicted (Snyder et al. 2000), the observed NAcc amphetamine induced decrease in PP1 activity led to a significant increase in phosphorylation of GluR1 at S845 (Fig. 3C; P<0.001, by post-hoc Scheffé comparison following ANOVA showing significant effects of amphetamine, F1,20=22.26, P<0.001, PF, F1,20=9.96, P<0.01, and a significant amphetamine-PF interaction, F1,20=19.12, P<0.001). Again, PF blocked this effect when infused with amphetamine into the NAcc (P<0.001). PF produced no significant effects on PP1 activity or pGluR1(S845) relative to saline when administered alone. Consistent with previous reports (Snyder et al. 2000; Nelson et al. 2009), phosphorylation of GluR1 at S831 and total NAcc GluR1 protein levels were unaltered by either amphetamine or PF (Fig. 3D-F).
Figure 3.
PF blocks NAcc AMPH induced inhibition of PP1 activity and phosphorylation of GluR1 at S845. (A) PP1 activity (**, P<0.01, vs Saline; ††, P<0.01, vs AMPH; Scheffé), and (C) pGluR1(S845) (***, P<0.001, vs Saline; †††, P<0.001, vs AMPH; Scheffé), (D) GluR1, and (F) pGluR1(S831) protein levels in the NAcc of rats in the four treatment groups (n/group=6). Data are shown as mean±SEM. Representative immunoblots of (B) pGluR1(S845) and (E) pGluR1(S831) and GluR1 in the NAcc of rats in the four treatment groups.
AMPA receptor phosphorylation at S845 is necessary for NAcc amphetamine-induced locomotion
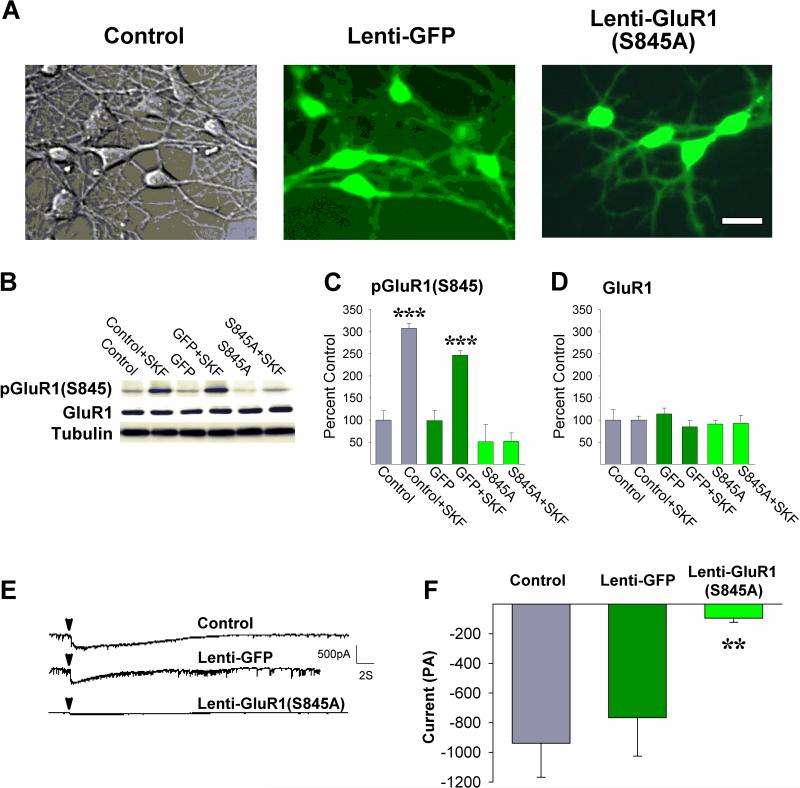
The above findings indicate that inhibiting Csnk1 in the NAcc blocks the ability of amphetamine in this site to increase locomotion possibly by preventing amphetamine-initiated phosphorylation of Darpp-32, inhibition of PP1 and phosphorylation of GluR1(S845). To directly assess this possibility, site-directed mutagenesis was used to generate a mutant form of GluR1 with the S845 residue replaced by an alanine [GluR1(S845A)] to prevent phosphorylation at this site and the construct inserted into an HIV-1-derived lentiviral vector containing GFP. In a first experiment, this vector was used to express the mutant construct [Lenti-GluR1(S845A)] in cultured medium spiny neurons (Fig. 4A). Incubating cultures with the D1 dopamine receptor agonist SKF81297 (10 μM) to activate PKA increased pGluR1(S845) as expected in neurons that were not infected (Control) or neurons infected with a vector containing GFP alone (Lenti-GFP) but not in neurons infected with Lenti-GluR1(S845A) 10-18 days earlier (Fig. 4C; P<0.001, by post-hoc Scheffé comparisons following ANOVA showing a significant effect of infection, F2,24=15.53, P<0.001, incubation, F1,24=26.46, P<0.001, and a significant infection-incubation interaction, F2,24=6.98, P<0.01). Relative to non-infected cells, Lenti-GluR1(S845A) also increased levels of GFP (Fig. 4A) but not total GluR1 protein levels (Fig. 4D). These findings, together with those showing increased GluR1 protein levels in HEK cells 48 hours post-infection (Fig. S1), suggest that post-transcriptional or post-translational homeostatic mechanisms reduced native GluR1 protein to maintain overall GluR1 protein levels constant in the medium spiny neurons 10-18 days post-infection. Electrophysiological recordings were then obtained in these three groups of cells before and after application of 0.5mM glutamate. Before application of glutamate, spontaneous transient currents with amplitudes between 5 and 200 pA, with occasional larger amplitude responses, were variously present in all preparations but no statistically significant differences between groups were detected in number of transient spikes, spike amplitude distributions or frequencies. In response to glutamate, both Control and Lenti-GFP infected medium spiny neurons exhibited larger currents with group mean amplitudes approaching 1000pA. Paralleling the lack of SKF81297-evoked GluR1 phosphorylation, glutamate-evoked currents were significantly smaller in the Lenti-GluR1(S845A) infected cells indicating that phosphorylation of GluR1 at S845 is necessary for medium spiny neurons to respond to glutamate (Fig. 4F; P<0.01, by post-hoc Scheffé comparison following ANOVA showing a significant effect of infection, F2,20=6.57, P<0.01).
Figure 4.
Viral mediated expression of GluR1(S845A) in cultured medium spiny neurons blocks SKF81297-evoked phosphorylation of GluR1 and glutamate-evoked currents. (A) Photomicrographs of neurons that were (left) not infected, (middle) infected with Lenti-GFP, or (right) infected with Lenti-GluR1(S845A). Scale bar = 20μm. (B) Representative immunoblots of pGluR1(S845) and GluR1, (C) pGluR1(S845) (***, P<0.001, vs remaining groups; Scheffé), and (D) GluR1 protein levels in the three groups of neurons either incubated or not incubated with SKF81297. n/group=4-7. (E) Representative current traces obtained in the three groups of neurons before and after focal application of glutamate (arrowheads). (F) Mean peak currents obtained following glutamate in the three groups of neurons (**, P<0.01, vs Control non-infected cells; Scheffé). n/group=5-12. Data are shown as mean±SEM.
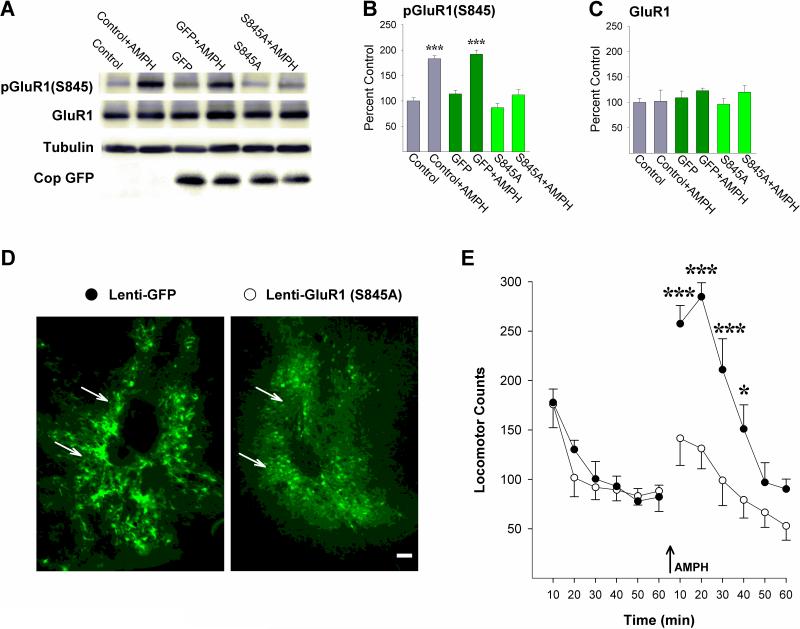
To directly test whether phosphorylation of GluR1 at S845 is necessary for NAcc amphetamine-induced locomotion, rats were administered bilateral microinjections into the NAcc of Lenti-GluR1(S845A) or Lenti-GFP. Immunohistochemical examination of brain sections three weeks later showed strong GFP expression limited to cells in close proximity to the NAcc injection site in both conditions (Fig. 5D). Administration of amphetamine (2.5μg/side) into the NAcc at this time increased pGluR1(S845) as expected in non-infected (Control) and Lenti-GFP-infected rats but not in rats infected with Lenti-GluR1(S845A) (Fig. 5B; P<0.001, by post-hoc Scheffé comparisons following ANOVA showing a significant effect of infection, F2,28=18.13, P<0.001, challenge, F1,28=62.49, P<0.001, and a significant infection-challenge interaction, F2,28=5.88, P<0.01). As with the NAcc cultured neurons, relative to non-infected rats, Lenti-GluR1(S845A) also increased levels of GFP (Fig. 5A) but not total GluR1 protein levels (Fig 5C), again suggesting homeostatic regulation of overall GluR1 protein levels to accommodate the Lenti-mediated increase in exogenous GluR1 3 weeks post-infection. Paralleling these findings, Lenti-GFP infected rats administered NAcc amphetamine (2.5μg/side) showed the expected close to 2-fold increase in locomotion but this effect was absent in Lenti-GluR1(S845A) infected rats. These rats showed significantly lower locomotor counts compared to rats infected with Lenti-GFP (Fig. 5E; Ps<0.05-0.001, by post-hoc Scheffé comparisons following ANOVA showing a significant effect of infection, F1,9=13.47, P<0.01, time, F5,45=33.49, P<0.001, and a significant infection-time interaction, F5,45=5.79, P<0.001). No significant differences were observed between the two groups before the NAcc amphetamine infusion.
Figure 5.
Viral mediated expression of GluR1(S845A) in the NAcc blocks AMPH-induced phosphorylation of GluR1 and AMPH-induced locomotion. (A) Representative immunoblots of GluR1(S845), GluR1, and Cop GFP, (B) pGluR1(S845) (***, P<0.001, vs remaining groups; Scheffé), and (C) GluR1 protein levels in rats not infected (Control), infected with Lenti-GFP, or infected with Lenti-GluR1(S845A) either challenged or not challenged with NAcc AMPH. n/group=5-6. (D) Photomicrographs of the NAcc obtained 3 weeks following infection with Lenti-GFP or Lenti-GluR1(S845A) illustrating GFP positive neurons in close proximity to the injection cannula tips (arrows). Scale bar = 50μm. (E) Locomotor activity counts obtained before and after (arrow) NAcc AMPH in the two groups (*, P<0.05, ***, P<0.001, vs Lenti-GFP; Scheffé). n/group=5-6. Data are shown as mean±SEM.
Discussion
In the present study, we show that inhibiting Csnk1 in the NAcc blocks amphetamine-induced locomotion as well as amphetamine's ability in this site to increase phosphorylation of Darpp-32 at S137 and T34, decrease PP1 activity, and increase phosphorylation of GluR1 at S845. Preventing GluR1 phosphorylation with the alanine mutant GluR1(S845A) reduces glutamate-evoked currents in cultured medium spiny neurons and mimics the effect of Csnk1 inhibition in the NAcc to block amphetamine-induced locomotion. These results indicate that Csnk1 is essential to amphetamine's ability to increase locomotion and that it enables this stimulant effect by acting on the Darpp-32-PP1 signaling pathway to regulate AMPA receptor phosphorylation in the NAcc.
Increased Csnk1δ/ε gene expression in mouse forebrain has been associated with increased basal locomotor activity and increased sensitivity to the locomotor activating effects of psychostimulant drugs (Zhou et al. 2010; Palmer et al. 2005). Similarly in humans, subjects with more copies of the rs135745C allele of the CSNK1ε gene are more sensitive to the subjective effects of amphetamine (Veenstra-VanderWeele et al. 2006). An association between a different SNP in CSNK1ε (rs1534891) and heroin addiction has also been reported (Levtan et al. 2008). Our findings provide a mechanism by which Csnk1 can promote these effects. Dopamine and glutamate interact in the NAcc to mediate the locomotor and incentive motivational effects of psychostimulant drugs (Wise and Bozarth, 1987; Meredith et al. 1993; Sesack et al. 2003; Pierce and Kalivas, 1997) and Darpp-32 integrates the intracellular signaling initiated by these neurotransmitters (Greengard, 2001; Fernandez et al. 2006). Darpp-32 knockout (Fienberg et al. 1998; Zachariou et al. 2002) and alanine Darpp-32(T34A) mutant (Zachariou et al. 2006) mice show reduced sensitivity to the locomotor activating and rewarding effects of cocaine. By phosphorylating Darpp-32 at S137, Csnk1 can regulate these pathways and the behaviors they mediate. The activation of Csnk1 and the subsequent increase in pDarpp-32(S137) by amphetamine in the NAcc is in fact critical as inhibition of Csnk1 in this site blocks amphetamine's ability to increase pDarpp-32(S137), maintain increased pDarpp-32(T34), and increase locomotion (Fig. 1-2). As shown for metabotropic glutamate receptors (Liu et al. 2001; 2002), amphetamine may activate Csnk1δ/ε via calcium-dependent stimulation of calcineurin and dephosphorylation of CsnK1 from its inhibited autophosphorylated state, but unlike metabotropic glutamate receptors, recruit calcium through a pathway initiated by activation of dopamine receptors leading to phosphorylation of L-type calcium channels by PKA (Surmeier et al. 1995). Our findings show that once initiated, the phosphorylation of Darpp-32 by Csnk1 supersedes up- and downstream actions of PKA in the NAcc. pDarpp-32(S137) is necessary to maintain amphetamine-induced phosphorylation of Darpp-32(T34) and GluR1(S845), both PKA residues (Esteban et al. 2003; Roche et al. 1996; Banke et al. 2000).
The phosphorylation of GluR1 at S845 promotes trafficking and synaptic insertion of GluR1-containing AMPA receptors (Ehlers, 2000; Esteban, 2003; Mangiavacchi and Wolf, 2004; Sun et al. 2005; Oh et al. 2006) and increases peak current and channel open probability (Roche et al. 1996; Banke et al. 2000) thereby potentiating neuronal excitability. We show that phosphorylation of this residue is indeed necessary for NAcc medium spiny neurons to respond to glutamate and for NAcc amphetamine to produce increased locomotion (Fig. 4-5). Thus, by regulating Darpp-32 and PP1 activity, CsnK1 regulates the phosphorylation state of GluR1(S845) and amphetamine-induced locomotor activity. These findings are consistent with a number of reports showing that AMPA receptor blockade in the NAcc blocks the acute locomotor stimulant effects of amphetamine and cocaine (Wolf, 1998) and show that conditions that increase medium spiny neuron activity also increase NAcc amphetamine-induced locomotion. In apparent contrast to these results, expression in the NAcc core of pore-dead GluR1, a Q582E GluR1 mutant designed to reduce synaptic AMPA currents, has been reported to decrease the locomotion induced by NAcc AMPA but increase the locomotion induced by cocaine (Bachtell et al. 2008). The finding of reduced NAcc AMPA induced locomotion is consistent with the expected reduction in AMPA currents, although the subcellular distribution of these mutant subunits was not examined in this report and they are not targeted to synapses under all conditions (Shi et al. 2001). In addition, cocaine was administered systemically in these experiments (Bachtell et al. 2008), raising the possibility that AMPA receptors in other brain regions contributed to the observed increase in cocaine-induced locomotion. Our findings show that glutamate-evoked currents are blocked in Lenti-GluR1(S845A) infected medium spiny neurons and that amphetamine is unable to increase locomotion when infused specifically into the site of expression of GluR1(S845A) in the NAcc. In the dorsal striatum, it has also been reported that Csnk1 does not regulate AMPA receptor activity but inhibits other ionotropic receptor currents by directly activating PP1 in this site (Chergui et al. 2005). No evidence was obtained for activation of NAcc PP1 by Csnk1 in the present study. Rather, inhibition of Csnk1 produced no effects on PP1 activity by itself and prevented the decrease in PP1 activity and subsequent increase in pGluR1(S845) produced by amphetamine in the NAcc (Fig. 3). These results indicate either that Csnk1 does not directly activate PP1 in the NAcc or that this action is overwhelmed by Csnk1 regulated Darpp-32 inhibition. These findings may reflect differences in Csnk1-associated signaling in dorsal and ventral striatum. For example, D1 dopamine receptor and Darpp-32 dependent activation of ERK1/2 by amphetamine is considerably more pronounced in the NAcc than the dorsal striatum (Gerfen et al. 2008). Our findings showing that Csnk1 regulates the Darpp-32-PP1 signaling pathway to promote AMPA receptor phosphorylation in the NAcc are consistent with a similar striatal organization.
The behaviors produced by psychostimulant drugs are complex. As such, their expression can be modulated by a number of signaling pathways. Csnk1 interacts with some of these pathways in addition to those involving Darpp-32 and PP1 and these interactions may also potentially influence psychostimulant-induced behaviors. For example, Csnk1δ/ε phosphorylates PERIOD proteins to influence the circadian rhythm (Knippschild et al. 2005; Badura et al. 2007; Walton et al. 2009) which is known to influence responding to psychostimulant drugs (Falcón and McClung, 2009). Csnk1 also regulates amphetamine-induced changes in GSK-3β phosphorylation (Svenningsson et al. 2003) which contribute to the locomotion produced by this drug (Beaulieu et al. 2004). Consistent with a modulating role, effects in these pathways are observed either in other sites but not the NAcc, as in methamphetamine-induced increases in PERIOD mRNA (Nikaido et al. 2001), or at time points following initiation of locomotor responding to the drug, as with PERIOD mRNA levels (Nikaido et al. 2001), amphetamine-induced decreases in pGSK-3β, and the decreased locomotor response to amphetamine exhibited by GSK-3β+/- mice (Beaulieu et al. 2004). In the present study, we show that inhibition of Csnk1 blocks the initiation of NAcc amphetamine-induced locomotion as well as those changes in Darpp-32-PP1-GluR1(S845) signaling that are necessary for its expression. These findings point to a pivotal role for NAcc Csnk1 in the generation of psychostimulant-induced behaviors. Considering that medium spiny neurons in the NAcc also integrate the contribution of dopamine and glutamate to the incentive motivational effects of a number of abused drugs (Wise and Bozarth, 1987; Meredith et al. 1993; Sesack et al. 2003; Pierce and Kalivas, 1997), Csnk1 is likely also a critical target for intervention in the treatment of drug craving and addiction.
Supplementary Material
Acknowledgements
This study was supported by grants R01-DA-09397 (PV) and F31-DA-022834 (JAL) from the NIH. The authors declare no conflicts of interest. The authors are also grateful to Dr. Sangram S. Sisodia for facilitating the PP1 assays.
Abbreviations
- AMPH
amphetamine
- Csnk1
casein kinase 1
- Darpp-32
cAMP-regulated phosphoprotein 32
- NAcc
nucleus accumbens
- PP1
protein phosphatase 1
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha J-HJ, Pierce RC. CaMKII: A biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nature Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Bachtell RK, Choi K-H, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in the nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur. J. Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- Badura L, Swanson T, Adamowicz W, et al. An inhibitor of casein kinase I epsilon induces phase delays in circadian rhythms under free-running and entrained conditions. J. Pharmacol. Exp. Ther. 2007;322:730–738. doi: 10.1124/jpet.107.122846. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee H-K, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J-M, Sotnikova TD, Yao W-D, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc. Natl. Acad. Sci. USA. 2004;101:5099–5104. doi: 10.1073/pnas.0307921101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Graham ME, Distler MG, Munoz MB, Li D, Vezina P, Sokoloff G, Palmer AA. A role for casein kinase 1 epsilon in the locomotor stimulant response to methamphetamine. Psychopharmacology. 2009;203:703–711. doi: 10.1007/s00213-008-1417-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Svenningsson P, Greengard P. Physiological role for casein kinase 1 in glutamatergic synaptic transmission. J. Neurosci. 2005;25:6601–6609. doi: 10.1523/JNEUROSCI.1082-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdouits F, Cohen D, Nairn AC, Greengard P, Girault J-A. Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase I in vitro and in vivo. J. Biol. Chem. 1995a;270:8772–8778. doi: 10.1074/jbc.270.15.8772. [DOI] [PubMed] [Google Scholar]
- Desdouits F, Siciliano JC, Greengard P, Girault J-A. Dopamine- and cAMP-regulated phosphoprotein DARPP-32: Phosphorylation of Ser-137 by casein kinase I inhibits dephosphorylation of Thr-34 by calcineurin. Proc. Natl. Acad. Sci. USA. 1995b;92:2682–2685. doi: 10.1073/pnas.92.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers MD. Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron. 2000;28:511–525. doi: 10.1016/s0896-6273(00)00129-x. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi S-H, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nature Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Falcón E, McClung CA. A role for circadian genes in drug addiction. Neuropharmacology. 2009;56:91–96. doi: 10.1016/j.neuropharm.2008.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg AA, Hiroi N, Mermelstein PG, et al. Darpp-32: Regulator of the efficacy of dopaminergic neurotransmission. Science. 1998;281:838–842. doi: 10.1126/science.281.5378.838. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Schiappa R, Girault J-A, Le Novere N. DARPP-32 is a robust integrator of dopamine and glutamate signals. PloS Comp. Biol. 2006;2:1619–1633. doi: 10.1371/journal.pcbi.0020176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Worley P. Differences between dorsal and ventral striatum in Drd1a dopamine receptor coupling of dopamine- and cAMP-regulated phosphoprotein-32 to activation of extracellular signal-regulated kinase. J. Neurosci. 2008;28:7113–7120. doi: 10.1523/JNEUROSCI.3952-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greengard P. The neurobiology of slow synaptic transmission. Science. 2001;294:1024–1030. doi: 10.1126/science.294.5544.1024. [DOI] [PubMed] [Google Scholar]
- Hemmings HC, Jr., Greengard P, Tung HYL, Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984;310:503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Kamens HM, Burkhart-Kasch S, McKinnon CS, Li N, Reed C, Phillips TJ. Sensitivity to psychostimulants in mice bred for high and low stimulation to methamphetamine. Genes Brain Behav. 2005;4:110–125. doi: 10.1111/j.1601-183X.2004.00101.x. [DOI] [PubMed] [Google Scholar]
- Knippschild U, Gocht A, Wolff S, Huber N, Löhler J, Stöter M. The casein kinase 1 family: Participation in multiple cellular processes in eukaryotes. Cell Signal. 2005;17:675–689. doi: 10.1016/j.cellsig.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Levran O, Londono D, O'Hara K, et al. Genetic susceptibility to heroin addiction: A candidate gene association study. Genes, Brain and Behav. 2008;7:720–729. doi: 10.1111/j.1601-183X.2008.00410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ma X-H, Ule J, Bibb JA, Nishi A, DeMaggio AJ, Yan Z, Nairn AC, Greengard P. Regulation of cyclin-dependent kinase 5 and casein kinase 1 by metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 2001;98:11062–11068. doi: 10.1073/pnas.191353898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Virshup DM, Nairn AC, Greengard P. Mechanism of regulation of casein kinase 1 activity by group 1 metabotropic glutamate receptors. J. Biol. Chem. 2002;277:45393–45399. doi: 10.1074/jbc.M204499200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löhler J, Hirner H, Schmidt B, Kramer K, Fischer D, Thal DR, Leithäuser F, Knippschild U. Immunohistochemical characterization of cell-type specific expression of CK1δ in various tissues of young adult BALB/c mice. PloS ONE. 2009;4:1–12. doi: 10.1371/journal.pone.0004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Singer BF, Baker LK, et al. Transient overexpression of α-Ca2+/calmodulin-dependent protein kinase II in the nucleus accumbens shell enhances behavioral responding to amphetamine. J. Neurosci. 2010;30:939–949. doi: 10.1523/JNEUROSCI.4383-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangiavacchi S, Wolf ME. D1 dopamine receptor stimulation increases the rate of AMPA receptor insertion onto the surface of cultured nucleus accumbens neurons through a pathway dependent on protein kinase A. J. Neurochem. 2004;88:1261–1271. doi: 10.1046/j.1471-4159.2003.02248.x. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Pennartz CM, Groenewegen HJ. The cellular framework for chemical signaling in the nucleus accumbens. Prog. Brain Res. 1993;99:3–24. doi: 10.1016/s0079-6123(08)61335-7. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Milovanovic M, Wetter JB, Ford KA, Wolf ME. Behavioral sensitization to amphetamine is not accompanied by changes in glutamate receptor surface expression in the rat nucleus accumbens. J. Neurochem. 2009;109:35–51. doi: 10.1111/j.1471-4159.2009.05911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikaido T, Akiyama M, Moriya T, Shibata S. Sensitized increase of Period gene expression in the mouse caudate/putamen caused by repeated injection of methamphetamine. Mol. Pharmacol. 2001;59:894–900. doi: 10.1124/mol.59.4.894. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkack VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Verbitsky M, Suresh R, et al. Gene expression differences in mice divergently selected for methamphetamine sensitivity. Mamm. Genome. 2005;16:291–305. doi: 10.1007/s00335-004-2451-8. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed. 3. Academic Press; New York: 1997. [DOI] [PubMed] [Google Scholar]
- Pellegrino LL, Pellegrino AS, Cushman AJ. A stereotaxic atlas of the rat brain. Plenum Press; New York: 1979. [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res. Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Roche KW, O'Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamatedopamine interactions: Evidence for specificity of connections and extrasynaptic actions. Ann. NY Acad. Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Allen PB, Fienberg AA, Valle CG, Huganir RL, Nairn AC, Greengard P. Regulation of phosphorylation of the GluR1 AMPA receptor in the neostriatum by dopamine and psychostimulants in vivo. J. Neurosci. 2000;20:4480–4488. doi: 10.1523/JNEUROSCI.20-12-04480.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J. Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Bargas J, Hemmings HC, Jr., Nairn AC, Greengard P. Modulation of calcium currents by a D1 dopaminergic protein kinase/phosphatase cascade in rat neostriatal neurons. Neuron. 1995;14:385–397. doi: 10.1016/0896-6273(95)90294-5. [DOI] [PubMed] [Google Scholar]
- Svenningsson P, Tzavara ET, Carruthers R, et al. Diverse psychotomimetics act through a common signaling pathway. Science. 2003;302:1412–1415. doi: 10.1126/science.1089681. [DOI] [PubMed] [Google Scholar]
- Utz AC, Hirner H, Blatz A, et al. Analysis of cell type-specific expression of CK1ε in various tissues of young adult BALB/c mice and in mammary tumors of SV40 T-Agtransgenic mice. J. Histochem. Cytochem. 2010;58:1–15. doi: 10.1369/jhc.2009.954628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenstra-VanderWeele J, Qaadir A, Palmer AA, Cook EH, Jr., de Wit H. Association between the casein kinase 1 epsilon gene region and subjective response to d-amphetamine. Neuropsychopharmacology. 2006;31:1056–1063. doi: 10.1038/sj.npp.1300936. [DOI] [PubMed] [Google Scholar]
- Walton KM, Fisher K, Rubitski D, et al. Selective inhibition of casein kinase 1ε minimally alters circadian clock period. J. Pharmacol. Exp. Ther. 2009;330:430–439. doi: 10.1124/jpet.109.151415. [DOI] [PubMed] [Google Scholar]
- Wise RA, Bozarth MA. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog. Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Benoit-Marand M, Allen PB, Ingrassia P, Fienberg AA, Ganon F, Greengard P, Picciotto MR. Reduction of cocaine place preference in mice lacking the protein phosphatase 1 inhibitors Darpp-32 or Inhibitor 1. Biol. Psychiatry. 2002;51:612–620. doi: 10.1016/s0006-3223(01)01318-x. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Sgambato-Faure V, Sasaki T, Svenningsson P, Berton O, Fienberg AA, Nairn AC, Greengard P, Nestler EJ. Phosphorylation of Darpp-32 at Threonine-34 is required for cocaine action. Neuropsychopharmacology. 2006;31:555–562. doi: 10.1038/sj.npp.1300832. [DOI] [PubMed] [Google Scholar]
- Zhou M, Rebholz H, Brocia C, Warner-Schmidt JL, Fienberg AA, Nairn AC, Greengard P, Flajolet M. Forebrain overexpression of CK1δ leads to down-regulation of dopamine receptors and altered locomotor activity reminiscent of ADHD. Proc. Natl. Acad. Sci. USA. 2010;107:4401–4406. doi: 10.1073/pnas.0915173107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.