Abstract
Breakdown in self-tolerance is due, in part, to a loss of regulatory T (Treg) cells. Recently, a controversy has surfaced about whether Treg cells are overwhelmingly stable, or if they can be reprogrammed in inflammatory and autoimmune environments. Those in the instability “camp” have shown that a fraction of Treg cells lose Foxp3 and acquire effector arm activities. Instability is coupled with IL-2 insufficiency and the inflammatory milieu that promote reprogramming. Here, we highlight the basic tenets of each viewpoint and discuss technical, biological and environmental differences in the models that may help yield a unifying hypothesis. Also considered is how Treg cell instability could link to development of autoimmune disease and the implications for Treg cell-based cellular therapy trials.
Background
In the mid 1990's a number of seminal papers by Sakaguchi et al. using a combination of cell depletion and adoptive transfer demonstrated the crucial role of thymically-derived CD4+ CD25+ cells for preventing and rescuing multi-organ autoimmunity and lethal systemic inflammatory and wasting disease [6, 7]. High expression of CD25, the alpha chain of the IL-2R, allowed the enrichment of regulatory suppressor T cells, termed Treg cells, in humans and mice [6, 8]. However, neither CD4 nor CD25 are uniquely expressed by Treg cells. These and other surface glycoproteins, such as CTLA-4, PD-1 and Nrp-1 are expressed on activated T effector cells as well making it impossible to fully trace the Treg cell lineage. Thus, investigators were limited to sorting the CD25 brightest cells with the risk of contaminating effector cells. A significant breakthrough in Treg cell research came with the discovery that the forkhead domain DNA-binding transcription factor (Foxp3), responsible for lethal scurfy disease in mice [9], was expressed specifically in mouse Treg cells [1, 10, 11] and essential for Treg cell development and function. In contrast, ectopic expression of Foxp3 in conventional CD4+ T cells conferred suppressive activity and induced the expression of the Treg cell-associated gene expression signature. Similarly, genetic mutation studies of Foxp3 in immunodysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome in humans confirmed the critical role of Foxp3 in human Tregs [12, 13].
However, the Foxp3 “marker” is not perfect. Foxp3 protein is expressed in most human CD4+ T cells after T cell activation with, in some cases, the cells developing transient suppressive activity [14, 15]. This is, at least in part, due to the fact that stable Foxp3 expression depends on DNAdemethylation of selected 5′ intronic regions, termed Treg-specific determining regions (TSDRs), of the Foxp3 gene [16]. In mice, if the TSDR that controls stable, inheritable Foxp3 is genetically deleted, Foxp3+ Treg cells are lost over time [17]. These results suggested that depending on the methylation status of Foxp3, protein expression might not be stable raising the possibility that Foxp3 expression is plastic potentially altering the balance of Treg cell to T effector cells. Foxp3 plasticity could also have direct pathogenic consequences as Treg cells and T conventional (Tconv) cell TCR repertoires are distinct in mice and humans [18, 19], with Tregs being skewed towards self-reactivity. If self-reactive Treg cells lose Foxp3 and gain effector function they could become pathogenic auto-reactive cells. Unfortunately, it has been problematic to easily distinguish Treg cells that have lost Foxp3 from those that never expressed Foxp3 in the first place. Thus, over the past several years, several groups have developed a number of novel Foxp3-reporter mice to track Treg cells with the goal of determining the stability of Treg cells and the potential of this cell subset to be reprogrammed under certain immune conditions.
Analysis of two strains of Foxp3-lineage tracer mice, revealed a subset of T cells that had lost Foxp3 expression and gained effector T cell attributes. This was initially surprising because Treg cells are a dominant facet of peripheral tolerance, thus, the loss of the Treg cell network would have devastating effects on immune homeostasis and potentially lead to systemic autoimmunity. However, full consideration of the results suggested that there might be an advantage at local sites of infection where “disabling” Treg cell function, even temporarily, might promote the adaptive immune response. Moreover, the finding that the majority of Treg cells are stable in healthy mice, while unstable Treg cells are most prominent under adverse conditions or genetic defects such as seen in autoimmune-prone mice, provide a potential unifying hypothesis that can accommodate the growing data in this area. Here, we highlight the conflicting data relating to Treg cell stability under normal and inflammatory situations, and discuss the relevance of this data within the context of our knowledge of Treg cell biology and in clinical circumstances where Treg cell instability may be contributing to immunity.
Is there direct evidence for Treg cell reprogramming?
Numerous studies have noted that adoptive transfer of GFP-tagged Treg cells, where the GFP fluorochrome is driven off the Foxp3 promoter, results in the appearance of GFP− T effectors cells. These experiments were carried out by adoptively transferring “purified” GFP+ Treg cells into immune deficient mice. This approach raised the concern that even small numbers of contaminating non-Treg cells might undergo homeostatic proliferation and account for the appearance of GFP− T cells [20]. Most recently, Foxp3 lineage marked mice have been developed using the Cre-loxP system. Specifically, Treg cells in bacterial artificial chromosome (BAC)-derived transgenic [3, 21] or knock-in [22, 23] mice express GFP-Cre driven off the Foxp3 promoter. By breeding these mice to specific reporter mice, which contain a transgene encoding an out-of-frame loxP site-flanked yellow fluorescent protein (YFP) or red fluorescent protein (RFP) inserted into the Rosa26 locus, the expressed Cre recombinase removes the loxP sites resulting in ‘gain of function’ permanent expression of reporter proteins [24, 25]. This ensures that a Foxp3+ cell that expressed the Cre will express heritable fluorescent protein for its lifetime, even if Foxp3 expression is extinguished [2, 3]. The Treg cells from these mice can be tracked over time in situ and after adoptive transfer. These mouse strains have been very useful in examining the stability of Treg cells.
The potential to visualize cells that had previously expressed Foxp3 but which were no longer Foxp3+, so called “exFoxp3” cells, was described recently [3]. In this study two YFP reporter mouse strains, either mixed background or non-obese diabetic (NOD) mice, showed that YFP+GFP-exFoxp3 cells could be detected in the spleen and lymph nodes. More importantly, the number of Treg cells that down-regulate GFP in the YFP+ exFoxp3 population was significantly increased during inflammation [3]. It was reported that 10-15% of Foxp3+ cells lose Foxp3 protein and a subset of these is permissive for IFNγ or IL-17 production. Furthermore, the YFP+ Foxp3− GFP− exFoxp3 cells are enriched in the inflamed pancreas of NOD mice at the onset of autoimmune inflammation [3].
The assertion that Treg cells can become unstable or reprogrammed, however, was recently challenged in a different study using Foxp3 lineage traced mice [2]. In this system, GFP-Cre-mutated human estrogen receptor fusion protein was knocked in to the endogenous Foxp3 locus (Foxp3GFP-Cre-ERT2). Foxp3-driven Cre translocated to the nucleus only after ligand binding by tamoxifen. Thus, Cre-recombinase functioned in a temporally controlled manner. As a consequence of Foxp3GFP-Cre-ERT2 activation, a subset of Treg cells was labeled and could be followed for their stability. In this setting, the investigators found >96% of the labeled Treg cells remained Foxp3-GFP+ over months under normal conditions and observed a decrease in Foxp3 expression only under an immune deficient setting. They studied the labeled polyclonal Treg cells in an infectious model, and TCR transgenic Treg cells in NOD mice, and concluded that there was minimal Foxp3 loss and no inflammatory cytokine production in Treg cells or exFoxp3 cells.
How can differences in the data from the two lineage tracing studies be explained? In the BACtransgenic studies, Cre was driven by the Foxp3 promoter from birth, and thus faithfully marks all Treg cells. In contrast, in the Foxp3GFP-Cre-ERT2 study, Cre activity was transiently induced in adult mice, marking approximately 30% of the Treg cells. The transient Cre recombinase expression likely labeled Treg cells that expressed high amounts of Foxp3, and these may be the most stable. Unstable Treg cells that are permissive for inflammatory cytokine production could have been present in the unlabelled Treg cells, but were not lineage-traced in the Foxp3GFP-Cre-ERT2 mice. On the other hand, Cre expression from birth may label cells that transiently express Foxp3-Cre during development, but which never become fully mature Treg cells and are interpreted as exFoxp3 cells (exTregs). However, BAC-Foxp3-GFP-Cre expression was consistent with normal Treg cell development in our studies. The transcript was expressed from the initiation of Treg cell development in the thymus and throughout maturation and T cell export. Indeed, GFP-Cre+ YFPlo cells arise in the thymus at the HSA-intermediate stage consistent with newly developing Treg cells [3]. It is thought that unlike human conventional T cells, mouse T cells lack promiscuous expression of Foxp3 during activation [26]. Our own data are consistent with this interpretation. When islet antigen-specific BDC2.5 TCR Tg CD4+ YFP− GFP− non-Treg cells are transferred into lymphopenic hosts, very few (0.3%) YFP+ cells were observed in the pathogenic effector T cell population infiltrating the islets [3]. We therefore conclude that the BAC-Foxp3-GFP-Cre lineage tracer marks Treg cells, and is not expressed in T effector cells.
Another caveat raised regarding the lineage tracing studies relates to the very nature of the lineage markers themselves – YFP, Cre and GFP. It has been suggested that the half-life of a reporter protein maybe an inaccurate readout of Foxp3 and Treg cell identity [27]. Thus, using the expression of GFP to reflect fluctuations in endogenous Foxp3 requires additional readouts. Additionally, it is possible that expression of non-self proteins, particularly the Cre recombinase enzyme which is toxic at high levels [28], may cause non-physiologic stress to the cell. This could result in increased apoptosis of Treg cells, or selective silencing of Cre-GFP transcription, or altered Foxp3 expression, resulting in an over-representation of YFP+ GFP− Treg cells. We do not believe that these caveats are likely to explain the differences observed between the Foxp3 lineage tracing studies. The number of Treg cells in the BAC-Foxp3-GFP-Cre was unchanged as compared to wild-type littermate controls, suggesting Treg cell death in the reporter mice was not significantly increased [21]. In addition, there is no evidence of the loss of immune regulation in our B6 BAC-Foxp3-GFP-Cre mice, or changes in autoimmune diabetes incidence on the NOD background. This suggests that the Treg cell network functions as normal in the reporter mice. Second, the proportion of GFP+ cells in the YFP+ population directly correlates with Foxp3 protein levels, the amount of Foxp3 protein in the Treg cells from reporter and wild type control mice were equivalent, and cytofluorometric cell sort purified YFP+ GFP− exTregs are >95% negative for Foxp3 protein by intracellular antibody detection [3]. This verifies that exTregs in the periphery of adult mice have down-regulated endogenous Foxp3 transcription and protein levels are low or absent, demonstrating faithful Foxp3-GFP-Cre expression.
It seems likely that the conflict is a consequence of looking at the same “elephant” from different perspectives. In fact, data from Foxp3GFP-Cre-ERT2 mice reported that after 5 months under homeostatic conditions, 2 – 4% of YFP marked Treg cells lost Foxp3-GFP expression. These results are more similar to, than different from, our study of BAC-Foxp3-GFP-Cre mice, which identified 10% exFoxp3 cells. Thus, whereas the focus of the Foxp3GFP-Cre-ERT2 study is that the majority of the Treg cells are stable, we highlight the small but significant population of cells that lose Foxp3 expression. This small population may be quite relevant as transfer of self-antigen reactive exFoxp3 cells caused rapid onset autoimmunity [3]. We don't know if the exFoxp3 cells carry auto-reactive-pathogenic potential in a mixed population, but this potential is important enough to investigate further. Add this to the fact that the Cre-recombinase lineage tracer, whether knocked into the endogenous Foxp3 locus, expressed transiently, or expressed on a BAC reveals exFoxp3 cells in each case, we believe they are a true population of exTregs.
Are subsets of Treg cells more susceptible to instability than others?
The Treg cell network is heterogeneous. In humans, bona fide Treg cells can be defined as activated or resting, the activated Treg cells have a high rate of turnover [18] and may generate exTregs. Treg cells are also comprised of two lineages; thymically-derived “natural” Treg (nTreg) cells, and peripherally-derived “adaptive” Treg (aTreg) cells. Several studies have implicated both lineages in the control of organ-specific autoimmunity. aTreg cells are derived from CD4+ conventional T cells, express Foxp3, and many but not all of the markers of nTreg cells (e.g. differential expression of the IKAROS family transcription factor, Helios) [29, 30]. In fact, it has been suggested that at least 30% of Foxp3+ Treg cells develop post-thymically [29]. Thus, fundamental differences may exist between the nTreg cells and aTreg cells that could lead to differential instability. For example, Treg cells induced from Tconv cells in vitro with exogenous TGFβ and IL-2, a model for mouse aTreg cells, are prone to Foxp3 instability and IL-17 production [31]. Interestingly, in our studies, about 30% of exTregs in the spleens of NOD mice produced IFNγ, and TCRα chain sequence analysis suggested that exTregs are derived from both nTreg cells and aTreg cells based on CDR3 sequences [3]. Thus, it is conceivable that the exTregs that are permissive for IFNγ production are generated from aTreg cells. Interestingly, a significant number of the TCR sequences identified in exTregs were not observed in either Treg cells or T conventional cells, suggesting that the environment pressures that cause Treg cell instability may alter the T cell repertoire.
Is the host environment key to the production of exTregs?
A number of extrinsic influences have been identified as affecting Treg cell stability. One example is the pro-inflammatory cytokine IL-6. IL-6 is crucial for the induction of αβ CD4+ IL-17+ cells in vitro and in vivo [32, 33], and is implicated in abrogating Treg cell control of T effectors in vitro [34], de-stabilizing Foxp3 expression in Treg cells in vitro [31], and re-programming Foxp3+ Treg cells in vivo to produce IL-17 [35, 36]. The opposite effect has been reported for the anti-inflammatory cytokine IL-10 that is important in retaining high Foxp3 expression in Treg cells during colonic inflammation, [20]. These are interesting observations because IL-6 and IL-10 signaling both depend on the activation of STAT3. Without STAT3, Treg cell function is abrogated: STAT3 deficiency in Treg cells results in loss of immune homeostasis and the selective alteration of genes implicated with suppressor function such as IL-10, Ebi3 and TGFβ [37]. How can a signaling molecule have such opposing effects? IL-10 also induces SOCS3 expression that suppresses IL-6 signaling pathways and is important for maintaining a regulatory phenotype. The subtlety of the phenotype of Treg cells will likely reflect the relative level of expression of transcription factors, which, in turn, are modulated by extracellular signals. In this regard, we reiterate that exTregs are most commonly observed at the site of inflammation consistent with a key role for inflammatory cytokines in controlling Treg cell stability.
Can the development of exTregs as a consequence of inflammation be linked to autoimmune disease progression?
There is ample evidence to suggest that loss of Treg cells can lead to autoimmunity. For instance, without hematopoietic stem cell transplants, IPEX patients die at a young age of a multi-organ autoimmunity caused by the abrogation of Treg cell suppression of autoreactive T effector cells. Similarly, attenuation of Foxp3 expression in mouse Treg cells results in altered expression of proteins key for Treg cell function and can be directly connected to loss of immune homeostasis. Anything that alters Foxp3 expression, even to a minor extent, can lead to autoimmunity [38]. Thus, as suggested above, inflammation plays a key role in creating Foxp3 instability leading to exacerbated autoimmune damage by autoreactive T effector cells. However, it is equally possible that the very T cells that down-regulate Foxp3, the exTregs, may play a direct role in the pathogenesis of autoimmunity. For instance, is it possible that increased Treg cell instability, perhaps as a consequence of genetic predisposition or the host environment, can act as a direct trigger for autoimmune disease? In the pancreas at the onset of autoimmune diabetes in non-obese diabetic (NOD) mice, the ratio of Treg:Teff cells is skewed towards Teff cells, indicating that Treg cells are losing the battle to destructive CD4+ T cells, specifically in the affected tissue [39]. The IL-2 axis is a key determinant for Treg cell stability and is altered in the NOD mouse genomic setting, which might lead to Treg cell instability and initiation of autoimmunity. In fact, Treg cells expressing the BDC2.5 transgenic TCR specific for an islet antigen have increased propensity for Foxp3 instability [3]. Moreover, Treg cells in NOD mice exhibit an increased loss of Foxp3 in the T cells in the islets of the pancreas, compared with other sites [3]. Islet resident Treg cells also display decreased expression of CD25, the IL-2Rα chain [39]. IL-2 is crucial for continued, stable expression of Foxp3 in Treg cells [40], and polymorphisms in loci containing IL-2 responsive genes are highly associated with T1D incidence which may be a consequence of Treg cell instability in the inflamed pancreas [41]. Lack of IL-2 has also been implicated in Treg cell reprogramming during infectious inflammatory responses. During lethal Toxoplasmosis, T effector cells lose the ability to produce IL-2 at the same time that Treg cell numbers drop and the remaining Treg cells produce IFNγ [42]. Inflammation has been implicated as triggering autoimmunity, and we recently showed that the Th1 promoting cytokine, IL-12, can induce IFNγ production from Treg cells, and that patients with type 1 diabetes have an increased number of IFNγ-producing Treg cells [43]. In this regard, the Th1-environment may also promote Treg cell instability in the in the affected tissue, contributing to autoimmunity.
Are exTregs that develop as a consequence of lymphopenia physiologically important?
Treg cells transferred into lymphocyte-deficient mice undergo rapid expansion, with approximately 50% of the cells losing Foxp3 expression within weeks of transfer. These exTregs become capable of developing into T cells of other lineages, such as Th1, Th17 and T follicular helper cells [44-46]. Treg cell instability is reduced when T effector cells are present, or in conjunction with IL-2 treatment. Thus, in a symbiotic relationship that is likely to ultimately control the balance of Treg cell to pathogenic T cells, activated conventional T cells inherently support the survival and function of Treg cells by producing IL-2 [47] and TNFα [48]. Thus, it is important to consider that Treg cells in lymphopenic patients may be more prone to instability. This can occur during bone marrow transplantation in two ways. First, any Treg cells that are transferred in the bone marrow inoculum might lose Foxp3 expression and potentially mediate graft versus host disease (GVHD). Alternatively, several studies have suggested that Treg cells are radioresistant and, thus, host Treg cells may expand after radiation treatment leading to an autoimmune syndrome in patients undergoing a bone marrow transplant. The latest concern in this regard, is a series of phase I trials currently underway examining the transfer of expanded polyclonal Treg cells into patients with advanced stage hemolytic malignancies undergoing bone marrow transplantation to prevent GVHD. In one study, patients received umbilical cord blood-derived expanded Treg cells as a phase I clinical trial to protect against acute GVHD [4]. Although no acute toxicity was reported in any patient receiving Treg cells [4], this may be a reflection of concomitant immune suppression or the use of cord blood Treg cells. Treg cells isolated from cord blood are essentially naive with high and stable Foxp3 expression. It will be crucial to determine if subtle changes of Foxp3 expression in the Treg cell compartment has adverse systemic affects in less acute disease states when Treg cell therapies are applied such as in IL-2-deficient settings such as type 1 diabetes, or other diseases where lymphopenia is common such as rheumatoid arthritis and systemic lupus erythematosus.
Does the development of exTregs make teleological sense?
What would be the teleological reason for Treg cell reprogramming? Treg cell instability may be important for the generation of effector T cells in response to pathogens. There are numerous examples of Treg cells suppressing effector responses giving advantage to the pathogen [49] [50]. Close examination of the TCR specificity of the Treg cells may reveal pathogen–specific Treg cells exhibit reduced Foxp3 expression and regulatory activity to allow more efficient T effector responses during priming. As mentioned in the autoimmune setting, the Treg cells or exTregs themselves may produce inflammatory cytokines to contribute to pathogen clearance. One example of this is during lethal infection with Toxoplasma gondi, Treg cell numbers drop and the remaining Treg cells produce IFNγ [42], IFNγ production by the Treg cells is likely an attempt to control the infection. Scenarios where inflammatory cytokines, such as IL-6 produced by toll-like receptor ligand activated antigen presenting cells is present locally, may promote Treg cell instability and efficient effector cell expansion [34]. Whether Foxp3 instability is stochastic, or reversible is not known. Reversible Treg cell instability may drive, or be in response to the resolution of inflammatory adaptive responses such as during chronic pathogenic infections or relapsing-remitting EAE [51].
Final thoughts
Recent concepts of plasticity in T effector cell subsets are extending to Treg cells. It makes sense that not all cells within a population are terminally differentiated, and can change in response to extrinsic cues. Reviewing the evidence we believe that the majority of Treg cells are stable and give rise to suppressor daughter cells, but that subsets of the Treg cell network possess the ability to down-regulate Foxp3 expression and regulatory function and become permissive for inflammatory cytokine production. In every example we are aware of, Treg cell therapy in animal models result in suppression of the T effector responses. No autoimmune inflammatory disease has been made worse by Treg cell transfer. Therefore, clinical therapies with Treg cells remain very attractive. However, after 30 years of research, we still don't understand the triggers of autoimmunity. The association of Treg cell instability at the onset of autoimmunity gives clues that this may be a factor in the progression of autoimmunity. Whether Treg cell instability is a cause or driver of autoimmunity should be determined to further our understanding of immune modulating therapeutics.
Box 1. Conditions that cause Treg instability.
| Model | Reference | Clinical consequence |
|---|---|---|
| Lymphopenia Transfer into RAGko mice |
Tsuji, M. et. al. 2009 Zhou, X. et. al. 2009 |
HIV : improve anti-viral response Chemical induced ; chemotherapy, improve anti-tumor response. BMT, promote GVHD response. Autoimmunity. |
| Infection and autoimmunity |
Oldenhove, G. et. al. 2009 Sharma, M.D. et al. 2010 Zhou, X. et. al. 2009 |
Improve anti viral response. Contribute to pathology. Promote autoimmunity |
| Treg sub-populations : aTreg, memory Treg, CD25low Treg |
Komatusu, N. et. al. 2009 Yang, X. O. et. al. 2008 Miyara, M. et. al. 2009 |
Unknown ; altered Treg ratio's after lymphopenia / lymphocyte ablation therapy |
Abbreviations ; Treg, CD4+ regulatory T cell. RAGko, recombinase activating gene knock out. HIV, human immunodeficiency virus. BMT, bone marrow transplant. GVHD, graft versus host disease.
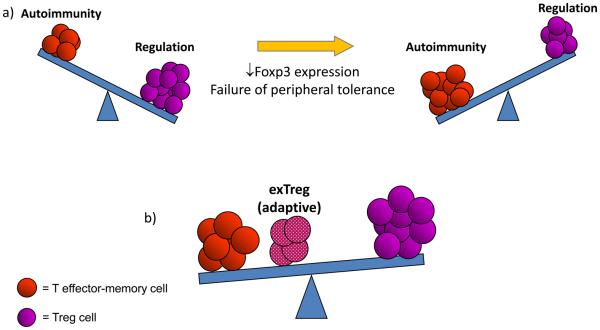
Figure 1. Balancing the immune system in health and disease.
This figure illustrates the fine balance between pathologic and regulatory pathways in immune homeostasis. a) The number and function of Treg cells, influenced by the stable expression of Foxp3, control this balance. A loss of Foxp3 expression can shift the balance leading to the development of autoimmunity. b) The production of exTregs from the adaptive Treg cell pool can combine with T effector-memory (TEM) cells and can shift the balance towards autoimmunity by helping lead to the failure of tolerance.
Acknowledgments
The authors would like to thank Lukas Jeker, M.D. Ph.D., and Mahesh Yadav, Ph.D. for critically reading this manuscript, and members of the Bluestone Laboratory past and present for contributing to the work discussed in this article. The work discussed in this commentary has been support by grants from NIAID, NIDDK and the JDRF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontenot JD, et al. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 2.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brunstein CG, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brusko TM, et al. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 7.Asano M, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baecher-Allan C, et al. CD4+CD25+ regulatory cells from human peripheral blood express very high levels of CD25 ex vivo. Novartis Found Symp. 2003;252:67–88. doi: 10.1002/0470871628.ch6. discussion 88-91, 106-114. [DOI] [PubMed] [Google Scholar]
- 9.Brunkow ME, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 10.Hori S, et al. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 11.Khattri R, et al. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4:337–342. [PubMed] [Google Scholar]
- 12.Bennett CL, et al. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 13.Wildin RS, et al. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 14.Pillai V, et al. Transient regulatory T-cells: a state attained by all activated human Tcells. Clin Immunol. 2007;123:18–29. doi: 10.1016/j.clim.2006.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tran DQ, et al. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110:2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huehn J, et al. Epigenetic control of FOXP3 expression: the key to a stable regulatory T-cell lineage? Nat Rev Immunol. 2009;9:83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 17.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Hsieh CS, et al. Recognition of the peripheral self by naturally arising CD25+ CD4+ T cell receptors. Immunity. 2004;21:267–277. doi: 10.1016/j.immuni.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 20.Murai M, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J Exp Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 23.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 24.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luche H, et al. Faithful activation of an extra-bright red fluorescent protein in “knockin” Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- 26.Sakaguchi S, et al. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol. 2010;10:490–500. doi: 10.1038/nri2785. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt-Supprian M, Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 28.Loonstra A, et al. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci U S A. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feuerer M, et al. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci U S A. 2010;107:5919–5924. doi: 10.1073/pnas.1002006107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang XO, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 33.Ghoreschi K, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, et al. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 36.Sharma MD, et al. Reprogrammed foxp3(+) regulatory T cells provide essential help to support cross-presentation and CD8(+) T cell priming in naive mice. Immunity. 2010;33:942–954. doi: 10.1016/j.immuni.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 39.Tang Q, et al. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Setoguchi R, et al. Homeostatic maintenance of natural Foxp3(+) CD25(+) CD4(+) regulatory T cells by interleukin (IL)-2 and induction of autoimmune disease by IL-2 neutralization. J Exp Med. 2005;201:723–735. doi: 10.1084/jem.20041982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamanouchi J, et al. Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet. 2007;39:329–337. doi: 10.1038/ng1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McClymont SA, et al. Plasticity of human regulatory T cells in healthy subjects and patients with type 1 diabetes. J Immunol. 2011;186:3918–3926. doi: 10.4049/jimmunol.1003099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Komatsu N, et al. Heterogeneity of natural Foxp3+ T cells: a committed regulatory T-cell lineage and an uncommitted minor population retaining plasticity. Proc Natl Acad Sci U S A. 2009;106:1903–1908. doi: 10.1073/pnas.0811556106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 2009;323:1488–1492. doi: 10.1126/science.1169152. [DOI] [PubMed] [Google Scholar]
- 46.Duarte JH, et al. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur J Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 47.Almeida AR, et al. Homeostasis of peripheral CD4+ T cells: IL-2R alpha and IL-2 shape a population of regulatory cells that controls CD4+ T cell numbers. J Immunol. 2002;169:4850–4860. doi: 10.4049/jimmunol.169.9.4850. [DOI] [PubMed] [Google Scholar]
- 48.Grinberg-Bleyer Y, et al. Pathogenic T cells have a paradoxical protective effect in murine autoimmune diabetes by boosting Tregs. J Clin Invest. 2010;120:4558–4568. doi: 10.1172/JCI42945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–589. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 50.Grainger JR, et al. Helminth secretions induce de novo T cell Foxp3 expression and regulatory function through the TGF-beta pathway. J Exp Med. 2010;207:2331–2341. doi: 10.1084/jem.20101074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kohm AP, et al. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]