Abstract
Dysregulation of protein kinase A (PKA) activity, caused by loss of function mutations in PRKAR1A, is known to induce tumor formation in the inherited tumor syndrome Carney complex (CNC) and is also associated with sporadic tumors of the thyroid and adrenal. We have previously shown that Prkar1a+/− mice develop schwannomas reminiscent of those seen in CNC and that similar tumors are observed in tissue-specific knockouts (KO) of Prkar1a targeted to the neural crest. Within these tumors, we have previously described the presence of epithelial islands, although the nature of these structures was unclear. In this article, we report that these epithelial structures are derived from KO cells originating in the neural crest. Analysis of the mesenchymal marker vimentin revealed that this protein was markedly down-regulated not only from the epithelial islands, but also from the tumor as a whole, consistent with mesenchymal-to-epithelial transition (MET). In vitro, Prkar1a null primary mouse embryonic fibroblasts, which display constitutive PKA signaling, also showed evidence for MET, with a loss of vimentin and up-regulation of the epithelial marker E-cadherin. Reduction of vimentin protein occurred at the posttranslational level and was rescued by proteasomal inhibition. Finally, this down-regulation of vimentin was recapitulated in the adrenal nodules of CNC patients, confirming an unexpected and previously unrecognized role for PKA in MET.
Introduction
Protein kinase A (PKA) is an evolutionarily conserved serine threonine kinase that regulates diverse signal transduction pathways, including cellular development, proliferation, differentiation, apoptosis, and tumorigenesis. The PKA holoenzyme exists as a heterotetramer consisting of two regulatory and two catalytic subunits. In humans and mice, there are four regulatory subunit genes: PRKAR1A, PRKAR1B, PRKAR2A, and PRKAR2B. As shown elegantly in knockout (KO) mouse studies, these four genes function in a tissue and cell-type specific manner to regulate accurately the activity of the catalytic subunits ( 1– 3). Of the four regulatory subunits, PRKAR1A is the most highly and ubiquitously expressed.
Carney complex (CNC, OMIM 160980) is an autosomal dominant multiple endocrine neoplasia syndrome caused by loss of function mutations in PRKAR1A in at least 50% of the CNC patients characterized to date ( 4– 6). Tumors from these patients display increased PKA activity when compared with non-CNC tumors from the same tissue ( 4). Loss of PRKAR1A has also been reported from sporadic tumors of the thyroid, breast, and adrenal, indicating that this gene has tumor suppressor function in a variety of sporadic cancers ( 7, 8). To investigate the tumor suppressor function of Prkar1a in vivo, we generated a KO mouse model for Prkar1a and have shown that heterozygote mice develop a spectrum of tumors that overlap with the tumors seen in human CNC patients ( 9). Prkar1a+/− mice developed schwannomas, bony tumors, and thyroid cancer, whereas tissue-specific ablation of Prkar1a from a subset of cranial neural crest cells led to the development of schwannomas. These data confirm Prkar1a's role as a tumor suppressor gene and indicate that complete loss of the gene is sufficient for tumor development.
Vimentin is an intermediate filament protein involved in maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions and structural processes ( 10). It is the most abundant of the intermediate filament proteins and is widely expressed in a variety of mesenchymal cell types, such as fibroblasts and endothelial cells. It is also expressed in other cell types derived from mesoderm, such as mesothelium, ovarian granulosa cells, and monocyte/macrophages. In most nonmesenchymal cells, vimentin is replaced by other intermediate filament proteins during the process of differentiation ( 11).
In this article, we describe mesenchymal-to-epithelial transition (MET), which occurs in Prkar1a−/− schwannomas, as evidenced by the gain of cytokeratin staining (as observed previously; ref. 9) and the loss of vimentin. This biochemical alteration, which occurred only in cells that had undergone cre-mediated excision of Prkar1a, was mirrored in vitro in Prkar1a−/− mouse embryonic fibroblasts (MEFs). Finally, these findings were corroborated in adrenal tumors from patients with the CNC due to mutation of PRKAR1A. Overall, these observations suggest that PKA dysregulation caused by loss of PRKAR1A/Prkar1a leads to MET, a finding which has clear implications for the behavior of these tumors.
Materials and Methods
Animal studies
The generation of the Prkar1a conditional null line ( 9) and the TEC3 (cre) line ( 12) have previously been described. Genetically modified mice were housed in sterile microisolator racks on a 12-h light/dark cycle. All animals were cared for under an IACUC-approved animal protocol in accordance with the highest standards of ethical animal care.
Patient samples
All human samples were collected with informed consent at NIH from patients participating in research protocol 96-CH-0069. Samples used in this study were all previously shown to carry mutations in the PRKAR1A gene ( 13).
Cell culture and transfections
Wild-type (WT) and Prkar1a−/− MEFs were generated and maintained in DMEM supplemented with 10% fetal bovine serum (FBS; Hyclone) as described ( 14). HeLa and 293T cells were cultured in DMEM supplemented with 10% FBS in a humidified atmosphere containing 5% CO2. For transfections, cells plated 24 h before transfection were transfected with constitutively active PKA-C expression plasmid using Superfect (Qiagen) according to manufacturer's recommendations. Cells were harvested 48 h after transfection for protein preparation as described ( 14).
Immunoblot analysis
For vimentin immunoblotting, whole-cell lysates prepared from early (P10) and late passage MEFs (P23) were separated by 10% SDS/PAGE, transferred to nitrocellulose membrane (Pall), and probed with primary antibodies from the following sources: Santa Cruz Biotechnologies [phosphorylated vimentin (Ser55), vimentin, twist, N-cadherin (N-19), and fibronectin (H-300)], Spring Bioscience (αSMA), Abcam (Snail, E-cadherin), Cell Signaling, Inc. (extracellular signal-regulated kinase), and BD Biosciences (SHP2). Binding of primary antibodies was visualized after incubation with species-specific secondary antibodies using chemiluminescence reagents (Perkin-Elmer).
Immunofluorescence and immunohistochemistry
For immunofluorescence, frozen schwannoma sections obtained from Prkar1a tissue-specific KO mice were fixed in cold acetone for 10 min and blocked for 1 h with the blocking solution obtained from mouse-on-mouse kit (Vector Labs). The sections were serially stained with vimentin and phalloidin or vimentin and cytokeratin 18, and the binding of primary antibodies was visualized by incubation with the appropriate secondary antibodies conjugated with Alexa 488 or 594 dyes. For immunohistochemistry, paraffin-embedded adrenocortical tumor sections from CNC patients were bleached in 10% hydrogen peroxide for ~8 h or until pigmentation had faded. Slides were then subject to antigen retrieval and staining for vimentin as described ( 9).
Microarray and quantitative real-time PCR analyses
mRNA was isolated from Prkar1a+/+ and Prkar1a−/− MEFs and quantitative real-time PCR (qRT-PCR) was performed as described ( 14). Vimentin primers used were forward, ATGTGAAGTTCATTTCCAACCC and reverse, TTGACTCC AGAAGGGCTTCA. mRNA fold changes were calculated by the ddCt method using Gapdh as a standard. All PCR reactions were done in triplicate, and each analysis was representative of three Prkar1a−/− and one Prkar1a+/+ cell lines taken from the same litter. Microarray analysis was performed essentially as described ( 15) using the Affymetrix Mouse 430A and B chips to compare two independently isolated cell lines that were WT or KO for the Prkar1a gene. Full details of this microarray analysis will be published elsewhere.
Results
MET in Prkar1a null tumors
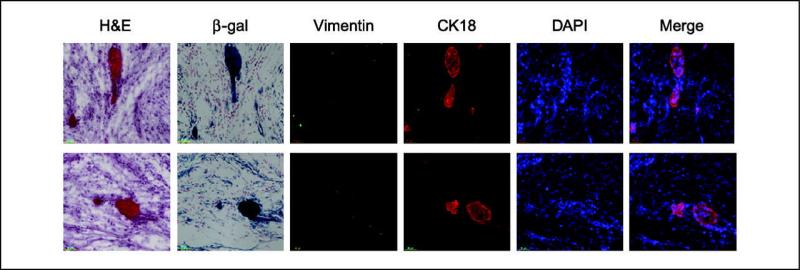
In our studies of schwannomas from Prkar1a+/− and tissue-specific Prkar1a−/− mice, we consistently noted the presence of small islands of epithelial-appearing cells that stained for cytokeratin 14 ( 9) and cytokeratin 18 ( Figure 1 ). To verify the connection between these cells and the loss of Prkar1a, we initially attempted to stain the tumors with an antibody specific for the Prkar1a protein. However, all anti-Prkar1a antibodies tested exhibited significant nonspecific staining in immunohistochemical testing, so results were felt to be unreliable (data not shown). To circumvent this problem, we introduced into the mouse line the Rosa26lacZ reporter allele ( 16), which enables β-galactosidase expression in the presence of cre activity. Because excision of the Prkar1aloxP allele seems to occur at high efficiency (data not shown), we used lacZ staining as a means to mark cells which had recombined the Prkar1a alleles. Staining of serial frozen sections of tumors showed that the epithelial islands also stained intensely for β-galactosidase activity, confirming that they arose from Prkar1a KO cells ( Figure 1). Because these tumors arose from neural crest cells, we also stained them for vimentin, an intermediate filament protein characteristic of mesenchymal-derived cells. Surprisingly, analysis of vimentin showed that the protein was essentially absent from the tumor, not only in the regions of the epithelial islands ( Figure 1), but also in the tumor as a whole ( Figure 2A and B ). In regions at the edge of the tumor, lacZ staining correlated both with neoplastic cells and with a lack of vimentin, both of which were clearly absent from the surrounding stromal tissue ( Figure 2A).
Figure 1.
Prkar1a null schwannomas exhibit gain of cytokeratin and loss of vimentin. Examples of epithelial islands observed in two different TEC3 tumors. Note the highly eosinophilic, cellular structures seen in H&E and 4′,6-diamidino-2-phenylindole (DAPI) correlate with intense β-galactosidase (β-gal) staining as a marker of cre activity. These structures exhibit loss of vimentin and gain of cytokeratin 18 (CK18) staining, indicating MET. Although all cells have lost vimentin, the most intense β-galactosidase staining occurs in the cytokeratin-positive epithelial islands.
Figure 2.
Loss of vimentin occurs in Prkar1a null schwannomas but not in tumor stroma. A, vimentin staining is lost only in cells with β-galactosidase staining as a marker for cre activity. B, immunofluorescence of vimentin and F-actin (stained by phalloidin) on frozen sections of Prkar1a null schwannomas. Vimentin staining is absent in the tumor compartment (T), whereas it is easily detectable in the stroma (S). Phalloidin, which stains F-actin, is present equally in both compartments. C, Western blotting of the tumor lysates confirmed the down-regulation of vimentin in tumors compared with normal Schwann cell lysates. β-Actin is used as a loading control.
In contrast, staining with phalloidin to detect structural filamentous actin (F-actin) showed uniform labeling of both stroma and tumor ( Figure 2B). Finally, to confirm the immunofluorescence data, cell lysates were prepared from snap-frozen tumors and shown to exhibit markedly reduced vimentin in the presence of unaltered levels of actin ( Figure 2C). These data suggested that all tumor cells had undergone MET, although only some of them had reached the state of full epithelial differentiation, including cytokeratin staining.
Down-regulation of vimentin in Prkar1a KO MEFs
To investigate the mechanisms by which loss of Prkar1a could result in the loss of vimentin and thus alter tumor behavior, we sought to determine if the same phenomenon was occurring in Prkar1a−/− MEFs. We have previously shown that these cells, which are generated in vitro from Prkar1aloxP/loxP MEFs, exhibit an increase in both free and total PKA activity ( 14). In confirmation of the in vivo data, we observed a striking down-regulation of vimentin in the cells, including both the total and phosphorylated forms of the protein ( Figure 3A ). This down-regulation was more pronounced in late passage MEFs, in which levels of phosphorylated vimentin are even more strongly suppressed. These results suggested that increased activity of PKA as a result of the loss of Prkar1a is able to modulate vimentin levels.
Figure 3.
Prkar1a KO MEFs exhibit characteristics of MET. A, Western blotting of early and late passage Prkar1a knockout and WT MEFs showing down-regulation of phosphorylated vimentin and vimentin in Prkar1a knockout MEFs. B, immunoblot analysis showing that MG-132 treatment of KO MEFs restores vimentin levels to those of WT cells. Note that MG-132 does not enhance vimentin levels in WT cells. C, Prkar1a KO MEFs exhibit marked up-regulation of E-cadherin. D, Western blotting for other markers of MET in KO and WT MEFs. Note the strong decreases in N-cadherin and αSMA, whereas levels of fibronectin are decreased slightly. The transcription factors Snail and Twist do not change in the KO cells compared with Shp2, which is shown as a loading control.
To explore the mechanism responsible for decreased vimentin in these cells, we first examined the levels of vimentin mRNA in both KO and WT MEFs by cDNA microarray analysis (Table 1). No significant differences were observed in the vimentin mRNA levels between Prkar1a KO MEFs and WT MEFs, and this observation was confirmed with qRT-PCR analysis (Supplementary Figure 1). These results indicated that the observed changes in vimentin protein levels occurred at the level of posttranscriptional control. To determine if the reduction in vimentin level was due to enhanced degradation, cells were incubated with the proteasome inhibitor MG132. Treatment of KO MEFs restored the levels of vimentin to that of WT controls in 6 h of treatment ( Figure 3B), whereas no significant changes in vimentin levels were observed in WT MEFs. These results indicate that a decreased vimentin level in KO MEFs is due to enhanced proteasomal degradation caused by excess PKA activation.
Table 1.
Mining of cDNA microarray data for the expression of epithelial and mesenchymal markers.
| Transcript | Fold expression change ± SD* | No. probesets | |
|---|---|---|---|
| Nuclear factors | Snai1 (Snail) | 1.3 | 1 |
| Snai2 (Slug) | –1.73 ± 0.08 | 2 | |
| Snai3 | 1.01 ± 0.04 | 2 | |
| Twist1 | 1.06 | 1 | |
| Twist2 | –2.04 | 1 | |
| Lef1 | –1.10 ± 0.03 | 2 | |
| Ets1 | –1.55 ± 0.30 | 4 | |
| Hnrpab (CBF-A) | 1.13 ± 0.20 | 6 | |
| Trim28 (KAP-1) | 1.16 ± 0.03 | 2 | |
| Hmga2 | –2.75 ± 1.10 | 4 | |
| Structural proteins | Vimentin | –1.16 ± 0.14 | 3 |
| E-cadherin (Cdh1) | 2.71 ± 2.34 | 2 | |
| N-cadherin (Cdh2) | –1.14 ± 0.07 | 3 | |
| P-cadherin (Cdh3) | 1.39 ± 0.21 | 2 | |
| R-cadherin (Cdh4) | 1.07 | 1 | |
| Acta2 (αSMA) | –2.42 ± 0.99 | 3 | |
| Fibronectin 1 | –1.10 ± 0.01 | 2 | |
| Fsp1 (S100A4) | –1.21 | 1 | |
| Tjp1 (ZO-1) | 1.54 | 1 |
Values of >1 indicate that the gene was more highly expressed in KO cells, whereas values of <–1 indicate the gene was down-regulated in KO cells. In cases where more than one probeset for the indicated gene was present, SD of the fold-changes is indicated. Bold cells indicate mRNAs altered at >1.5-fold, either up or down.
Loss of vimentin correlates with other signs of MET in Prkar1a−/− MEFs
To determine if loss of vimentin was an isolated event or if there were other molecular alterations associated with change in cell function, we next performed immunoblotting for the epithelial marker E-cadherin. As shown in Figure 3C, E-cadherin was markedly up-regulated in the KO MEFs. This up-regulation was mirrored at the mRNA level, as microarray hybridization studies indicated a 4.4-fold increase of the E-cadherin transcript (Cdh1) in KO cells compared with WT cells ( Table 1).
Finally, to confirm the alteration in cell fate, we performed Western blotting for other markers of the mesenchymal lineage ( 17). Analysis of N-cadherin and α-smooth muscle actin (encoded by Acta2) showed down-regulation of both of these mesenchymal structural proteins, whereas fibronectin was not altered. Analysis of the transcription factors Snai1 (Snail) and Twist did not show changes in expression level. To better understand these observations, we also mined our preexisting microarray data to determine the expression of these and other relevant mRNAs (Table 1). Using an arbitrary cutoff of a 1.5-fold change, we observed transcriptional up-regulation of the epithelial markers E-cadherin (Cdh1) and Tight junction protein 1 (Tjp1, also known as ZO-1), and transcriptional down-regulation of the mesenchymal markers Slug (Snai2), Twist2, Hmga2, Ets1, and α-SMA (Acta2). No alterations in transcript levels for other mesenchymal markers, such as Lef1, Ets1, Hnrpab (CBF-A), Trim28 (KAP-1), or Fsp1 (S100A4), were observed. Interestingly, there is no change in the transcript levels of N-cadherin, despite its reduction at the protein level. These data indicate that the protein changes characteristic of the MET phenotype occur by a combination of both transcriptional regulation (Acta2) and post transcriptional regulation (vimentin, N-cadherin).
Down regulation of vimentin is caused by increased PKA activity
Next, we wanted to examine if these changes were a general phenomenon associated with activation of PKA. To address this, we transfected the Protein Kinase A catalytic subunit (PKAC) into HeLa and 293T cells and measured the effects on levels of vimentin. Similar to the observations in Prkar1a KO MEFs, increasing PKAC expression led to the down regulation of vimentin protein in both HeLa and 293T cells ( Figure 4A and B ). These findings provided confirmatory evidence indicating that enhanced activation of PKA is able to suppress levels of the vimentin protein.
Figure 4.
Overexpression of PKA catalytic subunit in a heterologous system causes down-regulation of vimentin. Immunoblot analysis of 293T and HeLa cell lysates transiently transfected with PKA catalytic subunit revealed that increased expression of PKA-C led to down-regulation of vimentin protein. Note that this change occurs regardless if endogenous Prkar1a levels are low (293T cells) or high (HeLa cells). PTEN is shown as a loading control. Representative blot from among three experiments.
Loss of vimentin in adrenal nodules from CNC patients
The most common endocrine manifestation in CNC patients is primary pigmented nodular adrenocortical disease (PPNAD), in which multiple hypersecretory adrenal nodules are seen in the setting of an atrophic adrenal cortex ( 18). Adrenal nodules from these patients are highly pigmented due to the accumulation of lipofuscin, which has been reported to be seen only in nodule cells exhibiting loss of heterozygosity (LOH) of PRKAR1A ( 19, 20). Functionally, loss of PRKAR1A is associated with excess PKA signaling in these tumors, similar to Prkar1a KO MEFs ( 14, 21). To determine if CNC-associated adrenal tumors also exhibited changes in vimentin, paraffin embedded adrenocortical sections from patients were stained for vimentin. In agreement with the data presented above, human adrenal tumors exhibited essentially complete loss of vimentin in the adrenal nodules, whereas the internodular cortex stained intensely ( Figure 5 ). In contrast to human patients, adrenal nodules are not observed in Prkar1a+/− mice ( 9, 22), so a comparative study was not possible. These results show that vimentin is down regulated in response to loss of PRKAR1A/Prkar1a in both human and mouse tumors, suggesting that down regulation of vimentin is one of the consequences of increased PKA activity which might affect the behavior of tumor cells.
Figure 5.
Down-regulation of vimentin in adrenal nodules of primary pigmented nodular adrenocortical disease from CNC patients. PPNAD adrenals were stained with a human anti-vimentin antibody. Note the strong staining in the normal cortex (C), whereas staining in nodules (N) is essentially absent.
Discussion
Epithelial to Mesenchymal transition (EMT) and MET are normal embryonic processes involving interconversion of epithelial and mesenchymal cells, which serve to generate the proper variety of differentiated tissues during organogenesis ( 23). EMT has been proposed to be an important step in determining the behavior of cancers, as acquisition of the mesenchymal phenotype has been proposed to be important for allowing cancers to invade and cause metastasis ( 23, 24). Although the process of EMT has been studied carefully in the setting of cancer biology, much less is known about the role of MET. During development, MET occurs during somitogenesis, kidney development and coelomic cavity formation ( 25– 28), and a similar process plays a significant role in the repair of kidney damage ( 29). In tissue culture cells, MET can be induced by overexpression of the epithelial marker E-cadherin ( 30) or the proteoglycan versican ( 31). MET may also have a role in cancer progression different from that of EMT. In a model of bladder cancer metastasis, MET was shown to enhance the ability of injected cells to seed distant sites in a Fibroblast growth factor receptor 2 (FGFR2)-dependent manner, whereas the ability to metastasize from a primary tumor required a different set of cellular changes ( 29).
The connection between PKA signaling and epithelial-mesenchymal identity has not been elucidated in detail. PKA has been proposed as a mediator of EMT in response to transforming growth factor (TGF)-β1 in a murine hepatocyte cell line ( 32), although a direct role for PKA could not identified. Similarly, PKA activation was proposed to promote EMT in the developing neural crest downstream of BMP4/Sox9 signaling; in this model, PKA's role in this process was found to be complex ( 33). To the best of our knowledge, there have been no reports describing a role for PKA in MET, either in vitro or in vivo. It has been shown that PKA can regulate vimentin phosphorylation, and this posttranslational modification seems to affect intermediate filament formation. However, no effects on vimentin protein levels have been reported ( 34, 35). Phosphorylation of vimentin by PKA leads to changes in cell morphology, as observed in Prkar1a KO MEFs. PKA is also known to affect the recruitment and function of the vasodilator-stimulated phosphoprotein at tight junctions, and this may also play a role in modulating the epithelial phenotype ( 36).
Vimentin itself is considered a marker for mesenchymal cells, and recent data indicate that it also has a functional role in promoting the mesenchymal phenotype and invasiveness ( 24). This protein is typically expressed in neoplasms originating from mesenchymal tissues; sarcomas, lymphomas, malignant melanomas, and schwannomas are virtually always vimentin-positive ( 37). Additionally, mesoderm derived carcinomas, like renal cell carcinoma, adrenocortical carcinoma, and adenocarcinomas of the endometrium and ovary, usually express vimentin, as do many thyroid carcinomas ( 38).
In contrast with the finding in typical schwannomas, we observe a striking down-regulation of vimentin in Prkar1a−/− schwannomas, a finding recapitulated in PPNAD specimens from CNC patients. Similarly, although vimentin is normally easily detected in fibroblasts, we find that the protein is essentially absent from Prkar1a KO MEFs, which exhibit multiple molecular alterations consistent with MET. In each of these different models, we observed a consistent down-regulation of vimentin, which was corroborated by the activation of PKA by direct transfection of the catalytic subunit. Taken as a whole, the data suggest that direct activation of PKA causes MET. The role of PKA activation in this process is complex, however, as we have shown in the MEFs that changes in protein expression can occur at either the transcriptional or posttranscriptional level.
This physiologic change from a mesenchymal to an epithelial phenotype would be expected to play an important role in tumor cell biology ( 39), and the fact that malignant tumors are rarely seen in CNC patients may be a biological reflection of this cell fate alteration. Also, the fact that tumors associated with activation of PKA signaling, including thyroid tumors and adrenocorticorticotropic hormone (ACTH)–responsive adrenocortical lesions, are rarely malignant may be partly explained by the fact that activation of PKA signaling promotes epithelial differentiation, which must be overcome by other means before metastatic spread can occur. For these tumors to become invasive, molecular alterations causing loss of PKA signaling must occur, such as loss of the ACTH receptor in adrenocortical cancers ( 40).
Unlike other regulatory subunits of PKA, Prkar1a is the only isoform that is essential for early embryonic development ( 3, 9). Prkar1a−/− embryos fail to develop a functional heart tube at E8.5 and are resorbed before E10.5, highlighting the role of cyclic AMP/PKA regulation in early embryonic development. At the biological level, early Prkar1a−/− embryos show a reduced number of mesodermal cells emerging from the primitive streak and fail to develop normal mesoderm-derived structures. This developmental defect was shown to be due to excess PKA activity, as it was partially rescued by genetic disruption of PKA catalytic subunits ( 3). Based on the findings presented here, we propose that the defect in mesodermal specification in this model may be due to MET of these developing cells.
Given our observations suggesting that PKA activity causes MET, the exact role of PKA in development remains somewhat unclear. It is possible that the balance of EMT versus MET is controlled by cell type–specific factors so that the results of any experiment will depend strongly on the exact tissue/cell type chosen for study. Neither liver nor bladder tumors have been associated with loss of PRKAR1A in humans or mice, so the studies indicating that PKA is required for EMT in these tissues ( 32, 33) may contrast with findings in Schwann cells, which are well known to have a prominent growth response to PKA activation ( 41, 42). An alternate hypothesis is that there is a specific signaling effect of the PRKAR1A subunit that is required for EMT function of PKA. Individual PKA regulatory subunits have specific patterns of interaction with various A-kinase anchoring proteins, which can affect PKA localization and function, particularly as it affects cell structure and function ( 43). Therefore, it seems reasonable that specific loss of the PRKAR1A subunit, such as in CNC, shifts the balance toward MET, as observed in the experiments described here. Further experimental studies will be required to resolve this uncertainty.
In summary, we have shown that activation of PKA signaling is sufficient to cause a reduction in the mesenchymal marker vimentin as part of a process of MET. This occurs in a mouse model and in human patients with the CNC. The long-term implications of these findings, including the consideration that manipulation of the PKA system could be used for therapeutic effect, may bear further investigation.
Supplementary Material
Acknowledgments
NIH grants HD01323, CA112268-02 (L.S. Kirschner), and CA16058 (OSU Comprehensive Cancer Center) and intramural program Z01-HD-000642 (C.A. Stratakis).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
We thank Dr. Matthew Ringel for sharing reagents and for helpful discussions and Dr. Michael Oglesbee for his expert evaluation of tumor sections.
References
- 1.Cummings DE, Brandon EP, Planas JV, Motamed K, Idzerda RL, McKnight GS. Genetically lean mice result from targeted disruption of the RII β subunit of protein kinase A. Nature. 1996;382:622–6. doi: 10.1038/382622a0. [DOI] [PubMed] [Google Scholar]
- 2.Burton KA, Treash-Osio B, Muller CH, Dunphy EL, McKnight GS. Deletion of type IIα regulatory subunit delocalizes protein kinase A in mouse sperm without affecting motility or fertilization. J Biol Chem. 1999;274:24131–6. doi: 10.1074/jbc.274.34.24131. [DOI] [PubMed] [Google Scholar]
- 3.Amieux PS, Howe DG, Knickerbocker H, et al. Increased basal cAMP-dependent protein kinase activity inhibits the formation of mesoderm-derived structures in the developing mouse embryo. J Biol Chem. 2002;277:27294–304. doi: 10.1074/jbc.M200302200. [DOI] [PubMed] [Google Scholar]
- 4.Kirschner LS, Carney JA, Pack SD, et al. Mutations of the gene encoding the protein kinase A type I-α regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89–92. doi: 10.1038/79238. [DOI] [PubMed] [Google Scholar]
- 5.Kirschner LS, Sandrini F, Monbo J, Lin JP, Carney JA, Stratakis CA. Genetic heterogeneity and spectrum of mutations of the PRKAR1A gene in patients with the Carney complex. Hum Mol Genet. 2000;9:3037–46. doi: 10.1093/hmg/9.20.3037. [DOI] [PubMed] [Google Scholar]
- 6.Casey M, Vaughan CJ, He J, et al. Mutations in the protein kinase A R1α regulatory subunit cause familial cardiac myxomas and Carney complex. J Clin Invest. 2000;106:R31–8. doi: 10.1172/JCI10841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertherat J, Groussin L, Sandrini F, et al. Molecular and functional analysis of PRKAR1A and its locus (17q22-24) in sporadic adrenocortical tumors: 17q losses, somatic mutations, and protein kinase A expression and activity. Cancer Res. 2003;63:5308–19. [PubMed] [Google Scholar]
- 8.Sandrini F, Matyakhina L, Sarlis NJ, et al. Regulatory subunit type I-α of protein kinase A (PRKAR1A): a tumor-suppressor gene for sporadic thyroid cancer. Genes Chromosomes Cancer. 2002;35:182–92. doi: 10.1002/gcc.10112. [DOI] [PubMed] [Google Scholar]
- 9.Kirschner LS, Kusewitt DF, Matyakhina L, et al. A mouse model for the Carney complex tumor syndrome develops neoplasia in cyclic AMP-responsive tissues. Cancer Res. 2005;65:4506–44. doi: 10.1158/0008-5472.CAN-05-0580. [DOI] [PubMed] [Google Scholar]
- 10.Goldman BI, Frydman C, Harpaz N, Ryan SF, Loiterman D. Glandular cardiac myxomas. Histologic, immunohistochemical, and ultrastructural evidence of epithelial differentiation. Cancer. 1987;59:1767–75. doi: 10.1002/1097-0142(19870515)59:10<1767::aid-cncr2820591015>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 11.Katsumoto T, Mitsushima A, Kurimura T. The role of the vimentin intermediate filaments in rat 3Y1 cells elucidated by immunoelectron microscopy and computer-graphic reconstruction. Biol Cell. 1990;68:139–46. doi: 10.1016/0248-4900(90)90299-i. [DOI] [PubMed] [Google Scholar]
- 12.Tonks ID, Nurcombe V, Paterson C, et al. Tyrosinase-Cre mice for tissue-specific gene ablation in neural crest and neuroepithelial-derived tissues. Genesis. 2003;37:131–8. doi: 10.1002/gene.10242. [DOI] [PubMed] [Google Scholar]
- 13.Boikos SA, Stratakis CA. Carney complex: the first 20 years. Curr Opin Oncol. 2007;19:24–9. doi: 10.1097/CCO.0b013e32801195eb. [DOI] [PubMed] [Google Scholar]
- 14.Nadella KS, Kirschner LS. Disruption of protein kinase a regulation causes immortalization and dysregulation of D-type cyclins. Cancer Res. 2005;65:10307–15. doi: 10.1158/0008-5472.CAN-05-3183. [DOI] [PubMed] [Google Scholar]
- 15.Pavel E, Nadella K, Towns WH II, Kirschner LS. Mutation of Prkar1a Causes Osteoblast Neoplasia Driven by Dysregulation of PKA. Mol Endocrinol. 2007 doi: 10.1210/me.2007-0369. Epub 2007 Oct 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–1. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 17.Teng Y, Zeisberg M, Kalluri R. Transcriptional regulation of epithelial-mesenchymal transition. J Clin Invest. 2007;117:304–6. doi: 10.1172/JCI31200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041–6. doi: 10.1210/jcem.86.9.7903. [DOI] [PubMed] [Google Scholar]
- 19.Groussin L, Jullian E, Perlemoine K, et al. Mutations of the PRKAR1A gene in Cushing's syndrome due to sporadic primary pigmented nodular adrenocortical disease. J Clin Endocrinol Metab. 2002;87:4324–9. doi: 10.1210/jc.2002-020592. [DOI] [PubMed] [Google Scholar]
- 20.Mavrakis M, Lippincott-Schwartz J, Stratakis CA, Bossis I. Depletion of type IA regulatory subunit (RIα) of protein kinase A (PKA) in mammalian cells and tissues activates mTOR and causes autophagic deficiency. Hum Mol Genet. 2006;15:2962–71. doi: 10.1093/hmg/ddl239. [DOI] [PubMed] [Google Scholar]
- 21.Griffin KJ, Kirschner LS, Matyakhina L, et al. Down-regulation of regulatory subunit type 1A of protein kinase A leads to endocrine and other tumors. Cancer Res. 2004;64:8811–5. doi: 10.1158/0008-5472.CAN-04-3620. [DOI] [PubMed] [Google Scholar]
- 22.Veugelers M, Wilkes D, Burton K, et al. Comparative PRKAR1A genotype-phenotype analyses in humans with Carney complex and prkar1a haploinsufficient mice. Proc Natl Acad Sci U S A. 2004;101:14222–7. doi: 10.1073/pnas.0405535101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131–42. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 24.Vasko V, Espinosa AV, Scouten W, et al. Gene expression and functional evidence of epithelial-to-mesenchymal transition in papillary thyroid carcinoma invasion. Proc Natl Acad Sci U S A. 2007;104:2803–8. doi: 10.1073/pnas.0610733104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol (Berl) 1995;191:381–96. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- 26.Funayama N, Sato Y, Matsumoto K, Ogura T, Takahashi Y. Coelom formation: binary decision of the lateral plate mesoderm is controlled by the ectoderm. Development. 1999;126:4129–38. doi: 10.1242/dev.126.18.4129. [DOI] [PubMed] [Google Scholar]
- 27.Locascio A, Nieto MA. Cell movements during vertebrate development: integrated tissue behaviour versus individual cell migration. Curr Opin Genet Dev. 2001;11:464–9. doi: 10.1016/s0959-437x(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 28.Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol. 2002;3:155–66. doi: 10.1038/nrm757. [DOI] [PubMed] [Google Scholar]
- 29.Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Res. 2006;66:11271–8. doi: 10.1158/0008-5472.CAN-06-2044. [DOI] [PubMed] [Google Scholar]
- 30.Auersperg N, Pan J, Grove BD, et al. E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci U S A. 1999;96:6249–54. doi: 10.1073/pnas.96.11.6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheng W, Wang G, La Pierre DP, et al. Versican mediates mesenchymal-epithelial transition. Mol Biol Cell. 2006;17:2009–20. doi: 10.1091/mbc.E05-10-0951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang Y, Pan X, Lei W, et al. Regulation of transforming growth factor-{β}1-induced apoptosis and epithelial-to-mesenchymal transition by protein kinase A and signal transducers and activators of transcription 3. Cancer Res. 2006;66:8617–24. doi: 10.1158/0008-5472.CAN-06-1308. [DOI] [PubMed] [Google Scholar]
- 33.Sakai D, Suzuki T, Osumi N, Wakamatsu Y. Cooperative action of Sox9, Snail2 and PKA signaling in early neural crest development. Development. 2006;133:1323–33. doi: 10.1242/dev.02297. [DOI] [PubMed] [Google Scholar]
- 34.Lamb NJ, Fernandez A, Feramisco JR, Welch WJ. Modulation of vimentin containing intermediate filament distribution and phosphorylation in living fibroblasts by the cAMP-dependent protein kinase. J Cell Biol. 1989;108:2409–22. doi: 10.1083/jcb.108.6.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inagaki M, Nishi Y, Nishizawa K, Matsuyama M, Sato C. Site-specific phosphorylation induces disassembly of vimentin filaments in vitro. Nature. 1987;328:649–52. doi: 10.1038/328649a0. [DOI] [PubMed] [Google Scholar]
- 36.Kohler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 2004;165:175–80. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Azumi N, Battifora H. The distribution of vimentin and keratin in epithelial and nonepithelial neoplasms. A comprehensive immunohistochemical study on formalin- and alcohol-fixed tumors. Am J Clin Pathol. 1987;88:286–96. doi: 10.1093/ajcp/88.3.286. [DOI] [PubMed] [Google Scholar]
- 38.Nicoli S, Gilardelli CN, Pozzoli O, Presta M, Cotelli F. Regulated expression pattern of gremlin during zebrafish development. Gene Expr Patterns. 2005;5:539–44. doi: 10.1016/j.modgep.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Lee JM, Dedhar S, Kalluri R, Thompson EW. The epithelial-mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–81. doi: 10.1083/jcb.200601018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reincke M, Mora P, Beuschlein F, Arlt W, Chrousos GP, Allolio B. Deletion of the adrenocorticotropin receptor gene in human adrenocortical tumors: implications for tumorigenesis. J Clin Endocrinol Metab. 1997;82:3054–8. doi: 10.1210/jcem.82.9.4211. [DOI] [PubMed] [Google Scholar]
- 41.Kim HA, DeClue JE, Ratner N. cAMP-dependent protein kinase A is required for Schwann cell growth: interactions between the cAMP and neuregulin/tyrosine kinase pathways. J Neurosci Res. 1997;49:236–47. [PubMed] [Google Scholar]
- 42.Sobue G, Shuman S, Pleasure D. Schwann cell responses to cyclic AMP: proliferation, change in shape, and appearance of surface galactocerebroside. Brain Res. 1986;362:23–32. doi: 10.1016/0006-8993(86)91394-6. [DOI] [PubMed] [Google Scholar]
- 43.Scott JD. A-kinase-anchoring proteins and cytoskeletal signalling events. Biochem Soc Trans. 2003;31:87–9. doi: 10.1042/bst0310087. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.