Abstract
Background
Balance and gait problems have been detected among patients with HIV/AIDS. The extent to which these problems are exacerbated by either frailty or obesity has not been examined.
Objective
The purpose of this study was to compare participants who differed in body mass and the presence or absence of HIV/AIDS.
Design
This was a cross-sectional study.
Methods
Quantitative measurements were obtained from 86 participants who were HIV–type 1 (HIV-1) seronegative and 121 participants who were seropositive divided into subgroups based on their body mass index (BMI <21, 21–29, or >29 kg/m2).
Results
Participants who were seropositive were impaired relative to seronegative controls on several indices, including the limit of stability, sway amplitude and sway strategy, gait initiation time, and gait speed during a fast pace condition. Participants who were obese also exhibited impairments, which were evident during assessments of the limit of stability, nonpreferred leg stance time, sway strategy, normal and fast gait speed, fast gait initiation time, and 360-degree turn time. Importantly, the analysis revealed that participants with both attributes were more impaired than those with either or neither attribute: patients who were obese and seropositive were more impaired in fast gait initiation time and cadence, nonpreferred leg stance time, 360-degree turn time, and sway strategy scores.
Limitations
The validity of BMI as a measure of body mass can be challenged. In addition, the validity of chair rise time and 360-degree turn time as estimates of lower-extremity strength (force-generating capacity) can be argued.
Conclusions
The present findings have an obvious and unfortunate implication: as more patients who are HIV-1 seropositive join the seronegative community in becoming obese, the effects of obesity and their disease may summate and their risk for balance and gait problems may increase.
Patients living with HIV–type 1 (HIV-1) are more likely to exhibit balance and gait impairments than those without infection. More than 57% of patients who are HIV-1 seropositive and have symptoms of AIDS and 25% of their peers who are asymptomatic1–3 show clinical evidence of an impairment in motor function. Quantitative assessments have confirmed these clinical judgments. At least 4 studies2,4–6 have demonstrated statistically significant differences between seropositive and seronegative groups in either stance stability or the limit of stability (LOS).
Several factors are likely to contribute to the functional impairments shown by patients who are seropositive. The most obvious factor is the disease itself. HIV/AIDS with or without accompanying immunosuppression has been associated with peripheral neuropathy1 and myopathy,7,8 as well as decrements in cerebral white matter,9,10 which may additively or synergistically affect motor behavior. Human immunodeficiency virus–related fluctuations in pain, fatigue, nausea, and vertigo11 also may contribute.
A second factor contributing to functional impairment is antiretroviral treatment (ART). Many ART agents of the nucleoside reverse transcriptase inhibitor (NRTI)12 or protease inhibitor (PI)13 class are associated with mitochondrial toxicity and, therefore, can disrupt muscle structure and function. In addition, NRTI treatment, especially in combination with zidovudine,14 has been associated with a distal sensory neuropathy that may affect proprioception and coordination of movement.
A third potential contributor is body mass—a factor that varies markedly with both the severity of HIV disease and its treatment.15 From studies of patients who were seronegative, we know that patients who are underweight and weak16 have functional impairments. We also know that an excessive body mass is detrimental to balance,17 as well as to gait initiation and speed,18 in patients who are seronegative. Unfortunately, at the present time, we do not know whether an abnormally underweight or obese body mass is relevant to motor function in patients who are seropositive. We might hypothesize that an underweight body mass is associated with the greatest level of impairment because it implies diminished strength (force-generating capacity) as well as severe disease. We also might hypothesize that an obese body mass is detrimental because it presents a greater challenge to lower-extremity strength, especially among patients already compromised by a disease.
The goal of the present study was to test these hypotheses. The study compared 121 volunteers who were seropositive and 86 volunteers who were seronegative and who were assigned to subgroups defined by an underweight (body mass index [BMI] <21 kg/m2) versus normal-to-overweight (BMI=21–29 kg/m2) versus obese (BMI >29 kg/m2) body mass. The 6 groups were equivalent on many relevant background variables, including age and substance abuse.
Method
Participants
One hundred twenty-one participants who were HIV-1 seropositive were recruited via advertisements posted within outpatient infectious disease clinics in the greater Hartford, Connecticut, region. Eighty-six participants who were seronegative were recruited principally via word-of-mouth advertising provided by the patients who were seropositive.
Interested individuals were invited to telephone a member of the research staff for eligibility screening. Those who passed the telephone screen were asked to visit the health center on a subsequent day for further screening. At the beginning of this in-person visit, they reviewed and signed institutional review board–approved consent and HIPAA forms and a medical records release.
Participants were required to provide blood, urine, and breath samples for evaluation. From the blood sample, HIV-1 serostatus and viral load were determined. In addition, clinical blood chemistries, CD4 lymphocyte count and percent, VDRL, hepatitis B virus, hepatitis C virus, toxoplasmosis and cytomegalovirus antibody titers, renal and liver function, serum protein, albumin, and G-6-PD were examined. Urine (Ontrak*) and breath samples were tested for evidence of recent drug or alcohol use, respectively.
A structured psychiatric interview, the Computerized Diagnostic Interview Schedule for DSM-IV,19 was administered for the purpose of detecting psychiatric disorders. Participants completed additional questionnaires or brief interviews assessing medical history, medication use, family history, demographics, psychiatric symptoms, alcohol and drug use, and cognitive status. The list of questionnaires and interviews included the Addiction Severity Index,20 Michigan Alcoholism Screening Test,21 Drug Abuse Screening Test,22 and Beck Depression Inventory–version II.23
Participants were excluded for pregnancy, seizures, mental retardation, or a history of either neurosurgery or head injury with loss of consciousness for more than 10 minutes. In addition, they were required to have no acute illness and no major neurological disorders (eg, epilepsy, seizure disorder), psychiatric disorders (eg, DSM-IV–defined schizophrenia or bipolar disorder), or medical disorders (eg, chronic obstructive pulmonary disease, diabetes, cirrhosis, hepatic encephalopathy, ocular disorders). A positive urine toxicology test for cocaine, opiates, methadone, amphetamine, or marijuana and a positive breath test for alcohol also were reasons for exclusion.
Procedure
Estimates of body mass, mobility (gait speed, cadence, chair rise time, 360° turn time), balance (Sensory Organization Test [SOT], single-leg stance time, LOS), and sensory function (vibratory threshold) were derived from a standard assessment battery administered in a single session by 1 of 2 highly trained technicians. All but 2 measures were administered 2 or more times during the session. Their reliability was tested with intraclass correlation coefficients.
Body mass.
Body mass was calculated from measurements of height and weight obtained on the same day as all other tests.
SOT.
A computerized SOT of balance (Equi-Test System†) was used, consisting of 3 conditions (SOT4, SOT5, and SOT6). All of the conditions provided inaccurate somatosensory information in which the support platform was programmed to track changes in each participant's center of gravity (ie, sway-referenced). The differences across conditions related to the amount of visual information available to the participant regarding posture and sway. In the first condition (SOT4), visual input was normal. In the second condition (SOT5), the participant's eyes were closed (ie, visual input was absent). In the third condition (SOT6), the participant's eyes were open, but the visual horizon was sway-referenced (ie, visual input was inaccurate). Three 20-second trials were completed for each condition. The dependent variables calculated for each condition were the equilibrium quotient (EQ) and the sway strategy score (ie, the ratio of high-frequency ankle versus low-frequency hip corrections to balance).
LOS.
Another index of balance, the LOS, was calculated by the Equi-Test System. The system computed the maximum shift in the center of gravity, until loss of balance, during 2 forward lean (heels down) trials averaged with that measured during 2 backward lean (toes down) trials. The index was divided by foot length to correct for individual differences in the support surface.
Single-leg stance time.
Single-leg stance time was measured with a stopwatch while participants stood for as long as possible on one leg with the arms crossed over the chest. Four trials were conducted, alternating between preferred and nonpreferred legs. The single best time was retained for preferred and nonpreferred leg trials.
Gait speed and cadence.
To produce a measure of “normal” gait speed, participants were asked to walk 8 m at their usual pace. In a second condition, estimating “fast” gait speed, participants were asked to walk 8 m as quickly as possible without running. The times required to complete the first 0.5 m (initiation time) and the full 8 m during the 2 conditions were recorded with photocells and automatic timers positioned along the path. Cadence, defined as the time to complete 5 footfalls using the leading foot, was derived for both normal and fast pace conditions. Two trials were conducted and averaged.
360-degree turn time.
Participants were instructed to complete a 360-degree turn as quickly as possible. Stopwatch readings for right and left pivot turns were averaged.
Chair rise time.
Participants were instructed to alternate rapidly between standing and sitting 5 times without pauses and with arms crossed. Stopwatch readings were averaged over 2 trials.
Peripheral nerve vibrotactile threshold.
Participants were instructed to rest the great toe of the right foot upon a buzzer-like device (Bio-Thesiometer‡) which delivered a 120-Hz vibration of programmable amplitude. The sensory threshold for detecting the presence or absence of vibration was recorded. Thresholds were measured during an ascending and descending series and averaged.
Data Analysis
Group differences in background characteristics were evaluated using 2-factor analyses of variance for continuous measures and the Pearson chi-square test for categorical measures. Differences in balance and gait performance indexes were tested with 3-factor (serostatus × body mass rank × sex) univariate analyses of covariance. Age, number of alcohol and drug abuse problems, and depression symptoms were entered as covariates. All of the study participants provided complete data. Therefore, no listwise deletion or data imputation methods were needed.
Role of the Funding Source
This study was supported by grant R01MH61346 funded jointly by the National Institute of Mental Health and the National Institute on Drug Abuse. Additional support was provided by grants P60AA03510 and M01RR06192 funded by the National Institute on Alcohol Abuse and Alcoholism and the National Center for Research Resources, respectively.
Results
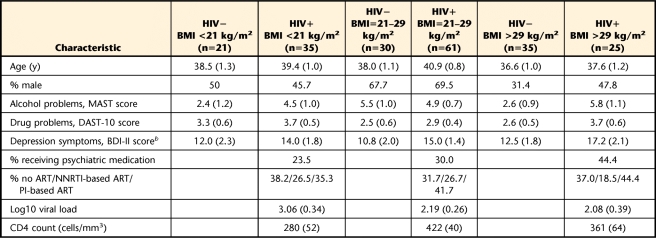
The analysis of background characteristics (Tab. 1) revealed small differences between the HIV seronegative and HIV seropositive groups in sex composition (χ2=16.7, P<.05) and Beck Depression Inventory scores (F=5.9, P<.05). There were otherwise no significant differences by group.
Table 1.
Background Characteristics by Participant Groupa
All values expressed and mean (standard error) unless otherwise indicated. HIV−=HIV seronegative, HIV+=HIV seropositive, BMI=body mass index, MAST=Michigan Alcoholism Screening Test, DAST-10=Drug Abuse Screening Test, BDI-II=Beck Depression Inventory–version II, ART=antiretroviral treatment, NNRTI=non-nucleoside reverse transcriptase inhibitor, PI=protease inhibitor. Main and interactive effects of sex were not statistically significant and, therefore, are not reported.
b HIV serostatus main effect.
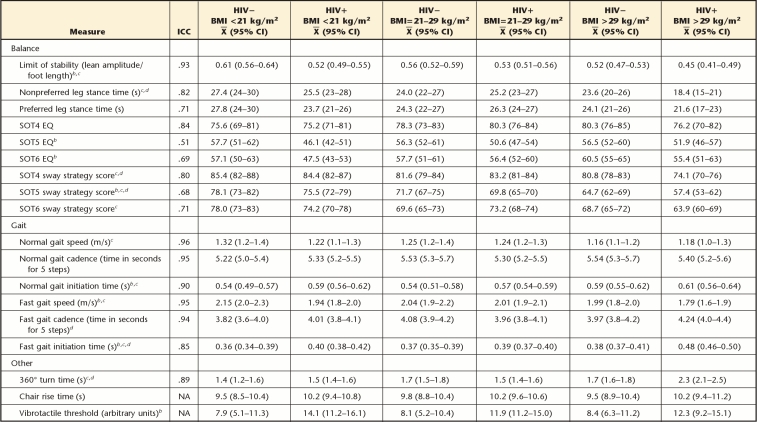
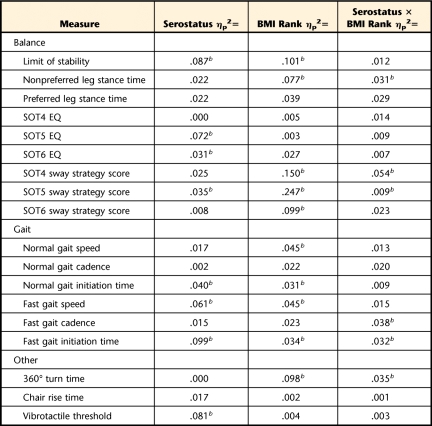
Against these similarities in the background characteristics of the groups, other analyses revealed differences across groups on specific measures of balance and gait (Tabs. 2 and 3). The balance and gait differences were variably associated with HIV serostatus, body mass rank, or the interaction. For example, the presence of HIV was associated with a reduced LOS (F=18.0, P<.001), lower EQ scores on the SOT5 subtest (F=15.3, P<.001) and the SOT6 subtest (F=5.7, P<.02), a lower sway strategy score on the SOT5 subtest (F=6.1, P<.01), delayed normal gait initiation time (F=6.4, P<.03), and reduced fast gait speed (F=9.8, P<.002). The BMI rank likewise was associated with many statistically significant effects, involving: LOS (F=12.3, P<.001); nonpreferred leg stance time (F=6.5, P<.002); sway strategy scores during the SOT4 subtest (F=15.5, P<.001), the SOT5 subtest (F=32.2, P<.001), and the SOT6 subtest (F=11.0, P<.001); normal gait speed (F=4.3, P<.02) and fast gait speed (F=4.1, P<.02); fast gait initiation time (F=3.3, P<.04); and 360-degree turn time (F=10.3, P<.001). Post hoc, pair-wise tests revealed that the overall effect of BMI rank was principally attributable to significant differences between the obese and normal-to-overweight groups on these measures.
Table 2.
Balance and Gait Test Performance by Participant Groupa
ICC=intraclass correlation coefficient, HIV−=HIV seronegative, HIV+=HIV seropositive, BMI=body mass index, CI=confidence interval, SOT=Sensory Organization Test, EQ=equilibrium quotient, NA=not available. Main and interactive effects of sex were not statistically significant and, therefore, are not reported.
b HIV serostatus main effect.
c BMI rank main effect.
d Serostatus × BMI rank interaction.
Table 3.
Estimates (ηp2=SSeffect/[SSeffect+SSerror]) of the Magnitude of Main and Interaction Effects for Each Variatea
SS=sum of squares, BMI=body mass index, SOT=Sensory Organization Test, EQ=equilibrium quotient.
b P<.05.
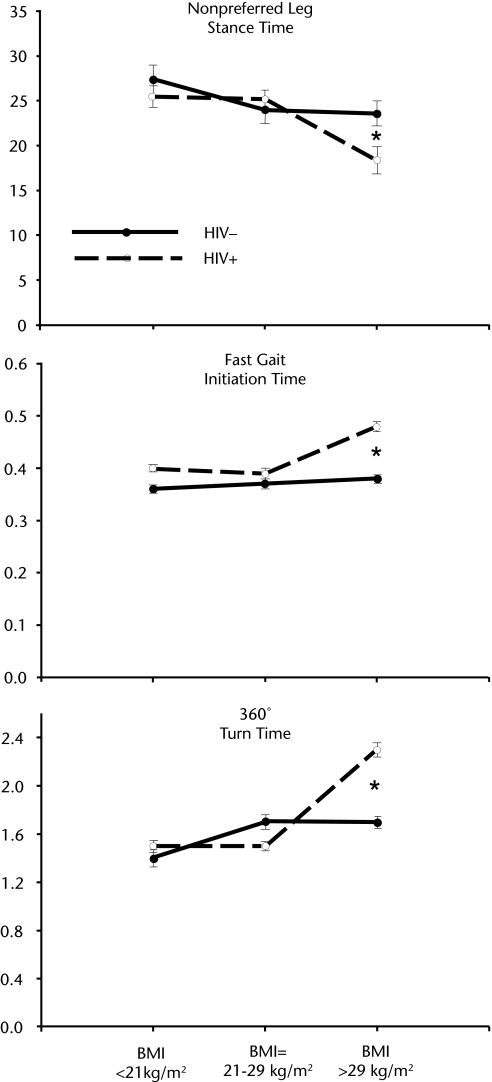
The analysis also revealed a synergistic interaction of serostatus and BMI rank affecting multiple measures. Participants who were seropositive with an obese body mass were impaired relative to those with an underweight body mass and the other participant groups in nonpreferred leg stance time (F=3.2, P<.04), fast gait cadence time (F=3.1, P<.05) and fast gait initiation time (F=3.1, P<.05), 360-degree turn time (F=3.7, P<.03), and 2 sway strategy scores (ie, during the SOT4 subtest [F=3.9, P<.01] and the SOT5 subtest [F=3.1, P<.05]). This interpretation was confirmed by the results of Scheffé post hoc tests of the effects of HIV serostatus in the obese versus underweight subgroups. Three examples of the interaction are shown in the Figure.
Figure.
Stance time, fast gait initiation time, and 360-degree turn time in seconds as a function of serostatus and body mass index (BMI). Note the significantly greater effects of HIV/AIDS among participants with a BMI >29 kg/m2. HIV−=HIV seronegative, HIV+=HIV seropositive. *P<.05.
Although the 3 seropositive groups with BMIs <21, 21 to 29, or >29 kg/m2 did not significantly differ in HIV disease severity, as reflected in CD4 count, or in the frequency of use of non-nucleoside reverse transcriptase inhibitors (NNRTIs) and PIs (Tab. 1), questions remain about the potential contribution of these variables to balance and gait. To further address the questions, we conducted additional analyses in the HIV seropositive groups only. The first set of analyses statistically removed the variance associated with BMI and tested the significance of the residual (partial) correlations between CD4 count and the balance, gait, and strength measures. The second set of analyses assigned the participants who were seropositive to subgroups based on the presence versus absence of either PI or NNRTI therapy. It used BMI as a covariate as well as the other covariates mentioned above.
The results of the first analysis revealed statistically significant correlations between CD4 count and 3 of the balance and gait measures. The SOT5 strategy score (r=.25, P=.01) improved, with a higher CD4 count. The vibrotactile threshold (r=−.22, P=.03) and 360-degree turn time (r=−.23, P=.02) also improved.
The second analysis revealed P values for NNRTI treatment across balance and gait measures that ranged from .10 to .96. For the effect of PI treatment on balance and gait, the P values also failed to reach the .05 threshold (P=.07–.84). The analysis of treatment effects, therefore, revealed no statistically significant results.
Discussion
The present study revealed a synergism in the statistical effects of HIV/AIDS and body mass on several measures of balance and gait, including nonpreferred leg stance time, fast gait cadence and initiation time, 360-degree turn time, and sway strategy scores during the SOT4 and SOT5 subtests. The direction of the synergism is counterintuitive in that it was not the group of patients with HIV/AIDS who were underweight or frail that was most impaired. Instead, patients with HIV/AIDS who were obese showed the greatest degree of impairment. The implication of this finding is important: as patients with HIV/AIDS join the healthier seronegative community in the national trend toward an obese body mass, their risk for significant balance and gait problems may increase.
Generating an explanation for the greater level of functional impairment among patients with HIV/AIDS who are obese is a challenge. We can start by discounting several possibilities. For example, from the absence of statistically significant effects in the ART subanalysis, we can argue that neither PI nor NNRTI use account for the differences. We also can discount disease severity as an explanation because it was not the obese group that showed the lowest CD4 count (ie, the most severe or acute form of the disease). Of course, we cannot dismiss these explanations with certainty, as a more-sensitive measure of adiposity, derived from a dual-energy x-ray absorptiometry scan or hydrodensitometry,24 might reveal associations with ART and disease severity that BMI does not capture.
In the absence of a compelling explanation defended by a group difference in either ART or disease severity, we searched for alternative explanations for the present results. One possibility is the known deleterious effect of HIV/AIDS on strength25,26 and the greater challenge to strength brought by an obese body mass. That is, it might be argued that the ratio of strength to body mass is compromised in patients with HIV/AIDS, leading to an impairment in their balance and gait. To the extent that nonpreferred leg stance time, fast gait cadence and initiation time, sway strategy scores, and 360-degree turn time reflect individual differences in strength, the explanation is compelling. Unfortunately, at the present time, we have no explanation for the absence of a group difference in chair rise time, which is heavily influenced by strength.
An alternative explanation is an adverse and synergistic effect of obesity and HIV/AIDS on brain regions contributing to mobility and balance. Indeed, recent literature shows that both of these conditions are associated with changes in brain white matter integrity.27–30 It is notable the predominant locus of the change is in frontal regions that indirectly contribute to mobility and balance.31,32 It would be valuable in a future study to collect structural magnetic resonance imaging data and to test for interactive effects of HIV serostatus and obesity on frontal white matter, balance, and gait simultaneously.
Additional explanations for the synergism are possible, but whatever the underlying explanation, the broader concern raised by the results remains: obesity is a factor affecting the mobility and balance of patients with HIV/AIDS. This concern is new because, at the outbreak of the HIV epidemic, obesity was not expected to be a medical problem for these patients. Yet, obesity rates in the seropositive community are now climbing33,34 for at least 3 reasons. First, antiretroviral treatment directly and indirectly adds body mass. Second, patients are living longer and, therefore, can experience the normal increase in BMI that comes with aging. Third, patients with HIV/AIDS are more impulsive than their peers who are HIV seronegative. They, therefore, are more likely than others to engage in risk-taking or impulsive behaviors,35 including loss of control over food intake.36
The clinical implications of our findings are numerous. A direct implication is the likely need for increased diet, exercise,37 and perhaps balance therapy among patients with HIV/AIDS who are obese, with the goal of reducing the physical risks from balance and gait deficits. Diet and exercise therapy also may be needed for reducing other risks, including possible synergistic effects of HIV/AIDS and obesity on risk for cardiovascular disease and stroke, metabolic syndrome, and type II diabetes.
Footnotes
Dr Bauer provided concept/idea/research design, writing, data collection and analysis, project management, fund procurement, and participants. Dr Wolfson provided facilities/equipment. All authors provided consultation (including review of manuscript before submission).
This study was supported by grant R01MH61346 funded jointly by the National Institute of Mental Health and the National Institute on Drug Abuse. Additional support was provided by grants P60AA03510 and M01RR06192 funded by the National Institute on Alcohol Abuse and Alcoholism and the National Center for Research Resources, respectively.
Varian Inc, 3120 Hansen Way, Palo Alto, CA 94304.
NeuroCom International Inc, 9570 SE Lawnfield Rd, Clackamas, OR 97015.
Bio-Medical Instrument Co, 15764 Munn Rd, Newbury, OH 44065.
References
- 1. Gilmer WS. Neurologic problems of the lower extremity associated with HIV and AIDS. Clin Podiatr Med Surg. 1998;15:281–303 [PubMed] [Google Scholar]
- 2. Arendt G, Maecker HP, Purrmann J, Homberg V. Control of posture in patients with neurologically asymptomatic HIV infection and patients with beginning HIV-1-related encephalopathy. Arch Neurol. 1994;51:1232–1235 [DOI] [PubMed] [Google Scholar]
- 3. Mitchell WG, Lynn H, Bale JF, Jr, et al. Longitudinal neurological follow-up of a group of HIV-seropositive and HIV-seronegative hemophiliacs: results from the hemophilia growth and development study. Pediatrics. 1997;100:817–824 [DOI] [PubMed] [Google Scholar]
- 4. Bauer LO, Ceballos NA, Shanley JD, Wolfson LI. Sensorimotor dysfunction in HIV/AIDS: effects of antiretroviral treatment and comorbid psychiatric disorders. AIDS. 2005;19:495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beckley DJ, Bloem BR, Martin EM, et al. Postural reflexes in patients with HIV-1 infection. Electroencephalogr Clin Neurophysiol. 1998;109:402–408 [DOI] [PubMed] [Google Scholar]
- 6. Fama R, Eisen JC, Rosenbloom MJ, et al. Upper and lower limb motor impairments in alcoholism, HIV infection, and their comorbidity. Alcohol Clin Exp Res. 2007;31:1038–1044 [DOI] [PubMed] [Google Scholar]
- 7. Sheikh RA, Yasmeen S, Munn R, et al. AIDS-related myopathy. Med Electron Microsc. 1999;32:79–86 [DOI] [PubMed] [Google Scholar]
- 8. Estanislao L, Thomas D, Simpson D. HIV neuromuscular disease and mitochondrial function. Mitochondrion. 2004;4:131–139 [DOI] [PubMed] [Google Scholar]
- 9. Sullivan EV, Rosenbloom MJ, Rohlfing T, et al. Pontocerebellar contribution to postural instability and psychomotor slowing in HIV infection without dementia. Brain Imaging Behav. 2011;5:12–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pfefferbaum A, Rosenbloom MJ, Adalsteinsson E, Sullivan EV. Diffusion tensor imaging with quantitative fibre tracking in HIV infection and alcoholism comorbidity: synergistic white matter damage. Brain. 2007;130:48–64 [DOI] [PubMed] [Google Scholar]
- 11. Fox MP, McCoy K, Larson BA, et al. Improvements in physical wellbeing over the first two years on antiretroviral therapy in western Kenya. AIDS Care. 2010;22:137–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haddow LJ, Wood CW, Ainsworth JG. Discontinuation of non-nucleoside reverse transcriptase inhibitor-based highly active antiretroviral therapy due to nucleoside analogue reverse transcriptase inhibitor-related metabolic toxicity. Int J STD AIDS. 2007;18:343–346 [DOI] [PubMed] [Google Scholar]
- 13. Richmond SR, Carper MJ, Lei X, et al. HIV-protease inhibitors suppress skeletal muscle fatty acid oxidation by reducing CD36 and CPT1 fatty acid transporters. Biochim Biophys Acta. 2010;1801:559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Simpson DM, Katzenstein DA, Hughes MD, et al. ; AIDS Clinical Group 175/801 Study Team Neuromuscular function in HIV infection: analysis of a placebo-controlled combination antiretroviral trial. AIDS. 1998;12:2425–2432 [DOI] [PubMed] [Google Scholar]
- 15. Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39:557–561 [PubMed] [Google Scholar]
- 16. Honeycutt PH, Ramsey P. Factors contributing to falls in elderly men living in the community. Geriatr Nurs. 2002;23:250–255 [DOI] [PubMed] [Google Scholar]
- 17. Colne P, Frelut ML, Peres G, Thoumie P. Postural control in obese adolescents assessed by limits of stability and gait initiation. Gait Posture. 2008;28:164–169 [DOI] [PubMed] [Google Scholar]
- 18. Grabowski A, Farley CT, Kram R. Independent metabolic costs of supporting body weight and accelerating body mass during walking. J Appl Physiol. 2005;98:579–583 [DOI] [PubMed] [Google Scholar]
- 19. Robins LN, Cottler LB, Bucholz KK, et al. Diagnostic Interview Schedule for the DSM-IV (DIS-IV). St Louis, MO: Washington University; 2002 [Google Scholar]
- 20. McLellan AT, Luborsky L, Woody GE, O'Brien CP. An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33 [DOI] [PubMed] [Google Scholar]
- 21. Selzer ML. The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry. 1971;127:1653–1658 [DOI] [PubMed] [Google Scholar]
- 22. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7:363–371 [DOI] [PubMed] [Google Scholar]
- 23. Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–597 [DOI] [PubMed] [Google Scholar]
- 24. Das SK. Body composition measurement in severe obesity. Curr Opin Clin Nutr Metab Care. 2005;8:602–606 [DOI] [PubMed] [Google Scholar]
- 25. Onen NF, Agbebi A, Shacham E, et al. Frailty among HIV-infected persons in an urban outpatient care setting. J Infect. 2009;59:346–352 [DOI] [PubMed] [Google Scholar]
- 26. Roubenoff R, Wilson IB. Effect of resistance training on self-reported physical functioning in HIV infection. Med Sci Sports Exerc. 2001;33:1811–1817 [DOI] [PubMed] [Google Scholar]
- 27. Haltia LT, Viljanen A, Parkkola R, et al. Brain white matter expansion in human obesity and the recovering effect of dieting. J Clin Endocrinol Metab. 2007;92:3278–3284 [DOI] [PubMed] [Google Scholar]
- 28. Stanek KM, Grieve SM, Brickman AM, et al. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring). 2011;19:500–504 [DOI] [PubMed] [Google Scholar]
- 29. Gazdzinski S, Kornak J, Weiner MW, Meyerhoff DJ. Body mass index and magnetic resonance markers of brain integrity in adults. Ann Neurol. 2008;63:652–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pfefferbaum A, Rosenbloom MJ, Rohlfing T, et al. Frontostriatal fiber bundle compromise in HIV infection without dementia. AIDS. 2009;23:1977–1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blahak C, Baezner H, Pantoni L, et al. Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. J Neurol Neurosurg Psychiatry. 2009;80:608–613 [DOI] [PubMed] [Google Scholar]
- 32. Nakamura T, Meguro K, Yamazaki H, et al. Postural and gait disturbance correlated with decreased frontal cerebral blood flow in Alzheimer disease. Alzheimer Dis Assoc Disord. 1997;11:132–139 [DOI] [PubMed] [Google Scholar]
- 33. Crum-Cianflone N, Tejidor R, Medina S, et al. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS. 2008;22:925–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bauer LO. Psychiatric and neurophysiological predictors of obesity in HIV/AIDS. Psychophysiology. 2008;45:1055–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bauer LO. A family history of psychopathology modifies the decrement in cognitive control among patients with HIV/AIDS. Brain Cogn. 2008;67:103–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nederkoorn C, Smulders FT, Havermans RC, et al. Impulsivity in obese women. Appetite. 2006;47:253–256 [DOI] [PubMed] [Google Scholar]
- 37. Engelson ES, Agin D, Kenya S, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55:1327–1336 [DOI] [PubMed] [Google Scholar]