Abstract
The public health burden of Alzheimer disease (AD), the most common neurodegenerative disease, threatens to explode in the middle of this century. Current FDA-approved AD treatments (e.g. cholinesterase inhibitors, NMDA-receptor agonists) do not provide a “cure”, but rather a transient alleviation of symptoms for some individuals. Other available therapies are few and of limited effectiveness so additional avenues are needed. Sphingolipid metabolism is a dynamic process that modulates the formation of a number of bioactive metabolites, or second messengers critical in cellular signaling and apoptosis. In brain, the proper balance of sphingolipids is essential for normal neuronal function, as evidenced by a number of severe brain disorders that are the result of deficiencies in enzymes that control sphingolipid metabolism. Laboratory and animals studies suggest both direct and indirect mechanisms by which sphingolipids contribute to amyloid-beta production and Alzheimer pathogenesis but few studies have translated these findings to humans. Building on the laboratory and animal evidence demonstrating the importance of sphingolipid metabolism in AD, this review highlights relevant translational research incorporating and expanding basic findings to humans. A brief biological overview of sphingolipids (sphingomyelins, ceramides, and sulfatides) in AD is first described, followed by a review of human studies including post-mortem studies, clinical and epidemiological studies. Lastly, the potential role of peripheral ceramides in AD pathogenesis is discussed, as well as the possible use of sphingolipids as biomarkers for AD.
Keywords: Alzheimer, Sphingolipid, Ceramide, Sulfatide, Sphingomyelin, CSF, Plasma, Biomarker, Humans
Introduction
The public health burden of Alzheimer disease (AD), the most common neurodegenerative disease (Mayeux 2003), threatens to explode in the middle of this century. The longevity of the US population and other developed countries is increasing and the prevalence of AD, comprising 60–70% of all dementia cases, doubles every decade after age 65 (Jorm and Jolley 1998). Unless new discoveries are made in the prevention or treatment of AD, the number of cases in the US alone is estimated to increase threefold, to 13.2 million by the year 2050 (Hebert et al. 2003). Since AD dementia is a lengthy and costly condition, this will create a large burden on the health care system in terms of both costs and services. Societal costs are estimated to be over $100 billion/year in the US alone (Fillit 2000). These costs will continue to skyrocket as the prevalence of dementia increases. By 2040, an estimated 81 million people worldwide will have dementia (Ferri et al. 2005).
Current FDA-approved AD treatments (e.g. cholinesterase inhibitors, NMDA-receptor agonists) do not provide a “cure”, but rather a transient alleviation of symptoms for some individuals. Other available therapies are few and of limited effectiveness. While the development of AD pathology in the brain is poorly understood, it is widely believed that it takes years, or even decades (Snowdon et al. 1996), after its onset to develop the clinical symptoms of AD dementia. The current causal hypothesis, known as the amyloid hypothesis, postulates that the initial step in the disease is the misprocessing of amyloid precursor protein (APP) leading to production of neurotoxic oligo-meric amyloid species which, slowly over time, initiates a complex downstream cascade eventually leading to synaptic dysfunction, neuronal death, loss of neuronal systems, and symptoms in the prodromal phases (e.g., mild cognitive impairment or mild behavioral impairment), followed by the fully symptomatic stage of the disease, dementia (Lyketsos et al. 2008).
By the time individuals develop dementia, the brain damage may be too extensive to be reversed by interventions targeted at early points of the cascade, such as the misprocessing of amyloid. The best example of this point is a randomized trial report showing that despite a greatly reduced amyloid-beta plaque load in actively immunized AD patients treated with AN1792 (Elan Pharmaceuticals), there was no evidence of improved survival or of an improvement in the time to severe dementia in the treated versus the placebo group (Holmes et al. 2008). Since the vaccine appeared to remove the amyloid-plaques, one hypothesis is that a better outcome would have ensued if given at an earlier timepoint (i.e. Mild Cognitive Impairment (MCI) or earlier) to patients that had a high likelihood of progressing to AD dementia (Lyketsos et al. 2008). This and other recent results from pharmaceutical trials have raised questions about the likely long-term value of anti-amyloid therapies, especially if delivered at the fully symptomatic phase of the disease (dementia). This has generated much interest in the pre-symptomatic phases of Alzheimer’s, and the development of biological markers to detect disease signatures at this stage. Additionally, interest has turned to non-amyloid aspects of the disease cascade since non-amyloid treatments may be necessary to reverse or slow disease progression.
The role of lipids in AD was first highlighted by the initial findings of Corder et al. (1993) and Strittmatter et al. (1993), and numerous validation studies, showing that the E4 allele of the APOE gene is the strongest known genetic risk factor for late-onset sporadic AD. ApoE, one of the primary apolipoproteins in the central nervous system (CNS; Merched et al. 1997; DeMattos et al. 2001; Koch et al. 2001), mediates the transport of lipids (e.g. cholesterol, phospholipids and sulfatides) (Pitas et al. 1987; Mahley 1988; Lomnitski et al. 1999; Han et al. 2003a) and plays a key role in the maintenance and repair of neurons (Mahley 1988; Nathan et al. 1994; Weisgraber and Mahley 1996). While lipids play an important role in the structure of neuronal cell membranes, directly affecting the solubility and fluidity of the membrane (reviewed by Mielke and Lyketsos 2006), lipids are also critical for proper cellular functioning. Alterations in sphingolipids, in particular, can lead to a variety of neuropsychiatric diseases.
Sphingolipid metabolism is a dynamic process that modulates the formation of a number of bioactive metabolites and second messengers critical in cellular signaling and apoptosis. In brain, the proper balance of sphingolipids is essential for normal neuronal function, as evidenced by a number of severe brain disorders that are the result of deficiencies in enzymes that control sphingolipid metabolism. For example, Niemann Pick disease (type I) involves a deficiency in sphingomyelinase (an enzyme that catalyzes the hydrolysis of sphingomyelin), and Tay-Sacks disease results from deficiency in hexosaminidase (involved in the hydrolysis of certain sphingolipids). While these severe disorders are the result of gross disruptions in sphingolipid metabolism, recent discoveries from a number of laboratories suggest that more subtle changes in sphingolipid balance may be intimately involved in neurodegenerative diseases including AD, Amyotrophic Lateral Sclerosis, Parkinson’s, and HIV-dementia (France-Lanord et al. 1997; Cutler et al. 2002, 2004; Haughey et al. 2004).
Building on the laboratory and animal evidence demonstrating the importance of sphingolipid metabolism in AD (reviewed by Mattson in this issue), the aim of this review is to highlight the relevant translational research incorporating and expanding basic findings to humans. The role of glycosphingolipids and gangliosides in the pathogenesis of AD has recently been reviewed extensively (see Yanagisawa 2007; Ariga et al. 2008) and will not be discussed here. Instead we highlight sphingomyelins, ceramides, and sulfatides.
Laboratory and Animal Studies Link Sphingolipid Metabolism to AD Warranting Translation to Humans
Both laboratory and animal studies suggest that perturbations in sphingomyelins (SM), ceramides, and sulfatides contribute to the pathophysiology of AD. Multiple studies suggest both direct and indirect connections between ceramides and Aβ(1–42), the hallmark AD pathology (Gulbins and Kolesnick 2003; Puglielli et al. 2003; Cutler et al. 2004; Jana and Pahan 2004; Lee et al. 2004; Grimm et al. 2005; Kalvodova et al. 2005; Mattson et al. 2005 and reviewed in this issue by Mattson). SM strongly affects membrane dynamics and is a critical component of lipid membrane rafts. The processing of APP by gamma-secretase, which is involved in the initial steps of AD according to the amyloid hypothesis, has specifically been associated with these lipid rafts (Hur et al. 2008). Sulfatides have been hypothesized to contribute to AD by increasing axonal damage through an apoE-dependent mechanism (Han 2005) or through their role as anti-inflammatory agents (Marinier et al. 1997). While ongoing animal and laboratory research is clearly needed to better understand the exact mechanisms by which these sphingolipids contribute to AD pathogenesis, the current evidence warrants translation of this research to humans. This is especially important because animal models of AD and corresponding therapeutic approaches have yet to demonstrate successful translation to the treatment of AD in humans as hoped.
The great majority of human studies examining sphingolipid metabolism in AD have focused on post-mortem analysis of lipid levels in specific brain regions. This is an important first step in describing perturbations of sphingolipid levels in brain regions by disease severity and has led to more recent studies, reviewed below, examining sphingolipid levels in CSF and blood.
Post-Mortem Studies of Sphingolipids in AD
Multiple post-mortem human studies have examined sphingolipids levels in AD. However, comparability is an issue as the several studies have examined different brain regions, white or grey matter (or combinations), as well as different compounds or enzymes within the sphingolipid pathway. These lipids have also been examined at different dementia stages, as defined by the Clinical Dementia Rating (CDR; Hughes et al. 1982) such that CDR = 0.5 is very mild dementia; CDR = 1 is mild dementia; CDR = 2 is moderate dementia; and CDR = 3 is severe dementia). Table 1 summarizes the study methodology, sample size and results of the published studies.
Table 1.
Post-mortem human studies examining the association between brain sphingolipids and Alzheimer’s disease
| Author | Brain regions and sample size | Findings |
|---|---|---|
| Pettegrew et al. (2001) | Grey matter from MFG, MTG, inferior parietal lobule, occipital cortex, and cerebellar cortex: 45 AD, 11 NC. | ↑ SM in AD vs. NC when all brain regions were combined or individually in cerebellum and inferior parietal cortex but not in Occipital, Superior/Middle Frontal or Superior Temporal Cortices. |
| Levels of SM positively correlated with the number of AB plaques, but not with neurofibrillary tangles. | ||
| Han et al. (2002) | Grey and white matter from MFG, MTG, Inferior parietal lobe, CA1 portion of hippocampus and subiculum, entorhinalcortex, cerebellum: 5 CDR = 0, 3 CDR = 0.5, 4 CDR = 1, 6 CDR = 2, 4 CDR = 3. | ↓ Sulfatide in grey and white matter of frontal, temporal and parietal lobes, and cerebellum, in CDR = 0.5–3 vs. CDR = 0. Sulfatide mass did not increase with increasing AD severity from CDR = 0.5 to CDR = 3. |
| ↑ Ceramide in both grey and white matter of all brain regions in CDR = 0.5 to CDR = 3 vs. CDR = 0 brains. | ||
| Ceramide mass of CDR = 2 or 3 ↓ compared to CDR = 0.5 or 1 but still higher than CDR = 0. | ||
| No differences in SM or galctosylceramide (GalC) mass or GalC sulfotransferase activity by disease severity. | ||
| Cutler et al. (2004) | Whole tissue from MFG and cerebellum: 7 AD, 7 NC. | ↑ Ceramide C24:0 & Galactoceramide C24:0 and ↓ SM C24:0 in MFG of AD vs. NC. No differences in the cerebellum. |
| Membrane raft preparations of Frontal Cortex: 2 NC, 5 mild AD,8 moderate AD, 12 severe AD. | ↑ Ceramide C18:0 & C24:0 from AD vs. NC. Both ceramides ↑ with disease severity. No differences in Galactocylceramide, sulfatide or SM. | |
| Huang et al. (2004) | Grey matter from frontotemporal areas of 10 AD and 10 NC. | ↑ Acid ceramidase (AC) in AD vs. NC. |
| Acid ceramidase colocalized in cell bodies of neurons with neurofibrillary tanges but not AB plaques. | ||
| Katsel et al. (2007) | Brain tissue from frontal, parietal, temporal and occipital areas; cingulate, caudate, hippocampus & putamen: 19 CDR = 0, 16 CDR = 0.5, 18 CDR = 1, 16 CDR = 2, 20 CDR = 0, 28 CDR = 4–5 | Temporal and fronal cortices had greatest number of transcripts with altered expression. |
| Several genes within sphingolipid pathway up- or down- regulated at specific disease stages vs. CDR = 0. | ||
| Enzymes controlling long-chain ceramide (C22:0, C24:0) synthesis upregulated early in disease process (CDR = 0.5) and glucosylceramide downregulated. | ||
| No alterations in gene expression for enzymes that control SM and glycosphingolipid turnover into ceramides. | ||
| He et al. (2010) | Grey matter from frontotemporal area: 9 AD, 6 NC. | ↑ Membrane, not cytosolic, acid sphingomyelinase (ASM) and acid ceramidase (AC) activity in AD vs. NC. |
| Divided samples into soluble (cytosolic) and membrane fractions. | No difference in neurtral sphingomyelinase (NSM) activity. | |
| ↑ Ceramide and sphingosine and ↓ SM and sphingosine-1- phosphate (S1P) levels in soluble (cytosolic) fractions. | ||
| Postive correlation between ASM activity and AB and phosphorylated tau protein (PHF-1). | ||
| Negative correlation between S1P levels and AB and phosphorylated tau protein (PHF-1). | ||
| Bandaru et al. (2009) | Grey and white matter from middle frontal gyrus (MFG), middle temporal gyrus (MTG), and cerebellum. 30 AD (15 APOE E4 & 15 APOE E3) 26 NC (6 APOE E4 & 20 APOE E3). | AD vs. NC comparison: ↑ SM C16:0, C18:0, C22:0 & C24:0, Ceramide C18:0, C24:0 and steraoyl in grey matter; ↓ Ceramide C16:0, C22:0, C24:1, steraoyl and sulfatide in white matter of MFG. No differences in MTG. |
| APOE E4 vs. E3: Among AD, E4 carriers had ↓ SM C22:0 and C24:0 and ↑ Ceramide C18:0, C24:1, and sulfatide in grey matter and ↑ Ceramide C22:0 in white matter of the MFG. No differences in the MTG or cerebellum by E4 status. Lipid levels in NC did not vary by APOE E4 allele status in any brain region. |
Ceramides
The majority of post-mortem studies have reported elevated ceramide levels in both grey and white matter brain regions of AD brain, even at the earliest clinically recognizable stage of AD (CDR of 0.5 at the time of death) relative to normal controls (Han et al. 2002; Cutler et al. 2004; Katsel et al. 2007; He et al. 2010; Bandaru et al. 2009). However, one study reported lower ceramide levels in the white matter of the middle frontal gyrus of AD patients compared to controls (Bandaru et al. 2009). This conflicting finding may be related to the stage of dementia examined as one study reported elevated ceramide levels across brain regions in patients with early AD (CDR 0.5 and 1) and lower levels in patients with more severe AD (CDR of 2 and 3) (Han et al. 2002). In a methodologically unique study examining the sphingolipid pathway in AD, Katsel et al. (2007) examined whether the expression of genes that control ceramide synthesis, metabolism and degradation were altered during the clinical and neuropathological progression of AD compared to what occurs during normal aging. Several genes within the sphingolipid pathway were significantly up- or down-regulated at specific disease severities compared to controls. The majority of altered gene expression occurred in temporal and frontal cortices, brain areas affected early in AD. The strongest findings were that enzymes controlling ceramide synthesis, particularly the long-chain ceramides (C22:0 and C24:0), were upregulated early in the disease process, while glucosylceramide was downregulated. These results suggest a shift in sphingolipid metabolism towards the accumulation of ceramides at the earliest stages of AD (CDR 0.5) and are consistent with the other studies described above.
Consistent with elevations in ceramide levels, acid sphingomyelinase (ASM) activity, which metabolizes SM into ceramides, has also been found to be increased in the frontotemporal grey matter of AD cases compared to controls (He et al. 2010). Importantly, a positive correlation was found between ASM activity and amyloid-beta or phosphorylated tau in this same region, further suggesting high ceramide levels are associated with AD pathology.
Sphingomyelins (SM)
While post-mortem AD studies of ceramides are consistent, studies of SM levels are less clear. SM levels have been found to be increased (Pettegrew et al. 2001; Bandaru et al. 2009) or decreased (Cutler et al. 2004; He et al. 2010) in AD vs. normal brains. One study reported that SM levels positively correlated with number of amyloid beta plaques, but not neurofibrillary tangles (Pettegrew et al. 2001). There are no clear differences between studies with regard to brain regions examined, or use of grey or white matter, so the reasons for these discrepancies are not understood. The only study to examine SM across disease severity found no change in SM levels versus controls or by disease severity (Han et al. 2002).
Sphingosine and Sphingosine-1-phosphate
Sphingosine, a pro-apoptotic lipid and metabolite of ceramide, has been found to be elevated in AD brains compared to normal controls (He et al. 2010). Two studies also reported that the enzyme activity of acid ceramidase, which converts ceramide to sphingosine, was elevated in AD brains versus normal controls (Huang et al. 2004; He et al. 2010). No studies have yet examined sphingosine or acid ceramidase in MCI or early AD to determine whether they are also perturbed early in the disease pathogenesis.
While ceramide and sphingosine are “pro-apoptotic,” S1P has been implicated in cellular proliferation and survival and therefore would be hypothesized to be reduced in AD. The only study that examined S1P found decreased levels in soluble (cytosolic) fractions of grey matter from the frontotemporal areas of AD cases versus controls (He et al. 2010). S1P levels were also negatively correlated with amyloid beta and phosphorylated tau protein levels in the frontotemporal area. Given the antagonistic effects between ceramide and S1P, future studies should examine the ratio of these lipids as potential indicators or predictors of ongoing neurodegeneration or AD progression.
Sulfatides
Compared to cognitively-normal controls, sulfatide levels were significantly decreased by up to 92% in the gray matter of all examined brain regions in persons with a Clinical Dementia Rating (CDR) of 0.5 (Han et al. 2002). Similarly, in the white matter of all brain regions, sulfatide levels were also substantially depleted (35–51%) in persons with a CDR of 0.5. There was no further decrease in sulfatide mass with AD disease severity (a CDR of 1 to a CDR of 3). These findings suggest that this lipid is altered in the earliest stages of AD, possibly during the preclinical stage of the disease. Since sulfatides did not further decrease with increasing AD severity, these findings imply that sulfatide content is permanently altered in early AD and maintained at a lower level relative to normal controls. The authors hypothesized that this altered state may be in response to amyloid plaques since the number of plaques have also been reported to not dramatically increase in number in AD brains after a CDR of 0.5 (Berg et al. 1998). This reduced sulfatide finding in AD was partially supported by Bandaru et al. (2009) who found decreased sulfatide content in white matter, but not grey matter, of the middle frontal gyrus. Sulfatide levels were not significantly altered in the middle temporal gyrus. Further, among AD cases, but not controls, sulfatide levels were higher in the middle frontal gyrus in APOE E4 allele carriers compared to non-carries, suggesting the APOE E4 allele may only be associated with sulfatide dysfunction among those with an underlying neurological disorder.
Lastly, one study reported that the sulfatide precursor, galactosylceramide, was increased in the middle frontal gyrus of AD patients compared to controls or the cerebellum of AD patients. Upon further examination of the frontal cortex lipid raft preparation isolated from AD and controls (Cutler et al. 2004), there were no differences in galactosylceramide or sulfatide concentrations. This finding highlights the importance of the exact brain region examined as well as whether the lipid is measured in grey or white matter, or more specifically in lipid rafts.
CSF Studies of Sphingolipids in AD
While post-mortem studies are important to examine lipid levels in autopsied-confirmed cases, in vivo studies are the only way to ascertain the role of these lipids in early AD pathogenesis and whether these lipids may be indicators or predictors of disease progression. Only two studies have examined CSF levels of sphingolipids. One examined CSF sulfatide and the sulfatide/phosphatidyinosital (PI) ratio in normal controls and patients with a CDR of 0.5 characterized as having “incipient dementia of the Alzheimer type” (Han et al. 2003b; see Table 2). Compared to controls (CDR = 0), patients with a CDR of 0.5 had a forty percent reduction in CSF sulfatide levels. There was no difference in PI levels. However, the sulfatide/PI ratio was the best at differentiating between a CDR of 0 and a CDR of 0.5. A cutoff between 0.68 and 0.72 in the sulfatide/PI ratio resulted in a sensitivity of 90% and specificity of 100%. While the sensitivity and specificity of CSF amyloid-beta (1–42), tau, and p-tau231 in these same samples were not given, the authors did find greater individual overlap between groups for each of these AD markers compared to sulfatides, suggesting that sulfatides may be a better diagnostic marker.
Table 2.
Studies examining the association between CSF sphingolipids and Alzheimer’s disease
| Author | Sample size | Findings |
|---|---|---|
| Han et al. (2003) | 19 with CDR = 0; 20 with CDR = 0.5 | 40% ↓ in CSF sulfatide for CDR 0.5 vs. CDR 0.0. No difference in phosphatidylinosital (PI). |
| Using sulfatide/PI ratio, 90% sensitivy & 100% specificy. | ||
| Satoi et al. (2005) | 16 AD, 14 NC, 13 ALS. Among AD: 6 had a CDR = 0.5–1 (mild); 4 had a CDR = 2 (moderate); and 5 had a CDR = 3 (severe) | ↑ Mean ceramide in AD vs. NC (P < 0.01). ALS was elevated but not sig different from NC. Among AD, CDR = 2 had ↑ ceramide vs. mild (CDR = 0.5–1) or severe (CDR = 3) dementia. |
NC Normal control, AD Alzheimer’s disease, ALS Amyotrophic Lateral Sclerosis, CDR Clinical Dementia Rating Scale
The only other CSF study published to date compared ceramide levels between AD and amyotrophic lateral sclerosis (ALS) patients, and controls with other neurological diseases (cervical spondylosis, tension-type headache, metabolic encephalopathy, or infarct) (Satoi et al. 2005; Table 2). Compared to neurologic controls, AD patients had significantly higher CSF ceramide levels. While the ALS group also had elevated levels compared to controls, this elevation did not reach statistical significance. To determine how ceramides varied by AD severity, the AD group was stratified into mild (CDR 0.5–1), moderate (CDR 2) and severe (CDR 3) AD. Ceramides levels were significantly higher in the moderate (CDR 2) group compared to either the mild or severe AD groups, suggesting that CSF ceramide levels do not continue to increase with severity but rather vary by disease severity in an inverted u-shape. Notably, neither of these studies examined the correlation between CSF sulfatides or ceramides and CSF amyloid-beta or tau levels. The examination of the relationship between CSF sphingolipids, amyloid-beta, tau, and phospho-tau at different AD stages is critical to help elucidate the role of these sphingolipids in the pathogenesis of AD.
Peripheral Studies of Sphingolipids in AD
Blood-based biomarkers for AD have been difficult to develop and often criticized because it is difficult to ascertain direct links between peripheral markers and brain processes. Despite this challenge, a blood-based biomarker would be a giant leap forward as it would meet expert criteria of being “non-invasive, simple to perform, and inexpensive” (Growdon et al. 1998), and be superior to more invasive and costly CSF or brain imaging markers. Moreover, a blood-based biomarker would be a routine tool that could be repeated over time and easily be incorporated into patient care at the general practitioner level or developing counties. While gangliosides have been examined in fibroblasts of AD patients (Pitto et al. 2005), the examination of peripheral ceramide levels has been lacking. We first measured serum ceramides, and other sphingolipids, among 100 women in the Women’s Health and Aging Study (WHAS) II followed up to six times over 9 years to assess cognitive outcomes (Mielke et al. 2010a). Cross-sectionally, lower levels of serum ceramides and SM were associated with memory impairment, but longitudinally higher ceramide levels predicted memory impairment over the follow-up. In fact, none of the participants with blood levels of ceramide C22:0 in the lowest tertile, and only one participant with blood levels of ceramides C16:0 and C24:0 in the lowest tertile, developed memory impairment over 9-years of follow-up. We have replicated the cross-sectional findings in a well-characterized clinical sample (Mielke et al. 2010b). Participants with amnestic MCI cases had significantly lower plasma ceramide C22:0 and C24:0 levels compared to both NC and AD. Together, these results suggest that blood ceramides vary by disease state with high levels several years prior to onset of memory impairment and low levels in the early stages of memory impairment. Thus, blood ceramides could be used as a biomarker or indicator of AD progression. Ongoing research is pursuing this avenue.
Are Peripheral Ceramides Part of the Causal Chain for AD?
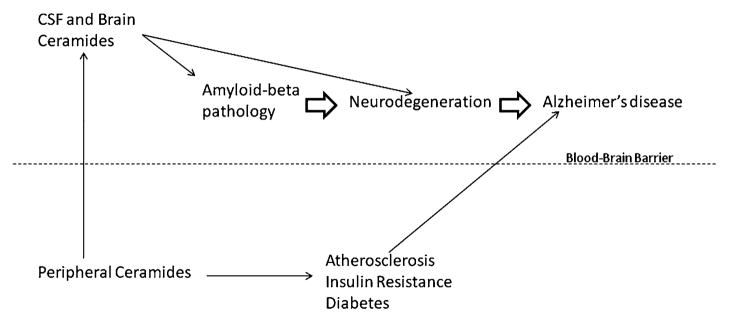
As mentioned above, it is difficult to interpret the association between blood-based measures and brain processes. There could be a direct correlation or, due to the blood brain barrier, blood measures may be indirectly related to brain processes, for example through vascular pathways (see Fig. 1 describing how blood ceramides may be related to AD). It is intriguing that ceramide levels are elevated in brain tissue and CSF of early AD, but appear to be decreased in blood levels. An explanation for these findings was recently provided in a study of how plasma and CSF ceramides in HIV infected subjects was associated with cognitive function. In this study it was found that plasma ceramides showed an inverse relationship to CSF ceramides (Norman Haughey, personal communication). Thus, the lower peripheral ceramide levels that have been observed cross-sectionally in the cognitively impaired and clinically-diagnosed MCI groups described above (Mielke et al. 2010a, b) may be indicative of higher brain tissue and CSF ceramide levels. Ongoing research will further examine the plasma-CSF ceramide correlation in a group of normal control, MCI and AD patients to further understand the utility of plasma ceramides as a biomarker of AD progression.
Fig. 1.
Peripheral ceramides may be associated with Alzheimer’s disease through either direct or indirect mechanisms. Direct mechanism: Peripheral ceramides reflect CSF and brain ceramide levels, both of which have been associated with amyloid-beta levels and neurodegeneration in animal and laboratory studies. Indirect mechanism: Peripheral ceramides have been found to be associated with atherosclerosis, insulin resistance and diabetes, all of which are known risk factors for AD
A key aspect of determining the role of blood ceramides in AD pathophysiology will also be to determine the relationship between blood and CSF ceramides and CSF amyloid-beta and tau, the hallmark AD pathology. If there is a strong correlation between blood and CSF ceramide levels, as well as blood ceramides and CSF amyloid-beta or tau, blood ceramides can be utilized as a direct measure of brain ceramides and would be a good blood-based candidate biomarker. However, if a direct correlation is not found, this does not mean that blood ceramides will be useless as biomarkers or risk factors for AD. Despite the dearth of research directly relating SM and ceramides to cognitive impairment or extending the outcome to AD, epidemiological studies have examined blood levels of these lipids in relation to vascular outcomes, which are well-known to increase the risk of AD. High levels of plasma SM and ceramides have been correlated with subclinical atherosclerosis (Ichi et al. 2006; Nelson et al. 2006). Clinical studies also suggest the importance of these lipids in insulin resistance and diabetes (see recent review by Holland and Summers 2008). All of these vascular outcomes are known to increase the risk of AD (Hofman et al. 1997; Ott et al. 1999; Xu et al. 2004), and also affect rate of disease progression after an AD diagnosis (Mielke et al. 2007). Thus, even if future research does not find that peripheral and brain ceramide levels directly correlate, this research will be important to further establish the identification of other mechanisms, such as vascular pathways, by which ceramide can increase risk of AD.
Can Spingolipids be Utilized as Biomarkers of AD?
The criterion for a biomarker, defined by Frank and Hargreaves (2003), is “A characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention (an indicator of disease status, both clinical and sub-clinical).” There has been a recent push to identify “biomarkers” for AD, using blood, CSF and neuroimaging measures. Such biomarkers could be utilized for different means, including for differential diagnosis or for early pre-symptomatic diagnosis, to predict cognitive progression for the purposes of trial enrichment, to predict drug response, or to serve as surrogate markers in trials. One biomarker will not fit all categories so several are needed. As an objective marker of disease processes, the utility of a biomarker can only be determined after understanding of the proposed biologic pathways. Based on the current human studies examining sphingolipids in AD, we next highlight possible uses of sphingolipids as biomarkers.
Utility of Sphingolipids as a Diagnostic Biomarker for AD
The value of specific lipids, or enzymes, involved in the sphingolipid pathway as potential biomarkers for AD depends on the type of biomarker under consideration and the biomarker medium. As previously proposed in a Consensus Report of the Working Group on Biological Markers of Alzheimer’s Disease (Growdon et al. 1998), an ideal diagnostic biomarker for AD “should detect a fundamental feature of neuropathology and be validated in neuropathologically-confirmed cases; it should have a diagnostic sensitivity >80% for detecting AD and a specificity of >80% for distinguishing other dementias; it should be reliable, reproducible, non-invasive, simple to perform, and inexpensive.” While the collection of CSF via lumbar puncture is invasive, it is a useful indicator, albeit indirect, of brain metabolism. CSF amyloid-beta and tau are potential diagnostic markers with moderate to high sensitivity and specificity (Mattsson et al. 2009). The field is moving towards the acceptance of these markers in the diagnosis of AD brain disease in MCI or early dementia (Dubois et al. 2007). In this light, the finding that elevated CSF sulfatides in AD participants with a CDR of 0.5 compared to normal controls (CDR 0.0), and appeared to be better than amyloid-beta(1–42), tau, and p-tau231, is notable and suggests that sulfatides might be better diagnostic biomarkers (Han et al. 2003b). Additional research comparing the CSF sulfatide/PI ratio to these AD pathologic markers is clearly needed, as well as assessing whether the addition of sulfatides to these CSF markers produces a better diagnostic indicator than either alone. The assessment of CSF ceramides as a diagnostic marker is also warranted.
One potential limitation in sphingolipids as a diagnostic marker for AD may be in distinguishing AD from other dementias or neurological conditions. CSF ceramide levels did not significantly differ between AD and ALS patients in the study conducted by (Satoi et al. 2005) and CSF sphingolipid levels were found to be elevated in HIV-dementia patients (Haughey et al. 2004). Thus, in order to determine whether CSF sphingolipids are a diagnostic marker for AD, comparison to other dementias and neurological conditions is needed. However, it is important to point out that even if CSF sphingolipids are not found to be specific diagnostic markers of AD this would not obviate the utility of these measures as markers of neuronal injury or neurodegeneration. If CSF sphingolipids are altered across neurodegenerative conditions, it is possible they could be a marker of disease progression for several disease, as for example creatinine phosphokinase is a marker of myocardial injury from diverse etiologies, and therefore still useful to the study of mechanisms and therapeutics in neurodegenerative disease.
Utility of Sphingolipids as Other Biomarkers
To date, there are no established biomarkers of AD progression. Cognitive, affective and behavioral symptoms are imprecise indicators of disease progression in the brain, and only observed after substantial pathology has developed. There is a need to identify other indicators that can accurately assess the progression of ongoing pathological changes in a timely manner. It is possible that ceramides could be such a marker since levels appear to vary by disease severity, increasing in early stages and decreasing at more severe stages. However, longitudinal studies with serial measures are necessary to confirm whether ceramides vary by disease progression within individuals, determine how fast a person in progressing, and how far apart the assessment of the proposed markers is needed. Such studies are ongoing in our laboratory. A key aspect of this biomarker type is using a non-invasive medium that can be assessed multiple times over the course of a disease. We have therefore focused on sphingolipids in blood as repeatable collection of CSF, while possible in small subsets of individuals, is not feasible on a global scale.
While the development of AD pathology in the brain is poorly understood, it is widely believed that it takes years, or even decades (Snowdon et al. 1996) to develop the clinical symptoms of AD, MCI and dementia. One explanation for why current AD treatments do not “cure patients” is that by the time medications are given to patients, after a clinical diagnosis of AD, the pathology is too great to reverse. Identifying at an earlier timepoint (i.e., preclinical or at the mild cognitive impairment stage) who will progress to AD would be highly advantageous and likely more effective in halting the disease progression, as we have previously highlighted (Lyketsos et al. 2008). Thus, there is an important need to identify additional measures that more reliably predict which MCI cases will progress to dementia, or who among cognitive normal individuals will develop MCI or AD dementia. The finding that blood ceramides predicted onset of memory impairment in cognitively normal individuals (Mielke et al. 2010a), especially ceramide C22:0, in which no one in the lowest tertile developed impairment over nine years of follow-up, warrants further research.
In addition to biomarkers of disease progression, sphingolipids may be important biomarkers for therapeutics. It is difficult to determine whether a particular treatment is therapeutic for a specific patient, especially when such a treatment is “disease modifying” as opposed to “symptomatic”. Therefore, it is critical to develop biomarkers that can be used to develop new treatments, particularly in preclinical and early clinical phases when the impact of disease modifying agents will be greatest. Such biomarkers might be used to predict cognitive progression for the purposes of trial enrichment, predict drug response, or serve as surrogate markers in trials. While laboratory and animal studies suggest both direct and indirect interactions between ceramides and amyloid-beta, there is little research translating this finding to humans. It will be important to establish this relationship in humans as it is possible that reducing ceramide levels may be a therapeutic opportunity for reducing amyloid-beta levels in AD.
Conclusions
Alzheimer’s disease (AD) is a growing public health problem with a global burden that is expected to exceed 80 million cases by 2040 (Ferri et al. 2005). Several pharmaceutical trials have examined anti-amyloid therapies, with little success to date, raising questions about the likely long-term value of anti-amyloid therapies (Holmes et al. 2008), especially if delivered at the fully symptomatic phase of the disease. This has generated much interest in the pre-symptomatic phases of Alzheimer’s, and the development of biological markers to detect disease signatures at this stage, as well as the need for new therapeutic targets. Laboratory and animal studies have implicated sphingolipids in the development of amyloid-beta plaques, as well as neurodegenerative process, both critical features of AD. While little research has translated these findings to human, existing evidence suggests that sphingolipids are altered at the earliest recognizable clinical phase (CDR 0.5) of AD in both CSF and plasma and may be useful diagnostic biomarkers or biomarkers of AD progression. Research on plasma sphingolipids is especially warranted since the majority of AD cases over the next century will occur in the developing world and a blood-based biomarker would be non-invasive, inexpensive, accepted, and more adaptable to rural or developing areas. Examination of the correlation between blood and CSF sphingolipids, as well as measurement of sphingolipid levels early in the disease process will be critical to further understanding the means by which sphingolipids may be used as a biomarker or therapeutic target for AD.
Acknowledgments
This research was supported by grants from the National Institute on Aging (R21AG028754 and P50AG005146), the National Institute of Neurological Disorders and Stroke (R21NS060271-01) and a grant from the George and Cynthia Mitchell Fund.
References
- Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer’s disease–a review. Journal of Lipid Research. 2008;49:1157–1175. doi: 10.1194/jlr.R800007-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandaru VV, Troncoso J, Wheeler D, Pletnikova O, Wang J, Conant K, et al. ApoE4 disrupts sterol and sphingolipid metabolism in Alzheimer’s but not normal brain. Neurobiology of Aging. 2009;30:591–599. doi: 10.1016/j.neurobiolaging.2007.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer’s disease: Relation of histologic markers to dementia severity, age, sex, and apolipo-protein E genotype. Archives of Neurology. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cutler RG, Kelly J, Storie K, Pedersen WA, Tammara A, Hatanpaa K, et al. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. PNAS. 2004;101:2070–2075. doi: 10.1073/pnas.0305799101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler RG, Pedersen WA, Camandola S, Rothstein JD, Mattson MP. Evidence that accumulation of ceramides and cholesterol esters mediates oxidative stress-induced death of motor neurons in amyotrophic lateral sclerosis. Annals of Neurology. 2002;52:448–457. doi: 10.1002/ana.10312. [DOI] [PubMed] [Google Scholar]
- DeMattos RB, Brendza RP, Heuser JE, Kierson M, Cirrito JR, Fryer J, et al. Purification and characterization of astrocyte-secreted apolipoprotein E and J-containing lipoproteins from wild-type and human apoE transgenic mice. Neurochemistry International. 2001;39:415–425. doi: 10.1016/s0197-0186(01)00049-3. [DOI] [PubMed] [Google Scholar]
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, et al. Global prevalence of dementia: A Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillit HM. The pharmacoeconomics of Alzheimer’s disease. The American Journal of Managed Care. 2000;6:S1139–S1144. [PubMed] [Google Scholar]
- France-Lanord V, Brugg B, Michel PP, Agid Y, Ruberg M. Mitochondrial free radical signal in ceramide-dependent apoptosis: A putative mechanism for neuronal death in Parkinson’s disease. Journal of Neurochemistry. 1997;69:1612–1621. doi: 10.1046/j.1471-4159.1997.69041612.x. [DOI] [PubMed] [Google Scholar]
- Frank R, Hargreaves R. Clinical biomarkers in drug discovery and development. Nature Reviews. 2003;2:566–580. doi: 10.1038/nrd1130. [DOI] [PubMed] [Google Scholar]
- Grimm MO, Grimm HS, Patzold AJ, Zinser EG, Halonen R, Duering M, et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nature Cell Biology. 2005;7:1118–1123. doi: 10.1038/ncb1313. [DOI] [PubMed] [Google Scholar]
- Growdon JH, Selkoe DJ, Roses A, et al. Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. Neurobiology of Aging. 1998;19:109–116. [PubMed] [Google Scholar]
- Gulbins E, Kolesnick R. Raft ceramide in molecular medicine. Oncogene. 2003;22:7070–7077. doi: 10.1038/sj.onc.1207146. [DOI] [PubMed] [Google Scholar]
- Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimer’s disease: Implication of the role of lipids in the pathogenesis of Alzheimer’s disease. Current Alzheimer Research. 2005;2:65–77. doi: 10.2174/1567205052772786. [DOI] [PubMed] [Google Scholar]
- Han X, Cheng H, Fryer JD, Fagan AM, Holtzman DM. Novel role for apolipoprotein E in the central nervous system. Modulation of sulfatide content. The Journal of Biological Chemistry. 2003a;278:8043–8051. doi: 10.1074/jbc.M212340200. [DOI] [PubMed] [Google Scholar]
- Han X, Fagan AM, Cheng H, Morris JC, Xiong C, Holtzman DM. Cerebrospinal fluid sulfatide is decreased in subjects with incipient dementia. Annals of Neurology. 2003b;54:115–119. doi: 10.1002/ana.10618. [DOI] [PubMed] [Google Scholar]
- Han X, Holtzman D, McKeel DW, Jr, Kelley J, Morris JC. Substantial sulfatide deficiency and ceramide elevation in very early Alzheimer’s disease: Potential role in disease pathogenesis. Journal of Neurochemistry. 2002;82:809–818. doi: 10.1046/j.1471-4159.2002.00997.x. [DOI] [PubMed] [Google Scholar]
- Haughey NJ, Cutler RG, Tamara A, McArthur JC, Vargas DL, Pardo CA, et al. Perturbation of sphingolipid metabolism and ceramide production in HIV-dementia. Annals of Neurology. 2004;55:257–267. doi: 10.1002/ana.10828. [DOI] [PubMed] [Google Scholar]
- He X, Huang Y, Li B, Gong CX, Schuchman EH. Deregulation of sphingolipid metabolism in Alzheimer’s disease. Neurobiology of Aging. 2010;31(3):398–408. doi: 10.1016/j.neurobiolaging.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: Prevalence estimates using the 2000 census. Archives of Neurology. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer’s disease in the Rotterdam Study. Lancet. 1997;349:151–154. doi: 10.1016/S0140-6736(96)09328-2. [DOI] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: New insights from in vivo manipulation of sphingolipid metabolism. Endocrine Reviews. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, et al. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- Huang Y, Tanimukai H, Liu F, Iqbal K, Grundke-Iqbal I, Gong CX. Elevation of the level and activity of acid ceramidase in Alzheimer’s disease brain. European Journal of Neuroscience. 2004;20:3489–3497. doi: 10.1111/j.1460-9568.2004.03852.x. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. British Journal of Psychiatry. 1982;140:266–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- Hur JY, Welander H, Behbahani H, Aoki M, Franberg J, Winblad B, et al. Active gamma-secretase is localized to detergent-resistant membranes in human brain. FEBS Journal. 2008;275:1174–1187. doi: 10.1111/j.1742-4658.2008.06278.x. [DOI] [PubMed] [Google Scholar]
- Ichi I, Nakahara K, Miyashita Y, Hidaka A, Kutsukake S, Inoue K, et al. Association of ceramides in human plasma with risk factors of atherosclerosis. Lipids. 2006;41:859–863. doi: 10.1007/s11745-006-5041-6. [DOI] [PubMed] [Google Scholar]
- Jana A, Pahan K. Fibrillar amyloid-beta peptides kill human primary neurons via NADPH oxidase-mediated activation of neutral sphingomyelinase. Implications for Alzheimer’s disease. Journal of Biological Chemistry. 2004;279:51451–51459. doi: 10.1074/jbc.M404635200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Jolley D. The incidence of dementia: A meta-analysis. Neurology. 1998;51:728–733. doi: 10.1212/wnl.51.3.728. [DOI] [PubMed] [Google Scholar]
- Kalvodova L, Kahya N, Schwille P, Ehehalt R, Verkade P, Drechsel D, et al. Lipids as modulators of proteolytic activity of BACE: Involvement of cholesterol, glycosphingolipids, and anionic phospholipids in vitro. The Journal of Biological Chemistry. 2005;280:36815–36823. doi: 10.1074/jbc.M504484200. [DOI] [PubMed] [Google Scholar]
- Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: A shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochemical Research. 2007;32:845–856. doi: 10.1007/s11064-007-9297-x. [DOI] [PubMed] [Google Scholar]
- Koch S, Donarski N, Goetze K, Kreckel M, Stuerenburg HJ, Buhmann C, et al. Characterization of four lipoprotein classes in human cerebrospinal fluid. Journal of Lipid Research. 2001;42:1143–1151. [PubMed] [Google Scholar]
- Lee JT, Xu J, Lee JM, Ku G, Han X, Yang DI, et al. Amyloid-beta peptide induces oligodendrocyte death by activating the neutral sphingomyelinase-ceramide pathway. Journal of Cell Biology. 2004;164:123–131. doi: 10.1083/jcb.200307017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomnitski L, Oron L, Sklan D, Michaelson DM. Distinct alterations in phospholipid metabolism in brains of apolipoprotein E-deficient mice. Journal of Neuroscience Research. 1999;58:586–592. [PubMed] [Google Scholar]
- Lyketsos CG, Szekely CA, Mielke MM, Rosenberg PB, Zandi PP. Developing new treatments for Alzheimer’s disease: The who, what, when, and how of biomarker-guided therapies. International psychogeriatrics/IPA. 2008;20:871–889. doi: 10.1017/S1041610208007382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science. 1988;240:622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Marinier A, Martel A, Banville J, Bachand C, Remillard R, Lapointe P, et al. Sulfated galactocerebrosides as potential antiinflammatory agents. Journal of Medicinal Chemistry. 1997;40:3234–3247. doi: 10.1021/jm9606960. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Cutler RG, Jo DG. Alzheimer peptides perturb lipid-regulating enzymes. Nature Cell Biology. 2005;7:1045–1047. doi: 10.1038/ncb1105-1045. [DOI] [PubMed] [Google Scholar]
- Mattsson N, Zetterberg H, Hansson O, Andreasen N, Parnetti L, Jonsson M, et al. CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA. 2009;302:385–393. doi: 10.1001/jama.2009.1064. [DOI] [PubMed] [Google Scholar]
- Mayeux R. Epidemiology of neurodegeneration. Annual Review of Neuroscience. 2003;26:81–104. doi: 10.1146/annurev.neuro.26.043002.094919. [DOI] [PubMed] [Google Scholar]
- Merched A, Blain H, Visvikis S, Herbeth B, Jeandel C, Siest G. Cerebrospinal fluid apolipoprotein E level is increased in late-onset Alzheimer’s disease. Journal of the Neurological Sciences. 1997;145:33–39. doi: 10.1016/s0022-510x(96)00234-1. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VV, Haughey NJ, Rabins PV, Lyketsos CG, Carlson MC. Serum sphingomyelins and ceramides are early predictors of memory impairment. Neurobiology of Aging. 2010a;31:17–24. doi: 10.1016/j.neurobiolaging.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Bandaru VVR, Haughey NJ, Schech S, Chu M, Albert M, et al. Plasma ceramides are altered early in the course of Alzheimers disease. Alzheimer’s & Dementia. 2010b in press. [Google Scholar]
- Mielke MM, Lyketsos CG. Lipids and the pathogenesis of Alzheimer’s disease: Is there a link? International Review of Psychiatry. 2006;18:173–186. doi: 10.1080/09540260600583007. [DOI] [PubMed] [Google Scholar]
- Mielke MM, Rosenberg PB, Tschanz J, Cook L, Corcoran C, Hayden KM, et al. Vascular factors predict rate of progression in Alzheimer disease. Neurology. 2007;69:1850–1858. doi: 10.1212/01.wnl.0000279520.59792.fe. [DOI] [PubMed] [Google Scholar]
- Nathan BP, Bellosta S, Sanan DA, Weisgraber KH, Mahley RW, Pitas RE. Differential effects of apolipoproteins E3 and E4 on neuronal growth in vitro. Science. 1994;264:850–852. doi: 10.1126/science.8171342. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Jiang XC, Tabas I, Tall A, Shea S. Plasma sphingomyelin and subclinical atherosclerosis: Findings from the multi-ethnic study of atherosclerosis. American Journal of Epidemiology. 2006;163:903–912. doi: 10.1093/aje/kwj140. [DOI] [PubMed] [Google Scholar]
- Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology. 1999;53:1937–1942. doi: 10.1212/wnl.53.9.1937. [DOI] [PubMed] [Google Scholar]
- Pettegrew JW, Panchalingam K, Hamilton RL, McClure RJ. Brain membrane phospholipid alterations in Alzheimer’s disease. Neurochemical Research. 2001;26:771–782. doi: 10.1023/a:1011603916962. [DOI] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Hui D, Weisgraber KH. Lipoproteins and their receptors in the central nervous system. Characterization of the lipoproteins in cerebrospinal fluid and identification of apolipoprotein B, E(LDL) receptors in the brain. The Journal of Biological Chemistry. 1987;262:14352–14360. [PubMed] [Google Scholar]
- Pitto M, Raimondo F, Zoia C, Brighina L, Ferrarese C, Masserini M. Enhanced GM1 ganglioside catabolism in cultured fibroblasts from Alzheimer patients. Neurobiology of Aging. 2005;26:833–838. doi: 10.1016/j.neurobiolaging.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Puglielli L, Ellis BC, Saunders AJ, Kovacs DM. Ceramide stabilizes beta-site amyloid precursor protein-cleaving enzyme 1 and promotes amyloid beta-peptide biogenesis. The Journal of Biological Chemistry. 2003;278:19777–19783. doi: 10.1074/jbc.M300466200. [DOI] [PubMed] [Google Scholar]
- Satoi H, Tomimoto H, Ohtani R, Kitano T, Kondo T, Watanabe M, et al. Astroglial expression of ceramide in Alzheimer’s disease brains: A role during neuronal apoptosis. Neuroscience. 2005;130:657–666. doi: 10.1016/j.neuroscience.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, Markesbery WR. Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life. Findings from the Nun Study. JAMA. 1996;275:528–532. [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, et al. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. PNAS. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber KH, Mahley RW. Human apolipoprotein E: The Alzheimer’s disease connection. FASEB J. 1996;10:1485–1494. doi: 10.1096/fasebj.10.13.8940294. [DOI] [PubMed] [Google Scholar]
- Xu WL, Qiu CX, Wahlin A, Winblad B, Fratiglioni L. Diabetes mellitus and risk of dementia in the Kungshol-men project: A 6-year follow-up study. Neurology. 2004;63:1181–1186. doi: 10.1212/01.wnl.0000140291.86406.d1. [DOI] [PubMed] [Google Scholar]
- Yanagisawa K. Role of gangliosides in Alzheimer’s disease. Biochimica et Biophysica Acta. 2007;1768:1943–1951. doi: 10.1016/j.bbamem.2007.01.018. [DOI] [PubMed] [Google Scholar]