Abstract
In previous studies we have observed that, in comparison with wild type mice, Tg-CYP2D6 mice have increased serum levels of bufotenine [5-hydroxy-N,N-dimethyltryptamine] following the administration of 5-MeO-DMT. Furthermore, following the injection of 5-MeO-DMT, harmaline was observed to increase serum levels of bufotenine and 5-MeO-DMT in both wild-type and Tg-CYP2D6 mice. In the present investigation, 5-MeO-DMT-induced stimulus control was established in wild-type and Tg-CYP2D6 mice. The two groups did not differ in their rate of acquisition of stimulus control. When tested with bufotenine, no 5-MeO-DMT-appropriate responding was observed. In contrast, the more lipid soluble analog of bufotenine, acetylbufotenine, was followed by an intermediate level of responding. The combination of harmaline with 5-MeO-DMT yielded a statistically significant increase in 5-MeO-DMT-appropriate responding in Tg-CYP2D6 mice; a comparable increase occurred in wild-type mice. In addition, it was noted that harmaline alone was followed by a significant degree of 5-MeO-DMT-appropriate responding in Tg-CYP2D6 mice. It is concluded that wild-type and Tg-CYPD2D6 mice do not differ in terms of acquisition of stimulus control by 5-MeO-DMT or in their response to bufotenine and acetylbufotenine. In both groups of mice, harmaline was found to enhance the stimulus effects of 5-MeO-DMT.
Keywords: drug-induced stimulus control; harmaline; 5-methoxy-N,N-dimethyltryptamine; drug interaction; CYP2D6; humanized mice
1. Introduction
Man’s knowledge of plant sources of hallucinogens predates written history [Schultes and Hofmann 1980]. However, it is only recently that chemical identification of the active principles of these botanicals became possible and pharmacological studies undertaken. The object of the present investigation, 5-methoxy-N,N-dimethyltryptamine [5-MeO-DMT], together with its close relative, N,N-dimethyltryptamine [DMT], accounts in large measure for the hallucinogenic effects of a variety of South American snuffs. However, 5-MeO-DMT is inactive when taken orally, presumably due to rapid first-pass deamination. Thus, the oral efficacy of preparations such as Ayahuasca is thought to be due to the presence of harmaline, a naturally-occurring inhibitor of mono amine oxidase [Gambelunghe et al. 2008; Shulgin and Shulgin 1997]. Although Ayahuasca enjoys legal status both in the United States and South America for use by members of the O Centro Espirita Beneficenti Uniao do Vegetal [Gonzales 2006], 5-MeO-DMT is included in schedule I of the Controlled Substances Act of the USA [Federal Register 2010].
Our previous studies [Yu et al. 2003; 2004] using recombinant human cytochrome 450 isozymes demonstrated that the formation of bufotenine from 5-MeO-DMT is primarily catalyzed by the CYP2D6 enzyme. In addition, we observed [Shen et al. 2010a; 2010b] that CYP2D6-humanized [Tg-CYP2D6] mice have 60% higher bufotenine levels in serum than wild-type mice following the administration of 5-MeO-DMT. Furthermore, pretreatment with harmaline led to higher levels of both 5-MeO-DMT and bufotenine in wild-type and Tg-CYP2D6 mice.
The study of psychoactive drugs in animals gives rise to questions of interpretation and extrapolation to the human condition. However, with the demonstration that the hallucinogens, LSD and mescaline, can function as discriminative stimuli in rats [Hirschhorn and Winter 1971], it was suggested that drug-induced stimulus control in non-verbal species might provide insight into their mechanisms of action [Winter 1974; for recent reviews, see Nichols 2004; Fantegrossi et al. 2008; Winter 2009]. It has previously been reported that 5-MeO-DMT can function as a discriminative stimulus in the rat [Glennon et al. 1981; Spencer et al. 1987; Winter et al. 2000] but we are aware of no previous studies of stimulus control by 5-MeO-DMT in mice.
The present investigation examined the acquisition of stimulus control by 5-MeO-DMT in both wild-type and Tg-CYP2D6 mice and the potentiation of the stimulus effects of 5-MeO-DMT by harmaline. In addition, the hypothesis that 5-MeO-DMT acts via bufotenine as its active principle was tested.
2. Methods
The behavioral methods employed in the present investigation are essentially as described previously [Winter et al. 2005; Krall et al. 2008].
2.1 Subjects
Age-matched male wild-type FVB/N and Tg-CYP2D6 mice [Corchero et al. 2001] were used in the study. Mice were housed in a temperature-controlled room under a constant 12:12 h light-dark cycle. All experiments were conducted during the light phase. Access to water was restricted to 20 min per day immediately after training and test sessions. Animals used in these studies were maintained in accordance with the U.S. Public Health Service Guide for the Care and Use of Laboratory Animals, 8th ed. All experimental protocols were approved by the Institutional Animal Care and Use Committee of the University at Buffalo.
2.2 Discrimination training
Four small animal test chambers [MED Associates ENV-307W-CT] were used for all experiments. These were housed in larger light-proof, sound-insulated boxes which contained a house light and an exhaust fan. Chambers contained two snout-poke modules [MED Associates ENV-3BM] mounted at opposite ends of one wall. Centered between the operanda was a dipper which delivered 0.1 ml of water. Sessions were managed by a micro-computer using operant control software [MED-PC State Notation, Version IV]. Subjects were trained to discriminate 5-MeO-DMT from saline using a pretreatment time of 15 min and a dose of 0.3 mg/kg [subcutaneous injection] extrapolated from previous work in our laboratory with 5-MeO-DMT in the rat [Winter et al. 2000]. A fixed ratio 10 [FR10] schedule of reinforcement was employed. Drug-induced stimulus control was assumed to be present when, in five consecutive sessions, 83% or more of all responses prior to the delivery of the first reinforcer were on the appropriate lever.
2.3 Test procedures
These were as previously described [Krall et al. 2005; Winter et al. 2005]. Briefly stated, after stimulus control was established with the training agents, tests were conducted once per week in each animal so long as performance did not fall below the criterion level of 83% correct responding in any one of the previous three training sessions. Half of the test sessions were conducted the day after saline training sessions with the remainder following 5-MeO-DMT training sessions. During test sessions, no responses were reinforced and the session was terminated after the emission of ten responses on either manipulandum. The distribution of responses between the two manipulanda was expressed as a percentage of total responses emitted on the drug-appropriate manipulandum. Response rate was calculated for each session by dividing the total number of responses emitted on both manipulanda by the elapsed time prior to 10 responses on either manipulandum. Pretreatment times refer to the elapsed time between drug administration and testing, e.g., a 20 min pretreatment time for harmaline means that it was given 5 min before 5-MeO-DMT when the latter was given using its usual 15 min pretreatment time.
2.4 Drugs
All drugs were dissolved in 0.9% saline solution and injected subcutaneously in a volume of 3.0 ml/kg bodyweight. 5-MeO-DMT and bufotenine were supplied by the National Institute on Drug Abuse [Rockville, MD, USA]. Acetylbufotenine was synthesized in our laboratories as previously described [Glennon et al. 1979].
2.5 Statistical analysis
Behavioral data were assessed for statistical significance using individual applications of unpaired and paired Student’s t-test and 1-way repeated measures analysis of variance [ANOVA] followed by pair-wise comparisons using the Holm-Sidak method. Differences were considered statistically significant if the probability of their having arisen by chance was < 0.05. All analyses were conducted using SPSS for Windows [SPSS Inc.].
3. Results
3.1. Acquisition of stimulus control in wild-type and Tg-CYP2D6 mice
Preliminary experiments determined that a dose of 5-MeO-DMT of 0.3 mg/kg did not alter response rates relative to control values. All mice then began training at that dose. Of the 6 WT mice trained, 5 reached criterion performance in a mean of 59 sessions [SE 4.3; range = 45–70]. The WT mouse which failed to reach criterion after 85 sessions was removed from the study. Response rates expressed as responses per minute in the mice reaching criterion were not significantly different following treatment with 5-MeO-DMT [mean = 54, SE 5.3] as compared with vehicle administration [mean = 52, SE 4.5]. Stimulus control in Tg-CYP2D6 mice was established in all 6 mice trained [52 sessions to criterion, SE 3.0; range = 42–60]. Mean response rates during acquisition in Tg-CYP2D6 mice were identical for the two treatment conditions [mean = 61, SE’s V 4.1, 5-MeO-DMT 4.9]. Excluding the one wild-type mouse which failed to reach criterion, there was no statistically significant difference between wild-type and Tg-CYP2D6 mice in terms of sessions to criterion. Likewise, rates of responding were not significantly different in the wild-type and Tg-CYP2D6 mice for either treatment condition. Following acquisition of stimulus control, the dose-response relationship ED50 values for 5-MeO-DMT were determined for each group of mice. The slopes of the regression lines were very similar and the ED50’s in the two groups were visually determined to be virtually identical at a dose of approximately 0.1 mg/kg.
3.2 Generalization to bufotenine and acetylbufotenine
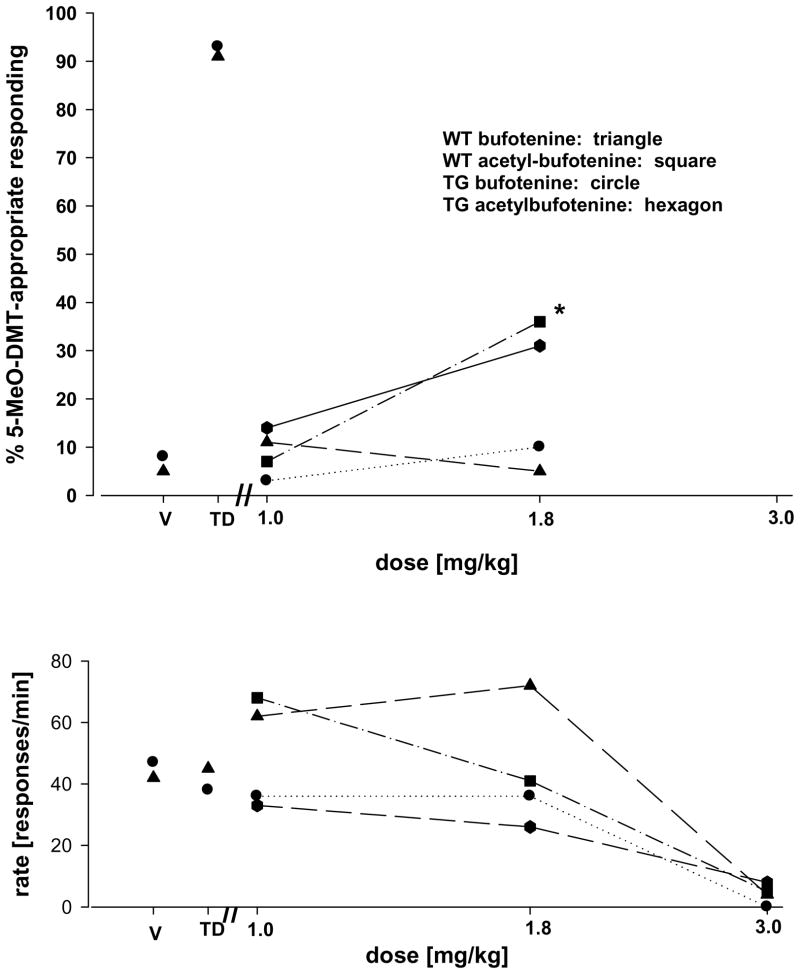
The results of generalization tests to bufotenine and to acetylbufotenine in wild-type and Tg-CYP2D6 mice are shown in Fig. 1. Following bufotenine, responding did not differ from the vehicle control. In contrast, visual inspection suggests a modest increase in 5-MeO-DMT-appropriate responding in both groups following a dose of 1.8 mg/kg of acetylbufotenine. However, our criteria for intermediate responding, i.e., significantly different from both training conditions, were achieved only in the Tg-CYP2D6 mice [1-way RM ANOVA: F(5,2)= 22.888; P < 0.001; pair wise comparisons: vehicle versus 1.8 mg/kg 5-MeO-DMT: P < 0.05; TD versus 1.8 mg/kg 5-MeO-DMT: P < 0.03]. In both groups, responding was suppressed at a dose of 3.0 mg/kg of either bufotenine or acetylbufotenine to a degree incompatible with behavioral testing.
Figure 1.
Generalization of 5-MeO-DMT to bufotenine and acetylbufotenine in WT and Tg-CYP2D6 mice. Tests of generalization of 5-MeO-DMT to bufotenine [WT: triangle; TG: circle] and acetylbufotenine [WT: square; TG: hexagon] in mice trained with 5-MeO-DMT [0.3 mg/kg, 15 min] as a discriminative stimulus. Each point represents the mean of one determination in each of 5 WT or 6 TG mice. The asterisk indicates a significant difference from both training conditions. Values given at the points labeled V and TD in the upper and lower panels are for the training conditions, vehicle and 0.3 mg/kg 5-MeO-DMT, respectively. The triangle and the circle refer to WT and TG mice, respectively. Ordinate: upper panel: percent 5-MeO-DMT-appropriate responding; lower panel: rate expressed as responses per minute. Abscissa: dose plotted on a log scale.
3.3 Interactions with harmaline in wild-type mice and in Tg-CYP2D6 mice
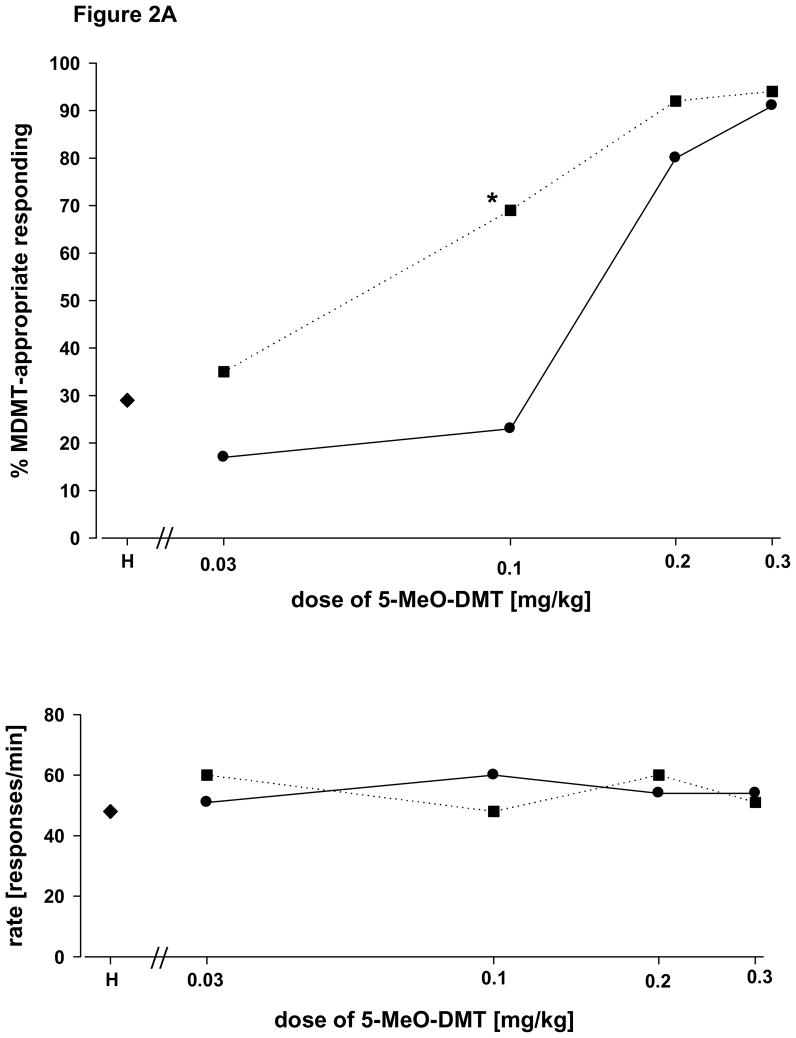
Figure 2A shows an orderly dose-related increase in 5-MeO-DMT-appropriate responding in WT mice trained and tested with 5-MeO-DMT. When the same doses of 5-MeO-DMT were tested in mice pretreated with a fixed dose of harmaline [0.1 mg/kg administered 5 min before 5-MeO-DMT], 5-MeO-DMT-appropriate responding increased for all doses of 5-MeO-DMT less than the training dose. At a dose of 0.1 mg/kg 5-MeO-DMT, the combination with harmaline yielded 5-MeO-DMT-appropriate responding which was significantly greater than that that following a dose of 0.1 mg/kg 5-MeO-DMT alone [paired t-test: P = 0.047]. No other comparisons reached statistical significance. It should be noted that in WT mice a dose of harmaline of 0.1 mg/kg when given alone was followed by a mean of 29% 5-MeO-DMT-appropriate responding.
Figure 2.
Figure 2A Interactions with harmaline in WT mice. Dose-response relationship for 5-MeO-DMT alone and in combination with harmaline. Circles represent the effects of 5-MeO-DMT alone in rats trained with 5-MeO-DMT as a discriminative stimulus [ mg/kg; 15 minute pretreatment time]. Squares represent the effects of 5-MeO-DMT in combination with harmaline [0.1 mg/kg; 20 minute pretreatment time]. Each point represents the mean of 2 determinations in each of 5 mice with the exception of the training dose where the mean of 6 determinations in each of the subjects is shown. The asterisk indicates a statistically significant difference between 5-MeO-DMT alone and in combination with harmaline [paired t-test; P = 0.047]. The point H on the abscissa is for harmaline alone at a dose of 0.1 mg/kg. Ordinate: upper panel: percent 5-MeO-DMT-appropriate responding. Lower panel: rate expressed as responses per minute. Abscissa: dose plotted on a log scale.
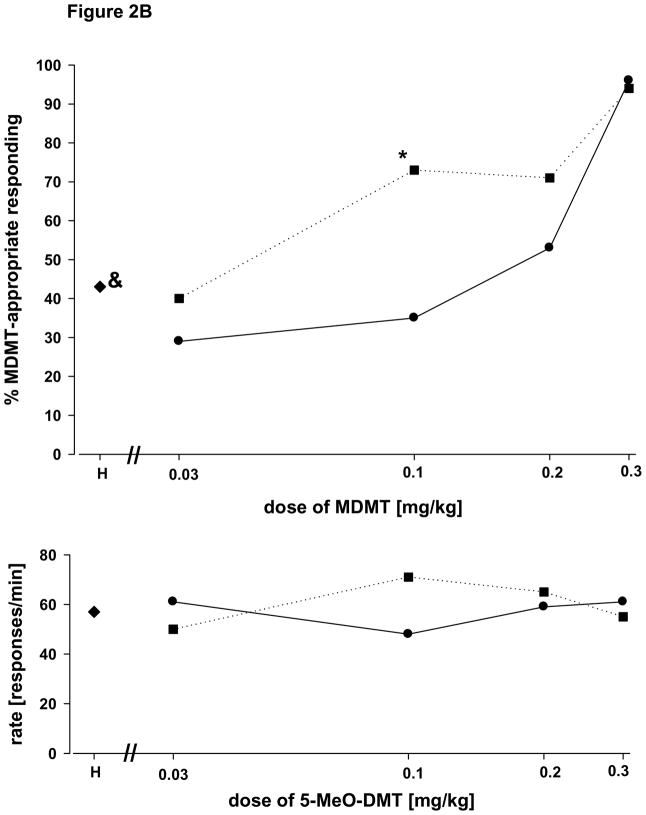
Figure 2B Interactions with harmaline in Tg-CYP2D6 mice. Each point represents the mean of 2 determinations in each of 6 mice with the exception of the training dose where the mean of 6 determinations in each of the subjects is shown. The asterisk indicates a statistically significant difference between 5-MeO-DMT alone and in combination with harmaline [paired t-test; P < 0.001]. The ampersand indicates a significant difference from both training conditions. All other details are as in figure 2A.
Figure 2B shows an orderly dose-related increase in 5-MeO-DMT-appropriate responding in Tg-CYP2D6 mice trained and tested with 5-MeO-DMT. When the same doses of 5-MeO-DMT were tested in mice pretreated with a fixed dose of harmaline [0.1 mg/kg administered 5 min before 5-MEO-DMT], 5-MeO-DMT-appropriate responding increased for all doses of 5-MeO-DMT less than the training dose. At a dose of 0.1 mg/kg 5-MeO-DMT, the combination with harmaline yielded 5-MeO-DMT-appropriate responding which was significantly greater than that following a dose of 0.1 mg/kg 5-MeO-DMT alone [paired t-test: P <0.001]. It should be noted that in Tg-CYP2D6 mice a dose of harmaline of 0.1 mg/kg when given alone was followed by a mean of 43% 5-MeO-DMT-appropriate responding. This value met our criteria for intermediate responding [1-way RM ANOVA: F(5,2)= 73,855; P < 0.001; pair wise comparisons: vehicle versus 0.1 mg/kg harmaline: P < 0.05; TD versus 0.1 mg/kg harmaline: P < 0.03]. In both WT and TG mice, the combination of 5-MeO-DMT at a dose of 0.1 mg/kg with harmaline at doses of 0.03 and 0.3 mg/kg resulted in no potentiation and suppression of responding, respectively. The data presented in figures 3A and 3B for the dose effect relationship for 5-MeO-DMT permit the determination of the ED50’s for the drug in WT and TG mice. The slopes of the regression lines very similar and the ED50’s in the two groups were visually determined to be virtually identical at a dose of approximately 0.1 mg/kg.
4. Discussion
The data obtained in training sessions indicate that 5-MeO-DMT at a dose of 0.3 mg/kg administered subcutaneously 15 min before training will establish stimulus control in both wild type and Tg-CYP2D6 mice. The fact that the two groups appear to be equally sensitive to 5-MeO-DMT in terms of rate of responding is in keeping with our previous observation [Shen et al. 2010] that wild-type and Tg-CYP2D6 mice do not differ in serum level of 5-MeO-DMT following IP administration of the drug.
The failure of bufotenine to mimic 5-MeO-DMT as seen in figure 1 is compatible with data previously obtained in the rat by Spencer et al. [1987]. Furthermore, bufotenine does not substitute for either LSD [Helsley et al. 1998] or psilocybin [Winter et al. 2007] in rats trained with the latter drugs. These results are plausibly explained on the basis of low lipid solubility [Gessner et al. 1968] with the consequence that only small amounts of bufotenine are found in rat brain following IV administration [Sanders and Bush 1967]. Furthermore, the hypothermic effects of bufotenine are antagonized by xylamidine, a peripherally acting serotonergic antagonist [Winter 1972].
The initial reports of hallucinogenic activity of bufotenine in man [Fabing 1956; Fabing and Hawkins 1956] were not replicated in a subsequent investigation [Turner and Merlis 1959]. Indeed, the issue of bufotenine as a hallucinogen has remained a matter of controversy [Shulgin and Shulgin 1997,pages 473–478; Ott 2001; Torres and Repke 2006]. If the production of bufotenine contributes to the stimulus effects of 5-MeO-DMT, we would predict, based on our previous observation that serum levels of bufotenine are 60% higher in Tg-CYP2D6 mice as compared with wild-type controls [Shen et al. 2010], that humanized mice would be more sensitive to 5-MeO-DMT. Based upon our acquisition data, this appears not to be the case. This conclusion in no way negates the hypothesis that bufotenine is pharmacologically active upon reaching the brain. Indeed, the intermediate results seen in Figure 1 for the more lipid soluble acetylbufotenine suggest greater activity for this drug in mice trained with 5-MeO-DMT and we have also observed an intermediate degree of substitution by acetylbufotenine for psilocybin in rats trained with the latter drug [Winter and Yu, unpublished]. A definite answer might be provided by [1] the training of acetylbufotenine as a discriminative stimulus and [2] the demonstration that acetylbufotenine is converted into bufotenine in brain.
In figures 2A and 2B it is seen that the combination of harmaline with 5-MeO-DMT results in an increase in 5-MeO-DMT-appropriate responding in both wild-type and Tg-CYP2D6 mice. Thus, for example, the addition of 0.1 mg/kg harmaline to a dose of 5-MeO-DMT of 0.1 mg/kg yields a statistically significant increase in both groups. However, interpretation of the data is confounded by the fact that harmaline alone at a dose of 0.1 mg/kg is followed by 5-MeO-DMT-appropriate responding of 29% and 43% in wild-type and Tg-CYP2D6 mice, respectively. Indeed, in Tg-CYP2D6 mice, the responding following harmaline is statistically significantly different from vehicle.
The simplest explanation for a potentiating effect of harmaline on the stimulus effects of 5-MeO-DMT is that harmaline acts to inhibit mono amine oxidase thus decreasing the inactivation of 5-MeO-DMT. Indeed, we have observed highly significant increases in serum levels of both 5-MeO-DMT and bufotenine following the administration of 5-MeO-DMT in combination with harmaline [Shen et al. 2010]. However, prior to the demonstration by Udenfriend et al. [1958] of the MAOI properties of harmaline, a variety of effects of harmaline were reported which, in retrospect, are quite possibly unrelated to inhibition of MAO. Most notable among these are tremorigenic actions as reported by Lewin [1928], Gunn [1935], and others. In a more recent example, we observed that, while the MAOI effects of harmaline, as indicated by the potentiation of the effects of tryptamine, persist for several hours in the rat, suppression of operant responding by harmaline is absent after 1 hour [Winter 1971]. Directly indicative of the psychoactivity of harmaline are reports in human subjects [Shulgin and Shulgin 1995] and the fact that it can be trained as a discriminative stimulus in the rat where it partially generalizes to the phenethylamine hallucinogen, DOM [Grella et al. 1998]. In our laboratories we observed that the stimulus effects of ibogaine, a naturally occurring psychoactive drug purported to be useful in the treatment of opiate addiction, generalize fully to harmaline [Helsley et al. 1997; 1998]. Furthermore, we have observed in rats trained with harmaline that LSD substitutes completely but clorgyline, a classic MAO inhibitor, at a range of doses up to and including a fully rate-suppressant dose does not [Winter and Yu, unpublished]. Taken together, these facts strongly suggest that harmaline per se is psychoactive and that these effects may be independent of inhibition of MAO.
5. Conclusions
The present investigation has demonstrated stimulus control by 5-MeO-DMT in both wild-type and Tg-CYP2D6 mice. The groups did not differ in sensitivity to the rate depressant effects of 5-MeO-DMT. Furthermore, the groups did not differ in their responses to the administration of either bufotenine or acetylbufotenine in that the former was without 5-MeO-DMT-like stimulus effects while the latter yielded intermediate effects. In both groups of mice, harmaline was found to increase the stimulus effects of an intermediate dose of 5-MeO-DMT, an effect consistent with activity as a mono amine oxidase inhibitor. However, previous findings as well as the present data suggest that harmaline possesses psychoactive properties in addition to inhibition of mono amine oxidase.
Research Highlights.
5-Methoxy-N,N-dimethyltryptamine is a naturally-occurring hallucinogen.
CYP2D6-humanized mice have increased levels of bufotenine following 5-MeO-DMT.
Stimulus control by 5-MeO-DMT was established in wild type and Tg-CYP2D6 mice.
Wild type and Tg-CYP2D6 mice did not differ in rate of acquisition of stimulus control.
5-MeO-DMT did not generalize to bufotenine and only partially to acetylbufotenine.
Harmaline, a mono amine oxidase inhibitor, enhanced the stimulus effects of 5-MeO-DMT.
5-MeO-DMT partially generalized to harmaline.
Acknowledgments
This study was supported in part by Award Number DA03385 [J.C. Winter] and DA021172 [A-M Yu] from the National Institute on Drug Abuse, National Institutes of Health. This report is dedicated to the memory of Stephen G. Holtzman [1943–2011], an esteemed colleague and a major contributor to the study of the stimulus effects of drugs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol. 2001;60:1260–7. doi: 10.1124/mol.60.6.1260. [DOI] [PubMed] [Google Scholar]
- Fabing HD. On going Berserk: a neurochemical inquiry. Am J Psychiatry. 1956;113:409–15. doi: 10.1176/ajp.113.5.409. [DOI] [PubMed] [Google Scholar]
- Fabing HD, Hawkins JR. Intravenous bufotenine injection in the human being. Science. 1956;123:886–7. doi: 10.1126/science.123.3203.886. [DOI] [PubMed] [Google Scholar]
- Federal Register 75 [#243] December 10, 2010. Schedules of controlled substances: Placement of 5-methoxy-N,N-dimethyltryptamine into Schedule I of the Controlled Substances Act.
- Fantegrossi WE, Murnane KS, Reissig CJ. The behavioral pharmacology of hallucinogens. Biochem Pharmacol. 2008;75:17–33. doi: 10.1016/j.bcp.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambelunghe C, Aroni K, Rossi R, et al. Identification of N,N-dimethyltryptamine and beta-carbolines in psychotropic ayahuasca beverage. Biomed Chromatogr. 2008;22:1056–9. doi: 10.1002/bmc.1023. [DOI] [PubMed] [Google Scholar]
- Gessner PK, Godse DD, Krull AH, McMullan JM. Structure-activity relationships among 5-methoxy-N,N-dimethyltryptamine, 4-hydroxy-N,N-dimethyltryptamine [psilocin], and other substituted tryptamines. Life Sci. 1968;7:267–77. doi: 10.1016/0024-3205(68)90200-2. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Gessner PK, Godse DD, Kline BJ. Bufotenine esters. J Med Chem. 1979;22:1414–16. doi: 10.1021/jm00197a025. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Rosecrans JA, Young R. Behavioral properties of psychoactive phenylisopropylamines in rats. Eur J Pharmacol. 1981;76:353–60. doi: 10.1016/0014-2999(81)90106-0. [DOI] [PubMed] [Google Scholar]
- Gonzales vs O Centro Espirita Beneficente União do Vegetal, United States Supreme Court, 2006.
- Grella B, Dukat M, Young R, Teitler M, Herrick-Davis K, Gauthier CB, Glennon RA. Investigation of hallucinogenic and related beta-carbolines. Drug alcohol Depend. 1998;50:99–107. doi: 10.1016/s0376-8716(97)00163-4. [DOI] [PubMed] [Google Scholar]
- Gunn JA. Relationship between chemical constitution, pharmacological actions, and therapeutic uses in the harmine group of alkaloids. Arch int Pharmacodyn Ther. 1935;50:379–96. [Google Scholar]
- Helsley SE, Rabin RA, Winter JC. The effects of noribogaine and harmaline in rats trained with ibogaine as a discriminative stimulus. Life Sci. 1997;60:PL147–53. doi: 10.1016/s0024-3205(96)00703-5. [DOI] [PubMed] [Google Scholar]
- Helsley SE, Fiorella D, Rabin RA, Winter JC. A comparison of N,N-dimethyltryptamine, harmaline, and selected congeners in rats trained with LSD as a discriminative stimulus. Prg Neuro-psychopharmacol Biol Psychiat. 1998;22:649–63. doi: 10.1016/s0278-5846(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Hirschhorn ID, Winter JC. Mescaline and lysergic acid diethylamide [LSD] as discriminative stimuli. Psychopharmacologia. 1971;22:64–71. doi: 10.1007/BF00401468. [DOI] [PubMed] [Google Scholar]
- Krall CM, Richards JB, Rabin RA, Winter JC. Marked decrease of LSD-induced stimulus control in serotonin transporter knockout mice. Pharmacol Biochem Behav. 2005;88:349–57. doi: 10.1016/j.pbb.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin L. Untersuchhungen uber Banisteria Caapi Spr. [Ein Sudamerikanisches Rauschmittel] N-SArchiv Expt Pathol Pharmakol. 1928;129:133–49. [Google Scholar]
- Nichols DE. Hallucinogens. Pharmacol Ther. 2004;101:131–81. doi: 10.1016/j.pharmthera.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Ott J. Pharmepéna-Psychonautics: Human intranasal, sublingual and oral pharmacology of 5-methoxy-N,N-dimethyl-tryptamine. J Psychoactive Drugs. 2001;33:403–7. doi: 10.1080/02791072.2001.10399925. [DOI] [PubMed] [Google Scholar]
- Sanders E, Bush MT. Distribution, metabolism and excretion of bufotenine in the rat with preliminary studies of its O-methyl derivative. J Pharmacol Exp Ther. 1967;158:340–52. [PubMed] [Google Scholar]
- Schultes RE, Hofmann A. The Botany and Chemistry of Hallucinogens. Springfield, IL, USA: Charles C. Thomas; 1980. [Google Scholar]
- Shen H-W, Wu C, Jiang X-L, Yu A-M. Effects of monoamine oxidase inhibitor and cytochrome P450 2D6 status on 5-methoxy-N,N-dimethyltryptamine metabolism and pharmacokinetics. Biochem Pharmacol. 2010a;80:122–8. doi: 10.1016/j.bcp.2010.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H-W, Jiang X-L, Winter JC, Yu A-M. Psychedelic 5-methoxy-N,N-dimethyltryptamine: metabolism, pharmacokinetics, drug interactions, and pharmacological actions. Curr Drug Metab. 2010b doi: 10.2174/138920010794233495. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulgin A, Shulgin A. TiHKAL, The Continuation. Transform Press; Berkeley: 1997. [Google Scholar]
- Spencer DG, Glaser T, Traber J. Serotonin receptor subtypes mediation of the interoceptive discriminative stimuli induced by 5-methoxy-N,N-dimethyltryptamine. Psychopharmacology. 1987;93:158–66. doi: 10.1007/BF00179927. [DOI] [PubMed] [Google Scholar]
- Torres M, Repke D. Anadenanthera: Visionary Plant of Ancient South America. New York: The Haworth Herbal Press; 2006. pp. 143–186. [Google Scholar]
- Turner WJ, Merlis S. Effect of some indolealkylamines on man. AMA Arch Neurol Psychiatry. 1959;81:121–9. doi: 10.1001/archneurpsyc.1959.02340130141020. [DOI] [PubMed] [Google Scholar]
- Udenfriend S, Witkop B, Redfield BG, Weissbach H. Studies with reversible inhibitors of monoamineoxidase: harmaline and related compounds. Biochem Pharmacol. 1958;1:160–5. [Google Scholar]
- Winter JC. Interaction of serotonin antagonists with harmaline-induced changes in operant behavior and body temperature in the rat. Arch int Pharmacodyn Ther. 1971;190:140–52. [PubMed] [Google Scholar]
- Winter JC. Xylamidine tosylate: Differential antagonism of the hypothermic effects of N,N-dimethyltryptamine, bufotenin, and 5-methoxytryptamine. Arch int Pharmacodyn Ther. 1972;198:61–6. [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli. Fed Proc. 1974;33:1825–1832. [PubMed] [Google Scholar]
- Winter JC. Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology. 2009;203:251–63. doi: 10.1007/s00213-008-1356-8. [DOI] [PubMed] [Google Scholar]
- Winter JC, Filipink RF, Timineri D, Helsley SE, Rabin RA. The paradox of 5-methoxy-N,N-dimethyltryptamine: A hallucinogen which induces stimulus control via 5-HT1A receptors. Pharmacol Biochem Behav. 2000;65:75–82. doi: 10.1016/s0091-3057(99)00178-1. [DOI] [PubMed] [Google Scholar]
- Winter JC, Kiers AK, Zimmerman MD, Reissig CJ, Eckler JR, Ullrich T, Rice KC, Rabin RA, Richards JB. The stimulus properties of LSD in C57BL/J mice. Pharmacol Biochem Behav. 2005;81:830–7. doi: 10.1016/j.pbb.2005.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter JC, Rice KC, Amorosi DJ, Rabin RA. Psilocybin-induced stimulus control in the rat. Pharmacol Biochem Behav. 2007;87:472–80. doi: 10.1016/j.pbb.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Herraiz T, Kupfer A, Gonzalez FJ. Screening for endogenous substrates reveals that CYP2D6 is a methoxyindoleamine O-demethylase. Pharmacogenetics. 2003;13:307–19. doi: 10.1097/01.fpc.0000054094.48725.b7. [DOI] [PubMed] [Google Scholar]
- Yu AM, Idle JR, Gonzalez FJ. Polymorphic cytochrome P4502D6: humanized mouse model and endogenous substrates. Drug Metab Rev. 2004;36:243–77. doi: 10.1081/dmr-120034000. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P4502D6: overview and update on pharmacology, genetics, biochemistry. N-S Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Masson J, Gingrich JA, Rayport S, Hen R. Targeted gene expression in dopamine and serotonin neurons of the mouse brain. J Neurosci Methods. 2005;143:27–32. doi: 10.1016/j.jneumeth.2004.09.020. [DOI] [PubMed] [Google Scholar]