Abstract
Cranioplasty following decompressive craniectomy is reported to result in improved blood flow, cerebral metabolism, and concomitant neurological recovery. We used multimodal functional imaging technology in a patient with marked neurological recovery after cranioplasty, specifically, imaging of functional MRI resting state networks, auditory responses, and cerebral metabolism before and after cranioplasty. Significant functional changes observed in the images correlated with the subject’s neurological recovery. Our results suggest a link between recovery of cerebral metabolism and intrinsic brain mechanisms of cerebral vascular integration and resting state networks identified with functional MRI following cranioplasty.
Keywords: Cranioplasty, resting state functional MRI, functional MRI, positron emission tomography
INTRODUCTION
Management of patients after severe brain injury often includes the temporary removal of skull bone (craniectomy) in the acute stage to mitigate the effects of brain compression due to a variety of processes that may increase intracranial pressure. Replacement of the skull (cranioplasty) may lag the acute phase considerably, and case reports and case series have indicated that the cranioplasty procedure results in improved blood flow, cerebral metabolism, and concomitant neurological recovery (1-3). More generally, recovery of integrative brain function after severe injuries resulting in a minimally conscious state (MCS) remains poorly understood with behavioral recovery typically occurring over many months (4) or even years (5). As a result of the slow time course of recovery and the potential for cognitive recovery to proceed in the absence of overt motor behavior (6) it is increasingly recognized that quantitative or neurofunctional measures are needed to more precisely track the evolution of recovery (7, 8). Here we measured fMRI auditory response, resting state networks (RSNs), and fluorodeoxyglucose positron emission tomography (FDG-PET) before and after right-hemispheric cranioplasty in a severely brain injured subject who emerged from MCS following the procedure.
In resting state fMRI, subjects undergo a conventional fMRI imaging protocol without performing specific cognitive or motor tasks. The resulting blood-oxygen-level-dependent (BOLD) signal contains various resting state network patterns, obtained by algorithms that compute and segment correlations in the signal (9-12), and putatively revealing information about connectivity of the cerebral neuro-vascular network. Although neither the physiological origins of resting state signals nor their potential clinical utility have been completely characterized yet, during the past five years a broad of clinical applications of resting state fMRI have been investigated (13). The most prominent resting state network, the “default mode,” was originally discovered in resting metabolism (PET) data (12) and later confirmed in conventional fMRI studies (14), and is thought to reflect the existence of an organized, baseline default mode of brain function. It is also thought to be involved in consciousness on a more basic level since it partially disintegrates during deep sleep (15). In addition to the default mode network, several other resting state networks have been identified more recently (16, 17), and a direct correspondence demonstrated between their BOLD signals and fluctuations in EEG oscillations (16, 18-20). Further, theoretical modeling attempts of the dynamics of RSNs, for example by means of numerical coupled oscillator networks (21), will lead to a deeper understanding of the relationship of clinical RSN observations and pathologies of intrinsic brain dynamics such as disturbances in conduction delays.
Case report
Six months prior to the first imaging study reported herein the subject, a 19 year old female, suffered a severe traumatic brain injury following a fall from the front of a moving vehicle. Initial examination in the field revealed signs of central herniation with bilateral pupillary dysfunction with a Glasgow Coma Scale (GCS) of 3. Emergency management included acute evacuation of a left epidural hematoma and bilateral craniotomies. Intracranial pressure monitoring showed average ICP of 30 mmHg. Over next five month period following acute injury the patient demonstrated inconsistent evidence of response to environmental stimuli as documented in medical records which did not improve after a placement of a ventriculoperitoneal shunt in the second month after injury. One month prior to the first imaging study (six months after injury) the patient underwent a left sided cranioplasty with subsequent recovery of reliable command following to simple motor commands. On admission to our study the patient’s neurological exam was notable for 4 mm pupils bilaterally reactive to 2 mm, a left upward gaze preference with occasional spontaneous nystagmus and increased range of movement to left with passive oculocephalic stimulation. Grip strength of 2/5 was noted bilaterally with no withdrawal of the right upper extremity to noxious stimuli and spontaneous withdrawal of the left upper extremity; lower extremities revealed bilateral spastic contractures with hyperreflexia. Formal quantitative behavioral assessment at the time of the first study reported here demonstrated an exam consistent with minimally conscious state (MCS) including reliable auditory command following and intermittent gestural communication. The Coma Recovery Scale Revised (CRS-R (22)) best total score was 14 (patient demonstrated consistent following of auditory commands, visual tracking despite a lack of blink to direct threat, object manipulation with the right hand, absence of vocalization or oral movement, inconsistent and inaccurate yes/no responses with right thumb, and eyes open state without stimulation). The second imaging study was done ten months after injury and two months following a right sided cranioplasty. At this time, the patient demonstrated further improvements on quantitative behavioral examination including recovery of functional object use (demonstrate using her right hand and upper extremity of the function use of common objects), vocalization to command, consistent communication (accurate yes/no responses through gesture), and improved attentional function with consistent responses to examiner queries (CRS-R total score of 20). At the time of this second evaluation formal testing indicated emergence from MCS based on sequential examination demonstrating consistent and accurate communication on simple situational accuracy questions.
MATERIALS AND METHODS
An institutional review board approved consent declaration was obtained from the patient’s legally authorized surrogate under active approved protocols. The imaging protocols for both study time points were identical. RS and auditory fMRI was performed, together with anatomical MRI, on a 3.0 Tesla General Electric Medical Systems (GEMS; Waukesha, WI) clinical MRI system with an eight-channel head coil using echo-planar imaging based functional MRI pulse sequences (repetition time TR = 2 s, echo time TE = 30 ms, flip angle 70, matrix size 64 × 64 × 28, axial field of view 24 cm, 5 mm slice thickness; RS fMRI was acquired with 180 samples, auditory fMRI with 128 samples). Before RS fMRI, the subject was instructed to think of nothing in particular; during auditory fMRI, the subject was instructed over headphones to imagine herself swimming and to stop imaging herself swimming, for eight times each. The data was analyzed with BrainVoyagerQX (Brain Innovation B.V., The Netherlands; motion correction, smoothing, detrending, re-sampling) and by using independent component analysis (RS-fMRI) and general linear modeling (auditory fMRI) including motion parameters as nuisance variables. In this model, the auditory instructions (and not the imagery periods in between) were used as stimuli. Fluorine-18 FDG-PET was performed on a GEMS combined PET-CT LS Discovery unit. Images were acquired in dynamic high-sensitivity emission mode (matrix size 128 × 128 × 35, axial field of view 25 cm, 4.25 mm slice thickness). Standard uptake values (SUV) were computed from the PET data including CT based skull attenuation corrections and then co-registered to high-resolution MRI images using PMOD (PMOD Technologies Ltd, Switzerland) and visualized using Mricron (Chris Rorden).
RESULTS
Anatomical changes
Before cranioplasty, anatomical MRI shows an overall loss of brain symmetry due to distortions with marked evidence of sunken skin flap depression on the side of the craniectomy (Figure 1, left panels). T1 weighted images show hyperintense cortical contusion with laminar necrosis. T2 weighted FLAIR images show anterior temporal, inferior frontal, and bilateral occipital injury. After cranioplasty, symmetry seems to be only slightly restored. Structural imaging shows serosanguinous collection underlining cranioplasty and new small subdural collections surrounding both hemispheres (Figure 1, right panels). A detailed comparison of the two brain images after co-registration reveals that ventricular spaces are markedly reduced after cranioplasty (not shown).
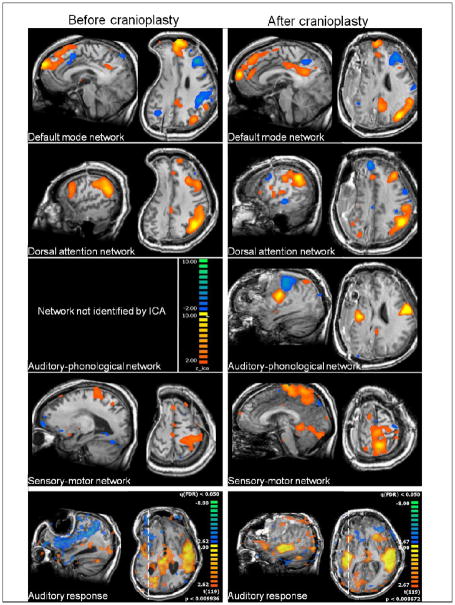
FIGURE 1.
Top four rows: Resting state networks before (left column) and after (right column) cranioplasty. The auditory-phonological network could not be identified by independent component analysis (ICA) before cranioplasty and emerges only post-surgery. Colors denote z-values for the independent components as shown on the scale in the third row on the left.
Bottom row: Auditory responses to stimulation with short spoken sentences before (left) and after (right) cranioplasty. Before cranioplasty, auditory response is absent on the ipsilateral side and restored after cranioplasty. Colors denote z-values of the general linear model used to fit the response. The threshold is defined as a false discovery rate of the multiple test problem of p = 0.05. Dashed lines on the axial cuts denote the position of the corresponding sagittal images. Axial cuts are shown in radiological convention in which the right side of the brain is shown on the left side of the image.
Resting state network changes
Out of the six resting state networks (16), in this subject three networks were found before and after cranioplasty, namely the default mode, the dorsal attention, and the sensory-motor network. The visual and the self-referential networks could not be found in either case. The auditory-phonological network only showed up post-cranioplasty. Parametric maps of resting state network connectivity are provided in Figure 1. (For comparison, a study with nine normal control subjects using the same methodology resulted in a 93% reliability of network identification (H.U.V., unpublished)). Overall, the networks that existed at both time points increased in volume or remained unchanged: default mode +40% (from 52 to 73 ccm), dorsal attention -2% (from 56 to 55 ccm), and sensory-motor +46% (from 67 to 98 ccm).
Auditory response changes
Before cranioplasty, auditory responses to short spoken sentences were found mainly in left primary auditory areas and were mostly absent on the side of the craniectomy. After cranioplasty, strong auditory responses were found bilaterally (Figure 1, bottom two rows).
Resting metabolism changes
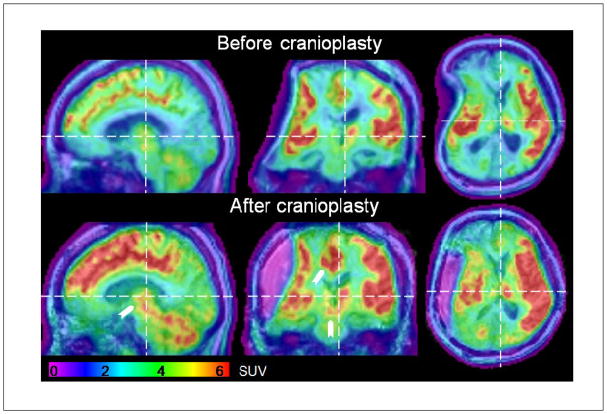
A marked increase in standard uptake values of FDG was observed after cranioplasty. Whole brain averaged SUVs (excluding the cavity at the second time point) increased from 2.5 ± 2.0 to 3.0 ± 2.4 g/ml [± standard deviation]. Regional changes were observed in left mesial frontal regions and within the mesodiencephalon (upper brainstem and thalamus) (Figure 2, arrows).
FIGURE 2.
FDG-PET images before and after cranioplasty overlaid onto anatomical MRI images. Color codes standard uptake values (SUVs) in units of g/ml as shown in the color bar at the bottom of the panel and arrows point towards changes in mesodiencephalic and mesial cortical regions. Dashed lines mark the position of the corresponding other cuts. Axial and coronal cuts are shown in radiological convention.
DISCUSSION
We have found signatures of increased functional activity using three different functional imagining modalities in a subject following cranioplasty to repair a sinking flap produced after decompressive craniectomy. FDG-PET measurements indicated both global increases in cerebral metabolism and marked local improvements in mesodiencephalic region; resting state and auditory functional MRI revealed more specific information about neuronal network functional connectivity. We have previously identified loss of RSNs in the setting of specific neurovascular injuries (13) with lesioned neuronal substrates, but the re-appearance of the auditory resting state network after cranioplasty along with a significantly enhanced auditory response on the ipsilateral side is novel and points towards further clinical significance of RSN imaging in addition to previous findings. Some RSNs are robust to anesthesia (23, 24) and light sleep (25) suggesting that intrinsic neurovascular coupling contributes to them. Prior studies have identified reversible changes in neurovascular coupling using electroencephalography measures and FDG-PET in the minimally conscious state (26). Taken together, these observations may relate to aspects of recovery of cerebral autoregulation mechanisms and raise the possibility that RSNs per se reflect a key aspect of the spatiotemporal regulation of intracranial blood volume, observed as the constancy of cerebral blood pressure across wide ranges of systemic blood pressures.
Our finding of reduced resting state network connectivity on the side of the craniectomy can be directly related to a prior demonstration of reduced EEG network coherence in the lesioned hemisphere in a vegetative patient with severe asymmetric subcortical brain damage associated with the loss of thalamic input (27). Measurement of RSNs is based on the coherence of BOLD signals and both findings may reflect damage to thalamo-cortical loop connections, likely a strong source of EEG coherence (28) as well as of resting state network connectivity (29, 30). Improvement in the auditory RSN seen here may thus associate with our findings of reversal of depressed resting metabolism in the thalamus following cranioplasty. Subject motion can conceal functional activations and resting state network components and should always be considered in interpreting data from subjects that are not fully cooperative and thus on average move more than healthy control subjects. In this study head motion was more pronounced at the second time point for the functional scan and at the first time point for the resting state scan. Therefore, it cannot account for the common trend observed in both scans alone.
The marked increase in regional cerebral metabolism (FDG-PET) in the left mesial frontal regions and within the mesodiencephalon of the left hemisphere before and after the cranioplasty is of particular note. This finding suggests a partial resolution of a component of “paradoxical herniation” originating from the earlier craniectomy. Prior studies have demonstrated a mesodiencephalic herniation syndrome resulting from the effects of atmospheric pressure and gravity in the setting of craniectomy (31-33).
Bilateral improvements of regional cerebral blood flow after cranioplasty have been shown before in perfusion (3), dynamic (34), and Xenon-enhanced (35) CT imaging. Our study demonstrates the potential utility of resting state MRI and functional MRI, both of which can be easily added to conventional clinical MRI protocols without the need of an additional imaging session. In our patient, significant increases in the volume of some RSN components and both global and regional cerebral metabolism measured using FDG-PET correlated with the subject’s neurological recovery. Notably, the auditory RSN, absent before cranioplasty, reappeared afterwards, a novel observation that suggests a link between intrinsic brain mechanisms of cerebral vascular integration and the RSN response. The findings support the role of cranioplasty in restoring aspects of integrative cerebral function after severe brain injuries and provide further insight into the physiological basis of the RSN.
Our results suggest the potential utility of these functional measures to track recovery following decompressive craniectomy procedures; the procedure has become an increasingly frequent part of the neurocritical care of patients with severe brain injury and has wide application in the clinical management of increased intracranial pressure arising in the setting of different types of brain insult (31). Such physiological assessments of recovery during post-operative management after craniectomies are likely to be particularly important for patients with severe brain injuries or the elderly who may recover slowly from their injuries (36).
Acknowledgments
Design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript were supported by the National Institutes of Health NIH-NICHD, the James S. McDonnell Foundation (N.D.S.), the Institute for Biomedical Imaging Sciences (IBIS), and the Fleming award from Weill Cornell Medical College (H.U.V). We acknowledge data analysis support from Lauren Rissman.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Henning U. Voss, Email: hev2006@med.cornell.edu, Department of Radiology and Citigroup Biomedical Imaging Center, Weill Cornell Medical College, 516 E 72nd Street, New York, NY 10021, Tel. 001-212 746-5216 (office, msg. box), 6-5702 (lab), Fax. 001-212 746-6681.
Linda A. Heier, Email: laheier@med.cornell.edu, Department of Radiology, Weill Cornell Medical College, 1300 York Avenue, New York, NY 10065.
Nicholas D. Schiff, Email: nds2001@med.cornell.edu, Department of Neurology and Neuroscience, Weill Cornell Medical College, 1300 York Avenue, New York, NY 10065.
Bibliography
- 1.Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004 Sept;53(3):288–292. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 2.Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. The influence of cranioplasty on postural blood flow regulation, cerebrovascular reserve capacity, and cerebral glucose metabolism. Neurosurg Focus. 2000;8(1):e9. doi: 10.3171/foc.2000.8.1.1920. [DOI] [PubMed] [Google Scholar]
- 3.Sakamoto S, Eguchi K, Kiura Y, Arita K, Kurisu K. CT perfusion imaging in the syndrome of the sinking skin flap before and after cranioplasty. Clin Neurol Neurosurg. 2006 Sept;108(6):583–585. doi: 10.1016/j.clineuro.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Lammi MH, Smith VH, Tate RL, Taylor CM. The minimally conscious state and recovery potential: a follow-up study 2 to 5 years after traumatic brain injury. Arch Phys Med Rehabil. 2005 Apr;86(4):746–754. doi: 10.1016/j.apmr.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 5.Voss HU, Ulug AM, Dyke JP, Watts R, Kobylarz EJ, McCandliss BD, Heier LA, Beattie BJ, Hamacher KA, Vallabhajosula S, Goldsmith SJ, Ballon D, Giacino JT, Schiff ND. Possible axonal regrowth in late recovery from the minimally conscious state. Journal of Clinical Investigation. 2006;116(7):2005–2011. doi: 10.1172/JCI27021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owen AM, Coleman MR, Boly M, Davis MH, Laureys S, Pickard JD. Detecting awareness in the vegetative state. Science. 2006 Sept;313(5792):1402. doi: 10.1126/science.1130197. [DOI] [PubMed] [Google Scholar]
- 7.Laureys S, Giacino JT, Schiff ND, Schabus M, Owen AM. How should functional imaging of patients with disorders of consciousness contribute to their clinical rehabilitation needs? Current Opinion in Neurology. 2006 Dec;19(6):520–527. doi: 10.1097/WCO.0b013e3280106ba9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schiff ND. Measurements and models of cerebral function in the severely injured brain. Journal of Neurotrauma. 2006 Oct;23(10):1436–1449. doi: 10.1089/neu.2006.23.1436. [DOI] [PubMed] [Google Scholar]
- 9.Formisano E, Esposito F, Kriegeskorte N, Tedeschi G, Di Salle F, Goebel R. Spatial independent component analysis of functional magnetic resonance imaging time-series: characterization of the cortical components. Neurocomputing. 2002 Dec;49:241–254. [Google Scholar]
- 10.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 11.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005 July;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America. 2001 Jan;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voss HU, Schiff ND. MRI of neuronal network structure, function, and plasticity. Prog Brain Res. 2009;175:483–496. doi: 10.1016/S0079-6123(09)17532-5. [DOI] [PubMed] [Google Scholar]
- 14.Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008 May;41(1):100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 15.Horovitz SG, Braun AR, Carr WS, Picchioni D, Balkin TJ, Fukunaga M, Duyn JH. Decoupling of the brain’s default mode network during deep sleep. Proc Natl Acad Sci U S A. 2009 July;106(27):11376–11381. doi: 10.1073/pnas.0901435106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. Electrophysiological signatures of resting state networks in the human brain. Proceedings of the National Academy of Sciences of the United States of America. 2007 Aug;104(32):13170–13175. doi: 10.1073/pnas.0700668104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006 Feb;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Goldman RI, Stern JM, Engel J, Cohen MS. Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport. 2002 Dec;13(18):2487–2492. doi: 10.1097/01.wnr.0000047685.08940.d0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences of the United States of America. 2003 Sept;100(19):11053–11058. doi: 10.1073/pnas.1831638100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proceedings of the National Academy of Sciences of the United States of America. 2007 Nov;104(46):18265–18269. doi: 10.1073/pnas.0705791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deco G, Jirsa V, McIntosh AR, Sporns O, Kotter R. Key role of coupling, delay, and noise in resting brain fluctuations. Proc Natl Acad Sci U S A. 2009 June;106(25):10302–10307. doi: 10.1073/pnas.0901831106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale-Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004 Dec;85(12):2020–2029. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 23.Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007 May;447(7140):83-U4. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- 24.Boly M, Phillips C, Tshibanda L, Vanhaudenhuyse A, Schabus M, ng-Vu TT, Moonen G, Hustinx R, Maquet P, Laureys S. Intrinsic brain activity in altered states of consciousness: how conscious is the default mode of brain function? Ann N Y Acad Sci. 2008;1129:119–129. doi: 10.1196/annals.1417.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horovitz SG, Fukunaga M, de Zwart JA, van GP, Fulton SC, Balkin TJ, Duyn JH. Low frequency BOLD fluctuations during resting wakefulness and light sleep: a simultaneous EEG-fMRI study. Hum Brain Mapp. 2008 June;29(6):671–682. doi: 10.1002/hbm.20428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman MR, Menon DK, Fryer TD, Pickard JD. Neurometabolic coupling in the vegetative and minimally conscious states: preliminary findings. Journal of Neurology Neurosurgery and Psychiatry. 2005 Mar;76(3):432–434. doi: 10.1136/jnnp.2004.045930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davey MP, Victor JD, Schiff ND. Power spectra and coherence in the EEG of a vegetative patient with severe asymmetric brain damage. Clin Neurophysiol. 2000 Nov;111(11):1949–1954. doi: 10.1016/s1388-2457(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 28.Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996 Nov;274(5288):771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- 29.Martuzzi R, Ramani R, Qiu M, Rajeevan N, Constable RT. Functional connectivity and alterations in baseline brain state in humans. Neuroimage. 2009 July; doi: 10.1016/j.neuroimage.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang D, Snyder AZ, Shimony JS, Fox MD, Raichle ME. Noninvasive Functional and Structural Connectivity Mapping of the Human Thalamocortical System. Cereb Cortex. 2009 Sept; doi: 10.1093/cercor/bhp182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akins PT, Guppy KH. Sinking skin flaps, paradoxical herniation, and external brain tamponade: a review of decompressive craniectomy management. Neurocrit Care. 2008;9(2):269–276. doi: 10.1007/s12028-007-9033-z. [DOI] [PubMed] [Google Scholar]
- 32.Oyelese AA, Steinberg GK, Huhn SL, Wijman CA. Paradoxical cerebral herniation secondary to lumbar puncture after decompressive craniectomy for a large space-occupying hemispheric stroke: case report. Neurosurgery. 2005 Sept;57(3):E594. doi: 10.1227/01.neu.0000170437.79760.df. [DOI] [PubMed] [Google Scholar]
- 33.Fields JD, Lansberg MG, Skirboll SL, Kurien PA, Wijman CA. “Paradoxical” transtentorial herniation due to CSF drainage in the presence of a hemicraniectomy. Neurology. 2006 Oct;67(8):1513–1514. doi: 10.1212/01.wnl.0000242889.02957.b6. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki N, Suzuki S, Iwabuchi T. Neurological improvement after cranioplasty. Analysis by dynamic CT scan. Acta Neurochir (Wien ) 1993;122(1-2):49–53. doi: 10.1007/BF01446986. [DOI] [PubMed] [Google Scholar]
- 35.Maekawa M, Awaya S, Teramoto A. Cerebral blood flow (CBF) before and after cranioplasty performed during the chronic stage after decompressive craniectomy evaluated by xenon-enhanced computerized tomography (Xe-CT) CBF scanning. No Shinkei Geka. 1999 Aug;27(8):717–722. [PubMed] [Google Scholar]
- 36.Crossley M, Shiel A, Wilson B, Coleman MR, Gelling L, Fryer T, Boniface S, Pickard J. Monitoring emergence from coma following severe brain injury in an octogenarian using behavioural indicators, electrophysiological measures and metabolic studies: a demonstration of the potential for good recovery in older adults. Brain Inj. 2005 Aug;19(9):729–737. doi: 10.1080/02699050400013733. [DOI] [PubMed] [Google Scholar]