Heteroblastic species change their leaf morphology due to changes in light environment. However, growth and biomass allocation pattern do not contribute to their better survival relative to homoblastic congeners in low light. Thus, shade does not select for leaf heteroblasty.
Abstract
Background and aims
Leaf heteroblasty involves dramatic phenotypic differences between adult and seedling leaves while leaves of homoblastic plants display only small differences. This study tested whether, in low-light environments, the marked difference in the morphology of seedling leaves that characterizes heteroblastic species confers advantages for seedling survival and growth compared with homoblastic congeners.
Methodology
Four pairs of heteroblastic and homoblastic species in genera Hoheria, Aristotelia, Pseudopanax and Melicope were grown in simulated full sunlight (100 % of light, red:far red ratio (R:FR) = 1.25) or in simulated forest understorey shade (5 % of full sunlight, R:FR ratio = 0.25) in a glasshouse.
Principal results
After 9 months, 100 % of seedlings of both homoblastic and heteroblastic species survived in full sun while in the understorey treatment there were 25 % fewer heteroblastic survivors than homoblastic congeners. Compared with homoblastic congeners, all heteroblastic species except for Pseudopanax crassifolius produced more and smaller leaves and branches, but grew more slowly in height, root collar diameter and total biomass both in full sun and in forest understorey treatments.
Conclusions
Homoblastic species survive and grow better in the forest understorey light treatment, suggesting that heteroblastic seedling leaf morphology does not give an advantage over homoblastic congeners under low light intensities.
Introduction
The term leaf heteroblasty defines the situation when leaf size and shape differ substantially between seedling and adult plants (Goebel 1900; Allsopp 1967). While heteroblasty is a worldwide occurrence, it is extremely common in the New Zealand flora, where ~10 % of seed plants are heteroblastic (either through changes in habit or leaf morphology; Cockayne 1912). The evolution of heteroblasty in New Zealand flora has been hypothesized to be an outcome of climatic change (Cockayne 1912; McGlone and Webb 1981) or of browsing by moa (flightless and now extinct birds known as ratites: Greenwood and Atkinson 1977; Mitchell 1980; Atkinson and Greenwood 1989). However, differences in light environment can also influence leaf morphology and this may select for heteroblasty (see Lee and Richards 1991; Gould 1993). For example, within a forest, changes in light quantity and/or quality occur in a predictable manner throughout plant ontogeny. These changes may be advantageous for plants that differentially express leaf morphology traits during development by maximizing growth and/or survival in low light in the forest understorey as seedlings and, later, in higher light intensities as adults. It is possible that in low-light environments, heteroblastic seedling leaf morphology would have an ecological advantage (shade tolerance) over homoblastic seedlings, which do not have a distinct juvenile morphology.
Few studies have examined the effect of light environment on leaf heteroblasty in New Zealand (Gould 1993; Day et al. 1997; Day 1998). For example, Gould (1993) studied the ontogenetic differences (seedling, juvenile, transitional and adult phases) in leaf morphology and anatomy of Pseudopanax crassifolius from plants collected in the field in New Zealand. He found that seedling leaves possessed many traits commonly observed in shade-acclimated plants, while the adult leaves were similar to those of sun-acclimated plants. Therefore, Gould (1993) concluded that heteroblastic changes during ontogeny are associated with changes in leaf construction costs, light interception and heat dissipation. Day (1998) examined the ontogenetic changes in phenotype and growth strategy of heteroblastic Elaeocarpus hookerianus, Carpodetus serratus (divaricating morphology) and P. crassifolius (non-divaricating). He suggested that a divaricating morphology in juveniles is likely to be an adaptation to moderate light intensity, while the non-divaricating morphology of juveniles of P. crassifolius is likely to be an adaptation to low light intensity in the understorey. Conversely, the adult leaf forms are adaptations to high light in the overstorey.
The above findings suggest that the morphology of heteroblastic juveniles may be an adaptation to low light. However, studies that have examined how these differences in foliar traits would influence the survival and growth of heteroblastic species are lacking. There are no published experimental studies comparing differences in the survival and growth of heteroblastic plants relative to their homoblastic congeners in response to changes in light environment at a comparable stage of plant development. Accordingly, we compared differences in seedling survival and growth of four pairs of congeneric homoblastic and heteroblastic species grown in full sun and in heavily shaded conditions. Phylogenetically independent contrasts were selected to examine whether the juvenile morphology of heteroblastic species allows for better survival and growth than the morphology of their homoblastic relatives to contrasting light environments.
In a previous paper (Gamage and Jesson 2007), we presented the results of an experiment examining the foliar traits of four congeneric pairs of homoblastic and heteroblastic species in simulated sun and shade light environments. The present findings suggested that the heteroblastic seedlings do not have leaf morphology consistent with shade tolerance. As a continuation of the above experiment, in this study, seedling survival, growth and biomass allocation patterns of congeneric homoblastic and heteroblastic species pairs were assessed to see if they matched the responses indicated by foliar traits. An attempt is made, in particular, to answer the question whether heteroblastic seedlings gain any advantage in terms of survival, growth and biomass allocation relative to homoblastic congeners in low light.
Materials and methods
Study species
Four pairs of homoblastic and heteroblastic species in genera Hoheria (Malvaceae), Aristotelia (Elaeocarpaceae), Pseudopanax (Araliaceae) and Melicope (Rutaceae) were used. These pairs differed in their primary growth habit. Two species pairs are trees (heteroblastic Hoheria sexstylosa and homoblastic Hoheria lyallii; heteroblastic P. crassifolius and homoblastic Pseudopanax arboreus), while the other two species pairs are shrubs or trees (heteroblastic Aristotelia fruticosa is a shrub, while homoblastic Aristotelia serrata is a tree; heteroblastic Melicope simplex is a small tree or shrub and homoblastic Melicope ternata is a small tree). The heteroblastic species produce either deeply toothed or lobed leaf margins at seedling stage, while their adult leaves are entire or serrately margined (Allan 1961; Dawson and Lucas 2000). Both the heteroblastic and homoblastic species can be found in lowland, montane and sub-alpine forests and are native to New Zealand (see Gamage and Jesson 2007 for details).
Design of the controlled-environment shelters
Enclosures were constructed within a single glasshouse to simulate deep-forest understorey and full-sunlight environments found in the temperate rainforests of New Zealand. Each enclosure comprised a wooden frame 80 × 215 × 120 cm (w × l × h). Two enclosures were constructed to simulate forest understorey light quality and quantity (2.5 % of full sun = 50 µmol m−2 s−1; red:far red (R:FR) = 0.25) achieved with dye-impregnated films (PANTHER 20; Lee et al. 1996) and shade cloth placed over the frames. Two further enclosures were constructed to simulate full-sunlight quality (R:FR = 1.25) and quantity (100 % of light = 1600 µmol m−2 s−1). These frames were covered with clear polythene. On sunny days during summer (November–January), the averaged maximum photosynthetic photon flux densities recorded in shade and full-sun enclosures were 51 µmol m−2 s−1 ± s.d. 6 and 1636 µmol m−2 s−1 ± s.d.102, respectively. The averaged maximum air temperatures were 18.2 °C ± s.d. 1.6 and 26.4 °C ± s.d. 2.1, respectively. Light intensity and air temperature measurements taken in an open area outside the glasshouse were 2027 µmol m−2 s−1 ± s.d. 112 and 25.5 °C ± s.d. 3.3.
Experimental design
Three-month-old seedlings of Hoheria, Aristotelia, Pseudopanax and Melicope were grown in full-sun and forest understorey enclosures during spring. All seedlings were planted in 2-L circular polybags filled with potting mix (a blend of compost, bark, pumice, trace elements, osmocote and slow-release fertilizer) and were grown for 9 months from September to May (spring and autumn) in sun and shade light treatments. There were 12 seedlings for each species in each light treatment. The exception was Melicope. Due to the poor germination of M. simplex, only six pairs of Melicope were planted. In each enclosure, seedlings were arranged in two blocks (six replicates of seedlings per species within a block) in regular lines at 20 × 20 cm spacing between bag centres. Seedlings were well watered during the experiment.
Seedling height (measured from root collar diameter to the tip of the apical shoot) and number of branches and leaves were recorded at the beginning and at the end of the experiment for each seedling in each light treatment. After 9 months, the number of surviving seedlings was recorded, and seedlings were taken out from soil polybags by cutting the bags. Roots were washed and root collar diameter (stem diameter where shoot and roots separate) was measured using a digital vernier caliper. Seedlings were then dried at 85 °C to constant weight prior to dry mass determinations of leaves, shoots (main stem and branches) and roots (tap root and fine roots). The large midribs of P. crassifolius leaves were excluded from the leaf measurements as they are not photosynthetic, weighed separately and added to the stem dry mass. Midribs of P. arboreus were also treated in this manner.
Plasticity index
To assess whether homoblastic and heteroblastic species differed in the degree of their response to sun and shade, an index of plasticity was calculated for each trait as the ratio of the mean in sun and the mean in shade (see Ashton and Berlyn 1994). This index indicates both the magnitude and the direction of plasticity for homoblastic and heteroblastic species for each measured variable.
Statistical analysis
To test for differences between congeneric pairs of homoblastic and heteroblastic species, general linear models were applied for each of the growth and biomass allocation measures using MINITAB Version 12. All data were log transformed prior to analysis to meet assumptions of normality with the exception of biomass allocation ratios. The differences among light, block, genera, species (homoblastic/heteroblastic) nested within genera and all two-way interactions were tested. Block was considered as a random factor. The light × species (genera) interactions that were significant (P < 0.05) were further compared using a t-test between homoblastic and heteroblastic seedlings within sun and shade light treatments separately. Biomass allocation ratios (ratios of leaf, stem and root mass to total mass) were arcsine-square root transformed and significant differences among light, block, genera, species nested within genera were examined using the Wilk's test in multiple analysis of variance (MANOVA). The index of plasticity between homoblastic and heteroblastic species was analysed using t-tests between the homoblastic and the heteroblastic species.
Results
Seedling survival
Three of the four homoblastic species had 100 % survival in the shade. In contrast, heteroblastic species had only 75 % survival in the shade with the exception of P. crassifolius (85 % survival). However, both homoblastic and heteroblastic species had 100 % survival in the full-sun treatment.
Seedling growth
Analysis of variance revealed significant interactions between light and species nested within genera for all growth measures (Table 1), indicating that homoblastic and heteroblastic species within each genus differed in their response to light. Heteroblastic P. crassifolius does not produce branches and was excluded from this analysis. In the shade, heteroblastic H. sexstylosa and M. simplex produced more branches relative to their homoblastic congeners, while Aristotelia species were similar (Table 2). In full sun, heteroblastic species had more branches than their homoblastic congeners. The production of leaves by homoblastic species was greater in the shade than in full sun with the exception of H. lyallii, while heteroblastic species produced more leaves in full sun with the exception of P. crassifolius (Table 2). All heteroblastic species had a smaller root collar diameter compared with their homoblastic species pair. In addition, for three of the four species pairs, heteroblastic species were significantly shorter and had less total biomass (Table 2).
Table 1.
Summary of statistical analysis for growth measures. F-statistics for nested analysis of variance (general linear model) for growth traits (increment in height, number of branches, number of leaves, root collar diameter and total dry mass), and multivariate analysis of variance (general linear model) for biomass allocation to leaves, shoots and roots.
| df | Height | Branch no. | Leaf no. | Root collar diameter | Total biomass | Biomass allocation | |
|---|---|---|---|---|---|---|---|
| Light | 1 | 1159.3*** | 1324.2*** | 301.2*** | 1908.5*** | 3697.4*** | 106.4*** |
| Block | 3 | ns | ns | ns | ns | ns | ns |
| Genera | 3 | 53.7*** | 477.4*** | 127.4*** | 351.6*** | 274.4*** | 124.4*** |
| Species (genera) | 4 | 34.3*** | 94.2*** | 52.3*** | 155.1*** | 410.5*** | 43.7*** |
| Light × genera | 3 | 4.1* | 16.8*** | ns | 16.8*** | 14.3*** | 39.3*** |
| Light × species (genera) | 4 | ns | 11.3*** | 25.1*** | 8.2*** | 63.3*** | 36.3*** |
Light was a fixed factor. Species (either homoblastic or heteroblastic) were nested within a genus. Block was considered as a random factor. Degree of freedom, df; degree of significance: ***P< 0.001, **P < 0.01, *P < 0.05.
Table 2.
Mean and standard errors of growth measures: height, number of branches/seedling, number of leaves/seedling, root collar diameter, total dry mass for homoblastic and heteroblastic (hetero) species in full-sun and shade treatments.
| Height (cm) | Number of branches | Number of leaves | Root collar diameter (mm) | Total dry mass (g) | |
|---|---|---|---|---|---|
| Shade | |||||
| H. lyallii | 7.1 (2.12)a | 0.8 (0.20)b | 24.5 (2.9)b | 6.36 (0.22)a | 1.71 (0.040)a |
| H. sexstylosa (hetero) | 6.1 (4.42)a | 3.3 (0.47)a | 143.3 (22.1)a | 4.22 (0.15)b | 1.69 (0.085)a |
| A. serrata | 7.6 (1.03)a | 1.7 (0.51)a | 33.5 (5.56)a | 3.87 (0.31)a | 0.93 (0.108)a |
| A. fruticosa (hetero) | 1.4 (0.31)b | 1.6 (0.61)a | 7.0 (5.22)b | 1.97 (0.26)b | 0.56 (0.046)b |
| P. arboreus | 8.0 (0.88)a | NA | 9.9 (0.34)a | 4.01 (0.15)a | 2.68 (0.105)a |
| P. crassifolius (hetero) | 1.5 (0.22)b | NA | 5.2 (0.36)b | 1.09 (0.05)b | 0.03 (0.003)b |
| M. ternata | 2.8 (0.38)a | 0.3 (0.21)b | 11.8 (1.08)a | 1.71 (0.06)a | 2.01 (0.066)a |
| M. simplex (hetero) | 1.0 (0.23)b | 1.0 (0.37)a | 8.5 (1.06)b | 0.75 (0.04)b | 0.03 (0.004)b |
| Full sun | |||||
| H. lyallii | 122.8 (2.61)a | 21.8 (2.54)b | 332.0 (44.6)b | 18.7 (0.63)a | 106.3 (6.23)a |
| H. sexstylosa (hetero) | 116.9 (5.38)a | 57.3 (3.50)a | 2516.8 (162)a | 16.0 (0.65)b | 90.4 (7.00)a |
| A. serrata | 163.3 (2.21)a | 32.9 (1.52)a | 480.7 (45.1)b | 16.3 (0.55)a | 108.9 (5.98)a |
| A. fruticosa (hetero) | 57.3 (3.82)b | 37.4 (3.31)a | 1101.8 (152)a | 8.8 (0.70)b | 23.1 (1.80)b |
| P. arboreus | 70.3 (2.51)a | 3.1 (0.31) | 49.2 (2.54)a | 18.4 (0.38)a | 118.4 (3.17)a |
| P. crassifolius (hetero) | 27.9 (3.82)b | NA | 32.8 (1.75)b | 6.4 (0.52)b | 5.2 (1.24)b |
| M. ternata | 31.8 (3.68)a | 1.8 (0.48)b | 37.2 (2.95)b | 5.8 (0.48)a | 8.6 (1.37)a |
| M. simplex (hetero) | 15.4 (1.93)b | 21.2 (2.33)a | 185.0 (26.6)a | 3.7 (0.36)b | 4.1 (0.90)b |
Means are from 12 seedlings (standard errors in parentheses) of each Hoheria, Aristotelia and Pseudopanax species. Each Melicope species had only six seedlings. Species within a genus (homo vs. hetero) followed by the same letter are not significantly different at the 5 % significance level. NA, data not available.
Biomass allocation
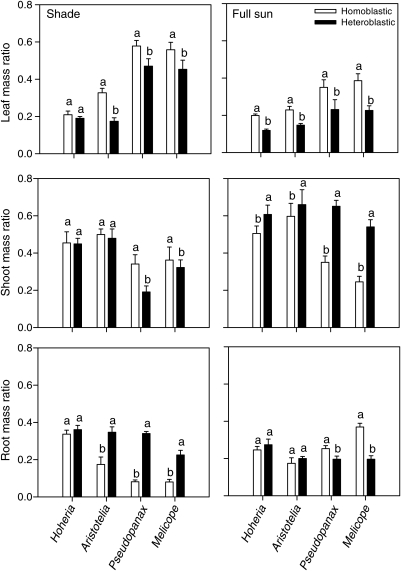
Wilk's test in MANOVA (general linear model) showed a significant interaction between light and species nested within genera for biomass allocation ratios (leaf mass ratio, shoot mass ratio and root mass ratio; Table 1). In the shade, t-tests showed that in three of the four species pairs, the heteroblastic species allocated less biomass to leaves and more to roots than the homoblastic pair (Fig. 1). In the full-sun treatment, all heteroblastic species had more biomass allocation to shoots but less to leaves than their homoblastic pair (Fig. 1).
Fig. 1.
Differences in biomass allocation to leaves (leaf mass ratio), stems (shoot mass ratio) and roots (root mass ratio) for homoblastic and heteroblastic Hoheria, Aristotelia, Pseudopanax and Melicope species in full-sun and shade light treatments. Bars indicate one standard error of the mean. Bars with the same letter are not significantly different at the P < 0.05 level between homoblastic and heteroblastic species within a genus.
Discussion
The heteroblastic leaf syndrome involves ontogenetically predictable changes in leaf morphology. These changes are likely to be a response to predictable changes in light environment. The ideal test of the function of heteroblasty at the seedling stage would be to examine the performance of juveniles with adult foliage. In the absence of the required mutants, other approaches, such as contrasting species with varying levels of heteroblasty, are required. In the present study, independent contrasts of homoblastic and heteroblastic species in four genera in four different families were used to assess whether plants with the heteroblastic syndrome respond to contrasting light environments in a similar manner. The results show that the heteroblastic seedlings do not perform well (in terms of survival or growth) in the shade, suggesting that heteroblasty is not an adaptation to changes in vertical light gradients such as found in the forest understorey. These findings are discussed further below in the context of other possible explanations for the evolution of heteroblasty.
In absolute terms, homoblastic species outperformed the heteroblastic species in both sun and shade. In the shade, the survival and growth (Table 2) of heteroblastic species were significantly reduced. Furthermore, in both sun and shade, heteroblastic species exhibited slower rates of growth in stem diameter than homoblastic species. In addition, for three of the four comparisons, heteroblastic species showed slower rates of growth in height and lower total biomass. Seedlings that are able to tolerate low light have often shown slow growth but high survivorship (King 1994; Kitajima 1994; Poorter and Arets 2003). Yet, this was not observed for heteroblastic species in the experiments, suggesting that heteroblastic seedlings were less able to acclimatize to deep-shade conditions compared with their homoblastic congeners.
Finding that the four heteroblastic species did not survive as well in the shade is inconsistent with studies on other heteroblastic plants (Cameron 1970; Ashton and Turner 1979). For example, Lee and Richards (1991) found that seedlings of heteroblastic vines were adapted to low-light environments in the tropical rainforest understorey. Gould (1993) also reported that seedlings of heteroblastic P. crassifolius possess many morphological and anatomical features similar to shade-acclimated plants. However, James and Bell (2000), who studied the leaf structure and growth of the heteroblastic tree Eucalyptus globulus in Australia found that juvenile leaves were similar to sun-adapted leaves. Thus, it seems that the association of heteroblastic seedlings with low light is not consistent across species or biomes.
The results of absolute survival and growth suggest that heteroblastic species may be more adapted to higher light environments. Yet, the relative response to differences in light environment is less clear. An examination of the ratio of performance in high light vs. low light (plasticity) gives an indication of whether heteroblastic species gain an advantage in the shade by having different juveniles. For all four contrasts, the relative changes in height and stem diameter were similar across environments for the homoblastic species (as indicated by a low ratio of performance in sun/performance in shade), while the heteroblastic species had a much greater response to the different light levels. However, the actual ratio of changes was very similar between the species pairs (Table 3), suggesting that the actual differences in response to sun and shade are not great. In addition, heteroblastic Aristotelia and Hoheria performed relatively better in the shaded environment (as indicated by a low ratio of performance in sun vs. shade). Thus, in some species, the heteroblastic syndrome or some other feature of these species does assist growth in shaded environments.
Table 3.
Summary of plasticity values (sun/shade) for various growth measures: increment in height, number of branches and leaves, root collar diameter, and total biomass gain for homoblastic and heteroblastic (hetero) species.
| Height | Branches | Leaves | Root collar diameter | Total biomass | |
|---|---|---|---|---|---|
| H. lyallii | 17.3a | 27.2 | 14.2a | 2.9a | 62.2 |
| H. sexstylosa (hetero) | 19.3 | 17.3a | 18.3 | 3.8 | 53.5a |
| A. serrata | 21.5a | 20.3a | 14.7a | 4.2a | 117.1 |
| A. fruticosa (hetero) | 40.4 | 23.7 | 157.3 | 4.5 | 41.3a |
| P. arboreus | 8.8a | NA | 5.3a | 4.6a | 44.1 |
| P. crassifolius (hetero) | 19.2 | NA | 7.3 | 5.9 | 174.7a |
| M. ternata | 11.6a | 5.5a | 3.2a | 3.4a | 4.3a |
| M. simplex (hetero) | 15.4 | 21.2 | 22.4 | 5.0 | 137.3 |
NA, data not available.
aRelatively better performance than congener in shade.
The species pairs selected here exhibited other differences in habit and morphology in addition to heteroblasty or homoblasty. It may be that these differences contribute to some of the variation in the present results. For example, the four homoblastic species are all small trees with large leaves. However, two heteroblastic species are small trees or shrubs. While these differences may contribute to the differences in responses found here, the results still suggest that, in general, heteroblastic species have low survival in the shade, and thus the characteristic leaf morphology is not consistent with an adaptation to shade. In addition, the simulated shade chambers were not completely representative of all factors found in the forest understorey, such as sunflecks, which are commonly utilized by many species (Kuppers et al. 1996). Further experimentation with other species pairs or different light environments is clearly warranted.
Heteroblastic and homoblastic species also had striking differences in their canopy architecture. Heteroblastic species produced more slender branches and smaller leaves than homoblastic relatives in both light environments with the exception of P. crassifolius. Branching pattern has a direct impact on leaf display and consequently on light capture (Valladares et al. 2002). It could also be that heteroblastic seedlings require more branches to foliate their smaller leaves than homoblastic congeners (see White 1983). This lateral growth could be an advantage for heteroblastic seedlings over homoblastic congeners when growing in open areas since it makes them mechanically more resistant to other environmental stresses (wind and snow) encountered in open sites (see Kempf and Pickett 1981; Ashton and De Zoysa 1989; King 1997; Gardiner and Hodges 1998). However, heteroblastic P. crassifolius does not branch at the seedling stage but had relatively longer leaves (45–50 cm in length) than the other heteroblastic species (3–8 cm in length) in the full-sun treatment. While the massive midribs of the lengthy leaves of P. crassifolius are likely to perform similar functions to branches, it does seem that there are different syndromes in plant growth among heteroblastic species.
This study suggests that some additional explanations for the evolution of the heteroblastic syndrome should be explored. For example, in the shade, only homoblastic species had a decrease in allocation to roots. Decreased allocation to roots is often associated with decreasing irradiance (Popma and Bongers 1988; Bongers and Popma 1990), and so the lower survival of heteroblastic plants in the shade could be associated with this greater root allocation since it is a respiratory cost (see Veneklaas and Poorter 1998). However, increased root allocation suggests that adaptations for enhanced water uptake may be important (see Ashton 1995; Ashton et al. 1995). It is also possible that heteroblastic species may be able to tolerate dry understorey sites in the forest better than their homoblastic relatives. It has also been hypothesized that heteroblasty could be an adaptation to defence against browsing birds, and the differences in branch number and leaf area could reflect this. Traits that are likely to reduce browsing by birds include small leaf size, thin twigs with high tensile strength, wide branch angle and zig-zag branches (Bond et al. 2004), and these traits were found in both New Zealand and Madagascan floras (which both had large bird browsers, now extinct; Bond and Silander 2007). The findings of small leaves and slender branches in the heteroblastic species fit with this syndrome. Interestingly, the differences in shoot allocation between the growth forms were more pronounced in the high-light environment, and clearly the interaction of water availability, herbivore browsing and environmental stresses needs to be investigated further.
Conclusions and forward look
The measured attributes of growth, biomass gain and allocation patterns of seedlings of heteroblastic species did not improve survival relative to homoblastic congeners in low light. Therefore, shade is not the selective mechanism generating the characteristic heteroblastic seedling leaf morphology in the species studied here. However, features of heteroblastic seedling morphology (slender branches and smaller leaves) and biomass allocation (leaf mass ratio and greater shoot mass ratio) may make heteroblastic seedlings less vulnerable to other environmental stresses (high temperature, low soil moisture, susceptibility to wind and snow) encountered in high light intensities. At present, the responses of heteroblastic plants, as seedlings or adults, to differences in temperature, soil moisture and wind are unknown. Further studies examining the combination effects of the above factors for both seedlings and adults would help us to understand more of the selective mechanisms that give rise to leaf heteroblasty and confer competitive advantage.
Sources of funding
Victoria University of Wellington, New Zealand provided funding for this project.
Contribution by the author
H.K.G. designed, carried out the project and wrote the manuscript.
Conflicts of interest statement
None declared.
Acknowledgements
I express sincere thanks to Victoria University of Wellington, New Zealand for awarding a Targeted PhD scholarship and annual research grants to carry out this project. I am also grateful to Lynley Jesson, Don Drake and Anna Richards for their comments on the manuscript.
References
- Allan HH. Flora of New Zealand. Vol. 1. Wellington, New Zealand: Government Printer; 1961. [Google Scholar]
- Allsopp A. Heteroblastic development in vascular plants. Advances in Morphogenesis. 1967;6:127–171. doi: 10.1016/b978-1-4831-9953-5.50008-1. [DOI] [PubMed] [Google Scholar]
- Ashton DH, Turner JS. Studies on the light compensation point of Eucalyptus regnans F. Muell. Australian Journal of Botany. 1979;27:589–607. [Google Scholar]
- Ashton PMS. Seedling growth of co-occurring Shorea species in the simulated light environments of a rain forest. Forest Ecology and Management. 1995;72:1–12. [Google Scholar]
- Ashton PMS, Berlyn GP. A comparison of leaf physiology and anatomy of Quercus (section Erythrobalanus-Fagaceae) species in different light environments. American Journal of Botany. 1994;81:589–597. [Google Scholar]
- Ashton PMS, De Zoysa ND. Performance of Shorea trapezifolia (Thwaites) Ashton seedlings growing in different light regimes. Journal of Tropical Forrest Science. 1989;4:356–364. [Google Scholar]
- Ashton PMS, Gunatilleke CVS, Gunatilleke IAUN. Seedling survival and growth of four Shorea species in a Sri Lankan rainforest. Journal of Tropical Ecology. 1995;11:263–279. [Google Scholar]
- Atkinson IAE, Greenwood RM. Relationships between moas and plants. New Zealand Journal of Ecology. 1989;(12(Suppl.)):67–95. [Google Scholar]
- Bond WJ, Silander JA. Springs and wire plants: anachronistic defenses against Madagascar's extinct elephant birds. Proceedings of the Royal Society of Biological Sciences. 2007;274:1985–1992. doi: 10.1098/rspb.2007.0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond WJ, Lee WG, Craine JM. Plant structural defences against browsing birds: a legacy of New Zealand's extinct moas. Oikos. 2004;104:500–508. [Google Scholar]
- Bongers F, Popma J. Leaf dynamics of seedlings in relation to canopy gaps. Oecologia. 1990;82:122–127. doi: 10.1007/BF00318543. [DOI] [PubMed] [Google Scholar]
- Cameron RJ. Light intensity and the growth of Eucalyptus seedlings. I. Ontogenetic variation in E. fastigata. Australian Journal of Botany. 1970;18:29–43. [Google Scholar]
- Cockayne L. Observations concerning evolution, derived from ecological studies in New Zealand. Transactions and Proceedings of the Royal Society of New Zealand. 1912;44:1–50. [Google Scholar]
- Dawson J, Lucas R. Nature guide to the New Zealand forest. New Zealand: Random House; 2000. [Google Scholar]
- Day JS. Light conditions and the evolution of heteroblasty (and divaricate form) in New Zealand. New Zealand Journal of Ecology. 1998;22:43–54. [Google Scholar]
- Day JS, Gould KS, Jameson PE. Vegetative architecture of Elaeocarpus hookerianus: transition from juvenile to adult. Annals of Botany. 1997;79:617–624. [Google Scholar]
- Gamage HK, Jesson LK. Leaf heteroblasty is not an adaptation to shade: anatomical and physiological responses to light in seedlings of heteroblastic and homoblastic species. New Zealand Journal of Ecology. 2007;31:245–254. [Google Scholar]
- Gardiner ES, Hodges ID. Growth and biomass distribution of cherry bark Oak (Quercus pagoda Raf.) seedlings as influenced by light availability. Forest Ecology and Management. 1998;108:127–134. [Google Scholar]
- Goebel K. Organography of plants. Oxford: Oxford University Press; 1900. Part 1. General organography. [Google Scholar]
- Gould KS. Leaf heteroblasty in Pseudopanax crassifolius: functional significance of leaf morphology and anatomy. Annals of Botany. 1993;71:61–70. [Google Scholar]
- Greenwood RM, Atkinson IAE. Evolution of divaricating plants in New Zealand in relation to moa browsing. Proceedings of New Zealand Ecological Society. 1977;24:21–33. [Google Scholar]
- James SA, Bell DT. Influence of light availability on leaf structure and growth of two Eucalyptus globulus ssp. globulus provenances. Tree Physiology. 2000;20:1007–1018. doi: 10.1093/treephys/20.15.1007. [DOI] [PubMed] [Google Scholar]
- Kempf JS, Pickett STA. The role of branch length and angle in branching pattern of forest shrubs along a successional gradient. New Phytologist. 1981;88:111–116. [Google Scholar]
- King DA. Influence of light level on the growth and morphology of saplings in a Panamanian forest. American Journal of Botany. 1994;81:948–957. [Google Scholar]
- King DA. Branch growth and biomass allocation in Abies amabilis saplings in contrasting light environments. Tree Physiology. 1997;17:251–258. doi: 10.1093/treephys/17.4.251. [DOI] [PubMed] [Google Scholar]
- Kitajima K. Relative importance of photosynthetic traits and allocation patterns as correlates of seedling shade tolerance of 13 tropical trees. Oecologia. 1994;98:419–428. doi: 10.1007/BF00324232. [DOI] [PubMed] [Google Scholar]
- Kuppers M, Timm H, Orth F, Stegemann J, Stober R, Schneider H, Paliwal K, Karunaichamy KSTK, Ortiz R. Effects of light environment and successional status on lightfleck use by understory trees of temperate and tropical forests. Tree Physiology. 1996;16:69–80. doi: 10.1093/treephys/16.1-2.69. [DOI] [PubMed] [Google Scholar]
- Lee DW, Richards JH. Heteroblastic development in vines. In: Mooney HA, Putz FH, editors. The biology of vines. Cambridge: Cambridge University Press; 1991. pp. 205–243. [Google Scholar]
- Lee DW, Baskaran K, Mansor M, Mohamad H, Yap SK. Irradiance and spectral quality affects Asian tropical rain forest tree seedling development. Ecology. 1996;77:568–580. [Google Scholar]
- McGlone MS, Webb CJ. Selective forces influencing the evolution of divaricating shrubs. New Zealand Journal of Ecology. 1981;4:20–28. [Google Scholar]
- Mitchell ND. A study of the nutritive value of juvenile and adult leaves of Pseudopanax crassifolius. New Zealand Journal of Ecology. 1980;3:159. [Google Scholar]
- Poorter L, Arets EJMM. Light environment and tree strategies in a Bolivian tropical moist forest: an evaluation of the light partitioning hypothesis. Plant Ecology. 2003;166:295–306. [Google Scholar]
- Popma J, Bongers F. The effect of canopy gaps on growth and morphology of seedlings of rain forest species. Oecologia. 1988;75:625–632. doi: 10.1007/BF00776429. [DOI] [PubMed] [Google Scholar]
- Valladares F, Skillman JB, Pearcy RW. Convergence in light capture efficiencies among tropical forest understory plants with contrasting crown architectures: a case of morphological compensation. American Journal of Botany. 2002;89:1275–1284. doi: 10.3732/ajb.89.8.1275. [DOI] [PubMed] [Google Scholar]
- Veneklaas EJ, Poorter L. Carbon partitioning strategies of tropical tree seedlings in contrasting light environments. In: Lambers H, Poorter H, Van Vuren MNI, editors. Inherent variation in plant growth: physiological mechanisms and ecological consequences. Leiden: Backhuys; 1998. pp. 337–361. [Google Scholar]
- White PS. Corner's rules in eastern deciduous trees: allometry and its implications of the adaptive architecture of trees. Bulletin of the Torrey Botanical Club. 1983;110:203–212. [Google Scholar]