Abstract
Pyruvate dehydrogenase complex is a key intramitochondrial multienzyme complex required for the conversion of pyruvate to acetyl-CoA. The majority of patients with pyruvate dehydrogenase deficiency have a defect in the E1 alpha subunit, associated with mutations in the PDHA1 gene. In this report, we submit detailed magnetic resonance images in four affected female patients with PDHA1 mutations, who present with severe cortical atrophy, dilated ventricles, and an incomplete corpus callosum. In one of these patients, the MRI pattern prompted molecular diagnostic testing when enzymatic testing was normal. We underscore that this constellation of features, which may be misdiagnosed as periventricular leukomalacia, illustrate a pattern highly suggestive of a deficiency of pyruvate dehydrogenase E1 alpha in female patients and should trigger appropriate diagnostic investigations.
Keywords: Lactic Acidosis, Malformations of Cortical Development, Mutation
Introduction
Pyruvate dehydrogenase (PDH) deficiency is a common cause of primary congenital lactic acidosis [1–5]. The PDH enzyme complex is a key intra-mitochondrial complex [6] which catalyzes the oxidative decarboxylation of pyruvate to acetyl-CoA, thereby controlling the flow of substrates from the glycolytic pathway to the citric acid cycle. Defects in the E1α subunit gene (PDHA1; EC 1.2.4.1) on Xp21.3 account for the majority of cases of PDH deficiency [3, 7].
The classical biochemical features of PDH deficiency consist of an elevated blood lactate and pyruvate level and a normal lactate-to-pyruvate ratio [3]. However, the neurological presentation of PDHA1 deficiency is variable, and often differs between males and females [1–5]. Imaging features in males with PDHA1 deficiency are well recognized, and include subependymal cysts [8]; agenesis of the corpus callosum [2]; or a Leigh-like picture frequently accompanied by leukoencephalopathy [2, 8–10]. Females with PDHA1 deficiency often have a more severe clinical phenotype [1–4]. Previous radiological or neuropathological [11, 12] reports describe severe structural brain anomalies in affected females, including absence or hypoplasia of the corpus callosum, very thin cerebral mantle, and ventriculomegaly. While MRI findings in PDHA1 deficient females have been described [3, 5, 11, 13–15], we underscore the importance of MRI pattern recognition as a diagnostic tool in this disorder.
We analyzed MR imaging features in four female patients with PDHA1 deficiency. In all 4 patients the phenotype was highly suggestive of the diagnosis.
Patient 1
Patient 1 was born after a full-term gestation. Birth weight was 2.9 kg and occipitofrontal circumference was 33.5cm (10th percentile). Due to developmental delay, the patient was evaluated by a pediatric neurologist at 4 months of age. Neurological exam revealed an occipitofrontal circumference of 36.5 cm (3–4 SD below the mean) and an appropriate suck and cry, but she did not smile or reach for toys. Visual tracking was absent. She also displayed significant axial and appendicular hypotonia. Initial serum lactate (7.3 mM; NR 1 – 1.7 mM), and pyruvate (0.65 mM; NR 0.03 – 0.17 mM), were both elevated, but the lactate/pyruvate ratio was normal. Brain MRI was performed at 4 months (Figure 1, Patient 1, A–C). Pyruvate dehydrogenase complex activity, assayed in fibroblasts, was decreased (29% of the mean). PDHA1 sequencing demonstrated a de-novo heterozygous invariant splice site mutation, c.899 + 1 g>c.
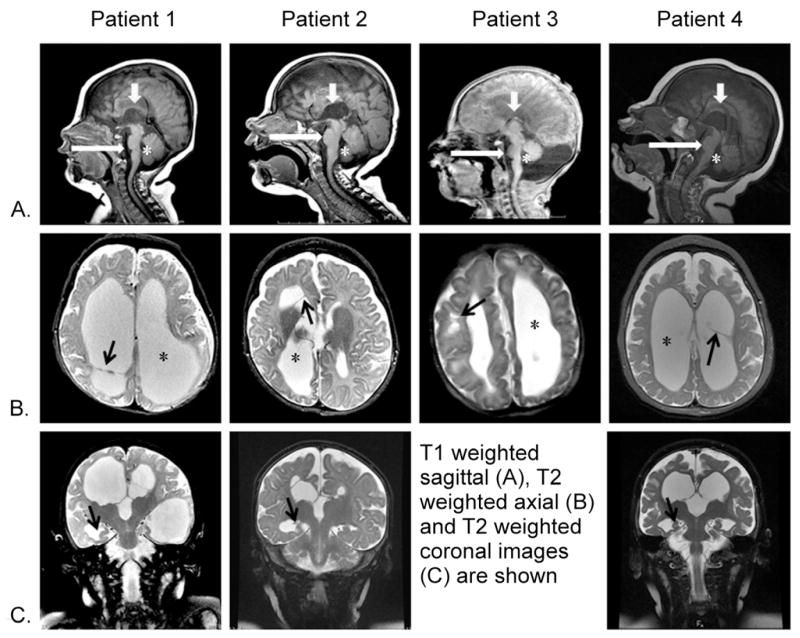
Figure 1. MRI characteristics in 4 female patients with PDHA1 deficiency.
Columns show patients 1, 2, 3, and 4 imaged at 4 months, 5 months, 1 day, and 11 months, respectively. Rows: A, sagittal midline T1-weighted images; B, axial T2-weighted images at the level of lateral ventricles; C, coronal T2-weighted images through the hippocampi.
Note the incomplete corpus callosum in all patients (Row A, short white arrows). All patients had asymmetrical ventriculomegaly (Row B, stars) with a normal 4th ventricle (Row A, stars). A small pons (Row A, long white arrows) can be seen. Ventricular septations were seen in 3/4 patients (row B, patients 1,2 and 4, black arrows) or parenchymal cysts in 1/4 patients (row B, patient 3, black arrow). Patients 1,2 and 4 displayed hyporotated hippocampi (row C, arrows).
Patient 2
Patient 2 was born at 35 weeks gestation. Asymmetric lateral ventricles, with progressive enlargement, had been observed on prenatal ultrasound. Birth weight was 2.5 kg, and occipitofrontal circumference at birth was not reported. In the neonatal period, she had marked hypotonia and feeding difficulties. At 5 months of age, she presented with infantile spasms and subsequently developed intractable epilepsy. Serum lactate (3.4 mM; NR 1–1.7 mM) and pyruvate (0.25 mM NR; 0.03–0.17 mM), were elevated at 5 months and the lactate/pyruvate ratio was normal. Brain MRI was performed at 5 months (Figure 1, Patient 2, A–C). Activity of the pyruvate dehydrogenase complex in fibroblasts was decreased (41% of the mean). Follow-up sequencing of PDHA1 demonstrated a de-novo heterozygous mutation, c.449 G>A.
Patient 3
Patient 3 was born at 36 weeks gestation. Birth weight was 2.2 kg, and occipitofrontal circumference was 30.5 cm (4–5 SD below the mean). In the neonatal period, she was noted to have hypotonia, contractures and feeding difficulties, but had a good suck and cry, and normal reflexes. Initial serum lactate (5.0 mM; NR 1 – 1.7 mM) was elevated. Repeat measurement revealed a lactate of 2.9 mM and pyruvate of 0.24 mM (NR 0.03 – 0.17 mM), with a normal lactate/pyruvate ratio of 12. Brain MRI was performed on day of life 1 (Figure 1, Patient 3, AB). Activity of the pyruvate dehydrogenase complex in fibroblasts was decreased (42% of the mean) and PDHA1 sequencing demonstrated a de novo heterozygous mutation, c.1011_c.1031duplication21.
Patient 4
Patient 4 was born at 37 weeks gestation after pre-term labor at 32 weeks. The neonatal period was significant for transient neonatal jaundice, as well as milk intolerance and reflux. Infancy was notable for global developmental delay, microcephaly, poor visual skills, irritability, obstructive sleep apnea and chronic congestion. Initial investigations revealed an elevated serum lactate (7.6 mM; NR 1.0–3.3 mmol/L) and elevated plasma alanine (1363 μM; NR 96–798). Repeated measurements showed elevated pyruvic acid (0.37 mM; NR 0.03–0.17), elevated lactate (4.7 mM; NR 1.0–1.7) and a normal lactate/pyruvate ratio of 13. Initial brain MRI was performed at 3 months of age and a repeat brain MRI/MRS was performed at 11 months (Figure 1). Activity of the pyruvate dehydrogenase complex was surprisingly normal in blood lymphocytes. However, because of typical MRI findings combined with a high clinical suspicion, PDHA1 sequencing was pursued prior to skin biopsy for fibroblast enzymatic testing. This detected a heterozygous novel missense variant, c. 680A>G (p.Y227C).
Brain Imaging
Brain MRI in all four patients (Figure 1) revealed asymmetric enlargement of the lateral and third ventricles, sparing the 4th ventricle. Patients 1, 2, and 4 presented with intraventricular septations. In all patients there was prominent paucity of cerebral white matter volume and a small pons. All patients exhibited an incomplete corpus callosum: specifically, parts of the genu and anterior body were present but the posterior body and splenium were absent, as confirmed by review of axial T2 and sagittal T1 images. Hyporotated hippocampi and small optic nerves with a low normal-sized optic chiasm were observed in patients 1, 2, and 4 but selected imaging of the optic nerves and hippocampi were not available for patient 3. Patient 3 also had subcortical cysts and asymmetrical subependymal cysts in the caudothalamic grooves. MR spectroscopy of the basal ganglia in patient 1 revealed a lactate peak (not shown). Head CT scans performed in patients 1 and 3 did not show calcifications.
Discussion
Female patients with PDHA1 deficiency often have more severe brain anomalies [1–4] than their male counterparts. This difference between the sexes is hypothesized to be as a result of differences in X-chromosome number. In affected males, who possess a single X-chromosome, a uniform population of cells with a deleterious PDHA1 allele that essentially abolishes enzyme activity is thought to be embryonically lethal [1, 2]. Only affected male fetuses with significant residual enzyme activity survive to the neonatal period [1, 2]. Consequently, newborn males with PDHA1 deficiency typically do not present severe structural brain anomalies at birth. In contrast, in females, random X-inactivation leads to expression of either the mutant or normal allele in neuronal cells [1, 2]. Therefore, even the most deleterious of PDHA1 alleles can result in a viable female pregnancy given a favorable lyonization: cells expressing the normal allele ensure fetal survival, whereas affected cells are non-viable, and their death results in malformations of the brain [1–4], such as those observed in our patients. Non-uniform patterns of lyonization may also result in diagnostic challenges, as is presumably the case in patient 4, in whom enzymatic evidence of PDH deficiency could not be established in lymphocytes.
Severe decrease in white matter volume, dilated ventricles with ventricular septations or parenchymal cysts and partial-to-complete agenesis of the corpus callosum have been previously reported in association with PDHA1 deficiency in female patients [3, 5, 11, 12, 14–16]. Based on MR imaging alone, it could be difficult to differentiate this pattern from other processes, such as cystic periventricular leukomalacia or postinfectious versus term hypoxic-ischemic injuries. However, the findings of markedly asymmetric white matter involvement are less likely to be seen in periventricular leukomalacia or hypoxic ischemic injury unless associated with a unilateral vascular event. Ventricular enlargement in PDH1 deficiency is probably the result of severe white matter volume loss. All four patients had a pattern of lateral and 3rd ventricle enlargement with normal size of the 4th ventricle. It is possible that in addition to decreased white matter volume, a structural abnormality of the aqueduct contributed to ventricular dilatation[17]. The observed small size of the pons may be due to hypogenesis/hypoplasia or secondary to developmental loss of cerebral white matter and decreased volume of descending long tracts. Similarly, the abnormalities of the corpus callosum may be due to hypogenesis/hypoplasia or related to prenatal white matter destruction. The difference between structural defects due to a prenatal destructive process secondary to a metabolic defect of selected cells[4] versus true hypogenesis/hyplasia is difficult to determine in this disorder. Ventricular septations and intraparenchymal cysts may also be the result of a destructive processes and may represent areas of incomplete porencephaly or cystic periventricular encephalomalacia.
Several other non-specific features were observed, including hyporotated hippocampi and small optic nerves. Hippocampal malrotation or “inversion” is seen in association with a variety of cortical malformations[18] or in the setting of hydrocephalus[19]. The significance of small size of the optic nerves in this setting is unclear. MR spectroscopy of the basal ganglia in patient 1 revealed a lactate peak, as has been previously described in PDH complex deficiency [15].
With regard to the genetics PDHA1, we add four novel mutations to the 116 currently described in the Human Gene Mutation Database PDHA1 mutation database (www.hgmd.cf.ac.uk). One of these mutations c899 + 1 g>c, affects the first nucleotide of intron 9 disrupting the canonical AGgt donor splice site. Only one of the other seven known PDHA1 splicing mutations is also intronic, and occurs in the acceptor splice site of intron 9 [20], leading to activation of a cryptic upstream splice site. The remaining known splicing mutations affect exonic splicing enhancer motifs in exons 5 [21], 6 [22], and 7 [23].
In summary, we emphasize the constellation of MRI findings in female patients with PDHA1 deficiency, including an abnormal corpus callosum, asymmetric ventricular dilatation, ventricular septations and/or parenchymal cysts, and a small pons. The recognition of this MRI pattern, especially in the context of an elevated blood lactate and a normal lactate-to-pyruvate ratio, should prompt further investigation, including molecular testing for PDHA1 mutations. Early diagnosis is important to facilitate supportive treatment and counseling for affected patients and their caregivers.
Abbreviations
- MR
Magnetic Resonance
- PDH
Pyruvate Dehydrogenase
- PDHA1
Pyruvate Dehydrogenase E1 alpha gene
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dahl HH. Pyruvate dehydrogenase E1 alpha deficiency: males and females differ yet again. Am J Hum Genet. 1995;56:553–557. [PMC free article] [PubMed] [Google Scholar]
- 2.Lissens W, De Meirleir L, Seneca S, Liebaers I, Brown GK, Brown RM, Ito M, Naito E, Kuroda Y, Kerr DS, Wexler ID, Patel MS, Robinson BH, Seyda A. Mutations in the x-linked pyruvate dehydrogenase (e1) alpha subunit gene (pdha1) in patients with a pyruvate dehydrogenase complex deficiency. Hum Mutat. 2000;15:209–219. doi: 10.1002/(SICI)1098-1004(200003)15:3<209::AID-HUMU1>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 3.De Meirleir L. Defects of pyruvate metabolism and the Krebs cycle. J Child Neurol. 2002;17(Suppl 3):3S26–33. discussion 23S33-24. [PubMed] [Google Scholar]
- 4.Chun K, MacKay N, Petrova-Benedict R, Federico A, Fois A, Cole DE, Robertson E, Robinson BH. Mutations in the x-linked e1 alpha subunit of pyruvate dehydrogenase: Exon skipping, insertion of duplicate sequence, and missense mutations leading to the deficiency of the pyruvate dehydrogenase complex. Am J Hum Genet. 1995;56:558–569. [PMC free article] [PubMed] [Google Scholar]
- 5.Knaap MSvd, Valk J, Barkhof F. Magnetic resonance of myelination and myelin disorders. 3. Chapter 28. Berlin; New York: Springer; 2005. pp. 228–230. [Google Scholar]
- 6.Patel MS, Roche TE. Molecular biology and biochemistry of pyruvate dehydrogenase complexes. FASEB J. 1990;4:3224–3233. doi: 10.1096/fasebj.4.14.2227213. [DOI] [PubMed] [Google Scholar]
- 7.Dahl HH, Brown GK, Brown RM, Hansen LL, Kerr DS, Wexler ID, Patel MS, De Meirleir L, Lissens W, Chun K, MacKay N, Robinson BH. Mutations and polymorphisms in the pyruvate dehydrogenase e1 alpha gene. Hum Mutat. 1992;1:97–102. doi: 10.1002/humu.1380010203. [DOI] [PubMed] [Google Scholar]
- 8.Soares-Fernandes JP, Teixeira-Gomes R, Cruz R, Ribeiro M, Magalhaes Z, Rocha JF, Leijser LM. Neonatal pyruvate dehydrogenase deficiency due to a R302H mutation in the PDHA1 gene: MRI findings. Pediatr Radiol. 2008;38:559–562. doi: 10.1007/s00247-007-0721-9. [DOI] [PubMed] [Google Scholar]
- 9.Tulinius M, Darin N, Wiklund LM, Holmberg E, Eriksson JE, Lissens W, De Meirleir L, Holme E. A family with pyruvate dehydrogenase complex deficiency due to a novel c>t substitution at nucleotide position 407 in exon 4 of the x-linked epsilon1alpha gene. Eur J Pediatr. 2005;164:99–103. doi: 10.1007/s00431-004-1570-2. [DOI] [PubMed] [Google Scholar]
- 10.Rahman S, Blok RB, Dahl HH, Danks DM, Kirby DM, Chow CW, Christodoulou J, Thorburn DR. Leigh syndrome: Clinical features and biochemical and DNA abnormalities. Ann Neurol. 1996;39:343–351. doi: 10.1002/ana.410390311. [DOI] [PubMed] [Google Scholar]
- 11.De Meirleir L, Lissens W, Denis R, Wayenberg JL, Michotte A, Brucher JM, Vamos E, Gerlo E, Liebaers I. Pyruvate dehydrogenase deficiency: Clinical and biochemical diagnosis. Pediatr Neurol. 1993;9:216–220. doi: 10.1016/0887-8994(93)90088-t. [DOI] [PubMed] [Google Scholar]
- 12.Michotte A, De Meirleir L, Lissens W, Denis R, Wayenberg JL, Liebaers I, Brucher JM. Neuropathological findings of a patient with pyruvate dehydrogenase E1 alpha deficiency presenting as a cerebral lactic acidosis. Acta Neuropathol. 1993;85:674–678. doi: 10.1007/BF00334680. [DOI] [PubMed] [Google Scholar]
- 13.Wada N, Matsuishi T, Nonaka M, Naito E, Yoshino M. Pyruvate dehydrogenase E1alpha subunit deficiency in a female patient: evidence of antenatal origin of brain damage and possible etiology of infantile spasms. Brain Dev. 2004;26:57–60. doi: 10.1016/s0387-7604(03)00072-x. [DOI] [PubMed] [Google Scholar]
- 14.Cross JH, Connelly A, Gadian DG, Kendall BE, Brown GK, Brown RM, Leonard JV. Clinical diversity of pyruvate dehydrogenase deficiency. Pediatr Neurol. 1994;10:276–283. doi: 10.1016/0887-8994(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 15.Shevell MI, Matthews PM, Scriver CR, Brown RM, Otero LJ, Legris M, Brown GK, Arnold DL. Cerebral dysgenesis and lactic acidemia: An mri/mrs phenotype associated with pyruvate dehydrogenase deficiency. Pediatr Neurol. 1994;11:224–229. doi: 10.1016/0887-8994(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 16.Otero LJ, Brown GK, Silver K, Arnold DL, Matthews PM. Association of cerebral dysgenesis and lactic acidemia with X-linked PDH E1 alpha subunit mutations in females. Pediatr Neurol. 1995;13:327–332. doi: 10.1016/0887-8994(95)00222-7. [DOI] [PubMed] [Google Scholar]
- 17.Algin O, Hakyemez B, Parlak M. Phase-contrast MRI and 3D-CISS versus contrast-enhanced MR cisternography on the evaluation of the aqueductal stenosis. Neuroradiology. 52:99–108. doi: 10.1007/s00234-009-0592-x. [DOI] [PubMed] [Google Scholar]
- 18.Sato N, Hatakeyama S, Shimizu N, Hikima A, Aoki J, Endo K. MR evaluation of the hippocampus in patients with congenital malformations of the brain. AJNR Am J Neuroradiol. 2001;22:389–393. [PMC free article] [PubMed] [Google Scholar]
- 19.Baker LL, Barkovich AJ. The large temporal horn: MR analysis in developmental brain anomalies versus hydrocephalus. AJNR Am J Neuroradiol. 1992;13:115–122. [PMC free article] [PubMed] [Google Scholar]
- 20.Ridout CK, Brown RM, Walter JH, Brown GK. Somatic mosaicism for a PDHA1 mutation in a female with pyruvate dehydrogenase deficiency. Hum Genet. 2008;124:187–193. doi: 10.1007/s00439-008-0538-0. [DOI] [PubMed] [Google Scholar]
- 21.Boichard A, Venet L, Naas T, Boutron A, Chevret L, de Baulny HO, De Lonlay P, Legrand A, Nordman P, Brivet M. Two silent substitutions in the pdha1 gene cause exon 5 skipping by disruption of a putative exonic splicing enhancer. Mol Genet Metab. 2008;93:323–330. doi: 10.1016/j.ymgme.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Okajima K, Warman ML, Byrne LC, Kerr DS. Somatic mosaicism in a male with an exon skipping mutation in PDHA1 of the pyruvate dehydrogenase complex results in a milder phenotype. Mol Genet Metab. 2006;87:162–168. doi: 10.1016/j.ymgme.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Ridout CK, Keighley P, Krywawych S, Brown RM, Brown GK. A putative exonic splicing enhancer in exon 7 of the PDHA1 gene affects splicing of adjacent exons. Hum Mutat. 2008;29:451. doi: 10.1002/humu.9525. [DOI] [PubMed] [Google Scholar]