Abstract
Background & Aims
To restore fecal continence, the weakened pressure of the internal anal sphincter (IAS) must be increased. We bioengineered intrinsically innervated human IAS, to emulate sphincteric physiology, in vitro.
Methods
We co-cultured human IAS circular smooth muscle with immortomouse fetal enteric neurons. We investigated the ability of bioengineered innervated human IAS, implanted in RAG1−/− mice, to undergo neovascularization and preserve the physiology of the constituent myogenic and neuronal components.
Results
The implanted IAS was neovascularized in vivo; numerous blood vessels were observed with no signs of inflammation or infection. Real-time force acquisition from implanted and pre-implant IAS showed distinct characteristics of IAS physiology. Features included the development of spontaneous myogenic basal tone; relaxation of 100% of basal tone in response to inhibitory neurotransmitter vasoactive intestinal peptide (VIP) and direct electrical field stimulation of the intrinsic innervation; inhibition of nitrergic and VIPergic EFS-induced relaxation (by antagonizing nitric oxide synthesis or receptor interaction); contraction in response to cholinergic stimulation with acetylcholine; and intact electromechanical coupling (evidenced by direct response to potassium chloride). Implanted, intrinsically innervated bioengineered human IAS tissue preserved the integrity and physiology of myogenic and neuronal components.
Conclusion
Intrinsically innervated human IAS bioengineered tissue can be successfully implanted in mice. This approach might be used to treat patients with fecal incontinence.
Keywords: IM-FEN cells, enteric nervous system, tissue engineering, contraction
INTRODUCTION
Fecal incontinence due to degenerated or weakened internal anal sphincter (IAS) has a high incidence rate in aging populations [1, 2]. The smooth muscle cells of the IAS have a “sphincteric specialization,” in that they are tonic muscles that remain in a constant state of contracture (basal tone), different from the phasic circular smooth muscle of the adjacent rectum. This makes the fatigue-resistant IAS a major contributor to rectoanal continence [3, 4]. In fact, the inward radial pressure of the IAS is responsible for 70% of the anal canal pressure. Voluntary squeeze of the external anal sphincter (EAS) reinforces the primary closure produced by the IAS. EAS muscle damage is associated with urge-related fecal incontinence. Weakened IAS (due to injury, surgical or obstetric trauma or aging) directly results in lower anal canal pressures and passive incontinence even at resting states [5, 6]. Surgical options for the repair of degenerated IAS have relied on grafts of skeletal muscle, injectable silicone material or implantation of a variety of mechanical devices; all of which have a high complication rate and limited success in restoration of quality of life [7, 8]. Bioengineering could play a role in developing a translational approach to remedy fecal incontinence due to weakened IAS.
We have previously bioengineered IAS from isolated human IAS circular smooth muscle. Additionally, we have successfully implanted physiologically functional bioengineered mouse IAS constructs in wild type mice. These were neovascularized in vivo and maintained IAS physiology (i.e., generated basal tone, relaxation and contraction in response to physiologically relevant contractile and relaxant neurotransmitters) [3, 9]. However, each of these previous works lacked an intrinsic nervous population, which would mimic normal IAS function as well as anatomy.
In this study, we provide proof of concept that we can successfully implant an intrinsically innervated human IAS construct. These constructs are neovascularized and maintain their IAS functionality (i.e., myogenic and neuronal components after implantation). This is the first instance of implantation of a bioengineered intrinsically innervated human IAS, where both the myogenic and neuronal components are viable and synergistically responsive to cholinergic, VIP-ergic (Vasoactive Intestinal Peptide) and electrical stimulation. Fundamental electromechanical coupling of the constituent smooth muscle is also maintained during implantation, rendering the implanted IAS physiologically similar to in vivo IAS.
MATERIALS AND METHODS
Materials
All reagents were purchased from Sigma Aldrich (St. Louis, MO) unless specified otherwise. Growth media for smooth muscle consisted of Dulbecco’s modified Eagle medium (DMEM; Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (FBS), 1.5% Antibiotics-Antimycotics and 0.6% L-glutamine. Growth media for neuronal progenitor cells (IM-FENs) consisted of DMEM/F12 medium supplemented with 5μg/ml insulin, 1 μg/ml transferrin, 20ng/mL progesterone, 30nM sodium selenite, 100μM putrescine, 0.1mg/ml fetuin, 1mg/ml BSA fraction V, 10% FBS and 20U/ml Interferon-γ. Neuronal differentiation media is modified neurobasal medium –A (Invitrogen) supplemented with the B-27 serum free supplement, 1mM L-glutamine and 1% FBS. Type I rat tail collagen was purchased from BD Biosciences (Bedford, MA) and Collagenase was purchased from Worthington Biochemicals (Lakewood, NY). Hank’s Balanced Salt Solution (HBSS) was purchased from Hyclone (Logan, UT).
Cell Isolation
Human IAS circular smooth muscle was isolated as described before [3] from human IAS obtained after surgery from National Development and Research Institute (NDRI; New York, NY; project code: B1K1 001; protocol code: 001; IRBMED No. 1991-0297) and from organ donors through the Gift of Life Michigan (IRBMED No. HUM00023670). Specimens were collected after all organs for transplantation were procured, between 1 and 3 hours after cross-clamp of the aorta and infusion of organ preservation solution. Most procured specimens were processed within 2 hours of leaving the operating suite.
Briefly, IAS tissue was cleaned and washed in ice-cold HBSS. Connective tissue and striated muscle were stripped off and circular smooth muscle of the IAS was minced and enzymatically digested in 0.1% type II Collagenase and 20mg/ml DNAse-1 twice for an hour each. Digested cells were washed to remove traces of Collagenase and resuspended in growth media and plated on tissue culture flasks.
Neuronal cell line was established as described elsewhere [10]. Briefly, cells were isolated from a D13 embryo from H-2Kb-tsA58 immortomouse, using magnetic bead immunoselection with an antibody directed against the low affinity NGF receptor p75 NTR. These cells were called ImmortoMouse Fetal Enteric Neurons (IM-FENs) and were proliferated at 33°C in the neuronal growth media described above.
Bioengineering intrinsically innervated human IAS tissue
35mm tissue culture dishes were prepared with a central silicone post (5mm outer diameter) to define luminal space. 1 × 105 IM-FENs in a collagen/laminin mixture were laid down on the 35 mm dish surrounding the central post first, and allowed to gel for 15 minutes. After gellation was complete, 4 × 105 IAS cells in a collagen gel was laid down on top of the IM-FEN gel layer and allowed to gel. Following gellation, 1.5mL of growth media was added and the plates were incubated at 39°C to allow the smooth muscle cells to elongate. Gels were gently released from the edges of the tissue culture dish using a sterile needle. After 24 hours of plating, media was switched to neuronal differentiation media. Dishes were incubated at 39°C for 9 days allowing for neuronal differentiation and network formation before implantation or physiological testing.
Inhibitory and Excitatory neurotransmitters in bioengineered IAS constructs
RNA for RT-PCR was isolated from intrinsically innervated human IAS rings at Day 10 using Trizol reagent according to the manufacturer's instructions (Invitrogen, Carlsbad, CA). RNA was quantified spectrophotometrically. RT-PCR was performed using the SuperScript One-Step with Platinum Taq kit (Invitrogen). Each reaction included 50 ng of RNA. The following primer sets were used: Choline Acetyltransferase (ChAT) sense 5′-GTAACAGCCCAGGAGAGCAG-3′ and ChAT anti-sense 5’-GCAGGGCTAGAGTTGACTGG-3′ or VIP sense 5′-CTAGCCAGCTACAGCCAACC-3’ and VIP anti-sense 5′-CTGCTGTAATCGCTGGTGAA-3. The RT-PCR reaction products were electrophoresed through a 1.8% agarose gel at 100 V and the bands were visualized using ethidium bromide.
Intrinsically innervated human IAS rings were also fixed in 3.5% buffered formaldehyde and embedded in paraffin at Day 10. 10μm sections were cut from these paraffin-embedded rings using a microtome. The sections were subjected to a standard deparaffinization and gentle graded alcohol re-hydration protocol. Sections of the bioengineered IAS were stained with ChAT (Abcam, 68779) and VIP (Abcam, 78536). Slides were visualized by a Nikon Ti-E fluorescence microscope.
Implantation in RAG1−/− mice
Surgical procedure for implantation of bioengineered IAS constructs has been described by Hashish et al. [11]. Briefly, human recombinant Fibroblast Growth Factor was primed in micro osmotic pumps and implanted along with the bioengineered tissue. RAG1−/− mice were anesthetized using isofluorane inhalation. A subcutaneous pocket was created caudally in the upper back of the mouse into which the pump and catheter complex was inserted. The bioengineered IAS ring was inserted near the tip of the catheter, about 0.5 cm away from the skin incision. A 6-0 Prolene suture was placed loosely around the ring to mark its position. The animals were returned to an approved facility for recovery in standard fashion.
Ring harvest
Mice were euthanized 25–28 days post surgery. The area of previous incision was reentered and bioengineered rings were removed from the surrounding tissue after localization of the Prolene suture. This harvested IAS ring was assessed for physiology immediately thereafter following protocols outlined below.
Histology and immunofluorescence
Following physiological testing, half the ring was fixed in Zinc-formalin and the other half was embedded in optimal cutting temperature compound and frozen in liquid nitrogen. Masson’s Trichrome (Sigma) was used for routine morphological assessment following manufacturer’s protocols. Immunofluorescence assays were carried out for α-Smooth muscle actin (F3777, Sigma), smooth muscle specific heavy-Caldesmon (c-4562, Sigma), an endothelial specific antigen von Willebrand’s Factor (Factor VIII; sc-14014, Santa Cruz Biotechnology) and neuron specific β.-III tubulin (ab25770, Abcam). Slides were visualized by a Nikon Ti-E fluorescence microscope.
Measurement of physiological functionality
Physiological testing protocols were adapted from previous work on bioengineered human IAS and implanted mouse IAS constructs [3, 9]. The tissue bath held 4 mL of basal DMEM and was placed on a heated platform that maintained the temperature at 37°C ± 1°C. A stainless steel pin was fixed to the center of the bath. Before any measurements were made, the noise in the measurement system was assessed by recording tension with air and warm bath fluid only without hooking the tissue. The mean noise was subtracted from total tension recordings. Passive tension was obtained by hooking the tissue with no stretch at the end of the day and incubation in zero calcium phosphate buffered saline with 50 mM ethylene glycol tetraaceticacid for 30 minutes. This value was subtracted from total tension resulting in active tension readings generated by the bioengineered IAS. All values of force reported are values of active tension.
The IAS rings were separated from the silicone post and one end was anchored to the fixed pin. The other end was looped over the measuring arm of a force transducer (Harvard Apparatus, Holliston, MA). Stock solutions of drugs used were prepared and added as described previously [9]. Basal DMEM soaking the ring was changed every 30 minutes, or at the end of a set of experiments, whichever was earlier.
Testing protocol
Bioengineered rings or harvested post-implant rings were hooked on to the force transducer with no additional stretch. Basal tone was established spontaneously with no external stimulation or stretch over time as shown in Figure 4A,C. The effect of Tetrodotoxin (TTX; nerve blocker) on basal tone was observed (Figure 4B,D). A 20% stretch was applied on the constructs by a micromanipulator and this remained the length at which all tests were carried out. The baseline of force established by the rings at this length was arbitrarily set to zero, to observe effects of drugs on the constructs. A stable baseline was allowed to develop before treatments with the various drugs.
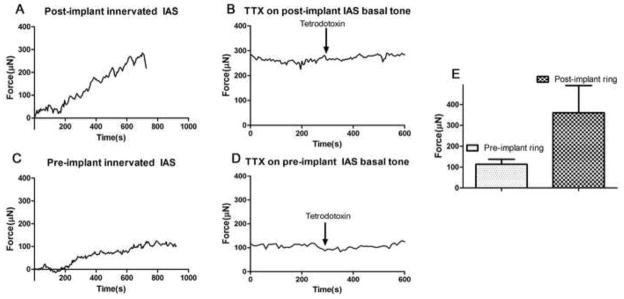
Fig. 4. Spontaneous myogenic basal tone.
Intrinsically innervated human IAS pre- and post-implant were tested for real-time force generation. IAS physiology was tested using a force transducer and recording of force generation in real-time. (4A & C): Generation of spontaneous basal tone is a key aspect of IAS physiology. All bioengineered pre-implant and post-implant IAS constructs generated active basal tone with no external stimulation. The implanted innervated IAS generated 360.2 ± 130.7μN of active basal tone. Pre-implant innervated IAS generated 113.4 ± 24.43μN of active basal tone. Noise due to air and bath fluid was cancelled from total tension first. Passive tension was determined by incubating the tissue in zero calcium + 50mM EGTA for 30 minutes at the end of the day. Passive tension values were subtracted from total tension to determine active basal tone. (4E): Summary of values of force generated by pre- and post-implant constructs. (4B & D): To confirm that the IAS basal tone is of myogenic nature, neuronal blocker, 1μM Tetrodotoxin (TTX), was added. TTX had no effect on the basal tone of the IAS. Reported values are means ± SEM of 3–5 experiments
Testing protocols were designed to study the maintenance of overall IAS physiology, including assessing the myogenic response and the neuronal contribution to the overall response. The neuronal contribution was assessed by studying the effect of drugs in the presence of nerve-blocker TTX. Comparisons of physiology of in vivo IAS were done by isolating the IAS from wild type mice and assessing their physiology. The generation of spontaneous basal tone was studied, along with the ability to relax basal tone in response to neurotransmitter 1μM Vasoactive Intestinal Peptide (VIP) or electrical field stimulation (EFS). Neurotransmitters involved in EFS-induced relaxation were assayed by pre-incubation with nNOS-blocker Nω-Nitro-L-arginine methyl ester hydrochloride (L-NAME; 300μM) and VIP-receptor antagonist [D-p-Cl-Phe6, Leu17]-Vasoactive Intestinal Peptide (VIP-Ra; 2μM). Receptor integrity and intracellular signaling mechanism preservation was studied by observing the effect of cholinergic stimulation. Maintenance of membrane characteristics and electromechanical coupling were studied by treatment with 30mM potassium chloride.
Data Analysis
Data was acquired by LabScribe2 (iWorx, Dover, NH) at a frequency of 0.02Hz. GraphPad Prism 5.01 for Windows (GraphPad Software, San Diego, CA, www.graphpad.com) was used for all further data analysis. Second order Savitsky-Golay smoothing was applied on raw data. Values were expressed as means and SEM of 3–10 experiments. Relaxation was quantified as a percentage of each individual construct’s basal tone. Ach- and KCl-induced contraction was expressed as absolute change in force from baseline in micro Newtons (μN). Mann-Whitney tests were used for testing significance.
RESULTS
Bioengineering and implantation of intrinsically innervated human IAS
Human IAS cells and immortomouse fetal enteric neuronal (IM-FEN) cells were first laid out on double-layered collagen/laminin matrices and cultured under neuronal differentiation conditions, including elevated temperature (39°C) and in a medium lacking interferon-γ. Fig. 1A shows the formation of a fully compacted ring at 60 hours. This process brought the IM-FEN cells into close association with the smooth muscle cells. The proximity to the IAS smooth muscle cells enhanced the differentiation of the IM-FENs. Axonal projections were observed protruding perpendicularly into the ring. Neuronal networks were visualized at Day 9 associated with the IAS (Fig. 1B).
Fig. 1. Bioengineering intrinsically innervated human IAS tissue.
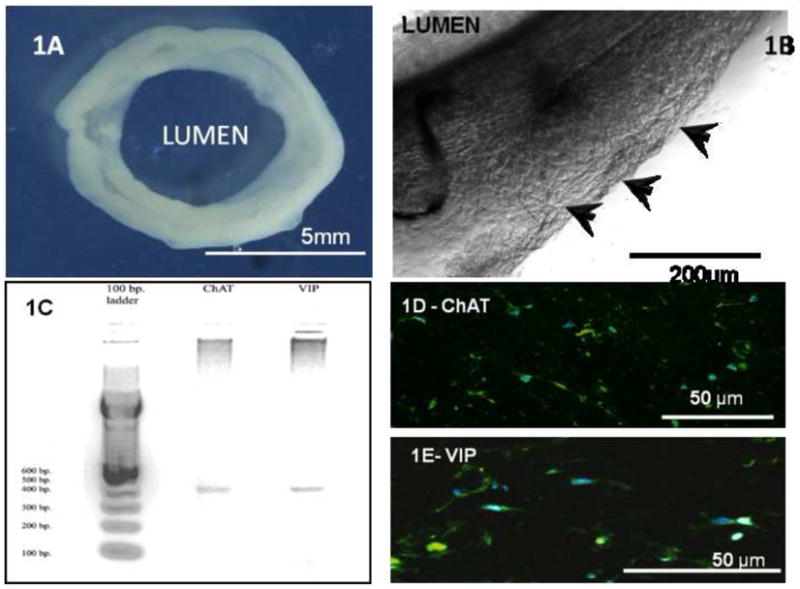
IAS smooth muscle cells and neuronal IM-FENs were plated in a collagen/laminin gel around a silicone post defining luminal space. (1A): Macroscopic view of the bioengineered intrinsically innervated ring at Day 10. The internal diameter of the ring is 5mm and the thickness is 2mm. (1B): The IAS circular smooth muscle cells and the IM-FENs were brought into close association during the ring formation process. In the proximity of the smooth muscle, the IM-FENs differentiated and formed neuronal networks (arrowheads) associated with the human IAS. (1C): Qualitative RT-PCR analysis for neurotransmitters—excitatory motor neurons and inhibitory motor neurons are present in the bioengineered constructs. Total RNA was isolated from 10-day constructs and subjected to PCR analysis using oligonucleotide primers designed to generate ~400 bp products for choline acetyltransferase (ChAT) and vasoactive interstinal peptide (VIP). mRNAs for both ChAT and VIP were present in the constructs. (1D): 10μm cross-section of the bioengineered construct, staining positive for excitatory neurons expressing ChAT. (1E): 10μm cross section of the construct, stained green for inhibitory motor neurons expressing VIP.
Qualitative analysis of neuronal networking using RT-PCR demonstrated the presence of mature excitatory (ChAT) and inhibitory (VIP) motor neurons (Fig. 1C) in the bioengineered ring. Immunofluorescence assays demonstrated populations of neurons that were excitatory (expressing ChAT; Fig. 1D) and inhibitory (expressing VIP; Fig. 1E). Intrinsically innervated rings were bioengineered for physiological assessment and implantation subcutaneously into immune suppressed RAG1−/− mice.
Detection of neovascularization and innervation
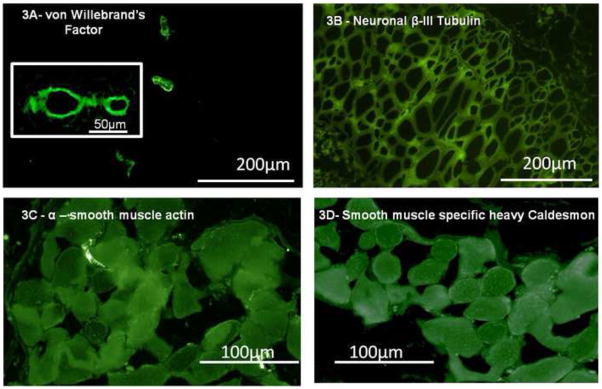
Implanted IAS rings were neovascularized in vivo. Constructs appeared pink in color from the abundant vascularization. There were no signs of inflammation or infection (Fig. 2A). Sections of implanted IAS were made on a cryostat. Fig. 2B is a cross-section of the ring stained with Masson’s Trichrome, showing intact alignment of the smooth muscle with no infiltration into the construct. Fig. 3A shows several blood vessels from the microvasculature embedded within cross sections of the implanted smooth muscle. Well-defined lumens of the blood vessels were visualized at higher magnification (Fig. 3A inset). Innervation was visualized by staining with neuron-specific β-III tubulin. Fig. 3B shows a reticulated pattern of a neuronal network associated with the IAS. Positive stains for smooth muscle markers, α-smooth muscle actin (Fig. 3C) and smooth muscle specific heavy caldesmon (Fig. 3D), indicated preservation of smooth muscle phenotype.
Fig. 2. Implanted IAS at Day 28.
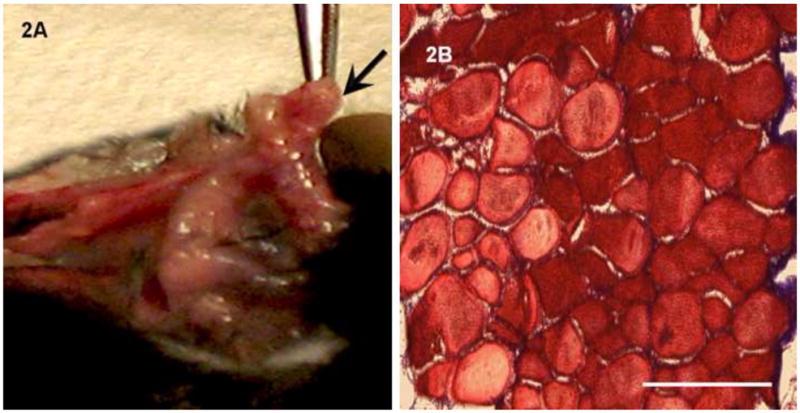
(2A): The implanted bioengineered IAS is neovascularized in vivo. The implanted IAS (arrow) presented pink color. There were no visible signs of abscess formations or infection in any of the implantations. (2B): Cross-section of the implanted IAS stained with Masson’s Trichrome to examine morphology. The smooth muscle alignment was preserved without any infiltration.
Fig. 3. Detection of neovascularization and neuronal networking with immunofluorescence.
A portion of the implanted innervated human IAS was flash frozen in liquid nitrogen and cryostat sections of 6μm are made. These sections were stained with markers for vascularization, neurons and smooth muscle. (3A): The implanted bioengineered intrinsically innervated human IAS was neovascularized in vivo. Staining with von Willebrand’s factor, an endothelial specific antigen revealed several blood vessels. Blood vessels were visualized at low magnification embedded within the implanted smooth muscle. At higher magnification (inset), the lumen of the blood vessel is clearly observed. (3B): Reticulated neuronal network is found embedded within the implanted human IAS, staining positive for neuron specific β-III tubulin. (3C & D): The phenotype of the implanted smooth muscle was also maintained staining positive for both α-smooth muscle actin and smooth muscle specific heavy caldesmon.
Spontaneous basal tone
Physiology of the bioengineered constructs was assessed pre- and post-implantation by measuring real-time force generated. All bioengineered IAS rings pre- and post-implantation generated spontaneous basal tone (Fig. 4A and 4C; i.e., an incremental force without external stimulation or stretch to reach a basal state of contracture). The addition of neuronal blocker 1μm tetrodotoxin (TTX) had no effect on the basal tone of the IAS (Fig. 4B & 4D), suggesting that the bioengineered implanted IAS constructs retain and maintain a similar and important characteristic of in vivo IAS to generate myogenic basal tone. Average values of active basal tone generated in the post-implant IAS was 360.2 ± 130.7μN (n=5), while it was 113.4 ± 24.43μN (n=8) in the bioengineered IAS pre-implant (Figure 4E). The high pressure due to basal tone is responsible for anal canal closure, thereby maintaining rectoanal continence.
VIP-ergic relaxation of basal tone
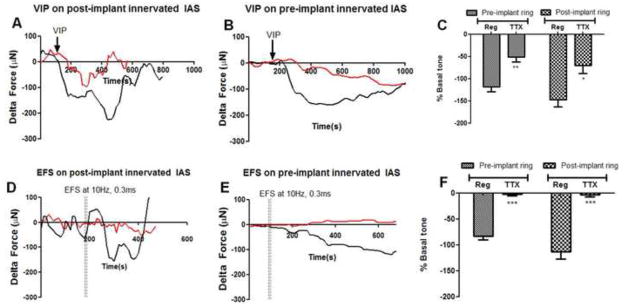
We next tested the effect of VIP on inducing relaxation of the IAS rings. Upon VIP treatment, the innervated IAS constructs pre- and post-implantation fully relaxed their active basal tone (Fig. 5A,B,C). The onset of relaxation was rapid, post addition of VIP, with maximal relaxation within 200s post stimulation. The IAS rings then subsequently returned to their previous state of contracture (i.e., they recouped the basal active tone). Addition of TTX attenuated the magnitude of relaxation (50–70% inhibition, red trace Fig. 5A,B), indicating that VIP-ergic relaxation has both neuronal, as well as myogenic, characteristics. The physiology of the post-implant and pre-implant IAS was similar to in vivo IAS, indicating the survival and maintenance of neuronal as well as myogenic component during implantation. Rapid relaxation of the innervated smooth muscle IAS in vivo during defecation and the return to basal tone for anal canal closure to maintain continence is an important characteristic of IAS physiology maintained by the bioengineered implanted IAS.
Fig. 5. Relaxation of basal tone by VIP and electrical field.
Relaxation and subsequent recovery of basal tone is one of the primary and necessary physiological functions of the IAS. Relaxation of active basal tone is studied by: i) addition of either neurotransmitter VIP (1μM; 5A,B,C) or ii) with electrical field stimulation (5D,E,and F). To evaluate the dual myogenic and neuronal characteristics involved in relaxation of basal tone, force generation was measured in the presence (“TTX,” red trace) or absence (“Reg,” black trace) of TTX. Basal tone was arbitrarily set to zero, to examine the effects of the stimulus. The arrow indicates the point in time after establishment of stable baseline where VIP was added. (A & B): Bioengineered IAS constructs responded to VIP by relaxing rapidly pre- (118 ± 10.8% of basal tone) and post-implantation (147 ± 16%). The magnitude of relaxation is significantly attenuated in the presence of TTX, with the extent of relaxation being 50.84 ± 11.2 %(pre-implant) and 70 ± 18% (post-implant) of active basal tone. IAS constructs recovered basal tone ~700s post VIP stimulation. (D & E): Pre- and post-implant IAS also relaxed rapidly in response to an electric field of 10Hz and 0.3ms on-time, switched on for 10s. The extent of relaxation was between 83 ± 7.2% (pre-implant) and 112 ± 14.5% (post-implantation). The relaxation of the muscle is completely neuronally mediated since TTX abolishes the rapid drop in active basal tone (red traces). Relaxation was quantified as a percentage of active basal tone, and represented as means ± SEM of 6 experiments. (C) VIP, (F) Electrical Field. **P<.01, ***P<.001 compared to regular by Mann-Whitney test.
Relaxation of basal tone by electrical field stimulation (EFS)
Selective stimulation of the intrinsic innervation with EFS at 10Hz, 0.3ms on-time produced a very rapid relaxation of active basal tone in the IAS constructs (Fig. 5D, E and F). The resultant relaxation was transient and recovery of basal tone occurred between 200–400s post-stimulation. In order to assay the chemical nature of EFS-induced inhibitory neurotransmission, innervated IAS constructs were pre-incubated with either L-NAME to block NO synthesis or [D-p-Cl-Phe6, Leu17]-Vasoactive Intestinal Peptide to antagonize the VIP receptors. EFS-induced relaxation was significantly inhibited by pre-incubation with both L-NAME (27 ± 4.5%; p<0.05) as well as VIP-receptor antagonist (23.28 ± 4.014 %; p<0.05) as shown in Figure 6. These results indicate that stimulation of the enteric nerves results in nitrergic as well as VIP-ergic inhibitory neurotransmission in bioengineered intrinsically innervated human IAS constructs. In the presence of neuronal blocker TTX, any relaxation obtained due to EFS was significantly abolished as shown by the red traces, indicating the neuronal mediation of relaxation (Fig. 5).
Fig. 6. Characterization of EFS-induced relaxation.
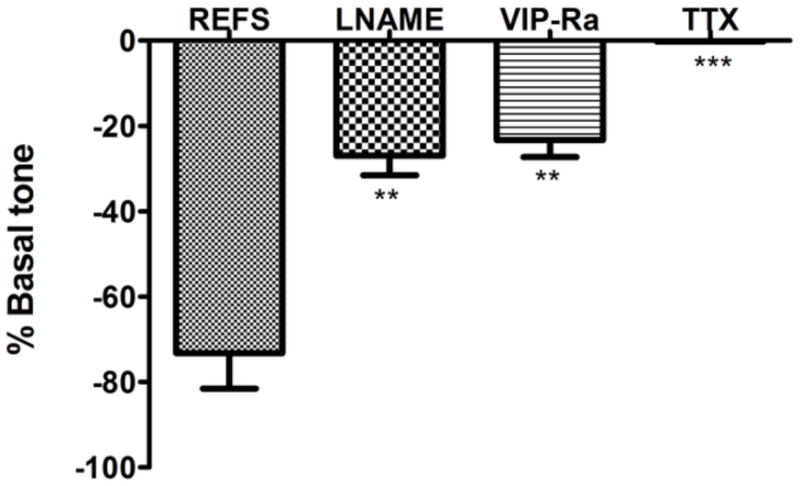
In order to characterize the chemical nature of inhibitory neurotransmission, bioengineered intrinsically innervated IAS constructs were pre-incubated with inhibitors before stimulation with electrical field. The inhibitors blocked nitric oxide synthesis (L-NAME) or antagonized VIP receptors (VIP-Ra). In the presence of L-NAME or VIP-Ra, the magnitude of EFS-induced relaxation was significantly reduced (P<.05). This implies involvement of both nitrergic and VIP-ergic inhibitory stimulation in response to electrical field. TTX abolished EFS-induced relaxation, indicating that the process was entirely neuronally mediated. Values are mean ± SEM of 3 experiments.
In the IAS in vivo, rectoanal inhibitory reflexes or transient relaxation of the IAS, an important continence mechanism, are mediated by the intrinsic enteric innervation in response to rectal distention. By mimicking the stimulation of the enteric neurons in the IAS constructs pre- and post-implantation with an electric field, the IAS rapidly relaxed transiently, displaying the capability of the myogenic and intrinsic innervation components to modulate the rectoanal inhibitory reflex similar to in vivo IAS.
Cholinergic contraction
Treatment with acetylcholine (Ach) resulted in rapid-rising contractions (~100% increase in basal tone) in IAS constructs pre- and post-implantation (Fig. 7A,B and C). Average contractions were 403 ± 89.8 μN (n=5) in post-implant IAS and 100.8 ± 30.8 μN (n=5) in pre-implant IAS. Peak maximal contractions were attenuated by 50%-70% in the presence of TTX. Contraction in response to the excitatory neurotransmitter Acetylcholine displayed distinct synergistic involvement of both neuronal and myogenic components. Cholinergic contraction is the major contractile pathway involved in the recovery of basal tone. Contraction of innervated IAS in vivo was emulated by the bioengineered IAS even after implantation.
Fig. 7. Cholinergic contraction and electromechanical coupling.
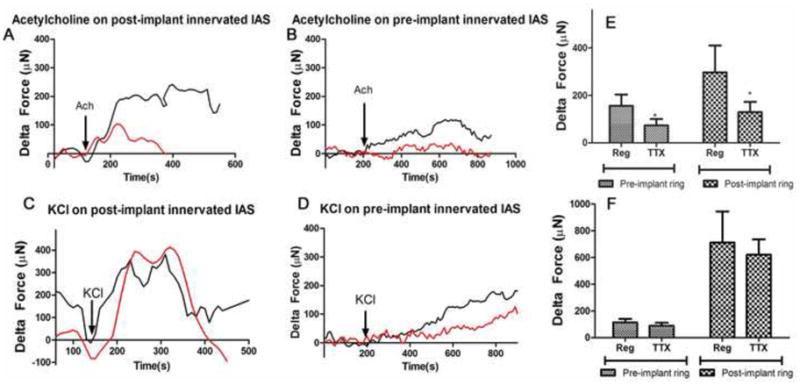
Contraction of the IAS was studied by treating either with: i) neurotransmitter-induced receptor mediated cholinergic response to 1μM Acetylcholine (Ach, A,B,C) ; or ii) 30mM potassium chloride (KCl; D,E,F) in the presence (red traces) and absence(“Reg,” black trace) of TTX. (A & B): Acetylcholine-induced rapid-rising sustained contractions in pre- and post-implant IAS. The cholinergic force generation observed was due to activation of both neurogenic and myogenic mechanisms in pre- and post-implant IAS constructs. TTX inhibited the magnitude of the peak contraction by 50–70%. (D & E): Treatment with KCl also resulted in rapid and sustained force generation of equal or higher magnitude than induced by Ach. TTX had no effect on either the time-course or the magnitude of KCl contractions. (C & F): Peak maximal force measured in response to either Ach- or KCl are summarized as means ± SEM of 5–10 experiments; *P<0.05 compared to regular, Mann-Whitney test.
Electromechanical coupling
To test the integrity of the smooth muscle cells of the IAS constructs, we used 30 mM potassium chloride. Immediate contractions were generated in the IAS constructs in response to 30 mM potassium chloride (KCl; Fig. 7D,E and F). KCl-induced contractions were between 90–100% of Ach-induced contraction. Nerve blocker TTX had no effect on KCl-induced contractions in the onset, time-course or magnitude of peak maximal force. The summarized bar graph (Fig. 7F) shows no major involvement of the intrinsic innervation in KCl-induced contraction. Direct response to KCl shows that the integrity of the constituent smooth muscle cells of the bioengineered IAS was maintained even after implantation.
DISCUSSION
Trauma (surgical or obstetric) or idiopathic degeneration due to aging all lead to weakened internal anal sphincter with lower anal canal pressures, resulting in incontinence [5]. Conventional therapies aim to ideally restore and augment smooth muscle sphincteric function, but have had limited efficacy [7, 8, 12]. Intestinal tissue engineering provides a translational therapy to remedy the weakened IAS in incontinent individuals. By implanting a physiologically functional innervated bioengineered smooth muscle IAS, the mechanical efficiency of the existing deficient sphincter can be augmented or replaced.
We have previously shown that bioengineered human circular smooth muscle IAS constructs display characteristics of IAS physiology [3]. We have also established that a bioengineered mouse IAS implanted in wild type mice displays physiological characteristics of in vivo IAS [9]. Fig. 1 displays the presence of both excitatory (ChAT) and inhibitory (VIP) neurotransmission within the intrinsically innervated bioengineered smooth muscle, both at the RNA and expression levels. The next logical step toward translational therapeutics was to provide proof of concept of implantation of bioengineered intrinsically innervated human IAS tissue. RAG1−/− mice were chosen as a model for implantation because of the deficiency of T and B lymphocytes in these animals, allowing a xenograft to survive without rejection.
Implanted IAS constructs showed no visible signs of inflammation or infection. In vivo vascularization was noted with the gross observation of blood vessels in and around the implanted IAS construct, as well as the pink color of the IAS (Fig. 2), and microscopically by deeply embedded vascularization shown by immunohistochemistry (positive stains for von Willebrand’s Factor, Fig. 3A).
The positive staining for neuron-specific β-III tubulin was further evidence of the preservation of the reticulated intrinsic neuronal network that appeared strikingly similar to a neuronal plexus in vivo. Positive stains for smooth muscle markers confirmed that the implanted IAS smooth muscle had not de-differentiated into a synthetic smooth muscle phenotype.
Physiological testing protocols were designed to evaluate the fundamental tenets of IAS physiology in the maintenance of rectoanal continence. These included: i) spontaneous generation of basal tone (i.e., high resting pressure required for anal canal closure); ii) relaxation of basal tone in response to the physiologically relevant neurotransmitter; iii) relaxation of IAS by stimulation of enteric neurons with electrical field to mimic the capability of a rectoanal inhibitory reflex in vitro; iv) analysis of neurotransmitters involved in ex-vivo response to EFS; v) cholinergic contraction as a major contractile pathway; and vi) electromechanical coupling in the smooth muscle.
The generation of spontaneous basal tone in all bioengineered IAS constructs—pre- and post- implantation (Fig. 4A, C)—is further evidence of their capability of existing in a state of contracture over extended periods of time, similar to in vivo IAS. Generation of basal tone of the IAS is known to have a myogenic molecular basis, and is independent of the extrinsic or intrinsic innervation [13]. In line with this, the addition of the nerve blocker TTX had no effect on the magnitude of basal tone generated in the IAS constructs (Fig. 4B, D).
Rapid “on-demand” relaxation of basal tone is another fundamental property of the IAS, important for defecation. Recovery of basal tone is equally important to maintain the closure of the anal canal post-defecation [14]. The implanted IAS, as well as the pre-implant IAS, rapidly relaxed in response to VIP, indicating the maintenance of receptors for VIP in the smooth muscle preserving the integrity of the myogenic smooth muscle component of VIP-ergic relaxation. The attenuation of relaxation in the presence of TTX indicated that VIP-ergic neurons were present in the bioengineered IAS, capable of mediating VIP-ergic neurotransmission to the smooth muscle, rendering maximal relaxation. The transient nature of the relaxation and the recovery of basal tone were retained during implantation.
The enteric innervation of the IAS plays a role in maintenance of continence by mediating a “sampling mechanism” [15]. Relaxation in response to a low frequency electric field in the bioengineered IAS pre- and post-implantation emulates the ability to transiently relax due to direct stimulation of the enteric innervation to release inhibitory neurotransmitters. These parameters have been previously known to produce relaxation of human IAS strips in vitro [16]. Since TTX abolished this transient relaxation, EFS-induced relaxation was purely neuronal mediated (Fig. 5D, E, F). To further characterize the neurotransmitters involved in EFS-induced relaxation of IAS, constructs were pre-incubated with inhibitors that either specifically blocked the synthesis of NO or antagonized the VIP receptor. Both these conditions resulted in a reduced magnitude of EFS-induced relaxation (Fig. 6). The intrinsic innervation of the bioengineered IAS, therefore, was viable and capable of mediating relaxation through nitrergic and VIP-ergic neurotransmission.
Contraction in response to Ach in the bioengineered IAS pre- and post-implantation is evidence of efficient neurotransmission in these constructs. Cholinergic contraction displayed myogenic and neuronal characteristics. Exogenous addition of neurotransmitter could have possibly resulted in further release of endogenous neurotransmitter from the intrinsic innervation, enhancing the magnitude of contraction (Fig. 7A & 7B, black trace). Effective electromechanical coupling in the bioengineered, implanted smooth muscle was evidenced by a direct contraction in response to potassium chloride. The integrity of the constituent smooth muscle membranes was maintained during implantation.
In this study, we have provided proof of concept for a successful implantation of bioengineered intrinsically innervated human IAS in immune suppressed mice. The ultimate step in translating this bioengineered sphincter to the clinical realm is that of in-situ implantation of this construct in humans. Our concept for a surgical implantation would be a circumferential dissection in the peri-anal area, distal to the levator complex. Single or multiple bioengineered constructs could then be placed on top of the patient's own IAS, without disrupting any intact muscle that is present. This technique will allow for the bioengineered construct to be placed exactly where the native IAS lies without breaking the plane of the rectum, thereby minimizing contamination of the implant. Further animal studies need to be performed in order to optimize surgical techniques prior to human studies.
Bioengineered IAS are a scalable process, and the construct sizes could be scaled up to match specific species of interest. Future challenges will be to ensure maintained viability to these scaled-up versions. Additionally, future work towards clinical translation also needs to focus on the isolation and culture of autologous human enteric neuronal progenitor populations so the bioengineered IAS can be completely autologous, eliminating any risk of rejection.
In conclusion, implantation of intrinsically innervated human IAS preserved key aspects of IAS physiology, including i) generation of spontaneous basal tone thereby existing in a resting state of contracture; ii) relaxation of 100% of basal tone by VIP-ergic and electrical stimulation and recovery of basal state of contracture, required for defecation and the subsequent return to continence; iii) cholinergic contraction and KCl-induced contraction; and iv) efficient VIP-ergic and nitrergic neurotransmission and appropriate contraction and relaxation to relevant neurotransmitters. The myogenic and neuronal components of the bioengineered IAS were viable and physiologically responsive even after implantation. This takes us one step closer towards realizing the goal of autologous implantation, thereby providing a bioengineering solution to remedy the weakened mechanical efficiency of the IAS in fecal incontinence.
Acknowledgments
This work was supported by NIH research grants R01DK080684, RO1DK071614 and 1RC1DK087151 and Training Program for Organogenesis T32HD007505.
Footnotes
Implantation and physiology of a bioengineered intrinsically innervated human internal anal sphincter construct as a proof of concept to potential therapy for fecal incontinence
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bharucha AE. Fecal Incontinence. Gastroenterology. 2003;124:1672–1685. doi: 10.1016/s0016-5085(03)00329-9. [DOI] [PubMed] [Google Scholar]
- 2.Bharucha AE, et al. Prevalence and Burden of Fecal Incontinence: A Population-Based study in Women. Gastroenterology. 2005;129:42–49. doi: 10.1053/j.gastro.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 3.Somara S, et al. Bioengineered Internal Anal Sphincter Derived from Isolated Human Internal Anal Sphincter Smooth Muscle Cells. Gastroenterology. 2009;137(1):53–61. doi: 10.1053/j.gastro.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 4.Frenckner B. Function of the anal sphincters in spinal man. Gut. 1975;16:638–644. doi: 10.1136/gut.16.8.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao SS. Pathophysiology of adult fecal incontinence. Gastroenterology. 2004;126(1 Suppl 1):S14–22. doi: 10.1053/j.gastro.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Lewicky-Gaupp C, et al. Anal sphincter structure and function relationships in aging and fecal incontinence. Am J Obstet Gynecol. 2009;200(5):559 e1–5. doi: 10.1016/j.ajog.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tjandra JJ, et al. Injectable silicone biomaterial for fecal incontinence caused by internal anal sphincter dysfunction is effective. Dis Colon Rectum. 2004;47(12):2138–46. doi: 10.1007/s10350-004-0760-3. [DOI] [PubMed] [Google Scholar]
- 8.Thornton MJ, et al. Long-term follow-up of dynamic graciloplasty for faecal incontinence. Colorectal Dis. 2004;6(6):470–6. doi: 10.1111/j.1463-1318.2004.00714.x. [DOI] [PubMed] [Google Scholar]
- 9.Raghavan S, et al. Successful implantation of physiologically functional bioengineered mouse Internal Anal Sphincter. Am J Physiol Gastrointest Liver Physiol. doi: 10.1152/ajpgi.00269.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anitha M, et al. Characterization of fetal and postnatal enteric neuronal cell lines with improvement in intestinal neural function. Gastroenterology. 2008;134(5):1424–35. doi: 10.1053/j.gastro.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hashish M, et al. Surgical implantation of a bioengineered internal anal sphincter. Pediatric Surgery. 2010;45:52–58. doi: 10.1016/j.jpedsurg.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ganio E, et al. Injectable synthetic calcium hydroxylapatite ceramic microspheres (Coaptite) for passive fecal incontinence. Tech Coloproctol. 2008;12(2):99–102. doi: 10.1007/s10151-008-0406-x. [DOI] [PubMed] [Google Scholar]
- 13.Rattan S. The internal anal sphincter: regulation of smooth muscle tone and relaxation. Neurogastroenterol Motil. 2005;17(Suppl 1):50–9. doi: 10.1111/j.1365-2982.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 14.Rattan S, Chakder S. Role of nitric oxide as a mediator of internal anal sphincter relaxation. Am J Physiol. 1992;262(1 Pt 1):G107–12. doi: 10.1152/ajpgi.1992.262.1.G107. [DOI] [PubMed] [Google Scholar]
- 15.O'Kelly T, Brading A, Mortensen N. Nerve mediated relaxation of the human internal anal sphincter: the role of nitric oxide. Gut. 1993;34(5):689–93. doi: 10.1136/gut.34.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burleigh DE, D'Mello A, Parks AG. Responses of isolated human internal anal sphincter to drugs and electrical field stimulation. Gastroenterology. 1979;77(3):484–90. [PubMed] [Google Scholar]