Abstract
In spite of continuous research efforts directed at early detection and treatment of pancreatic cancer, the outlook for patients affected by the disease remains dismal. With most cases still being diagnosed at advanced stages, no improvement in survival prognosis is achieved with current diagnostic imaging approaches. In the absence of a dominant precancerous condition, several risk factors have been identified including family history, chronic pancreatitis, smoking, diabetes mellitus, as well as certain genetic disorders such as hereditary pancreatitis, cystic fibrosis, familial atypical multiple mole melanoma, and Peutz–Jeghers and Lynch syndromes. Most pancreatic carcinomas, however, remain sporadic. Current progress in experimental molecular techniques has enabled detailed understanding of the molecular processes of pancreatic cancer development. According to the latest information, malignant pancreatic transformation involves multiple oncogenes and tumor-suppressor genes that are involved in a variety of signaling pathways. The most characteristic aberrations (somatic point mutations and allelic losses) affect oncogenes and tumor-suppressor genes within RAS, AKT and Wnt signaling, and have a key role in transcription and proliferation, as well as systems that regulate the cell cycle (SMAD/DPC, CDKN2A/p16) and apoptosis (TP53). Understanding of the underlying molecular mechanisms should promote development of new methodology for early diagnosis and facilitate improvement in current approaches for pancreatic cancer treatment.
Keywords: Pancreatic cancer, Risk factors, Molecular biology, Pancreatitis, Diabetes
INTRODUCTION
Pancreatic cancer is a fatal illness that is characterized by late diagnosis in the absence of early symptoms, which results in identification of the illness at an advanced stage. Despite all therapeutic efforts, the mortality of pancreatic cancer remains high, with the number of newly diagnosed patients nearly equaling that of deceased patients[1].
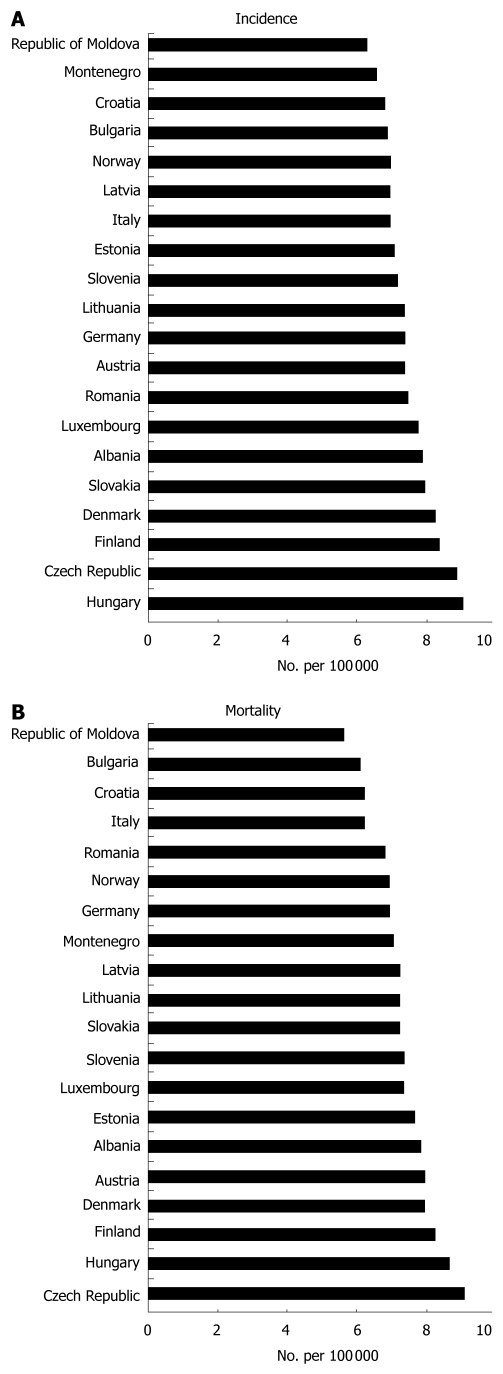
The incidence of pancreatic cancer is constantly on the rise, especially in regions of North America, Europe and Japan. In the United States, pancreatic cancer represents the fourth most frequent cause of death from cancer. In Europe it ranks fifth. In 2009, 42 470 new cases of this disease were diagnosed, and in the same year, 35 240 patients died of this disease[2]. One of the highest incidence and mortality rates among EU countries is observed in the Czech Republic, which, according to GLOBOSCAN 2008 data, ranks second in incidence (Figure 1A) and first in mortality (Figure 1B), followed by Hungary and Finland. In the Czech Republic, pancreatic cancer is the 10th most frequent malignancy, and accounts for 2.6% of all newly diagnosed neoplasms. In 2005, the absolute number of newly diagnosed pancreatic cancer cases was 901 in men and 876 in women. In that same year, the absolute number of deceased persons from the disease reached 1580 (819 men and 761 women)[3].
Figure 1.
Standardized incidence (A) and mortality (B) rates for the 20 European countries with the highest occurrence of pancreatic cancer (Adapted from GLOBOCAN 2008).
Five-year survival for pancreatic cancer is < 5%, mainly due to its late diagnosis, when it is already at an advanced stage[4]. At the time of diagnosis, < 5% of tumors are resectable. Median survival following surgical resection ranges from 13 to 21 mo. Without surgery, median survival is a mere 2.5-8 mo[2].
RISK FACTORS
Due to the relative rarity of pancreatic cancer, which is characterized by a complexity of underlying carcinogenesis, it is likely that a combination of multiple factors contributes to the initiation of the disease. Some factors, such as smoking or alcohol intake, can be controlled, while others such as age or family history cannot. Although most risk factors do not directly cause the disease, level of exposure often influences cancer development. As the treatment options are still limited and the survival prognosis remains poor, identification and evasion of the controllable risk factors becomes particularly important for individuals at high risk[5]. The most prominent pancreatic cancer risk factors are summarized in Table 1.
Table 1.
Pancreatic cancer risk factors
| Factor | Type | Group | Maximum risk to average ratio |
| Smoking | Exogenous | Behavioral | 3 |
| Alcohol | Exogenous | Behavioral | Non-significant |
| Diet/obesity | Exogenous | Behavioral | 1.72 |
| Occupational hazard | Exogenous | Environmental | - |
| Radiation | Exogenous | Environmental | Inconclusive |
| Age | Endogenous | Biological | - |
| Race | Endogenous | Biological/behavioral/environmental | 1.4 |
| Family history | Endogenous | Genetic | 32 |
| Peutz-Jeghers syndrome | Endogenous | Genetic | 132 |
| FAMMM syndrome | Endogenous | Genetic | 13.1 |
| HNPCC | Endogenous | Genetic | 7 |
| Hereditary pancreatitis | Endogenous | Genetic | 60 |
| Chronic pancreatitis | Endogenous | Behavioral | 26.3 |
| Diabetes mellitus | Endogenous | Biological/environmental | 2.0 |
| Hormonal | Endogenous | Biological | Inconclusive |
| Allergy | Endogenous | Biological | Non-significant |
FAMMM: Familial atypical multiple mole melanoma; HNPCC: Hereditary non-polyposis colorectal carcinoma syndrome.
Smoking and alcohol
Cigarette smoking represents one of the most significant and most widely studied risk factors for pancreatic cancer. The carcinogenic effect of tobacco smoke on pancreatic tissue is explained as the direct action of N-nitrosamines or their secretion into bile and their subsequent reflux into the pancreatic duct. Active smoking increases the relative risk of pancreatic cancer 1.5-3-fold, depending on the number of cigarettes smoked and the duration of this habit. Passive smoking has not been shown to be a risk factor[6].
A number of epidemiological studies have focused on the relationship between alcohol consumption and development of pancreatic cancer. An analysis of 14 prospective studies has not confirmed any association between alcohol consumption and a higher risk of pancreatic cancer[3]. Only a very weak association has been demonstrated in the case of alcohol consumption at a dose of ≥ 30 g/d, regardless of the alcohol source of beer, wine or spirits. There is also a significant association with body mass index (BMI), whereby a slight increase in cancer risk has been described in persons with an alcohol consumption of ≥ 30 g/d and a BMI of ≤ 25 kg/m2. From the aforementioned, it is possible to infer that the association between alcohol consumption and development of pancreatic cancer is only implied.
This association is apparently conditional on the development of chronic pancreatitis, for which alcohol is a known inducer, and chronic pancreatitis is an independent risk factor for this cancer. It thus appears that alcohol consumption at lower doses that do not damage pancreatic tissue does not carry a higher risk for developing pancreatic cancer.
Diet and obesity
Based on a number of relevant studies, it is possible to observe an association with a diet rich in animal fats and higher consumption of meat (roasted, grilled or fried). The results of studies that focus on the effect of cholesterol, eggs, milk and dairy product consumption on increased risk of pancreatic cancer are inconsistent[7]. In contrast, a diet rich in fiber, fruit, vegetables and vitamins, especially vitamin C, is considered to be a protective factor[8]. Omega-3 unsaturated fatty acids that are contained mainly in fish oil also act protectively. A similar protective effect has been shown for substances that influence DNA methylation and synthesis, such as folates. As demonstrated in an analysis of 14 prospective studies, a positive association between alcohol consumption and cancer has been discovered for alcoholics with a low daily folate intake. Similar results have also been observed in the case of methionine. No association with coffee or tea consumption has been demonstrated[3].
Obesity is a generally recognized risk factor for pancreatic cancer in men and women[9]. In cases in which prospective studies have evaluated BMI, men with BMI ≥ 30 kg/m2 had a higher relative risk compared to women with the same BMI[10]. One particularly interesting fact is that physical activity did not reduce the risk in persons with BMI < 25 kg/m2, but it was indirectly proportional to the risk in persons with BMI ≥ 25 kg/m2. Physical activity, which in its final effect leads to increased insulin resistance, decreases the degree of risk in obese patients.
Occupational hazards
The possible influence of aromatic and heterocyclic amines as well as exposure to chlorinated solvents in the carcinogenesis of pancreatic cancer remains unclear. The groups most at risk from this aspect include workers in the petrochemical and rubber industry, as well as barbers and hairdressers in whom exposure to these substances is higher compared to the general population[11]. In contrast, the influence of heavy metals and especially cadmium, in view of its accumulation in pancreatic tissue, has demonstrated an accentuation of neoplastic processes. Another element that is suspected of a carcinogenic effect on pancreatic tissue is chromium. Nonetheless, occupational exposure leads only to an imperceptible increase in the relative risk, in view of the minimal doses involved.
Radiation
A study published in 1990 has stated that ionizing radiation does not increase the risk of pancreatic cancer[9]. These findings are based predominantly on research in people who survived an atomic bomb explosion. The notion that the pancreas is relatively non-sensitive to ionizing radiation has been partially revised by a study of the increased incidence of pancreatic cancer among employees of nuclear research centers in the United States and other countries[12]. Nonetheless, this report[9] has stressed the significance of other concurrent risk factors such as smoking, diabetes, positive family history, or any other pancreatic disease.
Age and race
Age and race are the most prominent confounding factors of pancreatic cancer risk. During the first three decades, pancreatic cancer is a rarity, but from the age of 30 years onwards, its incidence increases significantly, peaking in the seventh to eighth decades, when 80% of adenocarcinomas are diagnosed. The mean age at diagnosis is 65 years. Pancreatic cancer is diagnosed before the age of 50 years only in 10% of patients[13].
With regard to racial differences, pancreatic cancer demonstrates the highest incidence in Afro-Americans in the United States, inhabitants of Northern Europe, in Polynesians in Hawaii, and Maoris in New Zealand. In the United States, the mortality of the Afro-American population is 1.4 times higher than that of the Caucasian population. This fact may be explained by a higher proportion of smokers and patients with diabetes, and at the same time, a positive family history of pancreatic cancer[14].
Hormonal factors and allergy
Pancreatic cancer demonstrates a different incidence in men and women. The cumulative risk in men is 0.2% and 0.1% in women. The lower incidence of pancreatic cancer in women may point to a link between hormonal factors and the development of cancer. The results of studies dealing with the relationship between female hormones and the development of cancer conducted to date have been inconclusive. As studies conducted to date confirm, this malignancy demonstrates minimal estrogen dependency. Parity and duration of hormonal exposure are negatively associated with the degree of risk of pancreatic cancer[15,16]. In the case of postmenopausal women, this risk is not influenced by hormonal substitution. In a number of studies, pancreatic cancer is associated with a higher number of deliveries, with earlier menarche and late menopause, higher age during the first delivery, or hormonal contraception. On the other hand, there exist studies in which the aforementioned factors were associated with a decrease in the risk of pancreatic cancer. An analysis of 10 case-control studies and five cohort studies did not demonstrate any link between hormonal factors and pancreatic cancer in women[17]. Indeed, additional factors, such as differences in life habits, may also contribute to the different cumulative risks between men and women.
Studies published to date have demonstrated a decrease in the risk of pancreatic cancer in individuals with allergies, especially respiratory allergy[18]. Longer survival has been described in allergic patients who have undergone resection procedures compared to resected patients with no allergies. The decrease in risk is most often associated with respiratory forms of allergy such as hay fever, and allergy to pollen and grass. The mechanisms of the possible protective effect of allergy in patients with a tumor is not exactly known[19].
CHRONIC PANCREATITIS
At present, acute and chronic pancreatitis are considered two pathogenically different disease entities; their relation to the genesis of pancreatic cancer is likewise quite different. Although acute pancreatitis is not considered a risk factor in terms of the index diagnosis, the concept of a causal association between alcoholic, hypercalcemic, trophic and hereditary forms of chronic pancreatitis and increased predisposition to developing pancreatic cancer is generally recognized. The obstructive type of chronic pancreatitis has been questioned as a risk factor by some authors[20].
Although no more than 5% of diagnosed cases of pancreatic cancer can be explained by recurrent attacks of chronic pancreatitis, the same genetic changes have been detected in individuals with chronic inflammation of the pancreas and pancreatic cancer. Chronic inflammation is thought to induce genetic alterations in tissue, while the ongoing healing process exposes defective cells to growth factors; the result is a pathological microenvironment in which stromal elements facilitate the neoplastic process in epithelial cells (the so-called feeder theory)[21]. According to this theory, the effector cells of the inflammatory response include active macrophages whose products, particularly the cytokines tumor-necrosis factor (TNF)-α, interleukin (IL)-1, IL-6, IL-8, epidermal growth factor (EGF), platelet-derived growth factor (PDGF), and transforming growth factor (TGF) α and β induce cell proliferation, angiogenesis and desmoplastic reaction, that is, processes that form part of the pathogenesis of chronic pancreatitis and pancreatic cancer. IL-6 promotes the maturation of myeloid precursors into macrophages, and TGF-α inhibits processes leading to apoptosis and stimulates progressive fibrosis. Additionally, the same cytokine activates the transcription factor nuclear factor (NF)-κB, a mediator of inhibition of programmed cell death. Its expression has been reported in cases of chronic inflammation of the pancreas and pancreatic cancer. In addition, NF-κB upregulation promotes the production of reactive nitrogen oxide and cyclooxygenase (COX)-2 and induces IL-8 expression. There have been reports of an autocrine growth promoting effect of IL-8, which is produced at increased rates in response to hypoxia; particularly in the center of tumor foci, thus having a pro-carcinogenic and pro-metastatic effect. COX-2 responds to increased prostaglandin production in cases of inflammation and cancer, facilitates cell proliferation and angiogenesis, and is a potent inhibitor of apoptosis. Additionally, COX-2 is involved in the transformation of chemical carcinogens into their mutagenic derivatives. This particular ability of COX-2 increases the risk of pancreatic cancer in smokers. Immunohistochemical investigations have shown that COX-2 expression is bound to pancreatic islet Langerhan’s cells; as a result, increased expression of the enzyme heralds islet inflammation.
One of the hypotheses of pancreatic cancer development is based on the key relations between islet of Langerhan’s inflammation, insulin resistance, growth promotion, and diabetes. Additional products of activated macrophages and neutrophil granulocytes in pancreatitis and pancreatic cancer include reactive forms of oxygen and nitric oxide, whose presence is causally related to DNA structural defects[22]. The risk for developing a malignancy in individuals with chronic pancreatitis is 16 times that of the healthy population. One study has reported in patients with chronic pancreatitis, an increased incidence of both extrapancreatic tumors (relative risk, 1.5) and pancreatic malignancy (relative risk, 18.5). When one considers relevant only conditions that develop during 4 years of chronic inflammation, then the relative risk increased by a factor of 15.6 for smokers, whereas there was no increase in non-smokers[23]. A prospective study of the French Cancer Registry, including 85% of cases of chronic pancreatitis of alcoholic etiology, has reported a relative risk of 19.0[24]. In a Czech study of 213 patients, 11 of whom had cancer, the prevalence of pancreatic cancer was 5.1%. The interval from establishing the diagnoses of chronic pancreatitis and pancreatic cancer was 6-13 years. The cumulative risk for malignancy in patients with chronic pancreatitis was shown to increase in a linear manner, and was 1.8 and as high as 4.0 after 10 and 20 years, respectively[25]. The priority of current clinical research is to identify patients with sporadic chronic pancreatitis who are at increased risk of developing pancreatic cancer.
GENETIC SUSCEPTIBILITY
Family history of pancreatic cancer
Familial pancreatic cancer (FPC) is defined as two or more first-degree relatives with pancreatic cancer. As an independent nosological unit, FPC represents only 3%-10% of the total number of pancreatic cancers. Nonetheless, the relative risk in such cases is 4.6-32 times higher, depending on the number of afflicted persons within the family[26]. Only 20% of FPC patients demonstrate a genetic abnormality. Nonetheless, individuals from families with FPC should undergo genetic testing for the presence of hereditary breast and ovarian syndrome (BRCA1, BRCA2). These mutations are most often identified in FPC. The relative risk of pancreatic cancer in carriers of the BRCA1 mutation increases to 2.26 and in the case of the BRCA2 mutation to 3.5-8[27].
Pancreatic cancer forms a component of a whole range of hereditary diseases and syndromes.
Cystic fibrosis is an autosomal recessive disease that is caused by mutations in the CFTR gene, and is characterized by the production of viscous mucus, which apart from blocking the airways, also leads to obstruction of the pancreatic duct, which increases the risk of inflammation. Patients with CF are at increased risk of chronic pancreatitis and of pancreatic tumors[28].
Familial atypical multiple mole melanoma (FAMMM) is an autosomal dominant disease that is characterized by the occurrence of > 50 atypical nevi and malignant melanoma in two or more first or second-degree relatives. Approximately 10% of melanomas have a familial incidence and the mutation of the CDKN2A gene is identified in -40% of these families[29].
Peutz-Jeghers syndrome is an autosomally dominant hereditary disease with characteristic hamartoma polyps of the gastrointestinal tract, and mucocutaneous melanin pigmentation. Almost half of these patients are carriers of a germinal STK11/LKB1 gene mutation. Thus, afflicted individuals have a 36% risk (cumulative lifetime risk) of developing pancreatic cancer[30].
Hereditary non-polyposis colorectal carcinoma syndrome (HNPCC) is another hereditary cancer syndrome, for which the incidence of pancreatic cancer is typical. This syndrome is caused by mutations in mismatch repair (MMR) genes MSH2, MLH1, MSH6 and PMS2. The average risk in carriers of MMR gene mutations is 5%-10%. Pancreatic cancer is approximately seven times more frequent in carriers of MMR gene mutations, and in these individuals, it is 15 times more often diagnosed before the age of 60 years[31]. Apart from the aforementioned, pancreatic cancer can occur in association with other diseases such as Li-Fraumeni syndrome, ataxia-teleangiectasia syndrome, multiple endocrine neoplasia type I syndrome (MENI) or Von Hippel-Lindau syndrome.
Hereditary pancreatitis
Hereditary pancreatitis is currently considered to be an independent nosological unit. This is an autosomally dominant disease with 80% penetrance. In patients with hereditary pancreatitis, trypsin becomes activated while still in the pancreas. This accounts for partial digestion of the pancreatic tissue, which causes irritation and inflammation. A strong genetic association exists with mutations found in the PRSS1, SPINK1 and CFTR genes[32]. Patients with this hereditary pancreatitis have a 40-60-fold higher risk of developing pancreatic cancer. If such predisposed individuals are smokers, then the development of pancreatic cancer, or rather its diagnosis, shifts to younger age categories, in which it occurs up to two decades earlier than in non-smokers. Similarly, alcohol consumption also leads to earlier diagnosis of cancer, also 20 years earlier[2].
DIABETES MELLITUS
A mutual association between pancreatic cancer and diabetes mellitus has long been monitored. However, the issue of mutual linkage is complicated by the fact that, while long-term diabetes is considered to be an etiological factor of the cancer, newly developed diabetes is an early manifestation of the cancer[33].
The pathogenesis of diabetes associated with cancer and the biochemical mediators involved have not been completely elucidated. Its development due to the mere destruction of pancreatic tissue by the tumor or as a consequence of chronic pancreatitis is less probable. The high prevalence of diabetes and disorders of glucose tolerance in small, early carcinomas (< 20 mm), and primary detection of diabetes nearly 2 years before the diagnosis of carcinoma, points to the influence of humoral markers rather than to local effects of the tumor. Further research is necessary to clarify the pathogenesis of carcinoma-associated diabetes, and to uncover new markers that can differentiate it from type 2 diabetes[33]. Newly developed diabetes during a period of < 2 years prior to the diagnosis of carcinoma is a promising sign of the presence of a completely asymptomatic carcinoma. This is why screening of sporadic, early pancreatic cancer in persons with newly diagnosed diabetes is being considered. The interval between primary detection of diabetes and the diagnosis of carcinoma ranges between 5 and 29 mo[34].
Primary detection of hyperglycemia and diabetes represent a reference point for the timely diagnosis of sporadic pancreatic carcinoma before symptoms develop. It is well known that the symptoms of pancreatic cancer occur only weeks or months before diagnosis, which usually means that an advanced, non-resectable tumor is present and expected survival is only 4-6 mo. If we monitor glycemia in patients with small, resectable carcinomas (< 20 mm), then most suffer from disorders of glucose tolerance. Studies have demonstrated in 55%-65% of patients with resectable carcinoma, a disorder of glucose tolerance or newly developed diabetes during a period of < 2 years prior to the diagnosis of carcinoma[34,35]. Approximately half of patients with sporadic carcinoma suffer from diabetes, and in almost 50%, diabetes is diagnosed at the time of carcinoma diagnosis. It is highly probable that diabetes precedes the diagnosis of the malignancy by several months or even years. The aforementioned facts indicate the application of pancreatic cancer screening in asymptomatic individuals with newly diagnosed diabetes[33].
Studies conducted to date have shown that the prevalence of diabetes (determined on the basis of the oral glucose test, fasting blood glucose, and meeting American Diabetes Association criteria) in patients with pancreatic cancer is 45%-65%. In the original study, diabetes was newly diagnosed concurrently with carcinoma in 40% of cases[34]. In other studies, the percentage of newly diagnosed diabetes was as high as 74%-88%. In summary, it could be concluded that the majority of diabetes associated with pancreatic cancer represents de novo diabetes, that is, diagnosed during a period of 2 years preceding pancreatic cancer, and almost half of patients with early carcinoma have diabetes. Moreover, diabetes that develops in this way usually improves following pancreatic resection.
MOLECULAR MECHANISMS
Model of pancreatic neoplasia
Molecular mechanisms of solid cancer are very complex with different mechanisms taking place and affecting the tissue at different stages of the disease. Detailed molecular mechanisms of initiation, development and progression of pancreatic cancer have been thoroughly studied since the basic principles of the disease were revealed in the 1970s and 1980s[36-40]. The classic model of pancreatic cancer development describes morphological as well as molecular transformation from precursor lesions into invasive carcinoma[41]. The standard nomenclature and diagnostic criteria for classification of duct lesions has primarily been based on grades of pancreatic intraepithelial neoplasia (PanIN)[42]. The grades 1A, 1B, 2 and 3 represent growing cytological atypia characterized by loss of polarity, nuclear crowding, enlarged nuclei, pseudo-stratification and hyperchromatism. Each PanIN stage is characterized by a distinct pattern of molecular processes that are characterized by genetic irregularities that affect specific genes and genetic pathways.
Proto-oncogenes and tumor suppressors
In the PanIN model, genetic alterations have a fundamental role affecting key guardians of cellular signaling, which induces instability of entire molecular systems such as cell growth, division, apoptosis and migration. Proto-oncogenes code for proteins that act as positive regulators for these systems, such as growth factors, signal transducers, transcription factors or apoptotic inhibitors. Their mutated forms, oncogenes, are often present in cancer cells. The mutation causes the protein products of oncogenes to be permanently activated, which results in uncontrolled cell proliferation. Oncogenic mutations have a dominant character; therefore, deficiency of one allele (i.e. heterozygous mutation) is sufficient for a fatal outcome. There are several key proto-oncogenes involved in pancreatic cancerogenesis, including KRAS, CTNNB1 (β-catenin), PIK3CA or AKT1. The most common oncogenic mutation types are point mutation, deletion, gene amplification, and gene rearrangement.
Tumor suppressor genes code for proteins that act against cell proliferation, such as signaling inhibitors, negative transcription factors, activators of apoptosis, or members of DNA repair systems. As a result of genetic alteration, their normal function may be reduced or eliminated completely. Mutations in tumor suppressor genes have a recessive character; hence, the cell looses their function only when both alleles are affected. In the most common case, described as a double hit model, one allele is initially mutated while the other is subsequently mutated or lost completely[43]. A separate mechanism of tumor suppressor deactivation is by hypermethylation[44]. In pancreatic cancer, the frequently affected tumor suppressors include TP53, APC, SMAD4 and TP16. The genes most frequently mutated in pancreatic cancer are listed in Table 2[45,46].
Table 2.
| Gene symbols | Protein name | Mutation frequency (%) | Type | Main signaling or system |
| KRAS | K-ras | 58 | Proto-oncogene | Ras/Raf/MAPK |
| TP53 | Tumor protein p53 | 37 | Tumor suppressor | Apoptosis Cell cycle control |
| CDKN2A | Tumor protein p16 (INK4A) | 29 | Tumor suppressor | Cell cycle control |
| CTNNB1 | β-catenin | 24 | Proto-oncogene | Wnt |
| SMAD4 DPC4 | Smad Dpc4 | 22 | Tumor suppressor | TNFbeta/SMAD |
| APC | Apc | 16 | Tumor suppressor | Wnt |
| PIK3CA | Phosphoinositide 3-kinase | 5 | Proto-oncogene | PTEN/PI3K/AKT |
DPC: Deleted in pancreatic cancer; APC: Adenomatous polyposis coli; MAPK: Mitogen-activated protein kinase.
Signaling pathways in pancreatic cancer c-MET/HGF signaling pathway
The c-MET/HGF (hepatocyte growth factor) signaling pathway is a key factor in early progression of pancreatic cancer. The pathway is responsible for invasive growth through activation of key oncogenes, angiogenesis and scattering (cell dissociation and metastasis). c-MET is a proto-oncogene that encodes an HGF receptor that has a primary function in embryonic development and wound healing[47]. Although c-MET mRNA is present at very low levels in normal human exocrine pancreas, it is upregulated in a majority of pancreatic cancers[48-50], as well as in pancreatitis-affected epithelial cells[51]. Overexpression of c-MET is also observed in regenerating tissue affected by acute pancreatitis[52], and it is seen as an early event in pancreatic cancerogenesis[51]. HGF is a primary ligand of c-MET. Upon c-MET/HGF interaction, several different signaling pathways are activated, including the Ras, phosphoinositide 3-kinase (PI3K), Janus kinase (JAK)/signal transducer and activator of transcription (STAT) and β-catenin (Wnt) pathways.
Ras/Raf/MAPK pathway
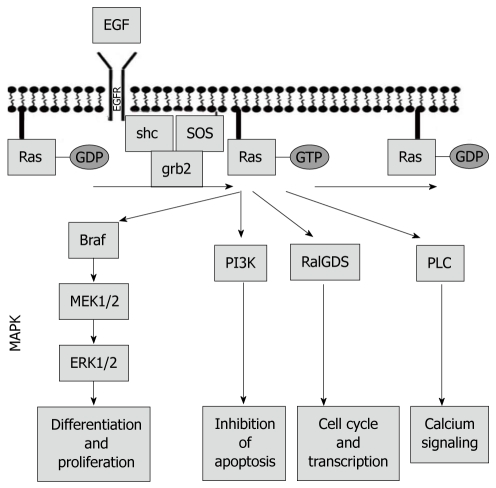
The Ras/Raf/mitogen-activated protein kinase (MAPK) pathway is one of the most studied and best described signaling pathways in cancer[53]. The role of Ras/Raf/MAPK signaling is critical for many cancerogenic processes, including cell growth and division, cell differentiation and migration, tissue healing and repair, and angiogenesis. The central regulator of the signal transduction from extracellular to intracellular environment is Ras protein, which is localized at the inner wall of the cellular membrane. Under normal physiological conditions, the hydrophobic Ras protein is inactive and bound to GDP. An extracellular signal coming through growth factor transmembrane receptors (such as growth factor receptors or cytokine receptors) promotes release of a guanidine exchange factor SOS, which initiates removal of GDP from Ras protein and its subsequent activation upon binding to GTP. Activated GTP-Ras complex triggers a kinase activity of Raf kinase, which ultimately results in activation of an MAPK, an important regulator of DNA transcription and mRNA translation. Mutations that affect any of the Ras/Raf/MAPK members produce an increase in tumorigenicity. Aside from Raf and MAPK, there are other downstream effectors of Ras protein, including PI3K, thus providing crosstalk between multiple pathways (Figure 2).
Figure 2.
Ras/Raf/mitogen-activated protein kinase pathway.
PTEN/PI3K/AKT pathway
PTEN/PI3K/AKT is a significant signaling pathway that is fundamentally based on regulated activation of AKT oncogene through its localization at the cell membrane[54]. The two important protein families involved in the membrane localization of AKT are PI3K and PTEN phosphatases.
PI3K is able to phosphorylate certain membrane-bound lipids known as phosphoinositides. The PI3K-mediated phosphorylation may progress in three stages, which produces phosphatidylinositol 3-phosphate (PIP), phosphatidylinositol (3,4)-bisphosphate (PIP2), and phosphatidylinositol (3,4,5)-trisphosphate (PIP3). The phosphorylated forms, PIP3 and, to a lesser extent, PIP2, attract important protein kinases to the cell membrane. The most prominent is AKT, a family of serine/threonine protein kinases that trigger a number of key cellular processes, including glucose metabolism, cell proliferation, apoptosis, transcription, and cell migration. AKT activity is strongly dependent on its proper localization on the cell membrane. The positioning of AKT at the membrane is achieved through its strong binding to PIP3. In pancreatic carcinogenesis, AKT1 acts as an oncogene that upholds cell survival by overcoming cell cycle arrest[55-57], blocking apoptosis[58-60], and promoting angiogenesis[61]. PTEN is a phosphatase that acts in opposition to PI3K. It has tumor suppression ability by converting PIP3 back to PIP2 and to PIP, hence disrupting membrane localization and reducing activity of AKT[62]. In most cancers, expression levels of PI3Ks and AKT are high, while PTEN is often deactivated by mutation, or deleted completely. Through its key role in pancreatic carcinogenesis, PI3K/AKT/PTEN signaling is an important target for anticancer therapy.
JAK/STAT pathway
The JAK/STAT signaling pathway has an important role in regulation of DNA transcription by transmission of chemical signals from cytokine receptors into the cell nucleus. The signal is passed upon phosphorylation of receptor tyrosine residues by JAK prompting activation and dimerization in a family of STAT proteins. Activated STAT dimers initiate DNA transcription inside the nucleus. It is known that inhibition of JAK/STAT signaling induces apoptosis in various human cancers, and is therefore, a primary focus for potential new drug candidates[63]. A recent study has reported reduced growth of pancreatic cancer cells in vitro when exposed to benzyl isothiocyanate (BITC), due to its suppression of STAT3 signaling and subsequent induction of apoptosis. This is suggested as a possible explanation of the anticarcinogenic effect of cruciferous vegetables (such as broccoli, cauliflower, cabbage or horseradish) that are rich in BITC[64].
TGF-β/SMAD signaling
TGF-β is a ligand that binds to type II cytokine receptor dimer, which then binds and activates type I cytokine receptor dimer, which triggers phosphorylation of receptor-regulated SMADs (R-SMADs), mainly SMAD2 and SMAD3. In phosphorylated form, the R-SMADs form a complex with SMAD4, which accumulates in the nucleus and interacts with other factors to stimulate transcription of genes that are important for cell cycle arrest and migration. SMAD4 is therefore a key mediator for TGF-β signals. Due to its frequent absence in proliferating pancreatic cancer tissue, it is also known as DPC or “deleted in pancreatic cancer”[65]. Relatively high frequency of SMAD4 mutations and loss of heterozygosity at the DPC4 locus (18q21.1) strongly suggest that the protein is a primary tumor suppressor that is involved in pancreatic cancerogenesis. However, reinstating SMAD4 expression results in tumor growth suppression only in vivo and not in vitro. It has also been found that SMAD4-independent pathways may be responsible for tumorigenic effect of TGF-β signaling[66].
Wnt signaling
Wnt signaling is crucial to formation and maintenance of endocrine pancreas[67]. During pancreatic carcinogenesis, Wnt triggers transcription of a number of genes that have a direct impact on cell proliferation, differentiation and migration[68]. Activation of Wnt signaling is by interaction of a family of membrane-bound receptors known as Frizzleds with Wnt ligands. Once activated, the downstream signals may proceed through separate pathways. In a canonical pathway, signal transduction is mediated by stabilization and translocation of β-catenin from the cytosol into the nucleus followed by its interaction with T-cell factor (HMG box) which activates transcription of target genes. The localization of high expression levels of β-catenin at the nucleus has been experimentally confirmed for various high grade PanIN lesions, as well as in advanced pancreatic cancer[69]. In a non-canonical, β-catenin-independent pathway, other signal mediators are involved, which block the β-catenin-assisted transcription. The nuclear localization of β-catenin and high expression levels of WNT5a, a gene involved in non-canonical Wnt pathways, suggests involvement of both pathways in pancreatic cancer progression[68].
CDKN2A and cell cycle control
The cell cycle control genes have profound importance in cancer and CDKN2A is one of key factors in its negative control. The CDKN2A has two promoters and alternative splicing sites that result in two alternative protein products: cyclin-dependent kinase inhibitor p16INK4a and p53-activator p14ARF. Although both proteins are active in negative control of the cell cycle, only the function of p16INK4a is frequently lost in pancreatic tumors due to point mutations, deletions or hypermethylation[70]. p16INK4a protein (also known as p16) inhibits key elements of cell cycle progression at the G1 checkpoint. p16 inactivation is an early event in pancreatic carcinogenesis, and low levels of p16 expression are associated with larger tumors, risk of early metastases and poor survival[71].
MOLECULAR DIAGNOSTICS
A whole range of findings regarding the molecular biological basis of malignant transformation in pancreatic cancer has been published in recent years, and certain progress has been achieved also in the diagnosis, staging and treatment of localized tumors. In the fields of prevention, early diagnosis, screening and treatment of advanced tumors, which represent the majority of newly diagnosed cases, research has failed to provide any fundamental discoveries that would significantly affect the prognosis of patients with pancreatic cancer. Better understanding of the mechanisms of molecular genetics involved in pancreatic carcinogenesis has enabled the identification of a number of hereditary syndromes that in probands represent an increased risk of cancer. An overview is shown in Table 3[72].
Table 3.
Overview of hereditary syndromes predisposing to pancreatic cancer
| Syndrome | Gene | Life-time risk | Relative risk |
| FAMMM | CDKN2A | 10%-15% | 20-34 |
| HBOC | BRCA2 | 5% | 10 |
| HBOC | BRCA1 | Not known | 2 |
| Hereditary pancreatitis | PRSS1/TRY1 | 30%-50% | 50 |
| Lynch syndrome | MLH1/MSH2 | Not known | Not known |
| Peutz–Jeghers syndrome | STK11/LKB1 | 36% | 136 |
| FAP | APC | Not known | 4 |
| Li–Faumeni syndrome | p53 | Not known | Not known |
| FPC | Not known | Up to 50% | 18-57 |
APC: Adenomatous polyposis coli; FAMMM: Familial atypical multiple mole melanoma; FAP: Familial adenomatous polyposis; FPC: Familial pancreatic cancer; HBOC: Hereditary breast and ovarian syndrome. Adapted from[72].
Carbohydrate antigen (Ca) 19-9 retains its dominant role among tumor markers. It is the only marker to have been applied in clinical practice, where it is used to detect early recurrence in patients with an already known diagnosis and those undergoing treatment[73]. Use of Ca 19-9 as a screening test has yielded unsatisfactory results. This marker is not specific for pancreatic cancer and may be elevated in various cholestatic syndromes, and not necessarily a tumor. Moreover, the levels of secreted Ca 19-9 are affected by positivity of the Lewis antigen a and b[74]. Table 4 presents an overview of other biomarkers being studied as potentially useful in the diagnosis of pancreatic cancer[75].
Table 4.
Overview of biomarkers in pancreatic cancer
| Biomarker | Sensitivity (%) | Specificity (%) |
| CEA | 45 | 75 |
| Carcinoembryonic antigen-related cell adhesion molecule-1 | 85 | 98 |
| Ca 19-9 | 80 | 73 |
| SPan-1 | 81-94 | 75 |
| DUPAN-2 | 48-80 | 75-85 |
| Macrophage inhibitory cytokine 1 | 90 | 62 |
| Alpha4GnT | 76 | 83 |
| PAM4 | 77 | 95 |
| Pancreatic juice DNA methylation | 82 | 100 |
| Fecal K-ras | 77 | 81 |
CEA: Carcinoembryonic antigen; CA19-9: Carbohydrate antigen 19-9; DUPAN-2: Pancreatic cancer-associated antigen 2; PAM4: Peptidylglycine alpha-amidating monooxygenase 4.
Currently, none of the listed biomarkers meets the criteria of utility for the detection of early carcinoma. Even the promising marker PAM4 has demonstrated a sensitivity of 54% in early stage 1a carcinoma and 75% in stage 1b carcinoma[75]. The common denominator of the failure of all biomarkers in early detection lies in their low sensitivity; in some cases, associated with difficult or an invasive collection of biological material.
Detection of early pancreatic cancer in the general population using currently available means is impossible. Interest is now focusing on at-risk groups; especially those in whom the risk of developing this cancer is at least 10-fold higher compared to the general population. This risk may be stratified into low, intermediate and high[76]. Table 5 summarizes this risk stratification.
Table 5.
Level of risk according to cumulative risk factors
| Factors | Risk level |
| Race/sex: Male; black; Ashkenazi Jewish descent. Exposures: obesity; smoking; diabetes mellitus; Helicobacter pylori infection.Family history: Cancer history in a first-degree relative; history of pancreatic cancer in one first-degree relative. Inherited conditions: Hereditary non-polyposis colorectal cancer; familial adenomatous polyposis; BRCA1 mutation carrier | Low (less than 5-fold) |
| Family history: History of pancreatic cancer in two first-degree relatives. Inherited conditions: cystic fibrosis; BRCA2 mutation carrier. Comorbidity: Chronic pancreatitis | Moderate (5- to 10-fold) |
| Inherited conditions: FAMMM kindreds with p16 germline mutation and at least one case of pancreatic cancer in a first-degree or second-degree relative; hereditary pancreatitis; Peutz–Jeghers syndrome; BRCA2 or BRCA1 mutation carrier with at least one case of pancreatic cancer in a first-degree or second-degree relative. Family history: Three or more first-degree; second-degree or third-degree relatives with pancreatic cancer | High (greater than 10-fold) |
FAMMM: Familial atypical multiple mole melanoma.
Several studies published recently have attempted to detect early and resectable carcinoma in high-risk groups with the aid of imaging methods[72,76]. Screening examinations usually include some type of imaging method, such as endoscopic ultrasound, magnetic resonance imaging, computer tomography, or genetic tests. Successful identification of small tumors or precursor lesions, cystic tumors and intraductal papillary mucinous neoplasia is now possible in the at-risk population to a higher degree than in the general population. In cases in which solid ductal adenocarcinoma is uncovered, these lesions are usually resectable. Nonetheless, according to the available literature, recurrence has been demonstrated in all such patients over a period of several months.
In recent years, clinical research has focused on identifying patients with chronic pancreatitis with a high risk of developing pancreatic cancer. As discussed above, the following risk factors have been identified to date: smoking[23], duration of chronic pancreatitis[25], status after surgery for chronic pancreatitis in symptomatic individuals (recurrent pain, jaundice, weight loss, loss of appetite)[77], presence of a mutated form of the K-ras oncogene in a sample obtained using fine-needle aspiration biopsy or pancreatic duct brush cytology[78], loss of suppressor gene p16 expression[79], and polymorphism of the uridine diphosphate glucuronyltransferase gene (presence of the UGT1A7 allele causing low detoxification activity of the enzyme)[80].
In view of the completely different pathogenesis of acute and chronic pancreatitis and thus the different relationship to the development of pancreatic cancer, acute pancreatitis currently is not considered to be a risk factor for the development of pancreatic cancer. On the contrary, the association between alcoholic, hypercalcemic, tropical and hereditary chronic pancreatitis and the increased risk of pancreatic cancer is generally valid. The risk of developing pancreatic cancer in patients with chronic pancreatitis is up to 16-fold higher compared to the healthy population. K-ras mutations are detectable in nearly 80% of patients with carcinoma. Detection of K-ras mutations in patients with chronic pancreatitis may thus be used in combination with other methods as a screening test for the detection of early carcinoma[81].
As described above, much attention is also being paid currently to the relationship between newly diagnosed type 2 diabetes mellitus and pancreatic cancer, whereby diabetes is considered to be an early manifestation of pancreatic cancer, preceding the usual clinical manifestations of this malignancy. Patients with newly diagnosed diabetes will probably be considered to be at higher risk than they are today and will be screened. However, patients with diabetes who are suitable for screening will need to undergo multilevel selection, and the diagnosis of diabetes itself will represent the first filter of such a process. The second level should be the presence of one of the current biomarkers, or preferably the identification of a new marker with a higher predictive value. No such marker is currently available, therefore, predictive computer models are also being envisaged.
Recent studies have demonstrated in pancreatogenic diabetes mellitus a protective effect of metformin for decreasing the risk of developing pancreatic cancer in patients with chronic pancreatitis. In contrast, treatment with insulin or its secretagogues increases the risk of carcinoma in these patients[82].
CONCLUSION
Uncovering the importance of basic risk factors such as chronic pancreatitis and diabetes mellitus, along with a detailed knowledge of fundamental molecular processes is expected to assist in reducing mortality of pancreatic cancer through development of new approaches for detection of early stages of the disease. This will mainly be applied to evaluation of the survival prognosis and rational selection of therapy; most importantly, with respect to options for radical surgical treatment. In addition, identification of specific genetic aberrations may serve as key molecular markers as predictors of response for targeted therapies. The response prediction should not only prolong survival, but also improve the quality of life for most advanced stages of the disease.
Acknowledgments
Authors would like to thank Dr. Lucie Benesova for her assistance with detailed description of signaling pathways and Professor Marcela Kopacova for helpful discussions and comments on the manuscript.
Footnotes
Supported by the Czech Ministry of Health Project 9809. It is a contribution No. 3 from CEGES (OPPK CZ.2.16/3.1.00/22213)
Peer reviewers: Jose JG Marin, Professor, Head of the Departament of Physiology and Pharmacology, University of Salamanca, CIBERehd, Campus Miguel de Unamuno, ED-S09, Salamanca 37007, Spain
S- Editor Tian L L- Editor Kerr C E- Editor Ma WH
References
- 1.Lowenfels AB, Maisonneuve P. Epidemiology and risk factors for pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:197–209. doi: 10.1016/j.bpg.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Chu D, Kohlmann W, Adler DG. Identification and screening of individuals at increased risk for pancreatic cancer with emphasis on known environmental and genetic factors and hereditary syndromes. JOP. 2010;11:203–212. [PubMed] [Google Scholar]
- 3.Dusek L, Muzík J, Gelnarová E, Fínek J, Vyzula R, Abrahámová J. Cancer incidence and mortality in the Czech Republic. Klin Onkol. 2010;23:311–324. [PubMed] [Google Scholar]
- 4.Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG, et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18:765–776. doi: 10.1158/1055-9965.EPI-08-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Decker GA, Batheja MJ, Collins JM, Silva AC, Mekeel KL, Moss AA, Nguyen CC, Lake DF, Miller LJ. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y) 2010;6:246–254. [PMC free article] [PubMed] [Google Scholar]
- 6.Harnack LJ, Anderson KE, Zheng W, Folsom AR, Sellers TA, Kushi LH. Smoking, alcohol, coffee, and tea intake and incidence of cancer of the exocrine pancreas: the Iowa Women's Health Study. Cancer Epidemiol Biomarkers Prev. 1997;6:1081–1086. [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 8.Nkondjock A, Krewski D, Johnson KC, Ghadirian P. Dietary patterns and risk of pancreatic cancer. Int J Cancer. 2005;114:817–823. doi: 10.1002/ijc.20800. [DOI] [PubMed] [Google Scholar]
- 9.BEIR V: implications for the nuclear workforce. Science. 1990;247:620–622. [PubMed] [Google Scholar]
- 10.Nöthlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Meat and fat intake as risk factors for pancreatic cancer: the multiethnic cohort study. J Natl Cancer Inst. 2005;97:1458–1465. doi: 10.1093/jnci/dji292. [DOI] [PubMed] [Google Scholar]
- 11.Ojajärvi A, Partanen T, Ahlbom A, Boffetta P, Hakulinen T, Jourenkova N, Kauppinen T, Kogevinas M, Vainio H, Weiderpass E, et al. Risk of pancreatic cancer in workers exposed to chlorinated hydrocarbon solvents and related compounds: a meta-analysis. Am J Epidemiol. 2001;153:841–850. doi: 10.1093/aje/153.9.841. [DOI] [PubMed] [Google Scholar]
- 12.Tilyou SM. BEIR V report. Experts urge cautious interpretation of higher risk estimates. J Nucl Med. 1990;31:13A–19A. [PubMed] [Google Scholar]
- 13.Li D, Morris JS, Liu J, Hassan MM, Day RS, Bondy ML, Abbruzzese JL. Body mass index and risk, age of onset, and survival in patients with pancreatic cancer. JAMA. 2009;301:2553–2562. doi: 10.1001/jama.2009.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverman DT, Hoover RN, Brown LM, Swanson GM, Schiffman M, Greenberg RS, Hayes RB, Lillemoe KD, Schoenberg JB, Schwartz AG, et al. Why do Black Americans have a higher risk of pancreatic cancer than White Americans? Epidemiology. 2003;14:45–54. doi: 10.1097/00001648-200301000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Kreiger N, Lacroix J, Sloan M. Hormonal factors and pancreatic cancer in women. Ann Epidemiol. 2001;11:563–567. doi: 10.1016/s1047-2797(01)00219-8. [DOI] [PubMed] [Google Scholar]
- 16.Skinner HG, Michaud DS, Colditz GA, Giovannucci EL, Stampfer MJ, Willett WC, Fuchs CS. Parity, reproductive factors, and the risk of pancreatic cancer in women. Cancer Epidemiol Biomarkers Prev. 2003;12:433–438. [PubMed] [Google Scholar]
- 17.Wahi MM, Shah N, Schrock CE, Rosemurgy AS 2nd, Goldin SB. Reproductive factors and risk of pancreatic cancer in women: a review of the literature. Ann Epidemiol. 2009;19:103–111. doi: 10.1016/j.annepidem.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Eppel A, Cotterchio M, Gallinger S. Allergies are associated with reduced pancreas cancer risk: A population-based case-control study in Ontario, Canada. Int J Cancer. 2007;121:2241–2245. doi: 10.1002/ijc.22884. [DOI] [PubMed] [Google Scholar]
- 19.Olson SH, Chou JF, Ludwig E, O'Reilly E, Allen PJ, Jarnagin WR, Bayuga S, Simon J, Gonen M, Reisacher WR, et al. Allergies, obesity, other risk factors and survival from pancreatic cancer. Int J Cancer. 2010;127:2412–2419. doi: 10.1002/ijc.25240. [DOI] [PubMed] [Google Scholar]
- 20.Cavestro GM, Comparato G, Nouvenne A, Sianesi M, Di Mario F. The race from chronic pancreatitis to pancreatic cancer. JOP. 2003;4:165–168. [PubMed] [Google Scholar]
- 21.Farrow B, Evers BM. Inflammation and the development of pancreatic cancer. Surg Oncol. 2002;10:153–169. doi: 10.1016/s0960-7404(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 22.Lowenfels AB, Maisonneuve P, Lankisch PG. Chronic pancreatitis and other risk factors for pancreatic cancer. Gastroenterol Clin North Am. 1999;28:673–685, x. doi: 10.1016/s0889-8553(05)70080-7. [DOI] [PubMed] [Google Scholar]
- 23.Talamini G, Falconi M, Bassi C, Sartori N, Salvia R, Caldiron E, Frulloni L, Di Francesco V, Vaona B, Bovo P, et al. Incidence of cancer in the course of chronic pancreatitis. Am J Gastroenterol. 1999;94:1253–1260. doi: 10.1111/j.1572-0241.1999.01075.x. [DOI] [PubMed] [Google Scholar]
- 24.Malka D, Hammel P, Maire F, Rufat P, Madeira I, Pessione F, Lévy P, Ruszniewski P. Risk of pancreatic adenocarcinoma in chronic pancreatitis. Gut. 2002;51:849–852. doi: 10.1136/gut.51.6.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dítĕ P, Pazourková M, Růzicka M, Precechtĕlová M, Novotný I, Dastych M. [Chronic pancreatitis as a risk factor for pancreatic carcinoma] Vnitr Lek. 2002;48:638–641. [PubMed] [Google Scholar]
- 26.Lynch HT, Lanspa SJ, Fitzgibbons RJ Jr, Smyrk T, Fitzsimmons ML, McClellan J. Familial pancreatic cancer (Part 1): Genetic pathology review. Nebr Med J. 1989;74:109–112. [PubMed] [Google Scholar]
- 27.Ferrone CR, Levine DA, Tang LH, Allen PJ, Jarnagin W, Brennan MF, Offit K, Robson ME. BRCA germline mutations in Jewish patients with pancreatic adenocarcinoma. J Clin Oncol. 2009;27:433–438. doi: 10.1200/JCO.2008.18.5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neglia JP, FitzSimmons SC, Maisonneuve P, Schöni MH, Schöni-Affolter F, Corey M, Lowenfels AB. The risk of cancer among patients with cystic fibrosis. Cystic Fibrosis and Cancer Study Group. N Engl J Med. 1995;332:494–499. doi: 10.1056/NEJM199502233320803. [DOI] [PubMed] [Google Scholar]
- 29.Lynch HT, Fusaro RM, Lynch JF, Brand R. Pancreatic cancer and the FAMMM syndrome. Fam Cancer. 2008;7:103–112. doi: 10.1007/s10689-007-9166-4. [DOI] [PubMed] [Google Scholar]
- 30.Latchford A, Greenhalf W, Vitone LJ, Neoptolemos JP, Lancaster GA, Phillips RK. Peutz-Jeghers syndrome and screening for pancreatic cancer. Br J Surg. 2006;93:1446–1455. doi: 10.1002/bjs.5609. [DOI] [PubMed] [Google Scholar]
- 31.Yamamoto H, Itoh F, Nakamura H, Fukushima H, Sasaki S, Perucho M, Imai K. Genetic and clinical features of human pancreatic ductal adenocarcinomas with widespread microsatellite instability. Cancer Res. 2001;61:3139–3144. [PubMed] [Google Scholar]
- 32.Keiles S, Kammesheidt A. Identification of CFTR, PRSS1, and SPINK1 mutations in 381 patients with pancreatitis. Pancreas. 2006;33:221–227. doi: 10.1097/01.mpa.0000232014.94974.75. [DOI] [PubMed] [Google Scholar]
- 33.Pannala R, Basu A, Petersen GM, Chari ST. New-onset diabetes: a potential clue to the early diagnosis of pancreatic cancer. Lancet Oncol. 2009;10:88–95. doi: 10.1016/S1470-2045(08)70337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pelaez-Luna M, Takahashi N, Fletcher JG, Chari ST. Resectability of presymptomatic pancreatic cancer and its relationship to onset of diabetes: a retrospective review of CT scans and fasting glucose values prior to diagnosis. Am J Gastroenterol. 2007;102:2157–2163. doi: 10.1111/j.1572-0241.2007.01480.x. [DOI] [PubMed] [Google Scholar]
- 35.Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol. 2007;34:284–294. doi: 10.1053/j.seminoncol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pour P, Althoff J, Krüger FW, Mohr U. Improvement of pancreatic cancer model by modified treatment with N-nitroso-bis (2-oxopropyl) amine. Cancer Lett. 1977;2:233–237. doi: 10.1016/s0304-3835(77)80027-x. [DOI] [PubMed] [Google Scholar]
- 37.Sayers HJ, Orloff MJ. Development of an animal model of pancreatic cancer. Surg Forum. 1976;27:456–458. [PubMed] [Google Scholar]
- 38.Morosco GJ, Goeringer GC. Lifestyle factors and cancer of the pancreas: a hypothetical mechanism. Med Hypotheses. 1980;6:971–985. doi: 10.1016/0306-9877(80)90049-3. [DOI] [PubMed] [Google Scholar]
- 39.Lin RS, Kessler II. A multifactorial model for pancreatic cancer in man. Epidemiologic evidence. JAMA. 1981;245:147–152. [PubMed] [Google Scholar]
- 40.Berlin NI, Williams M. Pancreatic cancer: an epidemiologic approach and model. JAMA. 1981;245:171. doi: 10.1001/jama.1981.03310270051026. [DOI] [PubMed] [Google Scholar]
- 41.Hruban RH, Goggins M, Parsons J, Kern SE. Progression model for pancreatic cancer. Clin Cancer Res. 2000;6:2969–2972. [PubMed] [Google Scholar]
- 42.Hruban RH, Adsay NV, Albores-Saavedra J, Compton C, Garrett ES, Goodman SN, Kern SE, Klimstra DS, Klöppel G, Longnecker DS, et al. Pancreatic intraepithelial neoplasia: a new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 43.Serra E, Puig S, Otero D, Gaona A, Kruyer H, Ars E, Estivill X, Lázaro C. Confirmation of a double-hit model for the NF1 gene in benign neurofibromas. Am J Hum Genet. 1997;61:512–519. doi: 10.1086/515504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Herman JG, Jen J, Merlo A, Baylin SB. Hypermethylation-associated inactivation indicates a tumor suppressor role for p15INK4B. Cancer Res. 1996;56:722–727. [PubMed] [Google Scholar]
- 45.Jackson MA, Lea I, Rashid A, Peddada SD, Dunnick JK. Genetic alterations in cancer knowledge system: analysis of gene mutations in mouse and human liver and lung tumors. Toxicol Sci. 2006;90:400–418. doi: 10.1093/toxsci/kfj101. [DOI] [PubMed] [Google Scholar]
- 46.Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177:151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebert M, Yokoyama M, Friess H, Büchler MW, Korc M. Coexpression of the c-met proto-oncogene and hepatocyte growth factor in human pancreatic cancer. Cancer Res. 1994;54:5775–5778. [PubMed] [Google Scholar]
- 49.Di Renzo MF, Poulsom R, Olivero M, Comoglio PM, Lemoine NR. Expression of the Met/hepatocyte growth factor receptor in human pancreatic cancer. Cancer Res. 1995;55:1129–1138. [PubMed] [Google Scholar]
- 50.Kiehne K, Herzig KH, Fölsch UR. c-met expression in pancreatic cancer and effects of hepatocyte growth factor on pancreatic cancer cell growth. Pancreas. 1997;15:35–40. doi: 10.1097/00006676-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Yu J, Ohuchida K, Mizumoto K, Ishikawa N, Ogura Y, Yamada D, Egami T, Fujita H, Ohashi S, Nagai E, et al. Overexpression of c-met in the early stage of pancreatic carcinogenesis; altered expression is not sufficient for progression from chronic pancreatitis to pancreatic cancer. World J Gastroenterol. 2006;12:3878–3882. doi: 10.3748/wjg.v12.i24.3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Otte JM, Kiehne K, Schmitz F, Fölsch UR, Herzig KH. C-met protooncogene expression and its regulation by cytokines in the regenerating pancreas and in pancreatic cancer cells. Scand J Gastroenterol. 2000;35:90–95. doi: 10.1080/003655200750024597. [DOI] [PubMed] [Google Scholar]
- 53.Molina JR, Adjei AA. The Ras/Raf/MAPK pathway. J Thorac Oncol. 2006;1:7–9. [PubMed] [Google Scholar]
- 54.Carnero A, Blanco-Aparicio C, Renner O, Link W, Leal JF. The PTEN/PI3K/AKT signalling pathway in cancer, therapeutic implications. Curr Cancer Drug Targets. 2008;8:187–198. doi: 10.2174/156800908784293659. [DOI] [PubMed] [Google Scholar]
- 55.Perugini RA, McDade TP, Vittimberga FJ Jr, Callery MP. Pancreatic cancer cell proliferation is phosphatidylinositol 3-kinase dependent. J Surg Res. 2000;90:39–44. doi: 10.1006/jsre.2000.5833. [DOI] [PubMed] [Google Scholar]
- 56.Lu X, Qian J, Yu Y, Yang H, Li J. Expression of the tumor suppressor ARHI inhibits the growth of pancreatic cancer cells by inducing G1 cell cycle arrest. Oncol Rep. 2009;22:635–640. [PubMed] [Google Scholar]
- 57.Roy SK, Srivastava RK, Shankar S. Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer. J Mol Signal. 2010;5:10. doi: 10.1186/1750-2187-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao Z, Okabayashi Y, Yutsudo Y, Kitamura T, Ogawa W, Kasuga M. Role of Akt in growth and survival of PANC-1 pancreatic cancer cells. Pancreas. 2002;24:42–46. doi: 10.1097/00006676-200201000-00006. [DOI] [PubMed] [Google Scholar]
- 59.Mortenson MM, Galante JG, Gilad O, Schlieman MG, Virudachalam S, Kung HJ, Bold RJ. BCL-2 functions as an activator of the AKT signaling pathway in pancreatic cancer. J Cell Biochem. 2007;102:1171–1179. doi: 10.1002/jcb.21343. [DOI] [PubMed] [Google Scholar]
- 60.Ripka S, Neesse A, Riedel J, Bug E, Aigner A, Poulsom R, Fulda S, Neoptolemos J, Greenhalf W, Barth P, et al. CUX1: target of Akt signalling and mediator of resistance to apoptosis in pancreatic cancer. Gut. 2010;59:1101–1110. doi: 10.1136/gut.2009.189720. [DOI] [PubMed] [Google Scholar]
- 61.Ma J, Sawai H, Ochi N, Matsuo Y, Xu D, Yasuda A, Takahashi H, Wakasugi T, Takeyama H. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol Cell Biochem. 2009;331:161–171. doi: 10.1007/s11010-009-0154-x. [DOI] [PubMed] [Google Scholar]
- 62.Maitra A, Hruban RH. A new mouse model of pancreatic cancer: PTEN gets its Akt together. Cancer Cell. 2005;8:171–172. doi: 10.1016/j.ccr.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–954. [PubMed] [Google Scholar]
- 64.Hanley AB, Burch R. Re: The role of STAT-3 in the induction of apoptosis in pancreatic cancer cells by benzyl isothiocyanate. J Natl Cancer Inst. 2009;101:893; author reply 893–894. doi: 10.1093/jnci/djp120. [DOI] [PubMed] [Google Scholar]
- 65.Schutte M, Rozenblum E, Moskaluk CA, Guan X, Hoque AT, Hahn SA, da Costa LT, de Jong PJ, Kern SE. An integrated high-resolution physical map of the DPC/BRCA2 region at chromosome 13q12. Cancer Res. 1995;55:4570–4574. [PubMed] [Google Scholar]
- 66.Levy L, Hill CS. Smad4 dependency defines two classes of transforming growth factor {beta} (TGF-{beta}) target genes and distinguishes TGF-{beta}-induced epithelial-mesenchymal transition from its antiproliferative and migratory responses. Mol Cell Biol. 2005;25:8108–8125. doi: 10.1128/MCB.25.18.8108-8125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dessimoz J, Grapin-Botton A. Pancreas development and cancer: Wnt/beta-catenin at issue. Cell Cycle. 2006;5:7–10. doi: 10.4161/cc.5.1.2293. [DOI] [PubMed] [Google Scholar]
- 68.Rulifson IC, Karnik SK, Heiser PW, ten Berge D, Chen H, Gu X, Taketo MM, Nusse R, Hebrok M, Kim SK. Wnt signaling regulates pancreatic beta cell proliferation. Proc Natl Acad Sci USA. 2007;104:6247–6252. doi: 10.1073/pnas.0701509104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Al-Aynati MM, Radulovich N, Riddell RH, Tsao MS. Epithelial-cadherin and beta-catenin expression changes in pancreatic intraepithelial neoplasia. Clin Cancer Res. 2004;10:1235–1240. doi: 10.1158/1078-0432.ccr-03-0087. [DOI] [PubMed] [Google Scholar]
- 70.Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, Goodman SN, Sohn TA, Hruban RH, Yeo CJ, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997;57:1731–1734. [PubMed] [Google Scholar]
- 71.Sasaki S, Yamamoto H, Kaneto H, Ozeki I, Adachi Y, Takagi H, Matsumoto T, Itoh H, Nagakawa T, Miyakawa H, et al. Differential roles of alterations of p53, p16, and SMAD4 expression in the progression of intraductal papillary-mucinous tumors of the pancreas. Oncol Rep. 2003;10:21–25. [PubMed] [Google Scholar]
- 72.Poley JW, Kluijt I, Gouma DJ, Harinck F, Wagner A, Aalfs C, van Eijck CH, Cats A, Kuipers EJ, Nio Y, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol. 2009;104:2175–2181. doi: 10.1038/ajg.2009.276. [DOI] [PubMed] [Google Scholar]
- 73.Harsha HC, Kandasamy K, Ranganathan P, Rani S, Ramabadran S, Gollapudi S, Balakrishnan L, Dwivedi SB, Telikicherla D, Selvan LD, et al. A compendium of potential biomarkers of pancreatic cancer. PLoS Med. 2009;6:e1000046. doi: 10.1371/journal.pmed.1000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vestergaard EM, Hein HO, Meyer H, Grunnet N, Jørgensen J, Wolf H, Orntoft TF. Reference values and biological variation for tumor marker CA 19-9 in serum for different Lewis and secretor genotypes and evaluation of secretor and Lewis genotyping in a Caucasian population. Clin Chem. 1999;45:54–61. [PubMed] [Google Scholar]
- 75.Bussom S, Saif MW. Methods and rationale for the early detection of pancreatic cancer. Highlights from the "2010 ASCO Gastrointestinal Cancers Symposium". Orlando, FL, USA. January 22-24, 2010. JOP. 2010;11:128–130. [PubMed] [Google Scholar]
- 76.Brand RE, Lerch MM, Rubinstein WS, Neoptolemos JP, Whitcomb DC, Hruban RH, Brentnall TA, Lynch HT, Canto MI. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–1469. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakorafas GH, Sarr MG. Pancreatic cancer after surgery for chronic pancreatitis. Dis Liver Dis. 2003;35:482–485. doi: 10.1016/s1590-8658(03)00221-4. [DOI] [PubMed] [Google Scholar]
- 78.Wong T, Howes N, Threadgold J, Smart HL, Lombard MG, Gilmore I, Sutton R, Greenhalf W, Ellis I, Neoptolemos JP. Molecular diagnosis of early pancreatic ductal adenocarcinoma in high-risk patients. Pancreatology. 2001;1:486–509. doi: 10.1159/000055852. [DOI] [PubMed] [Google Scholar]
- 79.Rosty C, Geradts J, Sato N, Wilentz RE, Roberts H, Sohn T, Cameron JL, Yeo CJ, Hruban RH, Goggins M. p16 Inactivation in pancreatic intraepithelial neoplasias (PanINs) arising in patients with chronic pancreatitis. Am J Surg Pathol. 2003;27:1495–1501. doi: 10.1097/00000478-200312000-00001. [DOI] [PubMed] [Google Scholar]
- 80.Ockenga J, Vogel A, Teich N, Keim V, Manns MP, Strassburg CP. UDP glucuronosyltransferase (UGT1A7) gene polymorphisms increase the risk of chronic pancreatitis and pancreatic cancer. Gastroenterology. 2003;124:1802–1808. doi: 10.1016/s0016-5085(03)00294-4. [DOI] [PubMed] [Google Scholar]
- 81.Salek C, Benesova L, Zavoral M, Nosek V, Kasperova L, Ryska M, Strnad R, Traboulsi E, Minarik M. Evaluation of clinical relevance of examining K-ras, p16 and p53 mutations along with allelic losses at 9p and 18q in EUS-guided fine needle aspiration samples of patients with chronic pancreatitis and pancreatic cancer. World J Gastroenterol. 2007;13:3714–3720. doi: 10.3748/wjg.v13.i27.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li D, Yeung SC, Hassan MM, Konopleva M, Abbruzzese JL. Antidiabetic therapies affect risk of pancreatic cancer. Gastroenterology. 2009;137:482–488. doi: 10.1053/j.gastro.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]