Abstract
Background and Objectives
Linxian in Henan Province, China, has among the highest rates of esophageal cancer worldwide. Little is known about long-term survival after esophagectomy for early neoplastic lesions found during early detection screening. A long-term survival analysis was performed for 315 patients from Linxian who received esophagectomy for early esophageal squamous cell carcinoma.
Methods
Cases that received esophagectomy for early esophageal squamous cell carcinoma were age- and gender-matched with two healthy controls, and Kaplan-Meier survival analyses were performed for both groups.
Results
10-year survival was 77% for cases and 64% for controls, and this difference was not statistically significant (p = 0.33). There were no significant differences in survival based on age or gender (p>0.05). Cases with esophageal squamous cell carcinoma-in-situ had significantly better survival than cases with invasive esophageal squamous cell carcinoma (p=0.035).
Conclusions
Survival of cases who received esophagectomy for early esophageal squamous cell carcinoma was not significantly different from survival of age- and gender-matched controls. Early intervention probably improved survival rates for these patients who otherwise would most likely have developed advanced esophageal carcinoma. Early screening and intervention are highly relevant in areas with a high risk of esophageal cancer such as Linxian, China.
Keywords: Esophageal Cancer, Esophageal Surgery, Statistics, survival analysis
Introduction
Esophageal cancer is the eighth most common cancer and the sixth most common cause of cancer deaths worldwide.[1,2] In the United States, the incidence of esophageal cancer is increasing, mostly due to an increase in adenocarcinoma.[3,4] In China, the overall incidence of esophageal cancer has been decreasing in past decades, but some regions still have among the highest rates of esophageal cancer in the world.[5-8] Linxian in Henan Province has long been a center of esophageal cancer research due to its extremely high rates of esophageal cancer (age-standardized incidence rates for both sexes of over 100 per 100,000/year).[8]
Esophageal cancer is often an insidious disease, and at presentation it is unresectable or has metastasized in about 50% of patients.[9] Since survival is highly dependent on disease stage, early detection and treatment can provide the best clinical outcomes.[10,11] Although screening programs can identify patients with early precursor lesions, little is known about the long-term results of esophagectomy performed on those patients. The clinical results of esophagectomy for early squamous cell carcinoma in 420 patients from Linxian, China have been previously reported.[12] Given that survival appeared to be favorable in these esophagectomy patients, a long-term survival analysis was conducted to see how their survival compared with healthy age- and gender-matched controls. This comparison between those with early esophageal squamous cell carcinoma (ESCC) and their healthy counterparts, as well as comparisons with previous reports of survival after esophagectomy in Chinese patients with advanced ESCC, can elucidate any possible survival benefit of esophagectomy for early ESCC lesions in Linxian.
Materials and Methods
The selection of a cohort of 420 esophagectomy patients from Linxian has been previously described.[12] In brief, population-based mass esophageal cancer screenings of asymptomatic patients were conducted since 1972 using esophageal balloon cytology and occult blood tests. Subjects identified as high-risk received endoscopy with iodine staining and multiple biopsies which were read by two independent pathologists who were unaware of the visual endoscopic results. 420 patients were found to have superficial esophageal squamous cell carcinoma, defined as disease limited to the mucosa or submucosa. Between 1974 and 2001, all of these subjects underwent resection of the esophageal carcinoma via left posterolateral thoracotomy. Thoracotomy was followed in the same stage with esophagogastrostomy covered by a tongue-like seromuscular flap of gastric wall as a manual anastomotic technique. The majority of lesions (73.1%) were in the midthoracic segment, and 9% of patients were found to have positive lymph nodes. Pathology results from resected specimens showed that 18% had carcinoma-in-situ, 30% had intramucosal infiltrating carcinoma, and 52% had submucosal infiltrating carcinoma. There was a 94% follow-up rate between 1974 and 2001.
In the current study, for each of the 420 patients who underwent esophagectomy, two age- and gender-matched controls were identified from a database of 3,692 patients with no history of malignancy or debilitating disease in the placebo arm of the Linxian Nutrition Intervention Trial (NIT).[6] The control matches were 40 to 69-years old when enrolled in the NIT, and were matched by gender and within 2 years of age with cases (two were matched within 5 years of age). All controls have been followed up since 1986, and were healthy and without other diagnoses at the time that their matched case underwent esophagectomy. The cases and controls were all from the same four communes of Linxian, Henan Province where the NIT was conducted. Since the NIT began in 1986, 79 esophagectomy patients who had surgery prior to 1985 were excluded because there were no available matched controls, and 26 cases were further excluded due to the availability of only one control that was matched for age and gender. Thus, 315 cases with age- and gender-matched controls were used for this analysis. This study was approved by the institutional review board at the Cancer Institute, Chinese Academy of Medical Sciences, and informed consent was obtained from each subject.
Survival analysis was performed using the Kaplan-Meier method[13] on 315 esophagectomy cases and 630 age- and gender-matched controls. All survival analyses were performed up to 10 years of follow-up, with the date of the case's operation considered as time zero. Survival was based on deaths from all causes. Follow-up times of controls were matched with the follow-up times of cases, such that a case who had follow-up for x years had controls with survival statuses also evaluated at x years. Cases were censored at the end of their follow-up period or when they were lost to follow-up, while controls were censored at the end of their follow-up period corresponding to the follow-up period of their case. Plots and statistical analyses using the log-rank test were performed using SPSS 15. To study the impact of age or gender on survival, the cases were divided into three age groups (≤50, 51-60, and >60 years old) or by gender, and the survival of each group was plotted by the Kaplan-Meier method. The relationship between pathologic diagnosis and death has been previously analyzed,[12] but the survival based on whether cases had invasive ESCC or ESCC-in-situ has not been performed. Thus, from the original cohort of esophagectomy patients, there were 270 cases with invasive ESCC and 56 cases with ESCC-in-situ available for this particular analysis.
Results
The two-to-one match consisted of 315 patients who underwent esophagectomy for early squamous-cell esophageal carcinoma, and 630 age- and gender-matched healthy controls from the same area of Linxian in Henan Province, China. Table I shows the baseline characteristics and survival rates for both the case and control groups. Approximately a quarter of the subjects were younger than 50, half were between 51 and 60 years old, about a quarter were older than 60, and 46 percent of cases and controls were female. The operative mortality rate of cases was 1.2% (4 of 315), and these cases were included in the survival analysis. Although data on the specific causes of death were sometimes unavailable, most cases died due to recurrence or metastasis, cardiovascular disease, or trauma; controls died primarily due to cancer (from any site), cerebrovascular accidents, or cardiovascular disease. Kaplan-Meier survival analysis revealed that mean survival time was 8.6 years for cases (95% CI, 8.1 to 9.0), and 8.2 years for controls (95% CI, 7.8 to 8.6). 5-year cumulative survival rates were 87 percent for cases and 83 percent for controls, while 10-year cumulative survival rates were 77 percent for cases and 64 percent for controls (Fig. 1). At 1 year, the numbers of deaths were 7 (cases) and 10 (controls), and the numbers remaining at risk were 225 (cases) and 442 (controls). At 5 years, the numbers of deaths were 24 (cases) and 50 (controls), and the numbers remaining at risk were 98 (cases) and 158 (controls). At 9 years, the numbers of deaths were 32 (cases) and 67 (controls), and the numbers remaining at risk were 27 (cases) and 34 (controls). There was no statistical difference between the survival rates of cases and controls (p=0.33).
Table I. Age, gender, and survival rates of esophagectomy cases and age- and gender-matched controls.
| Cases (% of total) | Matches (% of total) | |
|---|---|---|
| Number of Patients | 315 | 630 |
| Age at time of surgery | ||
| ≤50 | 87 (27%) | 125 (20%) |
| 51-60 | 142 (45%) | 326 (52%) |
| >60 | 86 (27%) | 179 (28%) |
| Sex | ||
| Female | 145 (46%) | 290 (46%) |
| Male | 170 (54%) | 340 (54%) |
| Survival Rates | ||
| 1 year | 97% | 98% |
| 5 years | 87% | 83% |
| 10 years | 77% | 64% |
Figure 1.
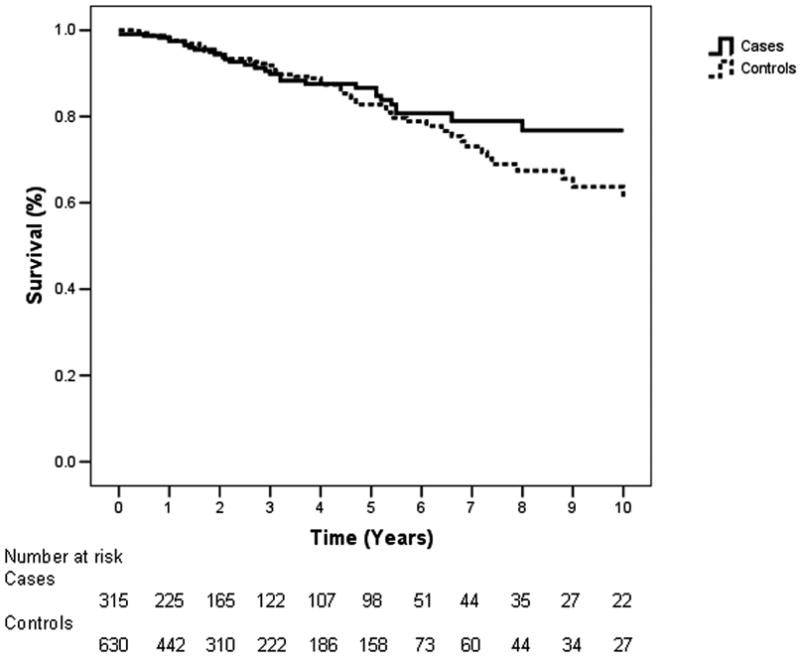
Kaplan-Meier 10-year survival curves of 315 Cases and 630 controls. There was no significant difference between the survival curves (p=0.33). The number at risk refers to the number of individuals remaining at a given time after deaths and censored individuals have been subtracted.
Figure 2 shows a survival analysis of the 315 cases stratified by age groups, in which 87 patients were ≤50 years old, 142 were 51-60, and 86 were >60. Mean survival times were 8.4 years for those ≤50 (95% CI, 7.6 to 9.2), 8.4 years for those 51-60 (95% CI, 7.8 to 9.1), and 9.1 years for those >60 (95% CI, 8.1 to 9.0). At 5 years, the numbers of deaths were 9 (≤50), 13 (51-60), and 3 (>60), while the numbers remaining at risk were 33 (≤50), 48 (51-60), and 16 (>60). There was no statistical difference in survival between the age groups (p=0.31).
Figure 2.
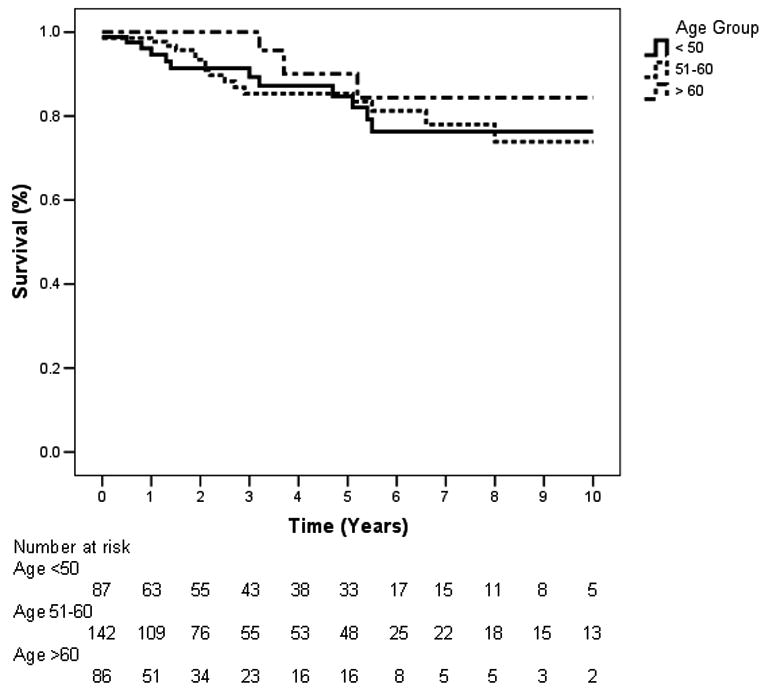
Kaplan-Meier 10-year survival curves of cases stratified by age (≤50, 51-60, >60). There was no significant difference between the survival curves (p=0.31).
An analysis according to gender was performed, in which neither males nor females had a significant difference in survival between cases or controls, and there was no significant difference between males and females within each group (data not shown). Figure 3 shows an analysis of 270 cases with invasive ESCC and 56 cases with ESCC-in-situ taken from the original esophagectomy cohort, which revealed mean survival times of 8.1 years for invasive cases (95% CI, 7.6 to 8.6) and 9.2 years for in-situ cases (95% CI, 8.6 to 9.9). The invasive ESCC and ESCC-in-situ groups both had a mean age of 54 at time of surgery. The 5- and 10-year survival rates for the invasive cases were 77 and 66 percent, respectively, while the 5- and 10-year survival rates for the in-situ cases were 94 and 85 percent, respectively. The difference in survival curves of the two groups was significant (p=0.035).
Figure 3.
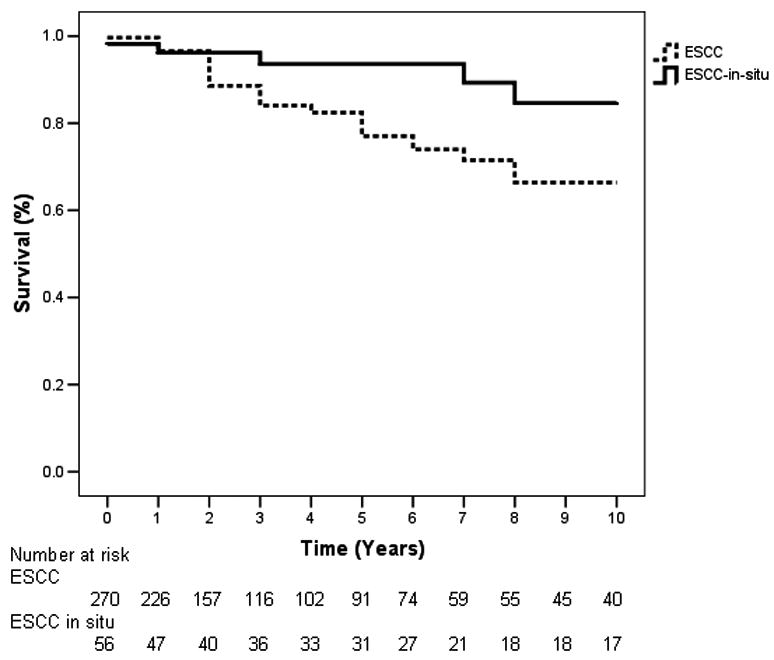
Kaplan-Meier 10-year survival curves of cases stratified by invasive vs. in-situ esophageal squamous cell carcinoma (ESCC). There was a significant difference between the survival curves (p=0.035).
Discussion
In recent decades, the incidence of esophageal cancer has been increasing in the West mostly due to a rise in adenocarcinoma, while in China, the incidence of esophageal cancer has been decreasing.[4,7,8] In 2008, age-standardized incidence rates of esophageal cancer were much higher in China (22.9/100,000 in males, 10.5/100,000 in females) than in the United States (5.8/100,000 in males, 1.2/100,000 in females).[2] The 5-year relative survival rate for esophageal cancer is approximately 19 percent in the US,[14] 10 percent in Europe,[1] and between 20-30 percent in China.[15,16] In the West, widespread population screening for esophageal cancer would not be reasonable due to the relatively low incidence rates.[9] This is not the case, however, in high-incidence areas such as the Taihang Mountain region in north central China, which includes the city of Linxian. Various etiologies have been implicated for the high incidence of esophageal squamous cell carcinoma seen in this region, including nutritional deficiencies, ingestion of nitrosamines and polycyclic aromatic hydrocarbons, consumption of hot beverages, and smoking and alcohol.[6,9,17] Mass population-based esophageal cancer screenings of asymptomatic patients have been performed in Linxian for several decades, and these screenings have identified patients with early ESCC who subsequently underwent esophagectomy and follow-up. We conducted survival analyses on these post-esophagectomy long-term follow-up patients in order to clarify the role that esophagectomy plays in esophageal cancer screening interventions in China.
Our analysis showed that esophagectomy cases had slightly higher overall survival rates than their age- and gender-matched controls at both 5 years (87 and 77 percent, respectively) and 10 years of follow up (83 and 64 percent, respectively). However, there was no statistically significant difference in survival rates between esophagectomy cases and controls. Thus, the survival of post-esophagectomy patients in this study is similar to the survival of healthy age- and gender-matched controls from the same geographical region in Linxian.
In our age-stratified analysis, there was no significant difference in survival due to age at time of surgery. While the survival of the oldest group (age >60 at the time of surgery) was the highest, this is probably not because older age is associated with higher survival but rather because the sample of patients over 60 years old did not have any deaths during the last five years of follow-up. While further studies are needed to clarify the role of age on esophagectomy results, this finding is similar to studies in other countries that have found no significant effects of advanced age on surgical outcomes or survival after esophagectomy.[18,19] Our analysis also found no significant difference in survival based on gender.
It is likely that the cases in this study would have experienced higher mortality if they had not received surgical intervention after screening that detected their early lesions. Another study from Henan Province following patients with esophagectomy performed for advanced to late cancers reported 5- and 10-year survival rates of 25 and 18 percent, respectively,[20] while a meta-analysis from Hebei Province, China, reported 5-year survival rates after esophagectomy of 20-30 percent.[16] In contrast, a survival analysis of patients from Henan Province in China who received esophagectomy for early ESCC showed 5- and 10-year survival rates of 93 and 72 percent, respectively, similar to the overall survival rates found in our study.[21] The current study also demonstrated a significant difference in survival after esophagectomy among cases with invasive versus in-situ carcinoma; esophagectomy performed on in-situ carcinoma was associated with a 1.1. year longer mean survival and about 20 percent higher 5- and 10-year survival rates. While the survival rates of in-situ carcinoma cases and matched controls from the NIT might be expected to be similar, the 56 in-situ carcinoma cases had a higher 10-year survival rate (85%) compared to that of the 630 matched controls (64%). This difference was not significant (p=0.073), and may have been due to closer medical attention that cases received following esophagectomy or may suggest the presence of a benefit to early screening, but future studies with larger sample sizes are warranted.
Therefore, in a high-risk region such as Henan Province, esophagectomies performed on early lesions found during screening are associated with longer survival than surgeries done on late lesions. Furthermore, there is no statistically significant difference in survival between the early esophagectomy patients and their age- and gender-matched counterparts from the general population, which suggests that the early removal of disease can help restore comparable survival rates to patients who would otherwise be susceptible to high mortality.
Factors other than early screening and early-stage esophagectomy may have contributed to the high survival rates observed in this study. Most of the literature on survival after esophagectomy deals with patients who underwent surgery for advanced disease. In contrast, the patients in this study were found through screening before presenting with any symptoms. The fact that our patients had long-term follow-up and were assessed with mortality as the endpoint makes it unlikely that lead-time bias was a significant factor in our results. Additionally, there is data from the West that increased surgical experience or hospital volume can be associated with better morbidity and mortality after esophagectomy;[4,27] while this issue remains to be studied in China, we speculate that the longstanding experience of the esophageal cancer hospitals in Linxian may have played a role in this study's survival rates.
A potential limitation of this study is that the 2:1 match used controls from the placebo group of the Linxian NIT, which provided a cohort of only 3,600 patients to draw controls from. However, this matching process provided controls with long-term follow-up without intervention bias, and it allowed controls to be matched for age, gender, and similar environmental and cultural exposures in the same area of Linxian. Also, the high follow-up rates and frequent surveillance of both cases and NIT controls in Linxian mitigate any effects on survival due to secular changes over time. Long-term data on quality of life in this study were limited, but indicated that 79% of cases had no dysphagia while 21% had mild dysphagia, and 80% of the cases returned to their normal work. Post-esophagectomy quality of life should be a subject of future research given the invasive nature of esophagectomy.[12,28]
One of the unresolved public health issues in esophageal cancer is the efficacy and need for early screening and intervention programs.[9] One of the reasons that esophageal cancer has high mortality and is difficult to treat is that it often does not cause symptoms until the cancer is already advanced.[9] While esophageal cancer remains an uncommon disease in most Western and developed countries, it is a common fatal disease in many developing countries, including China. In 2008, 83% of esophageal cancer deaths worldwide occurred in developing countries, and 52% of these deaths occurred in China.[2] Its high incidence in certain regions of China and other countries highlights the importance of early screening and intervention in these areas. The current study shows that esophagectomies performed on early lesions can lead to high survival rates that are comparable with those of the normal healthy population. This information on esophagectomies can also provide a useful baseline with which to compare endoscopic therapies which are growing in use. There may also be a potential role in Western countries for cytology-based screening that could be relevant for higher-risk patients with gastroesophageal reflux disease or Barrett's esophagus who might not be able to undergo endoscopic surveillance. The long-term results of screening and interventions must be closely followed-up in China and other countries with a high incidence of esophageal cancer, and these interventions should be accompanied by ongoing preventive efforts to lower the incidence of this disease. As China implements more esophageal cancer screening stations around the country, these advances in screening must be followed by the appropriate treatment interventions, as well as adequate training for clinicians and thoracic surgeons who will handle newly-discovered cases of early esophageal cancer.
Conclusions
In this study, the long-term survival of cases who received esophagectomy for early esophageal squamous cell carcinoma was not significantly different from the survival of healthy age- and gender-matched controls from the same geographical area. For these esophageal cancer patients, who otherwise would likely have developed advanced esophageal carcinoma, early intervention probably improved their survival rates. Early screening and intervention are highly relevant in areas with a high risk of esophageal cancer such as Linxian, China.
Acknowledgments
Financial Support: Fogarty International Clinical Research Scholars Program: Fogarty International Center, National Institutes of Health Office of the Director, through the International Clinical Research Fellows Program at Vanderbilt (R24 TW007988).
We thank the citizens of Linxian for their participation in this study, as well as the many resourceful and dedicated physicians or assistants who carried out long-term follow-up for many patients in Linxian. We also thank Dr. Philip R. Taylor and Dr. Sanford M. Dawsey for their helpful comments and insight on the manuscript.
This work was performed at the Department of Cancer Epidemiology, Cancer Institute: Chinese Academy of Medical Sciences, Beijing, China.
Acronyms Used
- ESCC
Esophageal squamous cell carcinoma
- NIT
Linxian Nutrition Intervention Trial
Footnotes
Disclosures: The authors declare no conflicts of interest, and had full control of the design of the study, methods used, outcome parameters, analysis of data and production of the written report.
References
- 1.Parkin D, Bray F, Ferlay J, Pisani P. Global Cancer Statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, et al. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No 10 [Internet] InLyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;55:74–108. [Google Scholar]
- 4.Khushalani NI. Cancer of the Esophagus and Stomach. Mayo Clin Proc. 2008;83:712–722. [PubMed] [Google Scholar]
- 5.Li YY. Epidemiology of esophageal cancer in China. J Natl Cancer Inst Monogr. 1982;62:113–120. [PubMed] [Google Scholar]
- 6.Qiao YL, Dawsey SM, Kamangar F, et al. Total and Cancer Mortality After Supplementation With Vitamins and Minerals: Follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–518. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly JM, Fry WA, Little AG, et al. Esophageal Cancer: Results of an American College of Surgeons Patient Care Evaluation Study. J Am Coll Surg. 2000;190:562–572. doi: 10.1016/s1072-7515(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 8.Ke L. Mortality and incidence trends from esophagus cancer in selected geographic areas of China circa 1970-90. Int J Cancer. 2002;102:271–274. doi: 10.1002/ijc.10706. [DOI] [PubMed] [Google Scholar]
- 9.Enzinger PC, Mayer RJ. Esophageal Cancer. N Engl J Med. 2003;349:2241–2252. doi: 10.1056/NEJMra035010. [DOI] [PubMed] [Google Scholar]
- 10.Reed CE. Surgical Management of Esophageal Carcinoma. The Oncologist. 1999;4:95–105. [PubMed] [Google Scholar]
- 11.Mclarty AJ, Deschamps C, Trastek VF, et al. Esophageal Resection for Cancer of the Esophagus: Long-Term Function and Quality of Life. Ann Thorac Surg. 1997;63:1568–1572. doi: 10.1016/s0003-4975(97)00125-2. [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Jiao G, Chang F, et al. Long-Term Results of Operation for 420 Patients With Early Squamous Cell Esophageal Carcinoma Discovered by Screening. Ann Thorac Surg. 2004;77:1740–1744. doi: 10.1016/j.athoracsur.2003.10.098. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Non-parametric estimation from incomplete observation. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006. InBethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 15.Dong Z, Tang P, Li L, Wang G. The Strategy for Esophageal Cancer Control in High-Risk Areas of China. Jpn J Clin Oncol. 2002;32:S10–12. doi: 10.1093/jjco/hye122. [DOI] [PubMed] [Google Scholar]
- 16.Liu JF, Wang QZ, Hou J. Surgical treatment for cancer of the oesophagus and gastric cardia in Hebei, China. British Journal of Surgery. 2004;91:90–98. doi: 10.1002/bjs.4402. [DOI] [PubMed] [Google Scholar]
- 17.Roth MJ, Strickland KL, Wang G, et al. High Levels of Carcinogenic Polycyclic Aromatic Hydrocarbons Present Within Food from Linxian, China may Contribute to that Region's High Incidence of Oesophageal Cancer. European Journal of Cancer. 1998;34:757–758. doi: 10.1016/s0959-8049(97)10071-5. [DOI] [PubMed] [Google Scholar]
- 18.Alibakhshi A, Aminian A, Mirsharifi R, et al. The effect of age on the outcome of esophageal cancer surgery. Ann Thorac Med. 2009;4:71–74. doi: 10.4103/1817-1737.49415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruol A, Portale G, Zaninotto G, et al. Results of esophagectomy for esophageal cancer in elderly patients: age has little influence on outcome and survival. J Thorac Cardiovasc Surg. 2007;133:1186–1189. doi: 10.1016/j.jtcvs.2006.12.040. [DOI] [PubMed] [Google Scholar]
- 20.Lian SY, Liu ZC, Cheng LP, et al. An Analysis of Survival Rate of Esophageal Carcinoma and Gastric Cardia Carcinoma from 1959 to 2002 in Linzhou City, Henan Province. China Tumor. 2007;16:77–78. [Google Scholar]
- 21.Shao LF, Gao SR, Li CC, Li GS. Long-term results of surgical resection of early esophageal and cardiac carcinoma. Zhonghua Surgery Journal. 1993;31:131–133. [PubMed] [Google Scholar]
- 22.Miller JD, Jain MK, de Gara CJ, et al. Effect of surgical experience on results of esophagectomy for esophageal carcinoma. J Surg Oncol. 1997;65:20–21. doi: 10.1002/(sici)1096-9098(199705)65:1<20::aid-jso4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 23.Headrick JR, Nichols FC, III, Miller DL, et al. High-Grade Esophageal Dysplasia: Long-Term Survival and Quality of Life after Esophagectomy. Ann Thorac Surg. 2002;73:1697–1703. doi: 10.1016/s0003-4975(02)03496-3. [DOI] [PubMed] [Google Scholar]


