Abstract
The multifunctional Ca2+-calmodulin-dependent protein kinase II (CaMKII) has emerged as a nodal point in cardiac muscle function and adaptation. This stems from CaMKII’s demonstrated role in phosphorylation and regulation of many key molecules known to be critical in excitation-contraction coupling and arrhythmias, and because it increases in expression and activation during hypertrophy and heart failure (HF) and CaMKII overexpression and suppression induce and suppress HF respectively. For many years, there were indications that intracellular Ca could activate altered gene expression, but only in the past ten years has it been recognized that CaMKII and a Ca-calmodulin-dependent phosphatase calcineurin are major Ca-dependent mediators of transcriptional regulation. Moreover, these pathways contribute to changes in gene expression of proteins involved in the HF phenotype, including some of the ion channels and Ca transporters that are acutely involved in systolic dysfunction and arrhythmias.
Keywords: Ca-Calmodulin dependent protein kinase, histone deacetylase, calcineurin
Introduction
Ca is a ubiquitous intracellular second messenger and calmodulin (CaM) mediates numerous aspects of Ca signaling in cardiac myocytes, as in other cells. Ca bound CaM (Ca-CaM) is the canonical activator of both the protein phosphatase calcineurin (CaN; PP2A) and Ca-CaM dependent protein kinase (CaMKII), although it is increasingly clear that there are other pathways to and modulators of activation for CaN and CaMKII. Other Viewpoints here address acute effects of CaMKII on key targets involved in myocyte Ca handling and electrophysiology that directly influence cardiac inotropy, chronotropy lusitropy and action potential (AP) configuration and excitability. These acute effects are undoubtedly intended to be adaptive short term compensations to perturbations associated with neurohumoral activation (e.g. sympathetic fight-or-flight response) and mechanical stress (pressure or volume overload). However, these acute CaMKII effects can also become maladaptive in leading to reduced SR Ca content, contractility and arrhythmias.
In addition to the acute modulatory effects of CaMKII on ion channels, transporters and myofilaments (in seconds to minutes), CaMKII activation can also alter gene transcription (on a time scale of minutes-hours-days). This could be considered a second line of defense in coping with stresses where the acute adaptations related CaMKII-dependent phosphorylation of existing proteins is not sufficient to deal with the stress. Of course this transcriptional regulation can be both a beneficial adaptation, but could also be maladaptive and contribute to worsening of cardiac function and increased propensity for arrhythmias. Indeed, when myocyte CaMKII is chronically activated, as occurs in HF or with CaMKII overexpression, it appears to be largely maladaptive by enhancing diastolic SR Ca leak (weakening contraction) and altering expression and gating of ion channels in ways that contribute to arrhythmogenesis. Thus, it seems that both acute and transcriptional actions of CaMKII require delicate balance. This already raises two important questions that are not well resolved in this field. 1) How is control normally achieved to prevent maladaptive CaMKII effects? 2) What are the real beneficial adaptive effects of CaMKII activation (acute and chronic)?
The process by which Ca-dependent signaling leads to transcriptional regulation we refer to as excitation-transcription (E-T) coupling by analogy to excitation-contraction (E-C) coupling. One key Ca-dependent E-T coupling pathway involves CaMKII-dependent phosphorylation of class II histone deacetylases (e.g. HDAC4 and HDAC5).1,2 When these HDACs are dephosphorylated they bind to and repress hypertrophic transcription factors such as MEF2 (myocyte enhancer factor 2) and the histone deacetylase activity also favors chromatin condensation. When these HDACs are phosphorylated by CaMKII or PKD, they are translocated out of the nucleus by 14-3-3 chaperone proteins, thereby relieving the repression and allowing MEF2 dependent transcriptional activation (Fig 1). A parallel Ca-dependent E-T coupling pathway involves the phosphatase CaN.3 When CaN is activated by Ca-CaM it dephosphorylates nuclear factor of activated T cells (NFAT) which drives nuclear import of NFAT which interacts with other transcription factors such as GATA4 to drive hypertrophic gene transcription. While some of the key upstream players in CaMKII and CaN activation are known, there are numerous questions that remain including: 3) Which phosphatases dephosphorylate HDAC4/5 and CaMKII, and which kinases phosphorylate relevant NFATs and CaN? 4) Which specific local/global Ca signals activate the CaMKII-HDAC vs. the CaN-NFAT pathway? 5) what cross-talk exists between these systems (e.g. CaMKII can phosphorylate and inhibit CaN)? The CaMKII-HDAC and CaN-NFAT pathways are probably not the only Ca-dependent E-T coupling factors. For example, the CaM binding transcription activator 2 (CAMTA2) is present in cardiac myocytes, but little is known about its functional role or Ca-CaM-dependent regulation in heart. My two goals here are to provide a summary framework for what is known about Ca-dependent activation of these transcriptional pathways (without detailed referencing) and also identify gaps in our knowledge (as in the questions raised above).
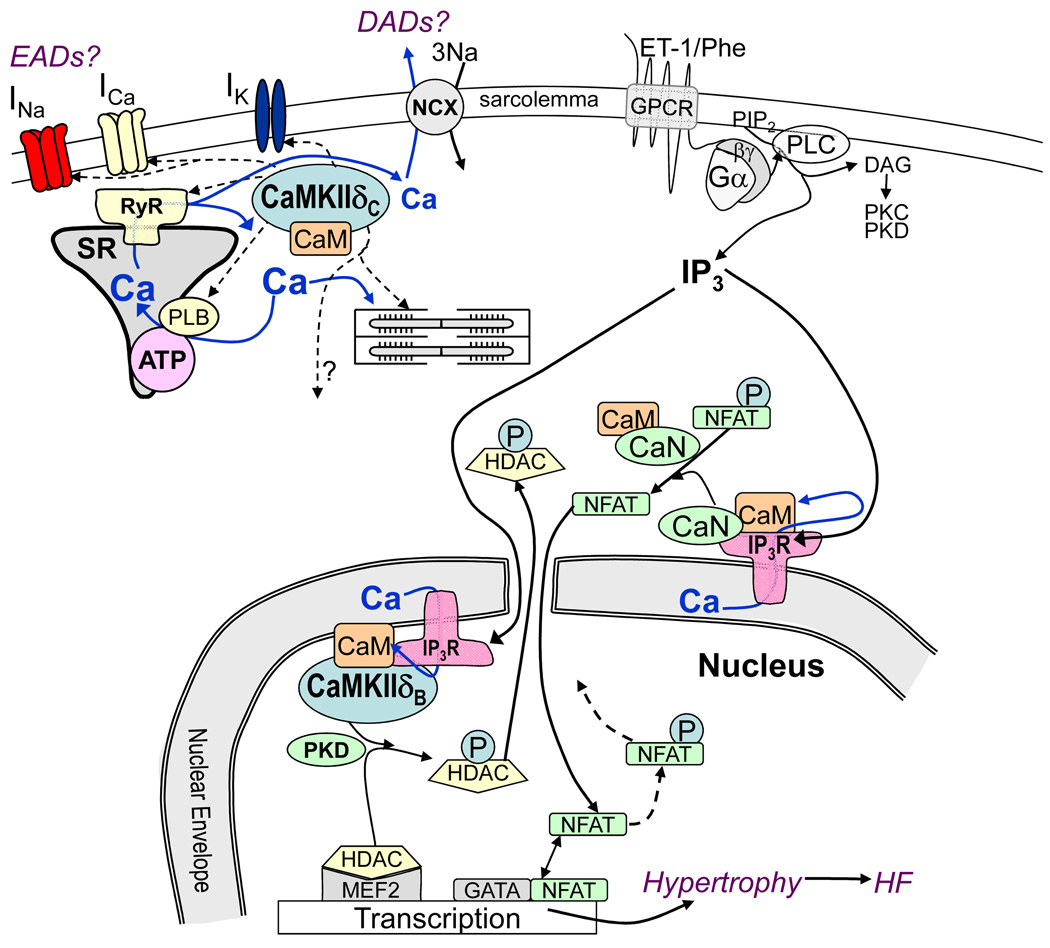
Figure 1.
CaMKIIδ can acutely regulate ion channels (INa, ICa, IK) and Ca handling proteins (RyR, IP3R, PLB) contributing to triggered arrhythmias such as early and delayed afterdepolarization (EADs and DADs). G-protein coupled receptor (GPCR) agonists (ET-1 and phenylephrine, Phe) activate Gα/βγ and phospholipase C to produce diacylglycerol (which can activate protein kinase C and D. PKC and PKD) and IP3. CaMKII and PKD can phosphorylate (P) HDAC and calcineurin (CaN) can dephosphorylate nuclear factor of activated T cells (NFAT) altering MEF2- and GATA-dependent transcription.
Localization of CaM and CaMKII
Both CaM and CaMKII are in many locations in the cardiac myocyte, including the nucleus. However, the highest concentrations appear at the transverse tubules where E-C coupling occurs. CaM can diffuse but even at diastolic [Ca]i, greater than 95% of the 6 µM total CaM in the myocyte is already tightly bound to cellular proteins (e.g. ryanodine receptors and Ca channels) and exhibits low mobility. When [Ca]i rises CaM binds even more tightly to target proteins, further limiting its mobility. Nevertheless, when either [Ca]i rises or G-protein coupled receptors are activated (e.g. by endothelin-1, ET-1) CaM translocates to the nucleus over tens of minutes, where it may participate in transcriptional regulation. My suspicion is that this CaM translocation is driven by an as yet unidentified Ca-CaM target protein that brings CaM to the nucleus. Otherwise, the very high affinity of free Ca-CaM for many cellular targets would dramatically slow such translocation. It may be most appropriate to visualize CaM as being 99% either prebound at or near specific Ca-CaM target sites or actually bound as Ca-CaM to a target site (with little CaM floating around). This sets the scene for very local Ca-CaM signaling as a normal modus operandi.
CaMKII exists mainly as a stable dodecamer, making its functional size ~12-fold larger, and consequently limiting its diffusional capability. It seems unlikely that it translocates appreciably in the short term (not like CaM). We know less about specific CaMKII binding partners and affinities in myocytes, but it makes sense to assume for now that CaMKII is normally localized very near its target phosphorylation sites, allowing for rapid signal transduction (see also below). For example, both CaM and CaMKII are known to bind to the ryanodine receptor (RyR), inositol 1,4,5-trisphosphate receptor (InsP3R) and L-type Ca channel in myocytes even at diastolic [Ca]i and both Ca-CaM itself and CaMKII-dependent phosphorylation can modulate gating of these channels.4
CaMKIIδ is the predominant isoform in cardiac myocytes and exists in two main splice variants CaMKIIδB and CaMKIIδC. The former contains a nuclear localization signal sequence, and these are often called nuclear and cytosolic forms, but because they readily hetero-multimerize both types occur in both loci. There are also smaller amounts of CaMKIIγ and CaMKIIβ in cardiac myocytes (which can also multimerize with CaMKIIδ).
CaMKII Activation and Memory
When [Ca]i increases and Ca binds to CaM it activates CaMKII by interfering with or opening an autoinhibitory domain of CaMKII. Once opened, a CaMKII monomer can be auto-phosphorylated by CaMKII, creating an autonomous active state even when [Ca]i declines. This creates molecular memory and allows CaMKII to serve as an integrator during repeated Ca pulses (and makes CaMKII activity sensitive to local phosphatase activity). Another site near the autophosphorylation site is also an oxidation target which under oxidative stress can also trap CaMKII in the autonomous state. We also recently identified two other forms of post-translational modification that function similarly and would also enhance CaMKII activation in high glucose conditions (e.g. diabetes) and oxidative stress. These multiple post-translational modifications add complexity to CaMKII regulation. It is possible that much physiological CaMKII regulation happens without progression to the autonomous state, but that different pathophysiological states can drive CaMKII increasingly into autonomous states and disrupt normal physiological regulation. This raises questions about: 6) how much autonomous CaMKII activation normally occurs under physiological conditions (as functions of heart rate and neurohumoral signaling)? 7) Which of the several autonomous states predominate in different pathophysiological states?
CaMKII has a relatively low affinity for Ca-CaM (Kd ~ 50 nM) compared to CaN (Kd <<1 nM) which has important functional consequences.5 First, activation of CaMKII requires relatively high [Ca]i and will de-activate more rapidly when [Ca]i declines. This makes CaMKII especially sensitive to Ca-CaM-dependent activation in environments where large local Ca transients occur (reaching local [Ca]i >20 µM), such as near the mouths of Ca channels (RyR, LTCC and InsP3R). The flip side of this is that bulk cytosolic CaMKII may not be significantly activated by beat to beat variations in [Ca]i.5 This raises questions about how CaMKII that is not near Ca channels is activated. The potential brevity of CaMKII activation might also limit the opportunity for autophosphorylation and how local CaMKII signaling integrates. For CaN the situation is very different because of a very high Ca-CaM affinity and slow off-rate.5 Thus, for CaN localized near Ca channels that open at each beat (e.g. clefts where E-C coupling occurs); one would expect CaN to be virtually fully activated at all relevant heart rates. In contrast, cytosolic CaN would be expected to serve as an intrinsically integrating signaling pathway (because of the slow off-rate of Ca-CaM).
Non-canonical pathways of myocyte CaMKII activation (in addition to new autonomous states) have also emerged recently. For example, β-adrenergic receptor (β-AR) are capable of activating CaMKII independent of either cAMP-dependent protein kinase or elevated [Ca]i. This adds new complexity to the understanding of CaMKII signaling dynamics in cardiac myocytes.
Pathways involved in CaMKII-dependent E-T coupling
Olson’s group has demonstrated the important role of class II HDACs in cardiac E-T coupling, and HDAC4 and 5 are phosphorylated in myocytes by CaMKII and PKD, and this mediates HDAC nuclear export in response to hypertrophic neurohumoral stimuli (e.g. ET-1 or phenylephrine).1 HDAC4 has a specific docking site for CaMKII, and as such nuclear export of HDAC4 appears to be mediated almost exclusively by CaMKII. HDAC5 lacks the CaMKII docking site and in cultured neonatal myocytes HDAC5 nuclear export is controlled by PKD rather than CaMKII. However, in adult ventricular myocytes, where PKD expression is lower and CaMKII expression is higher, these two kinases contribute equally to ET-1-induced HDAC5 nuclear export and MEF2 driven transcription.2 Moreover, we found that Ca release from InsP3R type 2 (which are primarily at the nuclear envelope in myocytes) was required for this ET-1-induced HDAC5 nuclear export (Fig 1). Furthermore, CaMKIIδ associates directly with this InsP3R, and the physical proximity may enhance the ability of Ca coming through nuclear envelope InsP3R channels to activate local CaMKII (as above). Indeed, global Ca transients throughout the cell were unable to activate the HDAC5 nuclear export, whereas the ET-1-induced InsP3R Ca release did not perturb the global [Ca]i.2 This shows how the myocyte can use different local Ca signaling pathways simultaneously for different functions (E-C coupling and E-T coupling) without appreciable crossover.
Mice lacking HDAC5 exhibit baseline cardiac hypertrophy and exaggerated hypertrophy in response to pressure overload or cardiac calcineurin activation (but not to chronic β-AR). CaMKIIδ knockout mice exhibit reduced HDAC4 phosphorylation and are partially protected from pressure overload-induced pathological hypertrophy or transition to HF (but not the initial phase of hypertrophy). There are many remaining unanswered questions, even in this Ca-dependent E-T coupling pathway, but it is clear that CaMKII is a key player in HDAC-dependent transcriptional regulation. Questions include: 8) Does HDAC4 phosphorylation by CaMKII work by the same local signaling pathway that was worked out for HDAC5?, 9) What about other class II HDACs (HDAC7 and 10)? Do different G-protein coupled (or other) receptor signals work via different pathways involving or not CaMKII? 11) Which specific downstream transcripts are altered by specific CaMKII-HDAC pathways? For this Journal’s audience, this may especially be relevant with respect to alterations in ion channels and Ca transporters. Indeed, CaMKII overexpression can alter the functional expression levels of certain potassium channel currents (reducing IK1 and fast Ito, but increasing slow Ito) and Ca handling alterations associated with HF and arrhythmogenesis.4 12) What is the functional extent of crosstalk between this CaMKII-HDAC signaling pathway and other cardiac hypertrophic signaling pathways (e.g. CaN-NFAT)? As discussed earlier, we would expect CaN-NFAT to respond to different Ca signals than CaMKII, but NFAT nuclear import is also clearly Ca-dependent, and differs among NFATc1 vs. NFATc3 and also in atrial vs. ventricular myocytes.6
In conclusion, this role of CaMKII in E-T coupling is a complicated area, and I have only discussed the very front end of this process with respect to the activation of Ca-CaM and CaMKII signaling that can activate HDAC translocation. As CaMKII has relatively recently emerged as a multifunctional nodal point in cardiac myocyte physiology and pathophysiology, the coming few years should provide valuable new insight into the questions that I have raised here and beyond.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health R37-HL30077 and P01-HL80101.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Backs J, Olson EN. Control of cardiac growth by histone acetylation/deacetylation. Circ Res. 2006;98:15–24. doi: 10.1161/01.RES.0000197782.21444.8f. [DOI] [PubMed] [Google Scholar]
- 2.Wu X, Zhang T, Bossuyt J, Li X, et al. Local InsP3-dependent Perinuclear Ca Signaling in Cardiac Myocytes Excitation-Transcription Coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilkins BJ, Molkentin JD. Calcium-calcineurin signaling in the regulation of cardiac hypertrophy. Biochem Biophys Res Commun. 2004;322:1178–1191. doi: 10.1016/j.bbrc.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 4.Bers DM, Grandi E. CaMKII regulation of cardiac ion channels. J Cardiovasc Res. 2009;54:180–187. doi: 10.1097/FJC.0b013e3181a25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saucerman JJ, Bers DM. Calmodulin mediates differential sensitivity of CaMKII and calcineurin to local Ca2+ in cardiac myocytes. Biophys J. 2008;95:4597–4612. doi: 10.1529/biophysj.108.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinne A, Kapur N, Molkentin JD, et al. Isoform- and tissue-specific regulation of the Ca2+-sensitive transcription factor NFAT in cardiac myocytes and in heart failure. Am J Physiol. 2010;298:H2001–H2009. doi: 10.1152/ajpheart.01072.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]