Abstract
It is well established that combination of heavy drinking and smoking has severe health consequences. However, at relatively low concentrations, both alcohol and nicotine may have beneficial effects including neuroprotection. Thus, protective effects of low alcohol concentration against beta-amyloid-induced toxicity in organotypic hippocampal slices and protective effects of nicotine against salsolinol-induced toxicity in human-derived neuroblastoma cells (SH-SY5Y) have been reported. In this study, we sought to determine whether alcohol might also be protective against salsolinol-induced toxicity in SH-SY5Y cells and whether the combination of low doses of alcohol and nicotine might have an additive or synergistic effect. Pre-exposure of SH-SY5Y cells to either ethanol (1 or 10 mM) or nicotine (20 or 50 μM) significantly attenuated salsolinol-induced toxicity. However, contrary to the expectation the combination of low doses of alcohol and nicotine not only did not provide any synergistic or additive protective effect, but exacerbated salsolinol-induced toxicity. Indeed, simple combination of low alcohol and nicotine resulted in significant toxicity in SH-SY5Y cells. This toxicity, reflected in a reduction in cell viability was associated with an increase in apoptosis as determined by caspase-3 measurement. These in vitro results suggest that combination of even low concentrations of alcohol and nicotine may activate apoptotic mechanisms that can lead to cell toxicity and detrimental consequences.
Keywords: Alcohol, Nicotine, Apoptosis, SH-SY5Y cell line, Caspase-3, Neurotoxicity, Neuroprotection
Introduction
Although detrimental effects of heavy drinking or smoking alone are well established, the combination of the two can lead to dramatic health consequences. For example, whereas either heavy drinking or smoking may increase the risk of cancers of the head, neck and esophagus or ulcer of the duodenum by 5–6-fold, their combination could raise these risks to as high as 25–30-fold (Rossini et al. 2008). However, a great majority of the population may be consuming alcohol and/or nicotine (via smoking) in a moderate or low amount. Recent evidence indicates that at a low or moderate level, consumption of alcohol may have a variety of health benefits including cardiovascular and/or neuroprotection (Collins et al. 2009). Similarly, nicotine or nicotinic agonists may prove of therapeutic potential in a number of neuropsychiatric, cognitive, or neurodegenerative disorders including Parkinson’s disease (Quik et al. 2008). Thus, neuroprotective effects of nicotine in several in vitro and in vivo experimental models have been observed (Guan et al. 2003; Hejmadi et al. 2003; Picciotto and Zoli 2008; Quik et al. 2008). Specifically, it has been shown that nicotine can protect against beta-amyloid induced damage in primary cell cultures (Liu and Zhao 2004) as well as against salsolinol-induced toxicity in SH-SY5Y cells (Copeland et al. 2005, 2007).
Salsolinol (1-methyl-6,7-dihydroxy-1,2,3,4-tetrahydro-isoquinoline), an endogenous dopamine metabolite has structural similarity to MPTP (1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine) which is especially toxic to nigral dopaminergic neurons, a cluster of cells implicated in Parkinson’s disease (Storch et al. 2002; Maruyama et al. 2004; Naoi et al. 2004). MPTP intake by humans or its administration to non-human primates can result in Parkinson-like syndrome (Storch et al. 2002; Maruyama et al. 2004; Quik et al. 2008). Since many Parkinson patients show high levels of salsolinol in their cerebrospinal fluid, it has been suggested that salsolinol might be involved in the etiology or loss of dopamine neurons in at least some of these patients (Storch et al. 2002; Maruyama et al. 2004). SH-SY5Y cells, derived from human neuroblastoma cells express high level of dopaminergic activity and are used extensively as a model to study nigral dopaminergic neurons (Storch et al. 2002; Maruyama et al. 2004; Naoi et al. 2004). It was reported earlier that nicotine at concentrations of up to 100 μM has protective effects against salsolinol-induced toxicity in SH-SY5Y cells (Copeland et al. 2005, 2007). Moreover, a combination of 50 μM nicotine and 5 μM donepezil, a cholinesterase inhibitor used clinically in Alzheimer’s disease, showed additive protection against salsolinol-induced toxicity in SH-SY5Y cells suggesting therapeutic potential of a combination of nicotine and donepezil in Parkinson’s disease (Das and Tizabi 2009).
In this study, we sought to determine whether alcohol might also be protective against salsolinol-induced toxicity in SH-SY5Y cells and whether the combination of low doses of alcohol and nicotine might have an additive or synergistic effect. We hypothesized that alcohol at low concentrations will also show protective effects against salsolinol-induced toxicity and that the combination of low alcohol and nicotine will have additive or synergistic effects. Moreover, since it has been reported that apoptosis is a major contributory pathway to salsolinol-induced toxicity in SH-SY5Y cells and that nicotine exerts an anti-apoptotic effect in these cell lines (Copeland et al. 2007), we postulated that any protective effect of alcohol, nicotine, or their combination would be mediated by inhibition of apoptosis and would be reflected in reductions of caspase-3 levels, a well-recognized marker of apoptosis. The data while confirming that both alcohol and nicotine alone at low concentrations have an anti-apoptotic and protective effect indicate that when these two agents are combined in such low concentrations apoptosis and cell toxicity instead of protection is manifested.
Materials and Methods
Drugs
Ethanol was obtained from EMD Chemicals Inc. (Gibbstown, NJ). Nicotine, salsolinol, and other analytical reagents were purchased from Sigma Chemical Company (Sigma-Aldrich, St. Louis, MO). The SH-SY5Y human neuroblastoma cell line was supplied by American Type Culture Collection (ATCC, Manassas, VA). The nitrocellulose membrane was purchased from BioRad (Hercules, CA). Caspase Kit and β-actin antibody were obtained from Cell Signaling (Danvers, MA). MTT reagent was purchased from Fisher Scientific (Pittsburgh, PA).
Cell Culture
SH-SY5Y cells in passage 37 were cultivated in a 1:1 mixture of Dulbecco’s Modified Eagle Medium and HAM’s F12 (Cellgro, Mediatech Inc. Manassas, VA) supplemented with 10% fetal bovine serum, penicillin/streptomycin (100 IU/ml), and gentamicin (50 ug/ml) at 37° C in a humidified incubator with 5% CO2 atmosphere.
Drug Treatment
The cells were harvested when confluent and plated in 96 well plates (1.2 × 104/well). Cells were allowed to adhere to bottom surface for 24 h. Then, fresh media containing various concentrations of drugs (ethanol, nicotine, or salsolinol) were added to the carefully aspirated wells. When testing the effects of ethanol or nicotine pretreatment on salsolinol-induced toxicity, these drugs were added 1 h prior to salsolinol. In combination studies, ethanol was added first followed immediately by nicotine. In all cases, the control group consisted of cells that were maintained in media alone and without any drug treatment. All treatments were carried out for 24 h and the effects on cell viability were determined following the 24 h incubation. Each assay was run in duplicate. For all cell viability studies 5 distinct experiments and for Western Blot experiments 3 distinct experiments were performed.
MTT Assay for Cell Viability
Cell viability was determined by 3, [4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay as detailed previously (Copeland et al. 2005, 2007). Briefly, following the 24 h incubation the medium was aspirated and 30 μl of MTT tetrazole (0.5 mg/ml) in PBS containing 10 mM HEPES was added to each well. The plates were incubated for three additional hours at 37°C followed by aspiration. The plates were then allowed to dry in the incubator for 1 h. The incorporated dye was solubilized in 100 μl of 0.04 N HCl in isopropanol. In order to determine cell number in each sample, the absorbance was measured spectrophotometrically in a plate reader at 570 nm with a background of 630 nm. Cell viability was determined by subtracting the test results from the background and is presented as a percentage of the control. The absorbance values for the controls in each experiment, reflecting the number of viable cells are provided in the “Results” section.
Caspase-3 Western Blot
To quantify caspase-3 levels, following the 24 h incubation cells were removed and were incubated in cell lysis buffer (10 mM Tris-buffer, 5 mM EDTA, 150 mM NaCl, 0.5% Triton X-100 (v/v) with protease inhibitors (Sigma-Aldrich, St. Louis, MO). Protein concentration was determined using Thermo Scientific protein assay reagents. The protein was loaded at 30 μg per well, as verified by β-actin and separated on a 12% SDS-polyacrylamide gel and then transferred to PVDF membrane (Immobilon-P: Millipore Corporation, Bedford, MA). After a 1/2 h block in Blocking Reagent, 5% non-fat milk in TBST buffer (TBS buffer with 2% Tween-20) the membranes were incubated with primary antibody (1:800) in TBST buffer overnight at 4°C. The following day, the membranes were rinsed five times in fresh TBST and incubated for 1 h at room temperature in secondary antibody (1:1,000). The membranes were then rinsed five times in fresh TBST and relative intensity of the bands was visualized and recorded using chemiluminescence.
Statistical Analysis
Data are expressed as mean ± SEM. Statistical differences within and between treatment groups were determined by one-way ANOVA followed by Tukey post-hoc where P < 0.05 was considered statistically significant. Data were analyzed using Graphpad Prism 3 (Graphpad Software Inc, San Diego, CA).
Results
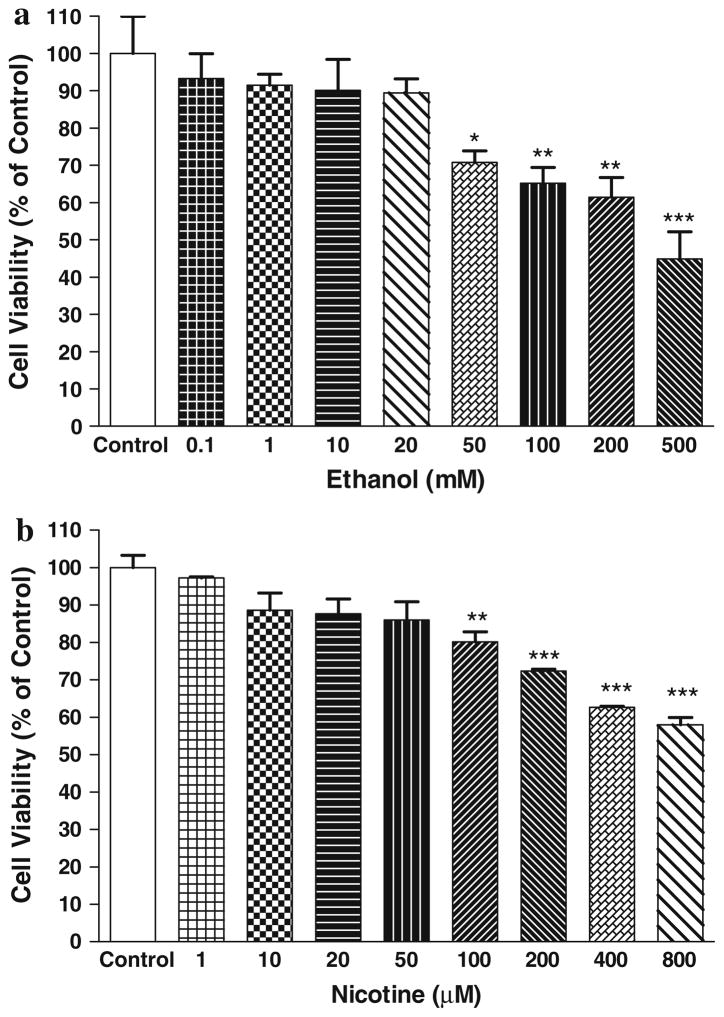
Figure 1a depicts the effects of various concentrations of ethanol on SH-SY5Y cell viability. There were no significant effects of ethanol on cell viability up to 20 mM concentration compared to control (MTT absorbance = 0.294 ± 0.010, mean ± SEM). At higher concentrations, there was a concentration-dependent toxicity where more than half of the cells were not viable at 500 mM ethanol. Based on these results, we chose 10 and 20 mM ethanol in combination studies with salsolinol.
Fig. 1.
a Dose–response effect of ethanol on SH-SY5Y cells after 24 h treatment. b Dose–response effect of nicotine on SH-SY5Y cells after 24 h treatment. Results represent mean ± SEM of five independent experiments *P <0.05, **P <0.01, ***P <0.001 compared to control
Figure 1b depicts the effects of various concentrations of nicotine on SH-SY5Y cell viability. There were no significant effects of nicotine on cell viability up to 50 μM concentration compared to control (MTT absorbance = 0.207 ± 0.014, mean ± SEM). At higher concentrations, there was a concentration-dependent toxicity where more than 40% of the cells were not viable at 800 μM nicotine. Based on these results, we chose 20 and 50 μM nicotine in combination studies with salsolinol. It is of relevance to note that in a previous study using SH-SY5Y cells nicotine at concentrations of 100 μM resulted in insignificant (approximately 11%) toxicity (Das and Tizabi 2009). The susceptibility of the SH-SY5Y cells to 100 μM nicotine in this study (approximately 19% toxicity) is most likely due to the use of late passage SH-SY5Y cells (passage 37) here versus the previous study where an early passage (less than 15) of these cells was utilized. Moreover, the refinement of technique and less variability in results might have also contributed to the detection of the effects of nicotine at 100 μM in this study.
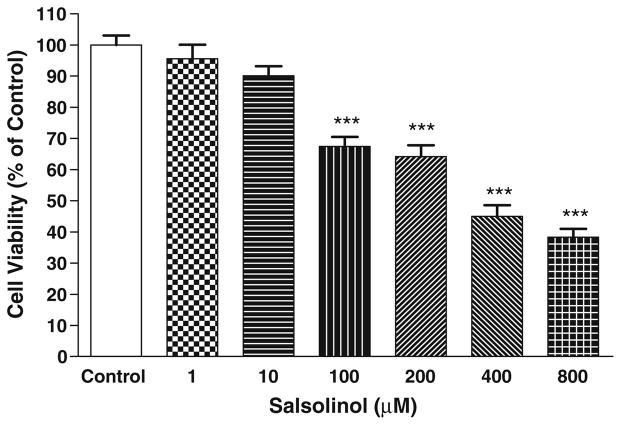
Figure 2 depicts the effects of various concentrations of salsolinol (SALS) on SH-SY5Y cell viability compared to control (MTT absorbance = 0.298 ± 0.017, mean ± SEM). There was a concentration-dependent toxicity where approximately 50% of the cells lost viability at 400 μM. Based on these results, we chose 400 μM SALS (EC50) to determine the effects of ethanol, nicotine, or their combination.
Fig. 2.
Dose–response effect of salsolinol on SH-SY5Y cells after 24 h treatment. Results represent mean ± SEM of five independent experiments ***P <0.001 compared to control
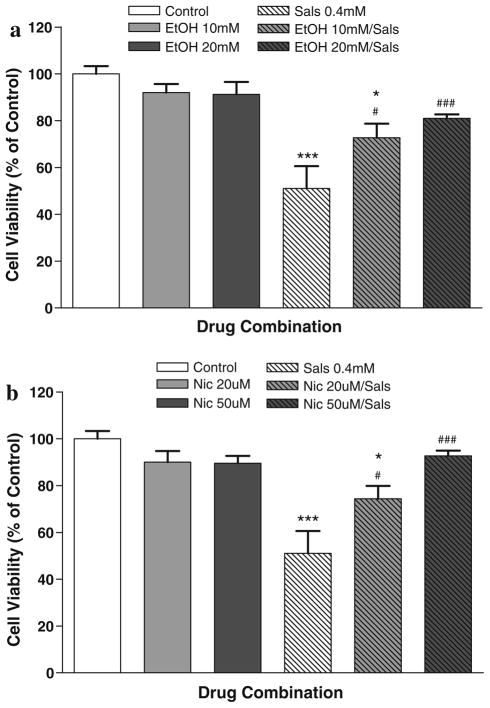
Figure 3a depicts the protective effects of two low concentrations of ethanol on SALS-induced toxicity in SH-SY5Y cells. At 10 mM concentration, ethanol reduced SALS-induced toxicity by 45% and at 20 mM ethanol there was a 60% reduction of SALS toxicity. Thus, there appears to be a dose-dependent protection by ethanol where the higher dose of 20 mM almost completely blocked salsolinol-induced toxicity as no statistically significant difference between this treatment and control was observed. Control MTT absorbance = 0.230 ± 0.021, mean ± SEM.
Fig. 3.
a Effects of 1 h pretreatment with two different concentrations of ethanol (10 and 20 mM) on salsolinol-induced toxicity after 24 h incubation. b Effects of 1 h pretreatment with 2 different concentrations of nicotine (20 and 50 μM) on salsolinol-induced toxicity after 24 h incubation. Results represent mean ± SEM of five independent experiments. *P <0.05, ***P <0.001 compared to control and #P <0.05, ###P <0.001 compared to salsolinol (SALS)
Figure 3b depicts the protective effects of two low concentrations of nicotine on SALS-induced toxicity in SH-SY5Y cells. At 20 μM concentration, nicotine reduced SALS-induced toxicity by 47% and at 50 μM nicotine there was an 86% reduction of SALS toxicity. Here also, there was an indication of dose-dependent protection by nicotine where the higher dose of 50 μM almost completely blocked salsolinol-induced toxicity as no statistically significant difference between this treatment and control was observed. Control MTT absorbance = 0.211 ± 0.044 mean ± SEM.
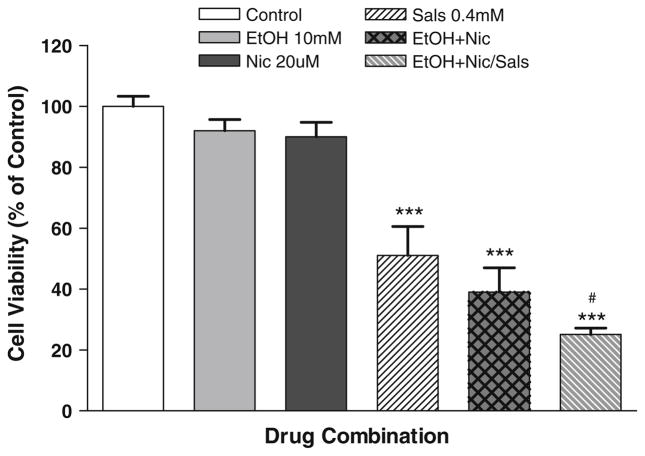
Figure 4 depicts the effects of a combination of a low ethanol (10 mM) and nicotine (20 μM) on SALS-induced toxicity. Contrary to the expectation, this combination not only did not provide any extra protection compared to individual treatment, but actually exacerbated SALS toxicity by 26%. Interestingly, the toxicity induced by the combination of low ethanol and nicotine was comparable to that induced by 400 μM SALS. However, no statistically significant differences between the ethanol and nicotine group alone and that in combination with salsolinol was observed. Control MTT absorbance = 0.211 ± 0.044, mean ± SEM.
Fig. 4.
Effects of combination of low concentration of ethanol (10 mM) and low concentration of nicotine (20 μM) alone or on salsolinol-induced toxicity. For salsolinol studies, ethanol and nicotine were added 1 h before SALS. In all cases, cell viability was assessed after 24 h incubation. Values are the mean ± SEM of five independent experiments. ***P <0.001 compared to control and #P <0.05 compared to salsolinol (SALS)
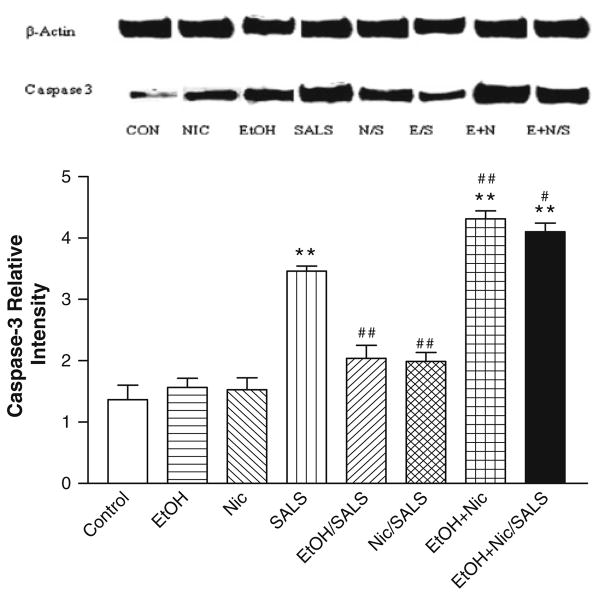
Figure 5 depicts the basal level of caspase-3 as well as the effects of ethanol, nicotine, and SALS on caspase-3 in SH-SY5Y cells. Neither ethanol alone (10 mM) nor nicotine alone (20 μM) had any significant effect on caspase-3 levels, whereas SALS (400 μM) caused approximately a 2.0-fold increase in caspase-3 levels which was significantly reduced (approximately 45%) by both 10 mM ethanol and 20 μM nicotine. The combination of ethanol and nicotine alone resulted in highest caspase-3 levels (2.6-fold increase), followed by ethanol + nicotine + SALS (2.5-fold increase).
Fig. 5.
Effects of low concentrations of ethanol, nicotine and salsolinol alone or in combination on caspase-3 and β actin protein in human neuroblastoma SH-SY5Y cells. Cells were treated with low concentrations of ethanol (10 mM) or nicotine (20 μM) alone or in combination or 1 h prior to salsolinol (0.4 mM). The bands indicate equal loading of proteins (beta-actin) and intensity of caspase-3. Values are the mean ± SEM of three independent experiments. **P <0.01 compared to control and #P <0.05, ##P <0.01 compared to salsolinol
Discussion
The results of this study indicate that alcohol at low concentrations of 10 or 20 mM can exert significant protection against SALS-induced toxicity in SH-SY5Y cells. The results also confirm a protective effect of nicotine at 20 and 50 μM concentration against SALS-induced toxicity. However, contrary to our expectations, a combination of low doses of alcohol and nicotine, not only did not provide any additional protection, but exacerbated SALS-induced toxicity. Indeed, the combination of low alcohol and nicotine alone resulted in significant toxicity in SH-SY5Y cells that were comparable or even higher than that induced by 400 μM (EC50) SALS. However, the toxicity induced by the combination of alcohol and nicotine alone was not significantly different than that obtained by the combination of alcohol, nicotine, and salsolinol suggesting a possible ceiling effect.
The results further demonstrate that the protective effects of low alcohol or nicotine against SALS are mediated by inhibition of apoptosis and the toxicity observed by their combination is mediated by induction of apoptosis. This contention is based on the observation that the pattern of changes in caspase-3 levels closely paralleled the pattern of cellular toxicity or protection induced by various treatments. Thus, the combination of alcohol and nicotine resulted in maximum cell toxicity as well as maximal increases in caspase-3 levels, whereas protective effects of alcohol and nicotine individually against SALS were associated with decreases in caspase-3 levels.
Although the exact implications of these in vitro results to actual human consumption of alcohol and nicotine is far from complete, the findings do raise a concern over concomitant use of alcohol and nicotine even in moderate or low amounts. It is a well-recognized clinical observance that the combination of high alcohol and nicotine intake (heavy drinking and smoking) can result in severe health consequences (Morita et al. 2010). However, alcohol use in low or moderate amounts may provide various beneficial effects including neuroprotection (Belmadani et al. 2004; Collins et al. 2009). Similarly, nicotine in relatively low concentrations may provide protective effects against various toxicants (Quik et al. 2008; Das and Tizabi 2009). Moreover, nicotine administered through patch, gum, or as an inhalant may provide various benefits such as aid in smoking cessation (Tønnesen 2009). Thus, it would be of immense relevance to determine whether a combination of low alcohol and nicotine may result in toxicity in an in vivo setting.
Other researchers have also reported toxic effects of a combination of alcohol and nicotine in vitro. Smith et al. (2006) found that exposure of pheochromocytoma (PC12) cells to alcohol (100 mM), nicotine (10 μM), or both resulted in cell loss and that the combination of alcohol and nicotine caused a significantly greater decrease in cell number when compared to either alcohol or nicotine alone. However, these investigators did not observe any changes in apoptosis as determined by caspase-3 or DNA fragmentation assay, suggesting that the reduction in PC12 cell numbers following alcohol and/or nicotine exposure may be due to factors other than apoptosis (e.g. interference with proliferation rates). On the other hand, Tizabi et al. (2003, 2004) reported protective effects of low nicotine concentrations (20 μM) against toxicity induced by high ethanol concentrations (100 mM) in cultured primary cerebellar or cortical cells. Moreover, the toxic effect of alcohol was associated with an increase in caspase-3 levels which was blocked by nicotine pretreatment (Tizabi et al. 2005). Hence, the type and nature of cellular cultures used might be a major reason for varied in vitro observations as different cells may show differential sensitivity to the toxicants and/or protectants.
Although the cellular mechanism leading to the observed toxicity by the combination of alcohol and nicotine in our paradigm is not clear, it is of relevance to note that ethanol can prolong the opening of the ionic channel gated by nicotinic receptors (Dopico and Lovinger 2009). Hence, prolonged ionic influx (e.g. Ca++) brought about by concomitant availability of alcohol and nicotine may result in enhanced cellular toxicity. However, a recent in vivo study in adolescent mice showed that whereas exposure to either ethanol or nicotine resulted in reductions in hippocampal neuronal and glial cell densities concomitant administration of these two drugs reduced the adverse effects compared to each drug alone (Oliveira-da-Silva et al. 2009). Moreover, the detrimental effects of all such treatments were abolished by prolonged withdrawal (Oliveira-da-Silva et al. 2010). Thus, the in vivo dynamics of nicotinic receptor subtypes and their modulation by a number of endogenous factors including acetylcholine as well as the complexity of alcohol interactions with numerous receptors may yield a different outcome in whole animal compared to the in vitro setting. These divergent findings underscore the need for further investigation of alcohol–nicotine interactions, particularly in lower dose ranges in various other in vitro (e.g. primary cell cultures) as well as in in vivo paradigms.
In summary, our results indicate a neuroprotective effect of low alcohol concentration against salsolinol-induced toxicity in neuroblastoma derived SH-SY5Y cell line. Similar results were obtained with low concentrations of nicotine. However, contrary to the expectation the combination of low alcohol and nicotine concentrations not only did not provide any additional protection against salsolinol, but resulted in significant toxicity that is likely to be apoptotically mediated.
Acknowledgments
Supported by NIAAA (P20 AA014643), NIH/NIGMS (2 SO6 GM08016-39), and NIH-RCMI 2 G12 RR003048.
References
- Belmadani A, Kumar S, Schipma M, Collins MA, Neafsey EJ. Inhibition of amyloid-beta-induced neurotoxicity and apoptosis by moderate ethanol preconditioning. Neuroreport. 2004;15:2093–2096. doi: 10.1097/00001756-200409150-00019. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, et al. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland RL, Jr, Leggett YA, Kanaan YM, Taylor RE, Tizabi Y. Neuroprotective effects of nicotine against salsolinol-induced cytotoxicity: implications for Parkinson’s disease. Neurotox Res. 2005;8:289–293. doi: 10.1007/BF03033982. [DOI] [PubMed] [Google Scholar]
- Copeland RL, Jr, Das JR, Kanaan YM, Taylor RE, Tizabi Y. Antiapoptotic effects of nicotine in its protection against salsolinol-induced cytotoxicity. Neurotox Res. 2007;12:61–69. doi: 10.1007/BF03033901. [DOI] [PubMed] [Google Scholar]
- Das JR, Tizabi Y. Additive protective effects of donepezil and nicotine against salsolinol-induced cytotoxicity in SH-SY5Y cells. Neurotox Res. 2009;16:194–204. doi: 10.1007/s12640-009-9040-2. [DOI] [PubMed] [Google Scholar]
- Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan ZZ, Yu WF, Nordberg A. Dual effects of nicotine on oxidative stress and neuroprotection in PC12 cells. Neurochem Int. 2003;43:243–249. doi: 10.1016/s0197-0186(03)00009-3. [DOI] [PubMed] [Google Scholar]
- Hejmadi MV, Dajas-Bailador F, Barns SM, Jones B, Wonnacott S. Neuroprotection by nicotine against hypoxia-induced apoptosis in cortical cultures involves activation of multiple nicotinic acetylcholine receptor subtypes. Mol Cell Neurosci. 2003;24:779–786. doi: 10.1016/s1044-7431(03)00244-6. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhao B. Nicotine attenuates beta-amyloid peptide-induced neurotoxicity, free radical and calcium accumulation in hippocampal neuronal cultures. Br J Pharmacol. 2004;141:746–754. doi: 10.1038/sj.bjp.0705653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama W, Yi H, Takahashi T, Shimazu S, Ohde H, et al. Neuroprotective function of R-(−)-1-(benzofuran-2-yl)-2-propyl-aminopentane, [R-(−)-BPAP], against apoptosis induced by N-methyl(R)salsolinol, an endogenous dopaminergic neurotoxin, in human dopaminergic neuroblastoma SH-SY5Y cells. Life Sci. 2004;75:107–117. doi: 10.1016/j.lfs.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Morita M, Kumashiro R, Kubo N, Nakashima Y, Yoshida R, et al. Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: epidemiology, clinical findings, and prevention. Int J Clin Oncol. 2010;15:126–134. doi: 10.1007/s10147-010-0056-7. [DOI] [PubMed] [Google Scholar]
- Naoi M, Maruyama W, Nagy GM. Dopamine-derived salsolinol derivatives as endogenous monoamine oxidase inhibitors: occurrence, metabolism and function in human brains. Neurotoxicology. 2004;25:193–204. doi: 10.1016/S0161-813X(03)00099-8. [DOI] [PubMed] [Google Scholar]
- Oliveira-da-Silva A, Vieira FB, Cristina-Rodrigues F, Filgueiras CC, Manhaes AC, Abreu-Villaca Y. Increased apoptosis and reduced neuronal and glial densities in the hippocampus due to nicotine and ethanol exposure in adolescent mice. Int J Dev Neurosci. 2009;27:539–548. doi: 10.1016/j.ijdevneu.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Oliveira-da-Silva A, Manhaes AC, Cristina-Rodrigues F, Filgueiras CC, Abreu-Villaca Y. Hippocampal increased cell death and decreased cell density elicited by nicotine and/or ethanol during adolescence are reversed during drug withdrawal. Neuroscience. 2010;167:163–173. doi: 10.1016/j.neuroscience.2010.01.060. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer’s and Parkinson’s disease. Front Biosci. 2008;13:492–504. doi: 10.2741/2695. [DOI] [PubMed] [Google Scholar]
- Quik M, O’Leary K, Tanner CM. Nicotine and Parkinson’s disease: implications for therapy. Mov Disord. 2008;23:1641–1652. doi: 10.1002/mds.21900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini AR, Hashimoto CL, Iriya K, Zerbini C, Baba ER, Moraes-Filho JP. Dietary habits, ethanol and tobacco consumption as predictive factors in the development of esophageal carcinoma in patients with head and neck neoplasms. Dis Esophagus. 2008;21:316–321. doi: 10.1111/j.1442-2050.2007.00769.x. [DOI] [PubMed] [Google Scholar]
- Smith AM, Zeve DR, Dohrman DP, Chen WJ. The interactive effect of alcohol and nicotine on NGF-treated pheochromocytoma cells. Alcohol. 2006;39:65–72. doi: 10.1016/j.alcohol.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Storch A, Ott S, Hwang YI, Ortmann R, Hein A, et al. Selective dopaminergic neurotoxicity of isoquinoline derivatives related to Parkinson’s disease: studies using heterologous expression systems of the dopamine transporter. Biochem Pharmacol. 2002;63:909–920. doi: 10.1016/s0006-2952(01)00922-4. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Al-Namaeh M, Manaye KF, Taylor RE. Protective effects of nicotine on ethanol-induced toxicity in cultured cerebellar granule cells. Neurotox Res. 2003;5:315–321. doi: 10.1007/BF03033151. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Smoot DT, Taylor RE. Nicotine inhibits ethanol-induced toxicity in cultured cerebral cortical cells. Neurotox Res. 2004;6:311–316. doi: 10.1007/BF03033441. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Manaye KF, Taylor RE. Nicotine blocks ethanol-induced apoptosis in primary cultures of rat cerebral cortical and cerebellar granule cells. Neurotox Res. 2005;7:319–322. doi: 10.1007/BF03033888. [DOI] [PubMed] [Google Scholar]
- Tønnesen P. Smoking cessation: how compelling is the evidence? A review. Health Policy. 2009;91:S15–S25. doi: 10.1016/S0168-8510(09)70004-1. [DOI] [PubMed] [Google Scholar]