Abstract
In general, faster infusions of cocaine are more likely to support behavior related to abuse than are slower infusions. However, some studies of cocaine self-administration in rats have failed to support this finding, possibly because the effect was masked by other factors. One such factor may be the pairing of a stimulus with the infusion, a procedure that is known to facilitate acquisition of drug self-administration. We compared fast and slow infusions by allowing groups of rats to acquire cocaine self-administration at a dose of 1 mg/kg/infusion, delivered over different durations (1.8 or 100 sec). Two groups were trained with either short or long infusions paired with a visual stimulus change (lights off), and two other groups were trained with short or long durations but with no stimulus change. Both groups trained with a paired stimulus acquired cocaine self-administration. With no stimulus change, the rats trained with the 1.8-sec infusion acquired cocaine self-administration at a rate comparable to the two groups that were trained with a paired stimulus. However, most rats in the group trained with the 100-sec infusion that was not accompanied by a stimulus change failed to acquire cocaine self-administration. The stimulus itself did not support responding. These results indicate that infusing a given dose of cocaine over a longer duration reduces its ability to support self-administration, but drug-paired stimuli can partially mask this effect by enhancing the effectiveness of slow infusions.
Keywords: Cocaine, self-administration, infusion duration, acquisition, rat
Substantial clinical and preclinical evidence supports the "rate hypothesis," which states that the speed of onset of a drug can have a substantial effect on behavior related to abuse (Gorelick, 1998). For example, clinical laboratory studies have shown that rapidly delivered drugs produce more positive subjective effects in humans. This has been shown with a variety of drugs, including cocaine (Abreu et al., 2001; Nelson et al., 2006; Volkow et al., 2000), diazepam (de Wit et al., 1993), pentobarbital (de Wit et al., 1992), methyphenidate (Kollins et al., 1998; Volkow et al., 2002), morphine (Marsch et al., 2001) and alprazolam (Mumford et al., 1995). These reports support clinical case reports that also suggest that drugs that have a more rapid onset are more likely to be abused or to produce more severe symptoms of dependence (e.g., Gossop and Strang, 1992; Hatsukami and Fischman, 1996).
Most research on this phenomenon in animals has focused on drug self-administration. In this context, drugs that are given more rapidly or that have an inherently faster onset are more likely to be self-administered or to maintain higher levels of self-administration. For example, when the same dose of cocaine is administered rapidly it will support higher rates of self-administration responding than cocaine given more slowly (Balster and Schuster, 1973; Kato et al., 1987; Panlilio et al., 1998; Schindler et al., 2009; Woolverton and Wang, 2004). Similar effects are observed with other drugs that have a mechanism of action similar to cocaine, but that have different inherent onset times. That is, compounds that have a slower onset than cocaine are not as likely to be self-administered, or they maintain lower rates of responding than cocaine (Kimmel et al., 2007, 2008; Woolverton et al., 2002). Winger et al. (2002) studied NMDA agonists with different inherent onset times and found that compounds with the fastest onset times supported greater self-administration behavior. Similarly, Wee et al. (2006) studied a number of cocaine analogs with different inherent onset times and compared them to cocaine in rhesus monkeys. They found that those compounds with slower onsets were associated with a weaker reinforcing effect as measured by self-administration. Unlike onset of action, duration of action does not appear to affect a drug’s ability to support self-administration (Ko et al., 2002; Panlilio and Schindler, 2000).
In addition to self-administration, speed of onset can also affect other behaviors that may be related to abuse. Cocaine is more likely to produce increases in locomotor activity when given rapidly (Brown and Kiyatkin, 2005; Desai et al., 2005) and locomotor sensitization to cocaine is more likely with a rapid infusion (Samaha et al., 2002, 2004). Further, it has recently been shown that addiction-like escalation of cocaine self-administration during long-access conditions is more likely if cocaine is given rapidly (Wakabayashi et al., 20010).
Despite this evidence, several research reports have failed to support the rate hypothesis. In rats, manipulations of cocaine infusion duration did not appear to affect either the acquisition or maintenance of cocaine self-administration (Crombag et al., 2008; Liu et al., 2005; Wakabayashi et al., 2010), although when given a choice, rats readily choose cocaine given more rapidly over cocaine given more slowly (Schindler et al., 2009). In primates a number of drugs that have relatively slow onset times are self-administered (Lile et al., 2003). These results suggest that factors other than the pharmacodynamic and pharmacokinetic effects of the drug may also play into whether a drug is self-administered.
One of these factors may be whether a stimulus change accompanies the presentation of the self-administered drug. For the relatively weak reinforcer nicotine, a stimulus paired with the nicotine seems to be crucial to whether it will be self-administered by rats (Caggiula et al., 2002). With a reinforcer like cocaine, the stimulus may not be as important (Carelli and Ijames, 2000; Derouche-Gamonet et al., 2002), although even for cocaine the stimulus can facilitate self-administration at lower self-administration doses (Schenk and Partridge, 2001). Lower cocaine doses are also more susceptible to manipulations such as a delay between the response and the reinforcer (Beardsley and Balster, 1993). When cocaine is delivered more slowly, it may be functionally similar to a lower cocaine dose or delayed reinforcement. Therefore, the presentation of a stimulus change with the cocaine injection may facilitate the self-administration of cocaine when given over a longer duration.
In the rat study by Liu et al. (2005) where delivery rate did not greatly affect cocaine self-administration, the cocaine injections were signaled by the retraction of the response lever. In the Crombag et al. (2008) study it was not clear if a stimulus was associated with the drug injection, but in the Wakabyashi et al. (2010) study there was no stimulus change associated with the injection. Overall, these finding suggest that a stimulus associated with the cocaine injection may facilitate the acquisition of self-administration when cocaine is given more slowly. To directly evaluate this possibility in the current study we investigated the acquisition of cocaine self-administration in rats trained with either a fast infusion speed or a slow infusion speed and with either an accompanying stimulus change (lights going off) or no stimulus change. The difference between acquisition under fast and slow infusion speeds was relatively small when there was a stimulus change, but robust when there was no stimulus change, suggesting that a stimulus can mask the effects of infusion speed. Testing of additional groups indicated that the stimulus change itself did not support responding under the conditions used here.
1. METHOD
1.1 Subjects
The subjects were experimentally naïve, male Sprague-Dawley rats (n=63), weighing approximately 350–380 g at the beginning of the experiment. Rats were individually housed in a room with a 12:12 h reverse light-dark cycle (lights on at 2200 h), at an average temperature of 23° C. The animals had free access to water, but food was restricted in order to maintain a weight of approximately 350 g. The guidelines of the Institutional Animal Care and Use Committee at the National Institute on Drug Abuse/Intramural Research Program and the Guide for the Care and Use of Laboratory Animals were followed at all times.
Jugular-vein catheters were implanted for intravenous (i.v.) drug administration. The catheters were made of approximately 3 cm of Silastic tubing (Dow Corning, 0.44 mm i.d., 0.9 mm o.d.) connected to vinyl tubing (Dural Plastics, 0.5 mm i.d., 1.0 mm o.d.) and were inserted into the right jugular vein. The tubing exited through the back in the mid-scapular region and was plugged with an obturator. All animals were given at least 7 days to recover before beginning training. The procedure followed for catheter implantation was described in detail by Panlilio et al. (1996). Immediately following catheter implantation, an incision was made posterior to the exit site of the catheter and a 20 mm nylon screw was inserted serving as a back mount. Catheters were flushed before and after each training session with 0.2 ml of a saline solution. If catheters became difficult to flush or it there was a question as to whether it remained operable, the catheter would then be tested using an injection 0.1 ml of 15 mg/ml of i.v. ketamine. An immediate loss of consciousness by the rat was taken as evidence of an operable catheter. If a catheter was discovered to be inoperable during the course of the experiment, an attempt was made to implant a second catheter in the left jugular vein. If this catheter also failed, the subject was dropped from the experiment. A total of 9 rats were dropped from the study due to catheter failure.
1.2 Apparatus
Eight training chambers (ENV-008CT, Med Associates, St Albans VT) were used, each of which had a grid floor and two nose-poke response holes, which could be illuminated from inside the hole by a dim yellow light. (ENV-114BM, Med Associates). Each of the chambers was individually enclosed in sound-attenuation chambers equipped with fans for ventilation and background noise (ENV-018M, Med Associates). A houselight (ENV-215M, Med Associates) that could illuminate the entire chamber was situated above the wall behind nose-poke holes. The offset of the houselight and nose-poke hole lights served as the stimulus.
A metal spring was suspended from the ceiling, from a single-channel fluid swivel (Instech, Plymouth Meeting, PA). Cocaine was delivered through Tygon tubing inserted through this metal spring. The metal spring was attached to the back mount on the rat to prevent the catheter from disconnecting during training sessions. The tubing from the fluid swivel was connected to a syringe that was controlled by a fast or slow motor-driven pump (PHM-100, Med Associates) located outside of the chamber. A 30 ml syringe was used for the fast pump, configured to administer a 1.8 s infusion and a 20 ml syringe was used for the slow pump, which was configured to administer a 100 s infusion. Both pumps delivered the same concentration and volume (0.3 ml/injection) of cocaine solution. All experimental events were controlled and recorded by a MED-PC computer system (Med Associates).
1.3 Procedure
1.3.1 Experiment 1
Rats were placed in training chambers once a day every weekday. At the beginning of each session, the houselight and the two nose-poke holes were illuminated. A response in the correct nose-poke hole was immediately reinforced with either a 1.8 sec fast (n = 8) or 100 sec slow (n = 9) infusion of 1.0 mg/kg of cocaine. Responses in the other nose-poke hole had no programmed consequence, but were counted. The nose-poke holes (left or right) that were reinforced were counterbalanced across subjects. Each response was also followed by a 110 s time out period, during which the houselights and nose-poke hole lights were turned off. The timeout and the injection began simultaneously. Responses during the timeout were counted, but had no programmed consequence. All rats completed 15 days of training. Sessions were two and a half hours in length.
1.3.2 Experiment 2
The procedures used in this experiment were identical to Experiment 1 with the exception of the stimulus change associated with the cocaine injection. Following the reinforced response, the houselights and nose-poke hole light remained illuminated for the duration of the 110 s timeout. Different groups of rats were trained with the fast (n = 10) and slow (n = 8) infusion durations.
1.3.3 Experiment 3
To determine whether the stimulus change itself could support nose-poking, a separate group of 11 rats were presented with only the stimulus change following active nose-pokes (stimulus-only group). For 8 other rats, nose-poking in either hole had no consequence (no-stimulus, no-drug group), but the holes were randomly assigned as active and inactive prior to the beginning of training, and for the purpose of data collection responses in the active hole produced unsignaled timeout periods to parallel the other five groups. All other stimulus conditions were the same as for the first 2 experiments and rats were trained for 15 sessions. These animals had surgery placing a nylon screw on the back to allow them to be connected to the tether, but did not have jugular vein catheters. No injections were given for active-hole responding for the rats in the stimulus-only group, but the pump did operate. There was no consequence of nose-poking in either hole for the no-stimulus, no-drug group.
1.4 Drugs
Cocaine hydrochloride (NIDA, Baltimore, MD) was dissolved in sterile saline. The concentration of the solution was adjusted according to the weight of each rat to give 1.0 mg/kg/injection (as the salt) during self-administration sessions.
1.5 Data Analysis
The primary dependent variable was the number of responses in the active and inactive nose-poke holes. Timeout responses were counted separately. Statistical analysis was performed on responses per session using SAS software (SAS Institute, Cary, NC) with the Proc Mixed procedure. Responses per session were analyzed in a full factorial design, with the factors being Hole (active vs inactive), Speed (Slow, Fast and No drug), Stimulus (stimulus change vs no stimulus change) and Session (1 – 15). Planned comparisons were performed using the Holms correction procedure, maintaining a significance level of 0.05 within each set of comparisons. Timeout responses were analyzed only for the drug groups in a manner that paralleled the analysis described above, with factors of Hole (active vs inactive), Speed (Slow vs Fast), Stimulus (stimulus change vs no stimulus change) and Session (1 – 15). For the trials-to-criterion analysis, groups were compared with an unequal variance t-test.
2. RESULTS
2.1 Experiment 1: Acquisition With a Cocaine Associated Stimulus
Figure 1 shows the active and inactive responses for those rats trained with a stimulus (light offset) paired with the infusion of cocaine. Rats trained with both the fast and slow infusion speed acquired the response as indicated by higher rates of active than inactive responding. However, there was some indication acquisition of cocaine self-administration occurred more rapidly and reached a higher level with the fast infusion. In the fast group active response rates were significantly increased relative to inactive responses beginning on day 4 and continuing to be significantly different on days 7–15. For the slow group active response were significantly higher than inactive first on day 8 and only one day thereafter. In addition, the overall level of active responses for the slow group was only 65% as high as in the fast group.
Fig. 1.
Active and inactive responses for those rats trained with a stimulus change following the reinforced response. Results shown in the top panel are for rats (n = 8) that received cocaine given over 1.8 sec (Fast). Results shown in the bottom panel are for rats (n = 9) that received cocaine given over 100 sec (Slow). Each point is the mean ± s.e.m. *p < 0.05 from inactive responses on that training day.
To further evaluate the speed of acquisition, we calculated the number of sessions to criterion based on when the subjects made at least 10 active hole responses with at least 80% of all responses in the active hole on two consecutive sessions. Seven of the 8 rats trained with the fast infusion met this criterion and 5 of the 9 rats trained with the slow infusion met this criterion. When a value of 16 sessions was given for any rat not meeting criterion, the fast infusion (6.8 ± 1.7) rats met the criterion in an average of nearly 3.5 days faster than the rats trained with the slow infusion (10.2 ± 2.0), however, this effect was not significant.
2.2 Experiment 2: Acquisition Without a Cocaine Associated Stimulus
Figure 2 shows the active and inactive responses for rats trained without the infusion-paired stimulus. Rats trained with the fast infusion again acquired the response, as indicated by the differences between active and inactive responses (see asterisks in Fig. 2). In contrast, rats trained with the slow infusion failed to show a difference between active and inactive responses, and average active responses remained below 10 throughout training. In general, inactive responses were initially higher in training for the no-stimulus groups when compared with the stimulus groups (compare Figures 1 and 2), while the final level of inactive responding was similar for both the stimulus and no-stimulus fast infusion groups.
Fig. 2.
Active and inactive responses for those rats trained without a stimulus change following the reinforced response. Results shown in the top panel are for rats (n = 10) that received cocaine given over 1.8 sec (Fast). Results shown in the bottom panel are for rats (n = 8) that received cocaine given over 100 sec (Slow). Each point is the mean ± s.e.m. *p < 0.05 from inactive responses on that training day.
Using the same trials-to-criterion measure described above, 8 of the 10 rats trained with the fast infusion met criterion by the end of training. Only 2 of the 8 slow infusion rats met criterion. Using a value of 16 sessions for any rat that failed to meet criterion, rats in the fast infusion group met criterion in an average of nearly 6 days sooner than the slow infusion rats (i.e., in 8.3 ± 1.6 days versus 14.3 ± 1.2 days, respectively), an effect that was significant (t14.5 = 2.25, p < 0.05).
2.3 Timeout Responding for Drug Groups
Rates of timeout responding were not significantly affected by Hole, Session, Speed, or any of their interactions in either of the two experiments. Since there was no signal for the beginning of the timeout in the no-stimulus groups, it may be expected that more timeout responses would have been observed with these groups. While a large amount of responding was observed on individual days for a few rats (as indicated by the larger variance), in general rates of timeout responding were not greatly affected by the absence of an infusion-paired stimulus for either the fast or slow infusion groups (data not shown).
2.4 Experiment 3: Responding for the Stimulus Alone
Over the course of training (data not shown), the responding of rats that received no cocaine but only a stimulus change (stimulus only group) or that received no stimulus change or cocaine (no-stimulus, no-drug group) was comparable to that rats that received slow cocaine infusions with no-stimulus change (Figure 2, lower panel). Using the same trials-to-criterion measure described above, only 2 of the 11 rats in the stimulus only group met criterion. Using a value of 16 sessions for any rat that failed to meet criterion, rats in the stimulus only group meet criterion in about the same number of days as the rats in the slow infusion, no-stimulus groups (i.e. 14.0 ± 1.4 days). Responding for the no-stimulus, no drug group was similarly low throughout training. One of these rats met criterion (group mean 14.5 ± 1.5).
2.5 Comparison of the Groups
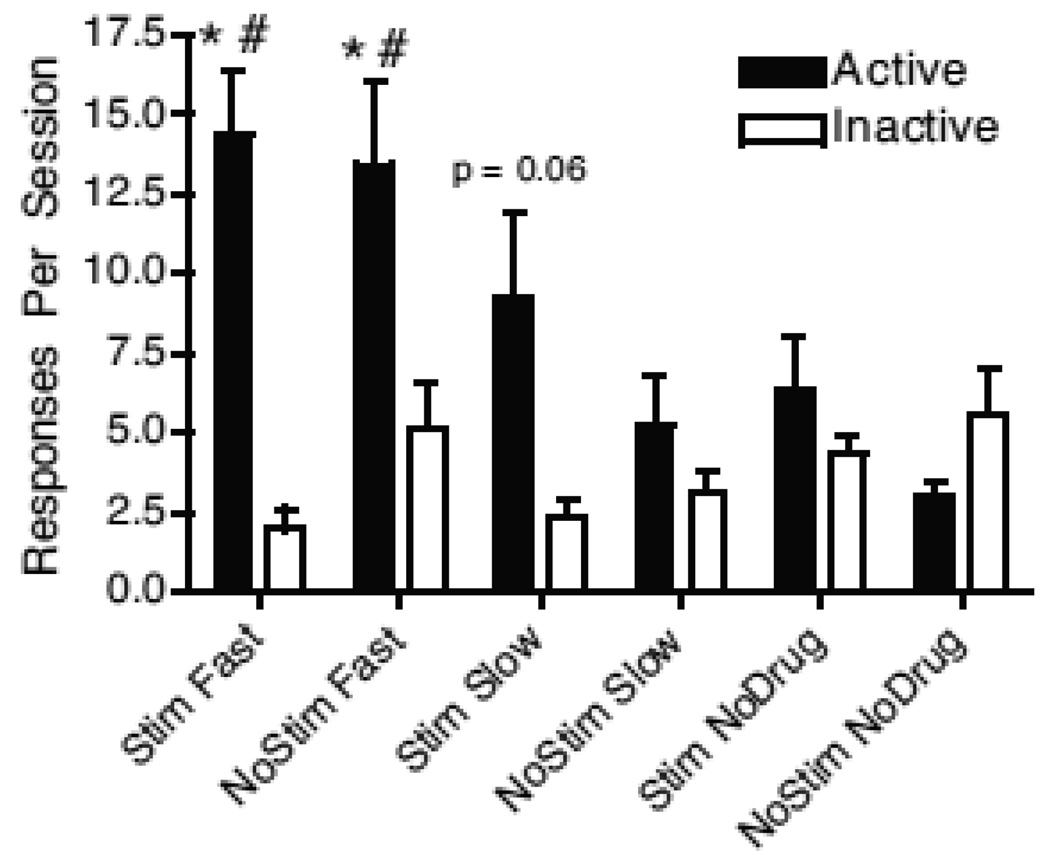
The first 4 pairs of bars in Figure 3 show the average responses per session for the 4 cocaine self-administration groups. The final 2 sets of bars in Figure 3 show the responses per session for those rats that received only the stimulus change for responding in the active hole or received neither drug nor the stimulus for responding. A factorial analysis revealed significant main effects of Speed (F2,43 = 4.17, p < 0.05), Hole (F1,43 = 64.2, p , 0.001) and Session (F14,602 = 2.49, p < 0.01). Significant interactions were also seen with Speed × Hole (F2,43 = 19.9, p < 0.001), Speed × Session (F28,602 = 1.72, p < 0.05) Stimulus × Session (F14,70 = 2.2, p < 0.05), Hole × Session (F14,600 = 1.73, p < 0.05) and a Speed × Stimulus × Session (F28,70 = 1.66, p < 0.05). When collapsed across sessions, follow-up comparisons revealed that active lever responding was higher than inactive lever responding for both fast infusion groups, with the effect for the slow infusion stimulus group approaching significance (p = 0.06). However, active and inactive responding was not different for the slow infusion no-stimulus group or for either of the no-drug groups. Comparing active lever responding, responding for both fast infusion groups was higher than for the no injection, no-stimulus group, but none of the other groups differed from the no drug, no-stimulus group.
Fig. 3.
Responses per session collapsed across sessions for the 4 drug self-administration groups and for the two no-drug groups. Statistical analysis was performed for the 6 groups as a factorial design. For that analysis, *p < 0.05 for active vs inactive within the same group, #p < 0.05 for active response in a group vs the no drug, no-stimulus group. The difference between active and inactive responses for the slow stimulus group approached significance (p = 0.06). Stim = Stimulus change associated with response, NoStim = No stimulus change associated with response, Fast = Fast infusion, Slow = Slow infusion, NoDrug = no infusion.
3.0 DISCUSSION
The primary finding of the current study is that rats acquire cocaine self-administration faster when the cocaine is given more rapidly. This effect was most evident for the no-stimulus training conditions. Only 2 rats trained with the slow infusion, no-stimulus condition even met the criterion for acquisition, and neither of those rats met the criterion in less than 8 days. In contrast 6 of the 8 rats that met criterion for the fast infusion, no-stimulus group met criterion in less than 8 days. Even for the stimulus groups, there was some evidence that rats trained with the faster infusion acquired cocaine self-administration sooner, showing significant differences between active and inactive responses earlier than the slow stimulus group. However, the effect of infusion duration was clearly less evident for the stimulus groups than for the no-stimulus groups. The finding that faster infusions of cocaine are more likely to be self-administered agrees with a number of research reports in animals, particularly non-human primates (Balster and Schuster, 1973; Kato et al., 1987; Kimmel et al., 2008; Panlilio et al., 1998; Woolverton and Wang, 2004). Although rats prefer cocaine delivered faster (Schindler et al., 2009), some recent studies of both acquisition and maintenance of cocaine self-administration in rats have failed to show a clear effect of infusion duration in rats (Crombag et al., 2008; Liu et al., 2005; Wakabayashi et al., 2010). The current results suggest that the effects of infusion speed may have been masked by other factors.
In the present study, the infusion-paired stimulus appeared to facilitate the reinforcing effects of a slow cocaine infusion. Rats trained with the fast infusion in the no-stimulus group acquired self-administration similarly to those rats trained with the fast infusion in the stimulus group. However, rats trained with the slow infusion with the stimulus clearly were more likely to acquire cocaine self-administration than those rats trained with the slow infusion without the stimulus. Research with nicotine has shown the importance of the external stimulus in facilitating acquisition of nicotine self-administration (Caggiula et al., 2002), although similar effects have not been observed with cocaine self-administration at higher cocaine doses (Carelli and Ijames, 2000). This suggests that infusing the same dose of cocaine over a longer period of time may be functionally equivalent to lowering the cocaine dose.
While the results discussed above suggest that the stimulus change paired with the slow infusion of cocaine facilitated the acquisition of self-administration, an alternative explanation could be that cocaine given as a slow infusion is not reinforcing even under the stimulus conditions, but that the stimulus change alone was itself reinforcing. In fact, previous studies in rats housed on a reverse light-dark cycle like that used here, do show acquisition of responding reinforced with only a stimulus where a component of that stimulus was turning off a houselight (Chaudhri et al., 2006; Palmatier et al., 2007). However, when that possibility was tested for the current stimulus conditions, the stimulus change only was not able to support the acquisition of nose-poking. The determination of the exact nature of the interaction between the stimulus change and the slow cocaine infusion that allows for the acquisition of self-administration is outside the scope of the current study. For example, it could be that the slow infusion of cocaine serves to enhance the reinforcing properties of the stimulus that may still be acting as a weak reinforcer, similarly to the effects of nicotine on a weak stimulus (Palmatier et al., 2007). Nevertheless, the results do show that the slow infusion clearly differed from the fast infusion in that is was not able to support self-administration under the current acquisition parameters in the absence of the stimulus.
While we observed a clear effect of the stimulus, Wakabayashi et al. (2010) also trained rats on cocaine self-administration without a paired stimulus change and failed to observe any effect of infusion duration on cocaine self-administration in rats. The dose of cocaine administered in the Wakabayashi et al. (2010) study was actually lower than that used here (0.4 mg/kg/injection). One difference between the studies that may have accounted for this difference is that Wakabayashi et al., (2010) pretrained rats on a food reinforcement task using the same response. Clemens et al. (2010) showed that pretraining with sucrose reinforcement could facilitate nicotine self-administration with both a nose-poke and lever response.
In addition to reaching the peak drug level faster, a higher drug level can be reached with a faster administration of the drug (see Panlilio et al., 1998). However, this would not appear to be a factor with the parameters used in this study. Using the parameters of the current study, Schindler et al. (2009) modeled peak whole-body cocaine levels produced by an acute injection at the slowest and fastest pump speeds and found that these peak levels would differ by only about 3 percent. In addition, changing the speed of infusion would not greatly affect the duration of the drug's actions when infusion durations are substantially less than the half-life of the drug (Panlilio et al., 1998). Therefore, it seems most likely that the rate of rise in whole-body drug levels, rather than the peak level or duration of effect, would be the primary factor responsible for the differences in behavior observed here. How this rate of rise might produce the differences in drug self-administration observed here is not clear. Porrino (1993) showed that giving cocaine i.v. to rats was more likely to produce cerebral metabolic effects in areas of the brain associated with cocaine self-administration than was an i.p. injection. Samaha et al. (2004) also showed that faster infusions of cocaine were more likely to induce c-fos and arc mRNA expression in the mesocorticolimbic regions of the brain. These results suggest that the faster infusions of cocaine are more likely to activate those areas of the brain that would lead to drug self-administration.
Given that the paired stimulus change interacts with cocaine dose to alter the acquisition of cocaine self-administration, then manipulations that alter that interaction might also change self-administration independent of cocaine or the stimulus themselves. For example, to the degree that a paired stimulus can mask the effect of infusion duration in rats, then paired stimuli may also mask the effect of a pretreatment that functionally antagonizes the direct effects of cocaine. That is, a pretreatment that effectively lowers the dose of cocaine through a direct effect on cocaine may not alter cocaine self-administration because of the masking effect of a paired stimulus. Alternatively, drugs that effectively antagonize the effects of the stimulus may appear more effective against cocaine than they actually are. Schenk et al. (2001) reported on the effect of a kappa agonist on cocaine self-administration and found antagonism of self-administration when the cocaine reinforcement was paired with a stimulus, but did not see antagonism when cocaine was not paired with a stimulus. This result would raise the question of whether the effect of the kappa agonist was specific to cocaine or would affect any other reinforcer paired with a stimulus. In the human drug abuse situation, a number of investigators have shown the importance of paired stimuli so a pretreatment drug that antagonizes self-administration only when the reinforcer is paired with a stimulus would not necessarily detract from its clinical utility. However, it would be useful to determine the specificity of that effect to the abused drug as opposed to a general effect of paired stimuli.
While the effect of delivery rate on acquisition and choice in cocaine self-administration seems clear, the effects of infusion duration on the maintenance of cocaine self-administration in rats have not been firmly established. Nevertheless, the results of this study clearly show that speed of infusion of cocaine can affect its ability to support self-administration, a finding in agreement with a number of both human and primate studies. These results also show that environmental factors can mask this effect of delivery rate on cocaine self-administration. While we showed here that a stimulus paired with cocaine partially masked the effect of changing the infusion duration, it is likely that other environmental factors may have similar effects.
ACKNOWLEDGEMENT
This research was supported by the Intramural Research Program of the NIH, NIDA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abreu ME, Bigelow GE, Fleisher L, Walsh SL. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology. 2001;154:76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–129. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL. The effects of delay of reinforcement and dose on the self-administration of cocaine and procaine in rhesus monkeys. Drug Alcohol Depend. 1993;34:37–43. doi: 10.1016/0376-8716(93)90044-q. [DOI] [PubMed] [Google Scholar]
- Brown PL, Kiyatkin EA. Brain temperature change and movement activation induced by intravenous cocaine delivered at various injection speeds in rats. Psychopharmacology. 2005;181:299–308. doi: 10.1007/s00213-005-2244-0. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology. 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Ijames SG. Nucleus accumbens cell firing during maintenance, extinction, and reinstatement of cocaine self-administration behavior in rats. Brain Res. 2000;866:44–54. doi: 10.1016/s0006-8993(00)02217-4. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caillé S, Cador M. The effects of response operandum and prior food training on intravenous nicotine self-administration in rats. Psychopharmacology. 2010;211:43–54. doi: 10.1007/s00213-010-1866-z. [DOI] [PubMed] [Google Scholar]
- Crombag H, Ferrario C, Robinson T. The rate of intravenous cocaine or amphetamine delivery does not influence drug-taking and drug-seeking behavior in rats. Pharmacol Biochem Behav. 2008;90:797–804. doi: 10.1016/j.pbb.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H, Bodker B, Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology. 1992;107:352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- de Wit H, Dudish S, Ambre J. Subjective and behavioral effects of diazepam depend on its rate of onset. Psychopharmacology. 1993;112:324–330. doi: 10.1007/BF02244928. [DOI] [PubMed] [Google Scholar]
- Deroche-Gamonet V, Piat F, Le Moal M, Piazza PV. Influence of cue-conditioning on acquisition, maintenance and relapse of cocaine intravenous self-administration. Eur J Neurosci. 2002;15:1363–1370. doi: 10.1046/j.1460-9568.2002.01974.x. [DOI] [PubMed] [Google Scholar]
- Desai RI, Kopajtic TA, French D, Newman AH, Katz JL. Relationship between in vivo occupancy at the dopamine transporter and behavioral effects of cocaine, GBR 12909 [1-{2-[bis-(4-fluorophenyl)methoxy]ethyl}-4-(3-phenylpropyl)piperazine], and benztropine analogs. J Pharmacol Exp Ther. 2005;315:397–404. doi: 10.1124/jpet.105.091231. [DOI] [PubMed] [Google Scholar]
- Gorelick DA. The rate hypothesis and agonist substitution approaches to cocaine abuse treatment. Adv Pharmacol. 1998;42:995–997. doi: 10.1016/s1054-3589(08)60914-x. [DOI] [PubMed] [Google Scholar]
- Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Brit J Addiction. 1992;87:1527–1536. doi: 10.1111/j.1360-0443.1992.tb02660.x. [DOI] [PubMed] [Google Scholar]
- Hatsukami DK, Fischman MW. Crack cocaine and cocaine hydrochloride. Are the differences myth or reality? J Am Med Assoc. 1996;276:1580–1588. [PubMed] [Google Scholar]
- Kato S, Wakasa Y, Yanagita T. Relationship between minimum reinforcing doses and injection speed in cocaine and pentobarbital self-administration in crab-eating monkeys. Pharmacol Biochem Behav. 1987;28:407–410. [PubMed] [Google Scholar]
- Kimmel HL, Negus SS, Wilcox KM, Ewing SB, Stehouwer J, Goodman MM, Votaw JR, Mello NK, Carroll FI, Howell LL. Relationship between rate of drug uptake in brain and behavioral pharmacology of monoamine transporter inhibitors in rhesus monkeys. Pharmacol Biochem Behav. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel HL, O'Connor JA, Carroll FI, Howell LL. Faster onset and dopamine transporter selectivity predict stimulant and reinforcing effects of cocaine analogs in squirrel monkeys. Pharmacol Biochem Behav. 2007;86:45–54. doi: 10.1016/j.pbb.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Terner J, Hursh S, Woods JH, Winger G. Relative reinforcing effects of three opioids with different durations of action. J Pharmacol Exp Ther. 2002;301:698–704. doi: 10.1124/jpet.301.2.698. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR, Pazzaglia PJ, Ali JA. Comparison of acute behavioral effects of sustained-release and immediate-release methylphenidate. Exp Clin Psychopharmacology. 1998;6:367–374. doi: 10.1037//1064-1297.6.4.367. [DOI] [PubMed] [Google Scholar]
- Lile JA, Wang Z, Woolverton WL, France JE, Gregg TC, Davies HML, Nader MA. The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307:356–366. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Liu Y, Roberts DCS, Morgan D. Sensitization of the reinforcing effects of self-administered cocaine in rats: effects of dose and intravenous injection speed. Eur J Neurosci. 2005;22:195–200. doi: 10.1111/j.1460-9568.2005.04195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK, Badger GJ, Rathmell JP, Swedberg MD, Jonzon B, Norsten-Höög C. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299:1056–1065. [PubMed] [Google Scholar]
- Mumford GK, Evans SM, Fleishaker JC, Griffiths RR. Alprazolam absorption kinetics affects abuse liability. Clin Pharmacol Ther. 1995;57:356–365. doi: 10.1016/0009-9236(95)90162-0. [DOI] [PubMed] [Google Scholar]
- Nelson RA, Boyd SJ, Ziegelstein RC, Herning R, Cadet JL, Henningfield JE, Schuster CR, Contoreggi C, Gorelick DA. Effect of rate of administration on subjective and physiological effects of intravenous cocaine in humans. Drug Alcohol Depend. 2006;82:19–24. doi: 10.1016/j.drugalcdep.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Goldberg SR, Gilman JP, Jufer R, Cone EJ, Schindler CW. Effects of delivery rate and non-contingent infusion of cocaine on cocaine self-administration in rhesus monkeys. Psychopharmacology. 1998;137:253–258. doi: 10.1007/s002130050618. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Schindler CW. Self-administration of remifentanil, an ultra-short acting opioid, under continuous and progressive-ratio schedules of reinforcement in rats. Psychopharmacology. 2000;150:61–66. doi: 10.1007/s002130000415. [DOI] [PubMed] [Google Scholar]
- Panlilio LV, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli. Psychopharmacology. 1996;125:202–208. doi: 10.1007/BF02247329. [DOI] [PubMed] [Google Scholar]
- Porrino LJ. Functional consequences of acute cocaine treatment depend on route of administration. Psychopharmacology. 1993;112:343–351. doi: 10.1007/BF02244931. [DOI] [PubMed] [Google Scholar]
- Samaha A-N, Li Y, Robinson TE. The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci. 2002;22:3244–3250. doi: 10.1523/JNEUROSCI.22-08-03244.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha A-N, Mallet N, Ferguson SM, Gonon F, Robinson TE. The rate of cocaine administration alters gene regulation and behavioral plasticity: implications for addiction. J Neurosci. 2004;24:6362–6370. doi: 10.1523/JNEUROSCI.1205-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Partridge B. Influence of a conditioned light stimulus on cocaine self-administration in rats. Psychopharmacology. 2001;154:390–396. doi: 10.1007/s002130000608. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. Effects of the kappa-opioid receptor agonist, U69593, on the development of sensitization and on the maintenance of cocaine self-administration. Neuropsychopharmacology. 2001;24:441–450. doi: 10.1016/S0893-133X(00)00190-1. [DOI] [PubMed] [Google Scholar]
- Schindler C, Panlilio L, Thorndike E. Effect of rate of delivery of intravenous cocaine on self-administration in rats. Pharmacol Biochem Behav. 2009;93:373–381. doi: 10.1016/j.pbb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BJ, Jones HE, Griffiths RR. Physiological, subjective and reinforcing effects of oral and intravenous cocaine in humans. Psychopharmacology. 2001;156:435–444. doi: 10.1007/s002130100740. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Ding YS, Gatley SJ. Role of dopamine in the therapeutic and reinforcing effects of methylphenidate in humans: results from imaging studies. Eur Neuropsychopharmacology. 2002;12:557–566. doi: 10.1016/s0924-977x(02)00104-9. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan J, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci. 2000;67:1507–1515. doi: 10.1016/s0024-3205(00)00731-1. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Weiss M, Pickup K, Robinson TE. Rats Markedly Escalate Their Intake and Show a Persistent Susceptibility to Reinstatement Only When Cocaine Is Injected Rapidly. J Neurosci. 2010;30:11346–11355. doi: 10.1523/JNEUROSCI.2524-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee S, Carroll FI, Woolverton WL. A reduced rate of in vivo dopamine transporter binding is associated with lower relative reinforcing efficacy of stimulants. Neuropsychopharmacology. 2006;31:351–362. doi: 10.1038/sj.npp.1300795. [DOI] [PubMed] [Google Scholar]
- Winger G, Hursh SR, Casey KL, Woods JH. Relative reinforcing strength of three N-methyl-D-aspartate antagonists with different onsets of action. J Pharmacol Exp Ther. 2002;301:690–697. doi: 10.1124/jpet.301.2.690. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Ranaldi R, Wang Z, Ordway GA, Paul IA, Petukhov P, Kozikowski A. Reinforcing strength of a novel dopamine transporter ligand: pharmacodynamic and pharmacokinetic mechanisms. J Pharmacol Exp Ther. 2002;303:211–217. doi: 10.1124/jpet.102.037812. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Wang Z. Relationship between injection duration, transporter occupancy and reinforcing strength of cocaine. Eur J Pharmacol. 2004;486:251–257. doi: 10.1016/j.ejphar.2004.01.003. [DOI] [PubMed] [Google Scholar]