Abstract
The 117-nucleotide (nt) RNA, called the packaging RNA (pRNA) of bacteriophage phi29 DNA packaging motor, has been shown to be an efficient vector for the construction of RNA nanoparticles for the delivery of small interfering RNA (siRNA) into specific cancer or viral-infected cells. Currently, chemical synthesis of 117-nt RNA is not feasible commercially. In addition, labeling at specific locations on pRNA requires the understanding of its modular organization. Here, we report multiple approaches for the construction of a functional 117-base pRNA using two synthetic RNA fragments with variable modifications. The resulting bipartite pRNA was fully competent in associating with other interacting pRNAs to form dimers, as demonstrated by the packaging of DNA via the nanomotor and the assembly of phi29 viruses in vitro. The pRNA subunit assembled from bipartite fragments harboring siRNA or receptor-binding ligands were equally competent in assembling into dimers. The subunits carrying different functionalities were able to bind cancer cells specifically, enter the cell, and silence specific genes of interest. The pRNA nanoparticles were subsequently processed by Dicer to release the siRNA embedded within the nanoparticles. The results will pave the way toward the treatment of diseases using synthetic pRNA/siRNA chimeric nanoparticles.
Introduction
Research in nanotechnology involves modification, engineering, and/or assembly of organized materials on the nanometer scale. RNA molecules can be designed and manipulated at a level of simplicity characteristic of DNA, while possessing the flexibility in structure and function or enzymatic activity similar to that of proteins. Thus, RNA is a suitable candidate for nanotechnological applications.1,2,3,4,5 The concept of RNA nanotechnology has been proposed for more than a decade2,4,6,7,8,9 (for review, see refs. 1,10,11). The first evidence was reported in 1998 showing that dimeric, trimeric, and hexameric RNA nanoparticles can be assembled through self-assembly of multiple reengineered natural RNA molecules.2 The field of RNA nanotechnology becomes more and more popular due to the recognition of the potential of RNA nanoparticles in the treatment of cancer, viral infection, genetics diseases, and other human ailments.1
Several RNA-based therapeutic approaches using small interfering RNA (siRNA)12,13,14,15 and ribozymes16,17,18,19,20 have been shown to downregulate specific gene expression in cancerous or viral-infected cells. RNA aptamer has been shown to bear functions similar to that of antibodies in their ability to recognize specific ligands (organic compounds, nucleotides, or peptides) for targeted delivery through the formation of binding pockets.21,22 This has led to heightened interest in the scientific community and the rapid development of RNA-based therapeutics. Although the methods for gene silencing with high efficacy and specificity have been achieved in vitro, the effective delivery of RNA to specific cells in vivo still remains challenging. Specific delivery of siRNA to target cells has been achieved using the packaging RNA (pRNA) of bacteriophage phi29,23,24,25 which forms dimers and trimers via the interaction of the left (L-loop) and right (R-loop) interlocking loops.2,26,27
Phi29 DNA packaging motor uses six pRNAs that form a ring to gear the DNA packaging motor.2,27,28,29,30 Each pRNA molecule contains two domains (Figure 1a). One of the domains, bases 23–97, located at the central region of pRNA, is for intermolecular interactions.27,28,31,32 The two interlocking loops reside within this domain. The second domain is for the binding of the DNA packaging enzyme gp16.33 This domain is located at the 5′/3′ ends that pair to form a double-stranded helical region.34 Removal of this domain does not affect the formation of dimer, trimer, and hexamer.27,32 Therefore, the pRNA 5′/3′ proximate double-stranded helical end34 can be replaced by a therapeutic siRNA (Figure 1a).23,24 Using this chimera technology, pRNA can escort the siRNA to silence genes and to destroy cancer cells of leukemia, lung, breast, as well as others.23,24,25,35,36,37,38
Figure 1.
Construction of bipartite RNA assemblies. (a) The structure of packaging RNA (pRNA) molecules and pRNA/siRNA chimera. (b) The design and sequence of three different bipartite modules for pRNA or pRNA/siRNA chimera. (c) 8% native polyacrylamide gel electrophoresis (PAGE) showing the self-assembly of two RNA fragments into the pRNA monomer and dimer formation of resulting bipartite RNA assemblies [lane 1, upper piece (P1); lane 2, lower piece (P2); lane 3, bipartite monomer; lanes 4 and 5, monomer control; lane 6, bipartite dimer; lane 7, dimer control]. The monomer control including (1–117) Ab′ pRNA and its dimer partner (1–117) Ba′ pRNA. The dimer control is the dimer formed by (1–117) Ab′ pRNA and Ba′ pRNA (D, dimer; M, monomer). (d) 8% native PAGE showing the self-assembly of two RNA fragments into the pRNA/siRNA chimera and dimer formation of resulting bipartite RNA assemblies [lane 1, upper piece (P1); lane 2, lower piece (P2); lane 3, bipartite monomer; lanes 4 and 5, monomer control; lane 6, bipartite dimer; lane 7, dimer control]. The monomer control including (1–117) Ab′ pRNA/ siRNA chimera and its dimer partner (1–117) Ba′ pRNA. The dimer control is the dimer formed by (1–117) Ab′ pRNA/siRNA chimera and Ba′ pRNA (D, dimer; M, monomer).
The pRNA system has several advantages including defined structure, controllable stoichiometry, multivalency, targeted delivery, ideal nanoscale size (~20–40 nm), and minimal induction of antibody response to enable repeated treatments of chronic diseases.1 In addition, the pRNA is remarkably stable in a wide range of pH (~4–9), temperature, and organic solvents.3 These unique features of pRNA have great potential to be applied not only for gene delivery but also for nanomachine fabrication and pathogen detection. However, one bottle neck in the RNA therapy and RNA nanotechnology is the requirement of relatively large quantities of RNA. The pRNA subunit is about 117 nucleotides, which is beyond the limit of currently available commercial chemical RNA synthesis technologies (maximum of 80 nucleotides with low yield). At this time, most of the pRNA or pRNA-related chimeras are synthesized enzymatically using RNA polymerase.
It has been previously reported that the pRNA can be assembled from two individual RNA oligonucleotides, one encompassing the R-loop and the other the L-loop, by annealing via a 6-nt duplex.39 However, one of these pieces is located in the middle of the pRNA; this made the resulting RNA not suitable for the construction of therapeutic RNA nanoparticles harboring siRNA or other modules, and also not feasible for applications in drug delivery. In this study, we further develop the bipartite chimeric constructs to generate full-length functional pRNAs that are not only competent for driving the phi29 DNA packaging motor but also proficient for therapeutic and diagnostic purposes.
Results
Nomenclatures
The pRNA constructs used in this work are identified by: (i) the R-loop and/or L-loop sequence(s); and (ii) the started/ended nucleotide (nt) number. A particular R-loop sequence is assigned an upper case letter (i.e., A, B,..), and a particular L-loop sequence is assigned a lower case letter with a prime (i.e., a′, b′,..). The same set of letters (i.e., Aa′) designates complementary sequences in the R/L loop, while different letters indicate lack of sequence complementarities. For example, Ab′ indicates that the pRNA assemblies contain a right-hand loop A and the left hand loop b′ for inter-RNA interaction with Ba′ in the assembly of the pRNA dimer.
Following the above rules, the 117-nt intact pRNA with various R-loop and L-loop is designated as (1–117) Rl′ (i.e., (1–117) Ab′). Three bipartite pRNA assemblages (Figure 1b) are designated as (1–28)/(30–117) Ab′, (1–55)/(56–117) Ab′, and (1–71)/(75–117) Ab′. Three bipartite pRNA chimeras (Figure 1b) are designated as (1–28)/(30–117) Ab′ pRNA/siRNA (eGFP), (1–55)/(56–117) Ab′ pRNA/siRNA (eGFP), and (1–71)/(75–117) Ab′ pRNA/siRNA (eGFP). pRNA/siRNA (eGFP) represents a pRNA chimera that harbors a siRNA targeting the enhanced green fluorescent protein (eGFP) gene while pRNA/siRNA (luciferase) and pRNA/siRNA (survivin) represent pRNA chimeras that harbor siRNAs targeting the firefly luciferase gene and survivin gene, respectively. (1–28) and (30–117) refer to start and stop of the RNA fragment one and fragment two, respectively, using phi29 pRNA sequence number as a reference.
Construction of pRNA assemblies by bottom-up approach using synthetic pRNA fragments
The goal of the construction of pRNA assemblies is twofold: driving the DNA packaging motor of phi29 and harboring RNA moieties, functionalities, or chemical groups for therapeutic purposes. In each RNA chimera, there are two important elements: the first one serves as the directing core to guide the folding and assembly of the resulting pRNA chimeras, whereas the second one functions to deliver these particles for medical applications. We constructed six pRNA assemblies using sets of RNA fragment pairs (P1 and P2) as building blocks shown in Figure 1b by three different bipartite approaches including Ab′(1–28)/(30–117), (1–55)/(56–117), and (1–71)/(75–117). The six assemblies were categorized into two classes: three with wild-type pRNA sequences, and three with siRNA sequences replaced 5′/3′ helical regions (Figure 1b). These three outlined bipartite approaches in pRNA construction overcome the size obstacle in RNA chemical synthesis, while maintaining the same bioactivity as the intact pRNA/siRNA particles. The design criteria for the bipartite assemblies were: (i) Each fragment of the bipartite RNAs should be <100 nt and suitable for chemical synthesis. (ii) The breaks on the pRNA chain to form functional bipartite particles basically followed the circular permutated pRNA design which closes the proximity of the wild-type pRNA 5′/3′ end and has a new 5′/3′ end opened at the different position along the pRNA chain. We adopted those new opening sites of functional circular permutated pRNAs which have viral DNA packaging ability and viral assembly activity40 as the selected breaks for bipartite design to ensure the assembled bipartite pRNAs maintain their biological activities. (iii) All the selected breaks are located at the less structural constraint and more flexible region. And the breaks should avoid the sequences involved in the intermolecular interaction, some important bulges responsible for viral packaging or the region which is for holding functional moieties such as siRNA insertion.
Assay for dimer formation to confirm the folding of the resulting bipartite RNA complex
Phi29 DNA packaging motor uses six pRNAs that form a ring to gear the DNA packaging motor.2,27,28,29,30 It has been reported previously that dimers are the building blocks in the assembly of the phi29 DNA packaging motor.28 Also, this self-assembling property can be used for the fabrication of reengineered dimeric pRNA chimeras that can serve as polyvalent vehicles for specific targeting and delivery of siRNA or ribozyme to cancer cells.10,23,24,25,36 Thus, it is crucial to find out whether pRNA constructs can form dimers, which would provide direct evidence that pRNA monomeric subunits constructed by the bipartite approach retain the self-assembling property of the intact pRNA.
As shown in the 8% native polyacrylamide gel electrophoresis (PAGE) (Figure 1c), three sets of RNA fragments annealed to form three types of bipartite Ab′ pRNA monomers, which migrated into the same position as wild-type monomer control [(1–117)Ab′ and (1–117)Ba′]. The bipartite monomeric Ab′ pRNA subsequently formed dimer, in the presence of its interacting partner (1–117)Ba′ pRNA and migrated into the upper dimer band which was at the same position as wild-type pRNA dimer Ab′-Ba′. The formation of dimers indicated that the bipartite pRNA assemblies folded into a conformation similar to that of the wild-type pRNA. Moreover, when the 5′/3′ paired helical region was replaced with a double-stranded siRNA, as with the three types of the bipartite pRNA/siRNA assemblies, the dimer formation pattern did not change, as revealed by an 8% native PAGE gel (Figure 1d) which also indicated the correct folding structure of the bipartite pRNA/siRNA assemblies.
DNA packaging and viral assembly activities of the bipartite pRNA assemblies
We used the phi29 system, with the known DNA packaging and the viral assembly assays, to further investigate the biological activity of the bipartite assemblies. Considering that the retention of the biological activity can be directly correlated with the retention of the structure, we used these two assays to confirm whether the bipartite assemblies can fold as the wild-type pRNA.
The phi29 DNA packaging assay41,42 was carried out by replacing one of the subunits of the pRNA dimer with the bipartite pRNA assemblies. After in vitro assembly of the functional DNA packaging motor, the double-stranded viral genome was packaged into the viral prohead. The mixture was then treated by DNase I and separated by 0.8% agarose gel. The DNA successfully packaged was protected from DNase digestion and can be observed on the gel (Figure 2a). Although the bipartite assemblies showed less amount of packaged viral genome in the gel (Figure 2a, lane 10–12) as compared to intact pRNA (Figure 2a, lane 4), we still found that all three bipartite Ab′ pRNA assemblies were proficient in driving the viral DNA packaging motor for packaging the viral genomic double-stranded DNA (Figure 2a), suggesting that bipartite pRNA maintained the structure and functions of wild-type pRNA.
Figure 2.
DNA packaging activity and viral assembly activity of bipartite packaging RNA (pRNA). (a) 0.8% agarose gel showing the procapsid protected viral DNA after packaging which indicated the active pRNA components. Lane1 is 1-kb DNA ladder; lane 2 indicated the total amount of input viral genome DNA for the packaging assay; lane 4 is the active packaging served as positive control. Lane 3 and lanes 5–9 served as negative control for background check, which only add monomeric (1–117) Ab′. (1–117) Ba′ or bipartite pRNA assemblies without presence of dimer partner. Lanes 10–12 showing the active packaging activity of all three bipartite pRNA assemblies. (b) Viral assembly activity is reflected by the plaque formation unit per milliliter (PFU/ml). The no RNA, no ATP, monomeric pRNA (Ab′ and Ba′ pRNA) or bipartite pRNA assemblies served as negative controls for checking the background plaque formation. All three bipartite pRNA assemblies together with their dimer partner (1–117) Ba′ pRNA can assemble mature virions to infect the host bacteria and form plaque which is comparable to the wild-type dimer (1–117) Ab′ pRNA plus (1–117) Ba′ which served as positive control.
The viral assembly assay also carried out by replacing one of the subunits of the dimer with the bipartite pRNA assemblies, the functional dimer will drive the DNA packaging motor to gear the viral genome into the procapsid and subsequently form the mature virions to infect the host bacteria and form plaques. The plaque-forming units (PFU)/ml was used to reflect the RNA activity during the phi29 viral assembly. As shown in Figure 2b, the control pRNA dimer showed 107 PFU/ml viral assembly activity whereas all three types of the bipartite Ab′ pRNA assembled from two RNA fragments still exhibited around 106 PFU/ml viral assembly activity, thereby demonstrating that the chimeric dimers were indeed functional.
The gene silencing effect of the pRNA assemblies constructed by the bipartite approach
Three RNA complexes, which include (1–28)/(30–117) Ab′ pRNA/siRNA(eGFP), (1–55)/(55–56) Ab′ pRNA/siRNA(eGFP), and (1–71)/(75–117) Ab′ pRNA/siRNA (eGFP) containing a siRNA functionality targeting eGFP, were constructed (Figure 3b) and shown competent in knocking down eGFP gene expression (Figure 3a). To verify the specificity in gene silencing, these bipartite pRNA assemblies have been also constructed with a single mutation either in the sense strand, the antisense strand or both strands. Although the incorporation of a single mutation at the sense strand is not sufficient to inhibit the gene silencing function of the constructions (Figure 3a), a single mutation on the complementary antisense strand resulted in a significant lost of the gene knockdown effects (Figure 3a). As expected, the incorporation of the mutation on both strands also resulted in the lost of the gene knockdown effects.
Figure 3.
Gene silencing assay for bipartite pRNA/siRNA (eGFP). (a) The enhanced green fluorescent protein (eGFP) gene silencing knockdown effects by bipartite pRNA/siRNA (eGFP) chimera and its mutant controls. Nucleotides in gray indicate the mutation. (b) 8% native polyacrylamide gel electrophoresis (PAGE) showing the self-assembly of two RNA fragments into the pRNA/siRNA chimera and its mutant controls [lane 1, monomer control; lane 2, pRNA/siRNA (eGFP); lane 3, pRNA/siRNA (eGFP) with sense strand mutant; lane 4, pRNA/siRNA (eGFP) with antisense strand mutant; lane 5, pRNA/siRNA (eGFP) with both sense strand and antisense strand mutant as well as dimer formation of resulting bipartite RNA assemblies accordingly; lane 6, pRNA/siRNA (eGFP) dimer; lane 7, dimer of pRNA/siRNA (eGFP) with sense strand mutant; lane 8, dimer of pRNA/siRNA (eGFP) with antisense strand mutant; lane 9, dimer of pRNA/siRNA (eGFP) with both sense strand and antisense strand mutant; and lane 10, dimer control.].
To further investigate the ability of the bipartite pRNA to be used as a therapeutic module in the construction of multivalent nanoparticle, we constructed another set of three RNA complexes containing a siRNA functionality targeting the experimental reporter, firefly luciferase gene (Figure 4). This include the constructions (1–28)/(30–117)Ab′ pRNA/siRNA (firefly luciferase), (1–55)/(55–56)Ab′ pRNA/siRNA (firefly luciferase), and (1–71)/(75–117)Ab′ pRNA/siRNA (firefly luciferase). Compared to the eGFP gene knockdown assay, the dual-Luciferase report system can quantitatively measure the gene silencing effects of these three bipartite pRNA/siRNA assemblies. The relative luciferase activity was used to reflect the expression level of firefly luciferase gene by normalizing the firefly luciferase activity with the internal control, renilla luciferase activity. The results indicated that all three constructs of the bipartite pRNA/siRNA (firefly luciferase) displayed a dramatic decrease in firefly luciferase gene expression which is comparable to the intact pRNA/siRNA (firefly luciferase) after transfection.
Figure 4.
Dual-luciferase assay for bipartite pRNA/siRNA (firefly luciferase) chimera. The no RNA, no plasmid DNA control served as the system blank for the assay. DNA1 is the plasmid pGL3 harboring firefly luciferase gene and DNA2 is the plasmid pRL-TK harboring renilla luciferase. The relative firefly luciferase activity is to normalize firefly luciferase activity using the internal control renilla luciferase activity, which reflected the level of luciferase gene expression.
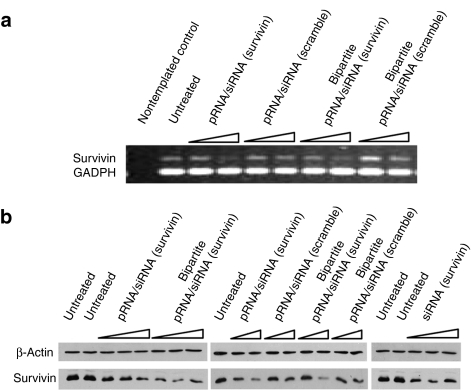
Furthermore, the bipartite (1–28)/(30–117) pRNA/siRNA (survivin) assembly showed similar silencing effects on the survivin gene expression as the intact pRNA/siRNA (survivin) which is demonstrated by reverse transcription-PCR (RT-PCR) assayed on mRNA level and western blot assayed on protein expression level; a bipartite pRNA/siRNA assembly harboring scrambled survivin siRNA served as negative control (Figure 5). This bipartite pRNA/siRNA was processed efficiently by Dicer in vitro to release the end RNA fragment (23–27 nt), as shown in Supplementary Figure S1. For details on Dicer processing of the two-piece pRNA/siRNA complex, see Supplementary Materials and Methods. The processed small RNA fragments were confirmed to harbor the siRNA sequence by northern blot assay (data not shown).
Figure 5.
The survivin silencing effects of bipartite pRNA/siRNA (survivin) chimera assayed by RT-PCR and western blot assay. (a) Cells were treated with different concentration of RNAs (5 and 20 nmol/l). The reduced survivin gene expression on mRNA level was displayed as the lighter band and GADPH served as loading control. (b) Cells were treated with different concentration of RNAs (5, 20, and 40 nmol/l), respectively including bipartite pRNA/siRNA (survivin), bipartite pRNA/scramble control as well as according intact pRNA/siRNA (survivin) and its scramble control. Column 2 of the figure only included two concentration of RNA treatment (5 and 20 nmol/l). The reduced survivin gene expression on protein level was displayed as the lighter band after blotting and β-actin served as loading control.
Cell binding and entry of the bipartite pRNA/folate therapeutic RNA nanoparticles
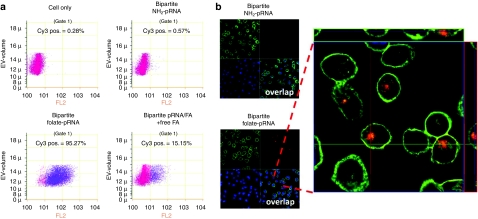
Many cancer cell lines, especially those of epithelial or myelocytic origin, overexpress the folate receptor (FR) on their surface.43 Folate has been used extensively as cancer cell delivery agent via FR-mediated endocytosis.25,37,44 Human nasopharyngeal epidermal carcinoma KB cells which have overexpressed FR on the cell surface37 were used as the cell model and the fluorescently labeled bipartite pRNA/folate was used to test its cell binding efficiency. A fluorescent bipartite pRNA/NH2 that did not contain folate group was used as the negative control. Flow cytometry data showed that the binding efficiency of the fluorescent bipartite pRNA/folate was close to 100% (Figure 6a). The binding specificity was also proved by free-folate competitive assay. Free folate can competitively bind to the FR positive KB cells and reduce the fluorescent signal from bipartite pRNA/folate binding. Confocal microscopy revealed strong binding of the fluorescent bipartite pRNA/folate complex, as well as efficient entry of the RNA into the targeted cells. The entry was demonstrated by excellent colocalization and overlap of the fluorescent bipartite pRNA/folate assembly (red) and cytoplasma (green) (Figure 6b).
Figure 6.
Flow cytometry and confocal microscopy imaging of KB cells showing the binding and entry of the bipartite pRNA/folate chimera. (a) The shifted cells in blue color indicated the strong binding of the bipartite pRNA/folate compared to the bipartite pRNA/NH2 control. (b) The green color indicated the region of the cell cytoplasmatic portion and blue color indicated the nucleus. The fluorescent labeled bipartite pRNA/folate shown in red.
Discussion
Unlike DNA, RNA molecules are diverse in structures due to numerous inter- and intramolecular interactions in RNA folding process.45 A single base change in RNA primary sequences could result in unpredictable folding alteration and function loss.46 It is also challenging to predict the RNA behavioral change upon changes within its primary sequence by current available tools. Thus, for rational design of our bipartite RNA assemblies, we followed multiple circular permutated pRNA designs.40 All these circular permutated pRNAs showed comparable DNA packaging and viral assembly activity which indicated the new opening/break along the pRNA chain have no or less affects on the correct folding as well as the function of pRNA. Other criteria were also considered as mentioned in Result section. The final assemblies generated by bipartite designs should: (i) still maintain the correct structure folding; and (ii) maintain the similar function as intact particles. We demonstrated that phi29 bacteriophage pRNA can be engineered to harbor therapeutic modules and efficiently assembled into higher order structures with defined stoichiometry using a bipartite construction approach. This method overcomes the current limitations in chemical synthesis of long RNA molecules, while retaining the structural and functional integrity and chemical stability of both wild-type pRNAs and the therapeutic pRNA chimeras.
The bipartite pRNA constructs were structurally competent, evidenced by efficient dimer formation. However, the bipartite modules showed lower DNA packaging and viral assembly activity (approximately 10-fold) while comparing to the wild-type pRNA. The reason might be due to the fact that the assembly of two RNA fragments resulted in unligated pRNA molecules and generated a nick that might interfere with pRNA function in gearing the DNA packaging motor.
The multivalent chimeric constructs harboring targeting, detection, and therapeutic moieties were functionally proficient, as demonstrated by gene knockdown, and dual-luciferase assays. Moreover, the (1–28)/(30–117) bipartite pRNA/siRNA chimera produced better silencing effects compared to the intact modules and the siRNA itself (Figure 5). Dicer in vitro processing results proved that the bipartite (1–28)/(30–117) pRNA/siRNA can be processed by Dicer more efficiently and precisely than intact pRNA/siRNA chimera. The intact pRNA/siRNA can only be processed to generate the RNA fragment larger than 23 nt, while the bipartite module could be processed to generate ~23 nt RNA fragment because the single nucleotide gap between nt 28 and 30 within the bipartite pRNA/siRNA molecule might facilitate the siRNA processing by Dicer. Meanwhile, siRNA alone might be unstable in cytoplasm compared to the bipartite pRNA/siRNA chimera which folded into the strong secondary/tertiary structure resistant to various conditions3 and might protect the embedded siRNA from fast degradation and resulting in an enhanced RNA interference function inside the cells.
Furthermore, flow cytometry and confocal images demonstrated that the therapeutic bipartite pRNA modules were strongly bound to the target cells and subsequently internalized into cancer cells with high efficiency.
In summary, our results showed the feasibility of the bipartite approach in assembling functional RNA nanoparticles with high yield. The self-assembly of pRNA using bipartite approach demonstrated the addressable and programmable nature of pRNA. The constructed bipartite pRNA/siRNA and bipartite pRNA-folate chimera can further assemble into dimeric particles for targeted delivery of therapeutics into FR positive cancer cells. This approach can be extended in the future to build more complex multifunctional nanoparticles for a wide range of therapeutic, detection, and diagnosis applications.
Materials and Methods
In vitro synthesis of RNA fragment and assembly of the pRNA complex using two RNA fragments. RNA fragments were transcribed by T7 RNA polymerase using double-stranded DNA templates from PCR, as described previously.3 To construct bipartite pRNA/siRNA (eGFP), pRNA/siRNA (luciferase), and pRNA/siRNA (survivin), the helical region at the 5′/3′ paired ends of pRNA was replaced with double-stranded siRNA that connects to bases 29 and 91. The chimeric pRNA/siRNA bipartite assemblies were synthesized in vitro using the similar principle.
The intact bipartite RNA complex pRNA/siRNA (eGFP), pRNA/siRNA (luciferase), and pRNA/siRNA (survivin) were assembled from the synthesized RNA fragments either by direct mixing of two fragments at 1:1 molar ratio in TMS buffer (50 mmol/l Tris, 100 mmol/l NaCl2, 10 mmol/l MgCl2) at room temperature for >30 minutes or by annealing the two fragments in TMS through heating at 75 °C for 5 minutes, followed by slow cooling to room temperature. Bipartite RNA complexes were then purified from 10% native polyacrylamide gel.28
Assay for pRNA dimer formation. The potential of dimer formation is one way to verify the correct folding of the bipartite pRNA. The pRNA construct Ab′ monomer was mixed with their interacting partner pRNA Ba′ in TBM buffer [89 mmol/l Tris–HCl (pH 7.6), 0.2 mol/l boric acid, and 5 mmol/l MgCl2] at equal mole ratio, and incubated at room temperature for 30 minutes. The dimer formation was then assayed, followed by purification in 10% native polyacrylamide gel.
Assay for DNA packaging and virion assembly using the bipartite pRNA assemblies. Methods for the assay of pRNA activity in DNA-packaging47 and in vitro virion assembly41,42 have been reported previously.
For DNA packaging assay, briefly, the synthesized and purified 100 ng bipartite pRNA assemblies and their dimer partner (1–117) pRNA Ba′, viral procapsid, gp16, and viral DNA-gp3, as well as the 10 mmol/l ATP (pH 7.0) were mixed and incubated for 1 hour at ambient temperature to let viral DNA translocate into preformed procapsid. Then the mixture was firstly treated with DNase I and followed by Protease K treatment. The treated mixture was separated by 0.8% agarose gel and the procapsid protected DNA could be observed after ethidium bromide staining.
For in vitro virion assembly assay, the mature viral particles could be obtained by mixing bipartite pRNA Ab′ monomer, (1–117) pRNA Ba′, viral procapsid, gp16, and DNA-gp3, 10 mmol/l ATP as well as other two components gp9 and gp11–14 were incubated at room temperature. The mixture was plated on host Bacillus subtilis su+44. After 12–14 hours incubation at 37 °C, the plaque formation per plate was counted and the viral assembly activity was calculated by PFU/ml.
Cell culture. Human nasopharyngeal carcinoma KB cells [American Type Culture Collection (ATCC), Manassas, VA] are routinely maintained in RPMI-1640 medium (Invitrogen, Carlsbad, CA) and supplemented with 10% fetal bovine serum. Cultures were incubated at 37 °C in a humidified 5% CO2 atmosphere.
GFP reporter assay to test the potential of the bipartite pRNA complex in escorting siRNA delivered into specific cells. For human nasopharyngeal epidermal carcinoma KB cells, 105 cells were seeded in 24-well plates. GFP-expressing plasmid pGFP-N2 (Clontech Laboratories, Mountain View, CA), bipartite Ab′ pRNA/siRNA (eGFP) and different kind of mutant controls were cotransfected into cells by using lipofactamine 2000 (Invitrogen) 24 hours after seeding. The effect was measured at the level of eGFP expression, as observed by fluorescence microscopy.23,24
Dual-luciferase assays to test the potential of the bipartite pRNA complex in escorting siRNA delivered into cells. For dual-luciferase assays,23 KB cells were seeded in 24-well plates. Gene silencing assays were performed by cotransfecting bipartite chimeric pRNA/siRNA (luciferase) with both plasmid pGL3 and pRL-TK (Promega, Madison, WI) coding for firefly and renilla luciferase, respectively. The latter served as an internal control to normalize the luciferase data (Dual-Luciferase Reporter Assay System; Promega). Cells were washed once with phosphate-buffered saline (PBS) and lysed with passive lysis buffer. The plates were shaken for 15 minutes at room temperature. 20 µl of lysate were added to 100 µl of luciferase assay reagent (LAR II) in a luminometer tube and firefly luciferase activity was measured. Upon addition of 20 µl of Stop & Glo Reagent, control measurements of renilla luciferase activity were then obtained. The previously obtained data was then normalized with respect to the renilla activity for determining the average ratio of firefly to renilla activity over several trials.
Cell transfection assay followed by RT-PCR and western blot analysis to test the potential of the bipartite pRNA complex in escorting siRNA for gene silencing. KB cells were seeded into 24-well plates overnight and transfected with 5, 20, and 40 nmol/l bipartite pRNA/siRNA (survivin) chimera as well as the scrambled control by Lipofectamine 2000. After 48 hours treatment, cells were collected and target gene silencing effects were assessed by both RT-PCR and western blot assays.
Cells were processed for total RNA using illustra RNAspin Mini kits (GE Healthcare, Buckinghamshire, UK). The first complementary DNA strand was synthesized on mRNA (500 ng) from KB cells using SuperScript III First-Strand Synthesis System (Invitrogen) according to manufacturer's instruction. PCR was performed using GoTaq Flexi DNA polymerase (Promega). Reactions were carried out in a final volume of 25 µl which contained complementary DNA from first-strand synthesis, 1× GoTaq Flexi colorless buffer, 2.5 mmol/l Mg2+, 0.2 mmol/l deoxynucleoside triphosphates, 0.2 µmol/l of each primer, and 0.02 U/µl GoTaq Flexi DNA polymerase. The PCR condition was 95 °C for 5 minutes then 25 cycles of 94 °C for 1 minute, 55 °C for 1 minute and 72 °C for 1 minute, followed by 72 °C for 10 minutes.
Primers for human GAPDH48 and survivin49 are:
GAPDH left: 5′-ACGGATTTGGTCGTATTGGGCG-3′
GAPDH right: 5′-CTCCTGAAGATGGTGATGGAA-3′
Survivin left: 5′-GCATGGGTGCCCCGACGTTG-3′
Survivin right: 5′-GCTCCGGCCAGAGGCCTCAA-3′.
Cells were rinsed and harvested in lysis buffer. Protein concentrations were determined and equal amounts of proteins were loaded onto a 15% polyacrylamide gel. Membranes were blocked, incubated with primary antibody to survivin and β-actin (R&D Systems, Minneapolis, MN), and conjugated to a secondary antibody (Sigma-Aldrich, St Louis, MO). Membranes were then blotted by ECL kit (Millipore, Billerica, MA) and exposed to film for autoradiography.
Flow cytometry to test cell receptor binding of the bipartite pRNA complex harboring ligands. Cell binding studies were performed on KB cells maintained in folate-free RPMI-1640 medium (Invitrogen). The folate-deficient KB cells were then trypsinized and rinsed with PBS. 500 nmol/l bipartite pRNA/folate and control bipartite pRNA/NH2 were each incubated with the 2 × 105 KB cells at 37 °C for 1 hour. After PBS wash, the cells were resuspended in PBS buffer. Flow cytometry (Beckman Coulter, Brea, CA) was used to observe the cell binding efficacy of the pRNA/folate complexes.
Confocal microscopy to test cell binding and entry. For confocal microscopy, KB cells were grown on glass coverslides in folate-free RPMI-1640 medium overnight. Bipartite pRNA/folate and control bipartite pRNA/NH2 with siRNA were each incubated with the cells at 37 °C for 2–3 hours. After PBS wash, the cells were fixed by 4% paraformaldehyde and stained by Alexa Fluor 488 phalloidin (Invitrogen) for cytoskeleton and TO-PRO-3 iodide (642/661) (Invitrogen) for nucleus, staining as per the manufacturer's instructions. Cells were then assayed for binding and entry of the RNA complexes by the Zeiss LSM 510 laser scanning confocal microscope (Carl Zeiss, Thornwood, NY).
SUPPLEMENTARY MATERIAL Figure S1. Autoradiogram showing the Dicer processing of the [32P] labeled bipartite pRNA/siRNA chimeras. Materials and Methods.
Acknowledgments
This work was supported by NIH Grant EB003730, GM059944, and CA151648 to P.G. We thank Shuhui Wan for the help with DNA-folate synthesis and Farzin Haque and Qixiang Li for their insightful comments. P.G. is a cofounder of Kylin Therapeutics, Inc.
Supplementary Material
Autoradiogram showing the Dicer processing of the [32P] labeled bipartite pRNA/siRNA chimeras.
REFERENCES
- Guo P. The emerging field of RNA nanotechnology. Nat Nanotechnol. 2010;5:833–842. doi: 10.1038/nnano.2010.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Zhang C, Chen C, Garver K., and, Trottier M. Inter-RNA interaction of phage phi29 pRNA to form a hexameric complex for viral DNA transportation. Mol Cell. 1998;2:149–155. doi: 10.1016/s1097-2765(00)80124-0. [DOI] [PubMed] [Google Scholar]
- Shu D, Huang LP, Hoeprich S., and, Guo P. Construction of phi29 DNA-packaging RNA monomers, dimers, and trimers with variable sizes and shapes as potential parts for nanodevices. J Nanosci Nanotechnol. 2003;3:295–302. doi: 10.1166/jnn.2003.160. [DOI] [PubMed] [Google Scholar]
- Shu D, Moll WD, Deng Z, Mao C., and, Guo P. Bottom-up Assembly of RNA Arrays and Superstructures as Potential Parts in Nanotechnology. Nano Lett. 2004;4:1717–1723. doi: 10.1021/nl0494497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansma HG, Oroudjev E, Baudrey S., and, Jaeger L. TectoRNA and ‘kissing-loop' RNA: atomic force microscopy of self-assembling RNA structures. J Microsc. 2003;212 Pt 3:273–279. doi: 10.1111/j.1365-2818.2003.01276.x. [DOI] [PubMed] [Google Scholar]
- Zhang F, Lemieux S, Wu X, St-Arnaud D, McMurray CT, Major F.et al. (1998Function of hexameric RNA in packaging of bacteriophage phi 29 DNA in vitro Mol Cell 2141–147. [DOI] [PubMed] [Google Scholar]
- Grabow WW, Zakrevsky P, Afonin KA, Chworos A, Shapiro BA., and, Jaeger L. Self-assembling RNA nanorings based on RNAI/II inverse kissing complexes. Nano Lett. 2011;11:878–887. doi: 10.1021/nl104271s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger L, Westhof E., and, Leontis NB. TectoRNA: modular assembly units for the construction of RNA nano-objects. Nucleic Acids Res. 2001;29:455–463. doi: 10.1093/nar/29.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chworos A, Severcan I, Koyfman AY, Weinkam P, Oroudjev E, Hansma HG.et al. (2004Building programmable jigsaw puzzles with RNA Science 3062068–2072. [DOI] [PubMed] [Google Scholar]
- Guo P. RNA nanotechnology: engineering, assembly and applications in detection, gene delivery and therapy. J Nanosci Nanotechnol. 2005;5:1964–1982. doi: 10.1166/jnn.2005.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger L., and, Chworos A. The architectonics of programmable RNA and DNA nanostructures. Curr Opin Struct Biol. 2006;16:531–543. doi: 10.1016/j.sbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE., and, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Li H, Li WX., and, Ding SW. Induction and suppression of RNA silencing by an animal virus. Science. 2002;296:1319–1321. doi: 10.1126/science.1070948. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R., and, Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. doi: 10.1126/science.1068999. [DOI] [PubMed] [Google Scholar]
- Carmichael GG. Medicine: silencing viruses with RNA. Nature. 2002;418:379–380. doi: 10.1038/418379a. [DOI] [PubMed] [Google Scholar]
- Kruger K, Grabowski PJ, Zaug AJ, Sands J, Gottschling DE., and, Cech TR. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell. 1982;31:147–157. doi: 10.1016/0092-8674(82)90414-7. [DOI] [PubMed] [Google Scholar]
- Guerrier-Takada C, Gardiner K, Marsh T, Pace N., and, Altman S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell. 1983;35 3 Pt 2:849–857. doi: 10.1016/0092-8674(83)90117-4. [DOI] [PubMed] [Google Scholar]
- Sarver N, Cantin EM, Chang PS, Zaia JA, Ladne PA, Stephens DA.et al. (1990Ribozymes as potential anti-HIV-1 therapeutic agents Science 2471222–1225. [DOI] [PubMed] [Google Scholar]
- Chowrira BM, Berzal-Herranz A., and, Burke JM. Novel guanosine requirement for catalysis by the hairpin ribozyme. Nature. 1991;354:320–322. doi: 10.1038/354320a0. [DOI] [PubMed] [Google Scholar]
- Sarver N, Cantin EM, Chang PS, Zaia JA, Ladne PA, Stephens DA.et al. (1990Ribozymes as potential anti-HIV-1 therapeutic agents Science 241222–1225. [DOI] [PubMed] [Google Scholar]
- Ellington AD., and, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Tuerk C., and, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Guo S, Tschammer N, Mohammed S., and, Guo P. Specific delivery of therapeutic RNAs to cancer cells via the dimerization mechanism of phi29 motor pRNA. Hum Gene Ther. 2005;16:1097–1109. doi: 10.1089/hum.2005.16.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled A, Guo S, Li F., and, Guo P. Controllable self-assembly of nanoparticles for specific delivery of multiple therapeutic molecules to cancer cells using RNA nanotechnology. Nano Lett. 2005;5:1797–1808. doi: 10.1021/nl051264s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Huang F., and, Guo P. Construction of folate-conjugated pRNA of bacteriophage phi29 DNA packaging motor for delivery of chimeric siRNA to nasopharyngeal carcinoma cells. Gene Ther. 2006;13:814–820. doi: 10.1038/sj.gt.3302716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo PX, Erickson S., and, Anderson D. A small viral RNA is required for in vitro packaging of bacteriophage phi 29 DNA. Science. 1987;236:690–694. doi: 10.1126/science.3107124. [DOI] [PubMed] [Google Scholar]
- Chen C, Zhang C., and, Guo P. Sequence requirement for hand-in-hand interaction in formation of RNA dimers and hexamers to gear phi29 DNA translocation motor. RNA. 1999;5:805–818. doi: 10.1017/s1355838299990350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Sheng S, Shao Z., and, Guo P. A dimer as a building block in assembling RNA. A hexamer that gears bacterial virus phi29 DNA-translocating machinery. J Biol Chem. 2000;275:17510–17516. doi: 10.1074/jbc.M909662199. [DOI] [PubMed] [Google Scholar]
- Chen C., and, Guo P. Sequential action of six virus-encoded DNA-packaging RNAs during phage phi29 genomic DNA translocation. J Virol. 1997;71:3864–3871. doi: 10.1128/jvi.71.5.3864-3871.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu D, Zhang H, Jin J., and, Guo P. Counting of six pRNAs of phi29 DNA-packaging motor with customized single-molecule dual-view system. EMBO J. 2007;26:527–537. doi: 10.1038/sj.emboj.7601506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid RJ, Bodley JW., and, Anderson D. Characterization of the prohead-pRNA interaction of bacteriophage phi 29. J Biol Chem. 1994;269:5157–5162. [PubMed] [Google Scholar]
- Garver K., and, Guo P. Boundary of pRNA functional domains and minimum pRNA sequence requirement for specific connector binding and DNA packaging of phage phi29. RNA. 1997;3:1068–1079. [PMC free article] [PubMed] [Google Scholar]
- Lee TJ., and, Guo P. Interaction of gp16 with pRNA and DNA for genome packaging by the motor of bacterial virus phi29. J Mol Biol. 2006;356:589–599. doi: 10.1016/j.jmb.2005.10.045. [DOI] [PubMed] [Google Scholar]
- Zhang C, Tellinghuisen T., and, Guo P. Confirmation of the helical structure of the 5'/3' termini of the essential DNA packaging pRNA of phage phi 29. RNA. 1995;1:1041–1050. [PMC free article] [PubMed] [Google Scholar]
- Hoeprich S, Zhou Q, Guo S, Shu D, Qi G, Wang Y.et al. (2003Bacterial virus phi29 pRNA as a hammerhead ribozyme escort to destroy hepatitis B virus Gene Ther 101258–1267. [DOI] [PubMed] [Google Scholar]
- Liu H, Guo S, Roll R, Li J, Diao Z, Shao N.et al. (2007Phi29 pRNA vector for efficient escort of hammerhead ribozyme targeting survivin in multiple cancer cells Cancer Biol Ther 6697–704. [DOI] [PubMed] [Google Scholar]
- Li L, Liu J, Diao Z, Shu D, Guo P., and, Shen G. Evaluation of specific delivery of chimeric phi29 pRNA/siRNA nanoparticles to multiple tumor cells. Mol Biosyst. 2009;5:1361–1368. doi: 10.1039/b903428e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HM, Su Y, Guo S, Yuan J, Lim T, Liu J.et al. (2009Targeted delivery of anti-coxsackievirus siRNAs using ligand-conjugated packaging RNAs Antiviral Res 83307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y, Shu D, Xiao F, Guo P., and, Qin PZ. Modular assembly of chimeric phi29 packaging RNAs that support DNA packaging. Biochem Biophys Res Commun. 2008;372:589–594. doi: 10.1016/j.bbrc.2008.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Trottier M., and, Guo P. Circularly permuted viral pRNA active and specific in the packaging of bacteriophage phi 29 DNA. Virology. 1995;207:442–451. doi: 10.1006/viro.1995.1103. [DOI] [PubMed] [Google Scholar]
- Lee CS., and, Guo P. A highly sensitive system for the in vitro assembly of bacteriophage phi 29 of Bacillus subtilis. Virology. 1994;202:1039–1042. doi: 10.1006/viro.1994.1434. [DOI] [PubMed] [Google Scholar]
- Lee CS., and, Guo P. In vitro assembly of infectious virions of double-stranded DNA phage phi 29 from cloned gene products and synthetic nucleic acids. J Virol. 1995;69:5018–5023. doi: 10.1128/jvi.69.8.5018-5023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., and, Low PS. Immunotherapy of folate receptor-expressing tumors: review of recent advances and future prospects. J Control Release. 2003;91:17–29. doi: 10.1016/s0168-3659(03)00215-3. [DOI] [PubMed] [Google Scholar]
- Lu Y., and, Low PS. Folate-mediated delivery of macromolecular anticancer therapeutic agents. Adv Drug Deliv Rev. 2002;54:675–693. doi: 10.1016/s0169-409x(02)00042-x. [DOI] [PubMed] [Google Scholar]
- Chen SJ. RNA folding: conformational statistics, folding kinetics, and ion electrostatics. Annu Rev Biophys. 2008;37:197–214. doi: 10.1146/annurev.biophys.37.032807.125957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Shcherbakova IV, Altman RB, Brenowitz M., and, Laederach A. High-throughput single-nucleotide structural mapping by capillary automated footprinting analysis. Nucleic Acids Res. 2008;36:e63. doi: 10.1093/nar/gkn267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P, Grimes S., and, Anderson D. A defined system for in vitro packaging of DNA-gp3 of the Bacillus subtilis bacteriophage phi 29. Proc Natl Acad Sci USA. 1986;83:3505–3509. doi: 10.1073/pnas.83.10.3505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha M, Plescia J, Leav I, Li J, Languino LR., and, Altieri DC. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res. 2009;69:4954–4958. doi: 10.1158/0008-5472.CAN-09-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulda S., and, Debatin KM. Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 2004;64:337–346. doi: 10.1158/0008-5472.can-03-1656. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Autoradiogram showing the Dicer processing of the [32P] labeled bipartite pRNA/siRNA chimeras.