Abstract
Recombinant adeno-associated virus (rAAV) are effective gene delivery vehicles that can mediate long-lasting transgene expression. However, tight regulation and tissue-specific transgene expression is required for certain therapeutic applications. For regulatable expression from the liver we designed a hepatospecific bidirectional and autoregulatory tetracycline (Tet)-On system (TetbidirAlb) flanked by AAV inverted terminal repeats (ITRs). We characterized the inducible hepatospecific system in comparison with an inducible ubiquitous expression system (TetbidirCMV) using luciferase (luc). Although the ubiquitous system led to luc expression throughout the mouse, luc expression derived from the hepatospecific system was restricted to the liver. Interestingly, the induction rate of the TetbidirAlb was significantly higher than that of TetbidirCMV, whereas leakage of TetbidirAlb was significantly lower. To evaluate the therapeutic potential of this vector, an AAV-Tetbidir-Alb-expressing interleukin-12 (IL-12) was tested in a murine model for hepatic colorectal metastasis. The vector induced dose-dependent levels of IL-12 and interferon-γ (IFN-γ), showing no significant toxicity. AAV-Tetbidir-Alb-IL-12 was highly efficient in preventing establishment of metastasis in the liver and induced an efficient T-cell memory response to tumor cells. Thus, we have demonstrated persistent, and inducible in vivo expression of a gene from a liver-specific Tet-On inducible construct delivered via an AAV vector and proved to be an efficient tool for treating liver cancer.
Introduction
Adeno-associated virus (AAV) is a naturally replication-defective single-stranded DNA parvovirus. The lack of pathogenicity of the virus, its persistence, long-term expression and relative lack of immune response have contributed to the increase in popularity of AAV vectors.1,2 The clinical efficacy and safety as well as the application range of AAV vectors will be broadened by developing systems capable of finely modulating the expression of therapeutic genes.3,4 In particular, treatment of malignancies by gene transfer of immunostimulatory proteins requires precise control over expression in order to avoid or reduce associated side effects. A regulatable system that can turn “on” and “off” therapeutic gene expression will not only be crucial for maintaining appropriate levels of a gene product within the therapeutic range, preventing any toxicity, but also allow the turning “off” of therapeutic gene expression to avoid harmful side effects. The development of drug-dependent transcription regulatory systems is thus of great importance.3,4
Tetracycline (Tet)-regulatable systems are based on the Escherichia coli Tn10 Tet-resistance operon, which consists of the Tet repressor protein (TetR) and the Tet operator DNA sequence (TetO DNA). In the absence of Tet or its derivate doxycycline (Dox) the TetR protein binds to the TetO DNA sequence and blocks gene expression, whereas in the presence of the drug, TetR changes its conformation and detaches from the DNA.3,4 This system has been modified to transform the Dox repressor system into a Dox inducible system (Tet-On system). In the Tet-On system, the reverse Tet transactivator (rtTA) fusion protein composed of the Dox-binding TetR mutant (now called rTetR) and the C-terminal activator domain from the herpes simplex virus VP16 protein was engineered to control gene expression with Dox. In the presence of Dox, the rtTA transactivator activates the activity of minimal promoters fused downstream of an array of seven TetO sequences.3,4
Tet-responsive regulatory systems are normally cloned in two different expression cassettes, one containing the regulatory protein (tTA) and the second one containing the Tet regulatory element fused to a minimal promoter, regulating the expression of the reporter or therapeutic transgene. These types of constructs are difficult to fit into a recombinant AAV (rAAV) vector due to the limited cloning capacity (~4.9 kb).5 For this reason, we developed a single Tet-inducible autoregulated expression system pTetbidirON, whose small size allowed regulated expression in AAV vectors.6,7 In this system, the transcription of both the rtTA and the transgene is initiated from a ubiquitous bidirectional, Tet-responsive promoter. Furthermore, a bidirectional polyadenylation sequence flanking the inducible system was included to insulate the expression cassette from the weak promoter activity of the inverted terminal repeats (ITRs). Using this system, an 80-fold induction in vivo in the rat brain has been obtained.6,7 Based on this principle, we have developed a hepatospecific Tet-On expression system. For this purpose, we replaced the minimal CMV promoter used in the original construct with the notably weak liver-specific murine albumin promoter.8,9 To characterize both inducible systems after systemic administration, expression cassettes-containing luciferase (luc) gene were packaged into AAV serotype 8 capsids (AAV8). AAV8, a serotype discovered in rhesus monkeys, is able to mediate robust transgene expression in various mouse tissues, being particularly efficient in liver transduction. Luc allows for monitoring of long-term in vivo expression and biodistribution analysis, where expression levels can be easily quantified using a cooled charge-coupled device camera or equivalent in vivo imaging systems.10
Interleukin-12 (IL-12) is a potent cytokine endowed with strong antitumor properties. IL-12 can serve as a link between the innate and adaptative immune responses because it can activate the proliferation of T lymphocytes and natural killer cells, the secretion of other inflammatory mediators such as interferon-γ (IFN-γ) and also activate the cytotoxic activity of these effector cells.11 In addition, IL-12 exerts an important antiangiogenic effect. These functions make this cytokine an attractive candidate to stimulate the immune response against cancer cells. The capacity of IL-12 to increase the number and activity of tumor-specific lymphocytes has been verified in humans, and specific antitumor effects have been observed.12 However, clinical responses tend to be poor, and intensification of the treatment is difficult due to the toxicity associated with systemic exposure to IL-12.12,13 The use of gene transfer strategies to obtain expression of the cytokine in specific locations is an attractive alternative.14,15,16 However, the clinical experience indicates that refinement of these vectors is needed. Intratumor administration of a replication-deficient adenoviral vector encoding the IL-12 gene was well tolerated in patients with hepatic metastases. Biological effect was demonstrated, but antitumor responses were very modest.16 The poor performance of the vector in terms of intensity and duration of expression was recognized as a key limitation in this approach. To circumvent these problems recombinant viral vectors capable of mediating long-term and sustained expression of IL-12 might be used. High-capacity adenovirus or AAV vectors represent ideal candidates to obtain sustained but controlled levels of the cytokine in serum.17
In this study, we demonstrate the ability of the hepatospecific Tet-regulatable system to establish long-term transgene regulation restricted to the liver following intravenous (i.v.) injection of AAV8 vector. Furthermore, the use of this vector encoding IL-12 cytokine plus Dox for 7 days prevents the development of MC38-derived liver metastasis and prolonged survival in 90% of mice with no side effects.
Results
Luc expression after i.v. administration of AAV-pTetbidir-CMV-luc and AAV-pTetbidir-Alb-luc
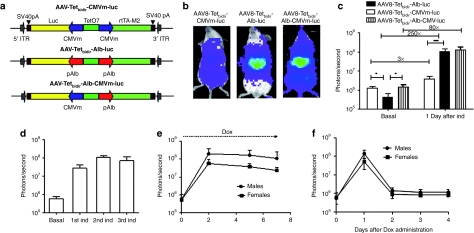
First, we tested luc expression biodistribution in vivo, after i.v. administration of an AAV8 vector harboring an autoregulated Tet-inducible system in which the expression of the transactivator and the gene of interest is under the control of TetO7 fused CMV minimal (CMVm) promoters (AAV8-Tetbidir-CMVm-luc) or the TetO7 fused albumin promoters8 (AAV8-Tetbidir-Alb-luc). A third vector was constructed in which the expression of the transactivator is under the control of the TetO7 fused albumin promoter and the expression of the gene of interest is under the control of TetO7 fused CMVm (AAV8-Tetbidir-Alb-CMVm-luc). All the constructs have been schematically represented in Figure 1a. Two groups of four BALB/c female mice received a dose of 5 × 1012 vector genomes (vg)/kg of either vector. Two weeks after vector injection luc expression was analyzed before (basal) and 24 hours after the intraperitoneal (i.p.) administration of Dox at a dose of 50 mg/kg. The optimal dose of Dox for transgene expression was experimentally determined (Supplementary Figure S1a). As shown in Figure 1b, luc expression in mice that received AAV8-Tetbidir-Alb-luc was confined to the liver, whereas a broader distribution of expression was detected in the animals AAV8-Tetbidir-CMVm-luc or AAV8-Tetbidir-Alb-CMVm-luc to quantify luc expression a region of interest covering the whole animal was drawn (as described in Supplementary Figure S1b). Quantification of the signal before induction revealed that leakage from the CMVm or the CMV-Alb promoter-inducible system was higher than from the albumin promoter-inducible system (Figure 1c). However, after Dox administration, luc expression was significantly higher in animals injected with AAV8-Tetbidir-Alb-luc or AAV8-Tetbidir-Alb-CMVm-luc than in the animal injected with AAV8-Tetbidir-CMVm-luc. The induction rate for AAV8-Tetbidir-pCMVm-luc, AAV8-Tetbidir-Alb-CMVm-luc, and AAV8-Tetbidir-Alb-luc was 3-, 80-, and 250-fold, respectively (Figure 1c). The viral genome copies in the liver were analyzed using a quantitative-PCR specific for the transactivator-expressing gene and no significant differences were observed (Supplementary Figure S2).
Figure 1.
In vivo characterization of AAV-pTetbidir-CMV-luc and AAV-pTetbidir-Alb-luc. (a) Schematic diagram of tetracycline-inducible adeno-associated viral (AAV) vectors used in this study. Alb, albumin promoter; CMVm, minimal early cytomegalovirus promoter; ITR, inverted terminal repeat; luc, luciferase gene; polyA, SV40 fragment containing the early and late polyadenylation signals; rtTAM2, mutated reverse tetracycline transactivator; TetO7, sequence-containing seven tetracycline operator sites. (b) BALB/c mice injected with 5 × 1012 vg/kg of AAV8-Tetbidir-CMVm-luc, AAV-Tetbidir-Alb-luc or AAV8-Tetbidir-Alb-CMVm-luc were analyzed with the charge-coupled device (CCD) camera before and 24 hours after administration of doxycycline (Dox). Shown are optical CCD images for luciferase expression of representative animal from each group, 1 day postinduction. (c) Luciferase expression was quantified and represented as photons/second. The data are shown as mean ± SD. The differences on luciferase expression was statistically evaluated by Student's t-test (*P < 0.05, ***P < 0.001). (d) Mice injected with AAV-Tetbidir-Alb-luciferase were reinduced 15, 30, and 60 days after vector injection and luciferase expression was measured. (e) AAV8-Tetbidir-Alb-luc, male and female BALB/c mice were injected intravenous (i.v.) with a dose of 5 × 1012 vg/kg. Two weeks after injection, luciferase expression was induced by a single intraperitoneal (i.p.) injection of Dox and expression induction was maintained by continuous administration of Dox in drinking water over the course of a week. Each dot represents the average of the decimal logarithm of bioluminescence for each group of animals at a time point. (f) The extinction time of luciferase expression was determined after removal of Dox.
Mice injected with AAV8-Tetbidir-Alb-luc were reinduced 15, 30, and 60 days after vector injection and luc expression reached similar levels after each induction (Figure 1d). To analyze whether sustained transgene expression can be obtained with the liver-specific vector, AAV8-Tetbidir-Alb-luc, male and female BALB/c mice were injected i.v. with a dose of 5 × 1012 vg/kg. Two weeks after injection, luc expression was induced by a single i.p. injection of Dox and expression induction was maintained by continuous administration of Dox in drinking water over the course of a week (2 mg/ml Dox + 5% sucrose). As shown in Figure 1e, luc expression can be maintained by administration of Dox for at least 1 week. Furthermore, the extinction of luc expression after removal of Tet was very rapid, as shown in Figure 1f, where 48 hours after removing Dox, expression of luc returned to basal levels.
Ex vivo biodistribution analysis
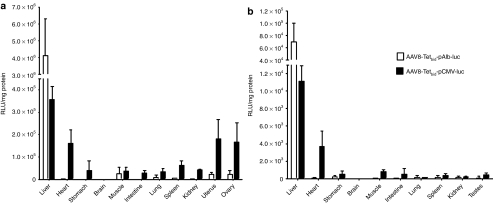
To corroborate the liver specificity of the vector, BALB/c and C57BL/6 mice of both genders (n = 7) were injected i.v. with 5 × 1012 vg/kg of AAV8-Tetbidir-pAlb-luc, AAV8-Tetbidir-CMV-luc. Twenty-one days later, luc expression was induced by i.p. injection of 50 mg/kg Dox in five animals per group, whereas two animals were left untreated. Twenty-four hours later, mice were analyzed for luc expression using the living image IVIS system and then sacrificed and organs harvested. Luc expression was analyzed from organ extracts using a luminometer. Results with C57BL/6 mice are shown in Figure 2, whereas similar results were also obtained with BALB/c mice (Supplementary Figure S3). Ex vivo luc expression analysis corroborates what was observed in vivo. Luc expression was restricted to the liver in all mice that received AAV8-Tetbidir-Alb-luc whereas luc expression was detected in several organs in addition to the liver in mice injected with AAV8-Tetbidir-CMV-luc. The biodistribution of luc expression of vector AAV8-Tetbidir-CMV-luc was similar to previous data obtained by our group using a rAAV8 encoding luc under the control of the constitutive and ubiquitous promoter:10 in female mice luc expression can be detected mainly in the liver followed by the heart, ovary, and uterus (Figure 2a) and at lower levels in the stomach, muscle, lung, intestine, and kidney. In males, luc expression was detected in the liver and in the heart, followed by intestine and stomach at lower levels, muscle (Figure 2b). Biodistribution of luc expression after AAV8-Tetbidir-Alb-CMVm-luc was similar to the one found for AAV8-Tetbidir-CMVm-luc vector in the absence of induction. However, after Dox administration luc was induced only in the liver in the animals that received AAV8-Tetbidir-Alb-CMVm-luc while was induced in all the transduced organs in animals injected with AAV8-Tetbidir-CMVm-luc (data not shown).
Figure 2.
Ex vivo biodistribution analysis of luciferase expression after AAV8-Tetbidir-Alb-luc or AAV8-Tetbidir-CMV-luc injection. (a) Male and (b) female C57BL/6 mice were intravenous (i.v.) injected with 5 × 1012 vg/kg of AAV8-Tetbidir-CMVm-luc or AAV-Tetbidir-Alb-luc. Twenty days after virus injection luciferase expression was induced by intraperitoneal (i.p.) administration of doxycycline (Dox) at a dose of 50 mg/kg and 24 hours later all the animals were sacrificed and organs dissected. The levels of luciferase activity (RLUs) were determined in organ homogenates and were normalized to protein content (RLU/mg). The data are shown as mean ± SEM. AAV, adeno-associated virus; CMV, cytomegalovirus; luc, luciferase gene.
In vitro and in vivo characterization of AAV8-Tetbidir-Alb-IL-12
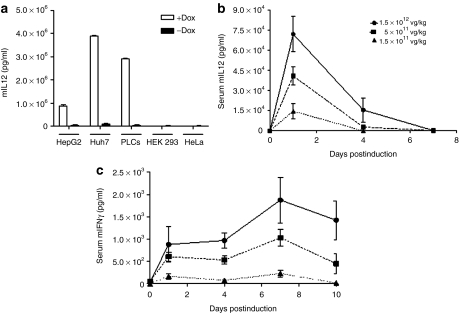
To construct AAV8-Tetbidir-Alb-IL-12, the luc reporter gene was replaced by the gene encoding single chain of IL-12.18 The inducibility and hepatospecificity of the system were first tested in vitro. Human HepG2, PLC/PRF/5, and HuH-7 cell lines of hepatic origin, as well as HEK293 and HeLa cells of nonhepatic origin were transfected with the pro-AAV plasmid containing the AAV-Tetbidir-Alb-IL-12 genome. Four hours after transfection, Dox was added to the culture medium at a concentration of 1 µg/ml and 48 hours later supernatants were harvested from induced and noninduced cells. As shown in Figure 3a, IL-12 was only detected in the supernatant of the induced hepatic cells and not in the supernatants from HEK293 or HeLa cells.
Figure 3.
In vitro and in vivo characterization of AAV8-Tetbidir-Alb-IL-12 construction. (a) Analysis of interleukin-12 (IL-12) expression by enzyme-linked immunosorbent assay (ELISA) in the supernatant of hepatic (HepG2, PLC/PRF/5, and HuH-7) and nonhepatic cells (HEK293, HeLa) after pAAV8-Tetbidir-Alb-IL-12 plasmid transfection and administration of doxycycline (Dox). (b, c) For in vivo characterization C57BL/6 female mice received three different doses of AAV8-Tetbidir-Alb-IL-12: 1.5 × 1012, 5 × 1011, 1.5 × 1011 vg/kg (n = 5). Twenty-one days after injection of the rAAV8 vector IL-12 expression was induced by the administration of 50 mg/kg of Dox and then maintained via the administration of Dox in drinking water at a concentration of 2 mg/ml. (b) IL-12 and (c) interferon (IFN)-γ levels in serum were measured by enzyme-linked immunosorbent assay (ELISA) before and 1, 4, 7, and 10 hours after administration of Dox. AAV8, adeno-associated virus serotype 8; Alb, albumin promoter; rAAV, recombinant AAV.
For in vivo analysis, C57BL/6 female mice received three different doses of pAAV8-Tetbidir-Alb-IL-12: 1.5 × 1012, 5 × 1011, 1.5 × 1011 vg/kg (n = 5 per group). Twenty-one days after virus injection, IL-12 expression was induced by i.p administration of Dox, and then maintained by the administration of Dox in drinking water (at a concentration of 2 mg/ml). IL-12 levels in serum were measured before and 1, 4, 7, and 10 days after i.p. administration of Dox. We had previously determined in the MC38 syngeneic tumor model that serum concentrations of IL-12 <20 ng/ml have no antitumor effect, and levels >1,000 ng/ml are associated with toxicity.17 The analysis of IL-12 expression before and after Dox administration showed that IL-12 cannot be detected by enzyme-linked immunosorbent assay in the absence of Dox. Twenty-four hours after administration of Dox, serum IL-12 levels reached maximal levels, which correlate with the dose of rAAV8 vector injected. After that, IL-12 levels leveled off reaching undetectable levels 7 days after administration of Dox (Figure 3b). In contrast, the same induction regime, given to mice injected with an equivalent vector expressing luc resulted in the expression of sustained levels of the transgene (Figure 1e). These results indicate that, as previously reported, the function of drug-inducible expression systems is influenced by the immunostimulatory properties of IL-12, acting at the transcriptional level.19
Since IFN-γ is the main mediator of IL-12 antitumoral activity, IFN-γ levels were measured before administration of Dox and 1, 4, 7, and 10 days after induction. Analysis of IFN-γ expression showed that the maximum levels were reached 7 days after administration of Dox and decreasing thereafter (Figure 3c). The levels of IFN-γ expression were proportional to the dose of virus with average levels of 2,000, 1,000, and 250 pg/ml for doses 1.5 × 1012, 5 × 1011, 1.5 × 1011 vg/kg, respectively. Regarding toxicity, very slight or no elevation in serum transaminases were detected during the induction period. However, higher viral dose, 5 × 1012 vg/kg, induces transaminase elevation and the dead of 30% the animals (Supplementary Figure S4).
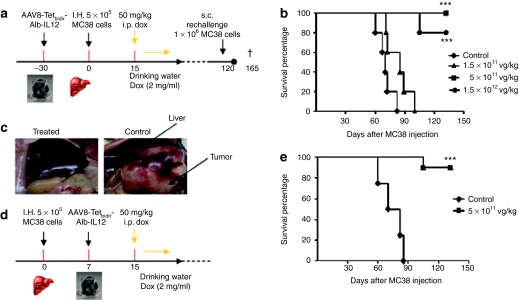
Antitumoral effect of AAV8-Tetbidir-Alb-IL-12 vector
C57BL76 female mice were injected with two different doses of AAV8-Tetbidir-Alb-IL-12 vector 1.5 × 1012, 5 × 1011, and 1.5 × 1011 vg/kg (n = 10). Other mice were administered saline solution instead of vector and Dox (negative control group). One month after vector administration, hepatic tumors were established by direct implantation of 5 × 105 MC38Luc120,21 murine colorectal cancer cells in the left liver lobe following medial laparotomy in isofluorane-anaesthetized animals. Cell engraftment was verified by bioluminescence 2–3 days later. Ten days after the inoculation of the tumor cells, IL-12 expression induction was performed as described previously, that is, by administration of 50 mg/kg i.p., followed by administration of Dox via drinking water for 6 days. A schematic representation of the experiments is provided in Figure 4a. IL-12 and IFN-γ expression levels before and after administration of Dox were in accordance with the result as described in Figure 3b,c (data not shown).
Figure 4.
Intravenous administration of AAV8-Tetbidir-Alb-IL-12 results in antitumoral activity. (a) Schematic representation of the antitumoral prophylactic study. (b) C57BL/6 female mice were injected with three different doses of AAV8-Tetbidir-Alb-IL-12 vector 1.5 × 1012, 5 × 1011, 1.5 × 1011 vg/kg (n = 10). A group of mice were inoculated with saline solution instead of vector and doxycycline (Dox) (control group) (n = 10). One month after vector administration, hepatic tumors were established by direct implantation of 5 × 105 MC38Luc1 cells in the left liver lobe following medial laparotomy of isofluorane-anaesthetized mice. Cell engraftment was verified by bioluminescence 2–3 days later. Ten days after the inoculation of the tumor cell, interleukin (IL)-12 expression induction was performed as described previously, that is, by intraperitoneal (i.p.) administration of 50 mg/kg, followed by administration of Dox via drinking waster for 6 days. (c) Representative images from a mouse belonging to the group treated with 5 × 1011 vg/kg AAV8-Tetbidir-Alb-IL-12 and a mouse treated with saline. (d) Schematic representation of the antitumoral therapeutic study. (e) Hepatic tumors were established by direct implantation of 5 × 105 MC38Luc1 cells in the left liver lobe following medial laparotomy of isofluorane-anaesthetized mice. Cell engraftment was verified by bioluminescence 2–3 days later. Seven days after tumor injection mice were treated with vg/kg of AAV8-Tetbidir-Alb-IL-12 vector or saline (n = 10). Fifteen days after the inoculation of the tumor cell, IL-12 expression induction was performed as described previously, that is, by i.p. administration of 50 mg/kg, followed by administration of Dox via drinking waster for 6 days. Kaplan–Meier survival analysis of mice with established MC38 tumors after treatment is assessed (***P < 0.01). AAV8, adeno-associated virus serotype 8; Alb, albumin promoter; s.c. subcutaneous.
Eighty and a hundred days after cell implantation all the animals from the control and from the group receiving the lowest dose of the AAV8-Tetbidir-Alb-IL-12, respectively, had died as a consequence of tumor progression. In contrast, none of the animals receiving 5 × 1011 vg/kg died and only two mice from the group receiving the highest dose died as a consequence of tumor progression at day 105. The rest of the mice were alive and free of tumor 100 days after cell implantation (Figure 4b). A representative image from AAV-Tetbidir-Alb-IL-12 (5 × 1011 vg/kg) treated and control mice was shown (Figure 4c).
After showing that AAV8-Tetbidir-Alb-IL-12 vector prevents tumor development, we analyzed the antitumoral efficacy of this vector in a therapeutic antitumoral assay. A schematic representation of the experiments is provided in Figure 4d. Hepatic tumors were established by direct implantation of 5 × 105 MC38Luc1 cells, 7 days after cell injection 10 animals were treated with 5 × 1011 vg/kg AAV-Tetbidir-Alb-IL-12 or saline. Eight days after vector injection IL-12 expression was induced by Dox administration, as previously described. Eighty-five days after cell implantation all the animals from the control group had died as a consequence of tumor progression whereas only one animal of the treated group died 105 days after cell injection (Figure 4e).
Protection against rechallenge
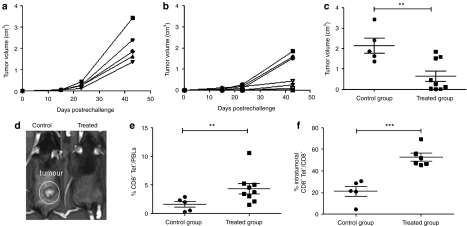
Protection from tumor relapse or from the appearance of new liver metastases is crucial to improve the prognosis of patients with colorectal cancer. Therefore, we studied the immunological protection against a tumor rechallenge. Animals that remained free from their hepatic tumors for more 120 days after cell implantation (n = 9) received a new inoculation of 1 × 106 MC38Luc1 cells in a (subcutaneous) distant location. Monitoring the tumor growth revealed that, on average, pretreated mice presented a delay in tumor progression compared with naive animals (Figure 5a,b). Furthermore, four out of nine mice were completely protected against tumor development while the five naive animals that were injected at the same time developed large tumors (Figure 5a,b). To appreciate this difference, in Figure 5c, we show the individual size of subcutaneous tumors at the end of the observation period. Photographs of representative liver tumors in control and AAV treated mice are shown in mice Figure 5c.
Figure 5.
AAV8-Tetbidir-Alb-IL-12 vector treatment induces a memory immune response and protects mice against rechallenge. Mice that remained free from hepatic tumors for >1 month after being treated with AAV8-Tetbidir-Alb-IL-12 vector (n = 9) (treated group) received a subsequent inoculation of 1 × 106 MC38Luc1 cells in a distant (subcutaneous) location as well as a group of five naive animals (control group). (a–b) Monitoring of tumor growth revealed that, on average, pretreated mice presented a delay in tumor progression compared with naive animals. (c) Individual size of subcutaneous tumors at the end of the observation period. (d) Representative images of subcutaneous tumor developed in a mouse from treated and control group. (e) Twenty-three days after tumor rechallenge the percentage of MC38-specific CD8+ T cells were determined in blood by tetramer staining in AAV8-Tetbidir-pAlb-IL-12-treated group and in the naive group The percentage of CD8+tetramer+ cells from total peripheral blood lymphocytes is represented. (f) At the end of the experiment, tumors were removed and lymphocytes were isolated to determine the proportion of MC38-specific CD8 T cells from the total number of infiltrating CD8 cells. AAV8, adeno-associated virus serotype 8; Alb, albumin promoter; IL, interleukin.
Twenty-three days after tumor rechallenge, the percentage of MC38-specific CD8 T cells were determined in blood. Higher expansion of CD8+ T lymphocytes specific for MC38 cells in peripheral blood were observed in the group previously treated with AAV8-Tetbidir-Alb-IL-12 (Figure 5d) compared with untreated mice.
We also evaluated the presence of CD8+ T lymphocytes specific for MC38 cells in the tumors at the time of sacrifice (45 days after rechallenge). We detected a significantly higher number of MC38-specific CD8 T cells in the tumor of mice previously treated being 50% of the CD8+ cells tetramer positive (Figure 5e), suggesting a major role of CD8 T-cell population in IL-12-mediated antitumor immune response, as previously described.
Discussion
The use of cytokines with strong antitumor effects for the treatment of malignancies will probably require repeated administrations to achieve effective levels, whereas for many patients the therapeutic effect is hampered by the systemic toxicity of these cytokines. Gene transfer strategies offer the possibility to express the therapeutic gene in the target organ reducing systemic toxicity. Furthermore, the use of a regulatable expression system will allow for controlling transgene expression levels with the ability to turn off expression once the therapeutic effect has been obtained or if severe toxicity is observed.3,4 rAAV vectors are promising gene delivery vehicles with distinct advantages in terms of being able to mediate long-term gene expression with minimal pathogenicity.1,2 Therefore, in this study, we pursued two main aims: (i) the construction of a Tet-On system to allow tight regulation of gene expression in the liver mediated by an AAV8 vector and (ii) application of such a vector encoding IL-12 to treat liver malignancies.
One of the limitations of the rAAV vectors system is a limited cloning capacity of ~4.9 kb,5 although for AAV5, there are reports of an increased capacity.22 For this reason regulated gene expression systems for AAV vectors usually consists of two AAV vectors: one that expresses a regulatory molecule which interacts with a small molecule inducer, and another, in which expression of the transgene of interest is controlled by a regulatory DNA region that reacts with the regulatory protein/inducer–molecule complex. The two AAV system requires coinfection by both vectors for a given cell to provide regulated expression.23 However, in this study, we have used a system previously described, in which both expression cassettes fits into one AAV. In this system, all necessary elements of a two-expression cassette system are contained within 3.5 kb of DNA. In this Tet-inducible AAV vector (AAV-TetbidirOn) the transcription of both the rtTA (rtTAM2) and the transgene is initiated from a bidirectional Tet-responsive promoter and terminated at bidirectional SV40 polyadenylation sites flanking both ITRs.8 In the present study, we have developed a liver-specific Tet-inducible AAV vector by replacing CMVm promoters by albumin promoters.9 We have also constructed a hybrid vector in which the expression of the transactivator is controlled by the albumin promoter and the expression of the transgene by the CMVm promoter.
When we compared in vivo the constitutive and liver-specific systems delivered by AAV vectors, the background expression in the absence of Dox was significantly higher when the expression system contains the minimal CMV promoter than with the liver-specific promoter in both male and female mice from BALB/c and C57BL/6 strains. The lower basal expression levels is most likely related with the lower transcriptional activity of the albumin promoter in comparison with the minimal CMV promoter, as has been previously reported.8,9 Thus, the introduction of the Alb promoter reduces basal expression from transduced tissues. In general, the background expression levels from an inducible system directly correlates with its' inducibility, where higher levels of expression in the absence of inducer is associated with higher rates of induction. Unexpectedly, we showed that the liver-specific inducible system led to very low basal expression, whereas the inducibility is significantly higher than the CMVm-based system (250-fold induction versus 3-fold induction). These results were corroborated by the use of the expression system containing both promoters, that showed mixed characteristics. The leakage of the system was higher and it has a wider biodistribution, however after induction the expression levels were similar to the ones obtained with the liver-specific expression cassette. For some therapeutic transgenes, it might be more important to obtain the highest possible level of induced expression, where higher levels of background expression may be tolerable. For other transgenes, especially those coding for toxic gene products, it might be critical to have the lowest possible background, with a high level of induced expression. The analysis of IL-12 expression showed that in the absence of Dox IL-12 is undetectable in serum whereas very high levels can be detected after Dox administration.
The molecular bases for the higher inducibility of the liver-specific inducible system are unknown. Both vectors contain exactly the same TetO7 sequence and we only found minor differences between both vectors at the 5′ and 3′ which are 9 and 17 nucleotides longer, respectively, in the liver-specific system than in the ubiquitous system. More experiments should be performed to elucidate the striking differences in the inducibility of both systems.
The in vivo characterization of the liver-specific inducible expression system demonstrated that expression of the luc transgene could be reinduced for several months by simply injecting Dox, or adding Dox to the animals' drinking water. Furthermore, transgene expression was rapidly turned off upon withdrawal of the antibiotic. Forty-eight hours after the removal of Dox, luc expression returned to basal levels.
When the luc gene was replaced with the therapeutic gene for IL-12, we saw no basal expression and a clear viral dose-dependent induction of cytokine expression with Dox. However, in contrast with the pattern of luc expression, continuous administration of the inducer resulted in a marked decrease of IL-12 expression, disappearing 7 days after the first day of Dox injection. This effect may be related to promoter silencing or loss of DNA by degradation. Previous studies performed by Reboredo et al.,19 have shown that biologically active IL-12 can transiently inhibit the function of drug-inducible systems plasmid vectors by reducing promoter activity, probably through IFN-γ and protein deacetylation-dependent mechanisms. Most likely, this will be also the situation after rAAV vector injection because IL-12 expression in IFN-γ receptor knockout mice resulted in sustained expression of IL-12 after administration of Dox (data not shown).
To test the antitumoral potential of AAV8-Tetbidir-pAlb-IL-12, the murine colorectal cancer cell line MC38 has been used as a model for poorly immunogenic tumors. Using this murine model of metastatic colon cancer to the liver in both prophylactic and therapeutic assays, we found a complete eradication of tumors in most of the animals treated with AAV8-Tetbidir-Alb-IL-12 and Dox for 7 days, whereas all animals that received saline, showed progressive tumor growth and death before 3 months. The antitumor treatment occurred without toxicity as indicated by the absence of a detectable rise in serum transaminase levels and long-term survival of all AAV8-Tetbidir-Alb-IL-12-treated mice. Furthermore, rechallenge experiments indicate that the mice have developed an MC38-specific memory T-cell response.24 The antitumoral efficacy of rAAV8 vector is better that the one showed by first generation adenoviral vector expressing the same cytokine and similar to the efficacy showed by Semliki forest virus expressing IL-12 under similar experimental settings.25,26,27
In conclusion, we have demonstrated long-term, persistent, and inducible in vivo expression of a gene from a liver-specific Tet-On inducible construct delivered via an AAV vector. Our system provides a powerful molecular tool to switch transgene expression on and off in vivo in the liver, allowing its delivery only for a desired period of time, thus improving the efficacy of therapeutic gene transfer and limiting their toxicity. Furthermore, this vector enabled tight regulation of the cytokine IL-12 and proved to be an efficient tool for treating liver cancer. These vectors combine a good antitumoral efficacy with a good safety profile.
Materials and Methods
Viral construct. The pAC1-M2 plasmid comprising AAV2 ITRs flanking the bidirectional Tet-responsive cassette expressing both rtTAM2 and enhanced green fluorescent protein has been described previously.6 To obtain the plasmid: AAV-Tetbidir-CMVm-luc, the luc cDNA was amplified using primers flanked by SalI and NotI sites, using pAlb-luc8 as template and was subsequently subcloned into pCR2.1 to obtain pCR2.1-luc. The SalI-NotI fragment obtained from pCR2.1-luc was inserted into pAC1-M2 previously digested with SalI and NotI, replacing the GFP gene with luc. To obtain the AAV-Tetbidir-Alb-luc, first a sequence-containing seven Tet operator sites fused to the albumin promoter was amplified by PCR using as template the plasmid pTonL2(T)-mIL-128 and primers containing AfeI and EcoRI sites at 5′ends and subcloned into pCR2.1 to obtain pCR2.1-TetO7-Alb. In a second step, the albumin promoter was amplified using primers containing AfeI site and subcloned into pCR2.1. To obtain pCR2.1-Alb, the albumin promoter was excised with AfeI and cloned into pCR2.1-TetO7-Alb to obtain the plasmid pCR2.1-Alb-Teto7-Alb. After sequencing, the fragment Alb-TetO7-Alb was excised with EcoRI and SalI and cloned into AAV-Tetbidir-CMVm-luc previously digested with the same enzymes and replacing CMV-TetO7-CMV by Alb-TetO7-Alb, obtaining the plasmid AAV-Tetbidir-Alb-luc. To obtain the plasmid, AAV-Tetbidir-Alb-IL-12, luc gene was excised from AAV-Tetbidir-Alb-luc by SalI-NotI digestion, the IL-12 single chain gene was excised using the same enzymes from pCR2.1-scIL-12,18 and ligated to AAV-Tetbidir-Alb.
AAV8 production, purification, and titration. rAAV8 vectors with wild-type AAV2 ITRs were produced by calcium phosphate-mediated co-transfection in HEK293 cells.28 For each production a mixture of plasmids, 20 µg of pro-AAV plasmid and 55 µg pDP8.ape (PlasmidFactory, KG, Bielefeld, Germany), was transfected into 293 T cells 15-cm plate using linear polyethylenimine 25 kDa (Polysciences, Warrington, PA) as described.29 The cells were harvested 48 hours after transfection and virus was released from the cells by three rounds of freeze–thawing. Crude lysate from all batches was then treated with Benzonase (50 U/ml crude lysate) for 1 hour at 37 °C and then kept at −80 °C until purification. Purification of crude lysate was performed by iodixanol gradients according to the method of Zolotukhin et al.30 The purified batches were concentrated and diafiltrated by cross-flow filtration (Spectrum Laboratories, Rancho Dominguez, CA) with a molecular mass cutoff of 400 kDa. The batches were then concentrated further by passage through Centricon tubes (YM-100; Millipore, Bedford, MA) to a final concentration of 1 × 1012 vg/ml, as determined by quantitative-PCR. After concentration, the viral batches were filtered (pore size, 0.22 mm) and stored at −80 °C. Viral titers in terms of genome copies/ml were determined by quantitative-PCR, performed three times in triplicate at three different dilutions.
Bioluminescence imaging. Mice were immobilized with i.p. anesthesia (a mixture of xylacine and ketamine). The substrate -luciferin (150 µg/kg dissolved in phosphate-buffered saline; Promega, Madison, WI) was injected i.p. Ten minutes later, animals were placed in the dark chamber for light acquisition in an IVIS charge-coupled device camera system (Xenogen, Alameda, CA) and analyzed with the Living Image 2.20 software package (Xenogen). A region of interest covering the whole animal was defined, and quantification of light emission was performed in photons/second. Time exposure ranged from 1 second to 5 minutes depending on light intensity.10
Luc measurement. Organ sections were frozen in liquid nitrogen until processed. Tissue was homogenized in Luciferase Lysis Reagent (Promega). Samples were centrifuged for 15 seconds at 12,000g. Supernatant was collected and measured in a tube luminometer. Total proteins were quantified using the Bradford assay using bovine serum albumin as a standard.10
Mice, tumor models, and treatment. Male and female BALB/c and C57BL/6 mice (6–8 weeks old) were obtained from Harlan Laboratories (Barcelona, Spain) and maintained according to the guidelines of our institution. For AAV biodistribution studies, mice received the virus via tail vein in a volume of 200 µl. Dox was administered i.p. in a 200 µl of saline solution. For all procedures, mice were anesthetized with ketamine–xylazine. Hepatic tumors were established by direct implantation of 5 × 105 MC38Luc1 cells in the left liver lobe of C57BL/6 mice following medial laparotomy in isofluorane-anaesthetized animals. Cell engraftment was verified by bioluminescence 2–3 days later. Characterization of this tumor model has been previously described.20,21 For subcutaneous tumor formation (rechallenge experiments) a total of 106 cells were injected in the right hind flank. In both cases, cells were resuspended in a total volume of 50 µl saline solution. Tumor size was monitored at indicated time points by measuring two perpendicular tumor diameters using a precision calliper. Tumor volume was calculated using the following formula: V = length × width2 × 0.5. Survival was checked daily and mice were euthanized if general status was deteriorated or subcutaneous tumors exceeded 20 mm in diameter. All in vivo studies were performed in accordance with the local animal commission.
Cell lines. HEK293, 293T, HeLa, HepG2, PLC/PRF/5, and HuH-7 cell lines were purchased from the ATCC (Manassas, VA) and were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 100 U/ml penicillin/streptomycin. One microgram of plasmid was transfected, using polyethylenimine with 1 µg of AAV-Tetbidir-Alb-IL-12.29 Briefly, 25-kDa branched polyethylenimine was used at an nitrogen to phosphate (N/P) ratio of 10 and using NaCl 150 mmol/l as the vehicle; 5 × 105 cells were plated in six-well plates and 24 hours later were transfected. After 24 hours, supernatant were collected and stored at −80 °C.
Determination of IL-12 and IFN-γ. Concentration of murine IL-12 and IFN-γ were determined by OptE1A mouse IL-12 (p70) and mouse IFN-γ enzyme-linked immunosorbent assay kits (BD Bioscience-Pharmingen, San Diego, CA).
Determination of serum levels of aspartate aminotransferase and alanine aminotransferase. The blood level of aspartate amino transferase and alanine aminotransferase were measured using commercial kits (Sigma Chemicals, St Louis, MO) in a Hitachi Automatic Analyzer (Boehringer Mannheim, Indianapolis, IN).
Antibodies and flow cytometry. Single-cell suspensions were pretreated with FcR-Block (anti-CD16/32 clone 2.4G2; BD Bioscience-Pharmigen). Afterward, cells were stained with the following antibodies: CD8a fluorescein (fluorescein isothiocyanate)-conjugated (53–6.7; eBioscience, San Diego, CA). To identify specific tumor CD8 T lymphocytes, cells were stained with the iTAg major histocompatibility complex class I tetramer loaded with the KSPWFTTL synthetic peptide and conjugated with PE (Beckmann Coulter, Madrid, Spain). Cells were analyzed with a FACSCalibur (BD) and FlowJo software.
Statistical analysis. Statistical analysis was performed with a two-tailed Student's t-test for unpaired samples or a Mann–Whitney nonparametric test (GraphPad Prism software). Survival curves were compared by logrank test (GraphPad Prism software).
SUPPLEMENTARY MATERIAL Figure S1. In vivo determination of the optimal dose of Dox to induce Luc expression and region of interest (ROI) drawn to analyze Luc expression. Figure S2. Viral genome copies were quantified by Q-PCR in the liver of mice injected with AAV8-Tetbidir-CMVm-luc, AAV8-Tetbidir-Alb-luc, and AAV8-Tetbidir-Alb-CMVm-luc. Figure S3. Ex vivo biodistribution analysis of luciferase expression after AAV8-Tetbidir-pAlb-luc or AAV8-Tetbidir-CMV-luc injection in female and male BALB/c mice. Figure S4. Analysis of serum AST levels in animals treated with 5 × 1012, 1.5 × 1012, and 1.5 × 1011 vg/kg AAV8-Tetbidir-Alb-IL-12 vector before and 1 and 7 days after Dox administration.
Acknowledgments
We thank Dr Ruben Hernandez–Alcoceba for fruitful discussion and critical review of the manuscript. We thank Pilar Alzuguren, Africa Vales, Cristina Olagüe, and Roberto Ferrero for technical assistance. L.V. was in receipt of an FPI grant, M.D.S. is in receipt of a fellowship from Fondo de Investigaciones sanitarias. This work was funded in part by grants from the UTE project CIMA, grants SAF 2006-03623 and SAF2009-08524 from the Spanish Department of Science and Fundación Mutua Madrileña.
Supplementary Material
In vivo determination of the optimal dose of Dox to induce Luc expression and region of interest (ROI) drawn to analyze Luc expression.
Viral genome copies were quantified by Q-PCR in the liver of mice injected with AAV8-Tetbidir-CMVm-luc, AAV8-Tetbidir-Alb-luc, and AAV8-Tetbidir-Alb-CMVm-luc.
Ex vivo biodistribution analysis of luciferase expression after AAV8-Tetbidir-pAlb-luc or AAV8-Tetbidir-CMV-luc injection in female and male BALB/c mice.
Analysis of serum AST levels in animals treated with 5 × 1012, 1.5 × 1012, and 1.5 × 1011 vg/kg AAV8-Tetbidir-Alb-IL-12 vector before and 1 and 7 days after Dox administration.
REFERENCES
- Grieger JC., and, Samulski RJ. Adeno-associated virus as a gene therapy vector: vector development, production and clinical applications. Adv Biochem Eng Biotechnol. 2005;99:119–145. [PubMed] [Google Scholar]
- Daya S., and, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21:583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M., and, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stieger K, Belbellaa B, Le Guiner C, Moullier P., and, Rolling F. In vivo gene regulation using tetracycline-regulatable systems. Adv Drug Deliv Rev. 2009;61:527–541. doi: 10.1016/j.addr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Yang H., and, Colosi P. Effect of genome size on AAV vector packaging. Mol Ther. 2010;18:80–86. doi: 10.1038/mt.2009.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chtarto A, Bender HU, Hanemann CO, Kemp T, Lehtonen E, Levivier M.et al. (2003Tetracycline-inducible transgene expression mediated by a single AAV vector Gene Ther 1084–94. [DOI] [PubMed] [Google Scholar]
- Chtarto A, Yang X, Bockstael O, Melas C, Blum D, Lehtonen E.et al. (2007Controlled delivery of glial cell line-derived neurotrophic factor by a single tetracycline-inducible AAV vector Exp Neurol 204387–399. [DOI] [PubMed] [Google Scholar]
- Kramer MG, Barajas M, Razquin N, Berraondo P, Rodrigo M, Wu C.et al. (2003In vitro and in vivo comparative study of chimeric liver-specific promoters Mol Ther 7375–385. [DOI] [PubMed] [Google Scholar]
- Zabala M, Wang L, Hernandez-Alcoceba R, Hillen W, Qian C, Prieto J.et al. (2004Optimization of the Tet-on system to regulate interleukin 12 expression in the liver for the treatment of hepatic tumors Cancer Res 642799–2804. [DOI] [PubMed] [Google Scholar]
- Pañeda A, Vanrell L, Mauleon I, Crettaz JS, Berraondo P, Timmermans EJ.et al. (2009Effect of adeno-associated virus serotype and genomic structure on liver transduction and biodistribution in mice of both genders Hum Gene Ther 20908–917. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Rakhit A, Yeon MM, Ferrante J, Fettner S, Nadeau R, Motzer R.et al. (1999Down-regulation of the pharmacokinetic-pharmacodynamic response to interleukin-12 during long-term administration to patients with renal cell carcinoma and evaluation of the mechanism of this “adaptive response” in mice Clin Pharmacol Ther 65615–629. [DOI] [PubMed] [Google Scholar]
- Leonard JP, Sherman ML, Fisher GL, Buchanan LJ, Larsen G, Atkins MB.et al. (1997Effects of single-dose interleukin-12 exposure on interleukin-12-associated toxicity and interferon-gamma production Blood 902541–2548. [PubMed] [Google Scholar]
- Barajas M, Mazzolini G, Genové G, Bilbao R, Narvaiza I, Schmitz V.et al. (2001Gene therapy of orthotopic hepatocellular carcinoma in rats using adenovirus coding for interleukin 12 Hepatology 3352–61. [DOI] [PubMed] [Google Scholar]
- Lui VW, Falo LD., Jr, and, Huang L. Systemic production of IL-12 by naked DNA mediated gene transfer: toxicity and attenuation of transgene expression in vivo. J Gene Med. 2001;3:384–393. doi: 10.1002/jgm.201. [DOI] [PubMed] [Google Scholar]
- Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I.et al. (2004Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors J Clin Oncol 221389–1397. [DOI] [PubMed] [Google Scholar]
- Wang L, Hernández-Alcoceba R, Shankar V, Zabala M, Kochanek S, Sangro B.et al. (2004Prolonged and inducible transgene expression in the liver using gutless adenovirus: a potential therapy for liver cancer Gastroenterology 126278–289. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Rao PK, Gately MK., and, Mulligan RC. Bioactive murine and human interleukin-12 fusion proteins which retain antitumor activity in vivo. Nat Biotechnol. 1997;15:35–40. doi: 10.1038/nbt0197-35. [DOI] [PubMed] [Google Scholar]
- Reboredo M, Zabala M, Mauleon I, De Las Rivas J, Kreppel F, Kochanek S.et al. (2008Interleukin-12 inhibits liver-specific drug-inducible systems in vivo Gene Ther 15277–288. [DOI] [PubMed] [Google Scholar]
- Zabala M, Alzuguren P, Benavides C, Crettaz J, Gonzalez-Aseguinolaza G, Ortiz de Solorzano C.et al. (2009Evaluation of bioluminescent imaging for noninvasive monitoring of colorectal cancer progression in the liver and its response to immunogene therapy Mol Cancer 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crettaz J, Berraondo P, Mauleón I, Ochoa L, Shankar V, Barajas M.et al. (2006Intrahepatic injection of adenovirus reduces inflammation and increases gene transfer and therapeutic effect in mice Hepatology 44623–632. [DOI] [PubMed] [Google Scholar]
- Allocca M, Doria M, Petrillo M, Colella P, Garcia-Hoyos M, Gibbs D.et al. (2008Serotype-dependent packaging of large genes in adeno-associated viral vectors results in effective gene delivery in mice J Clin Invest 1181955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Sanftner LH, Rendahl KG, Quiroz D, Coyne M, Ladner M, Manning WC.et al. (2001Recombinant AAV-mediated delivery of a tet-inducible reporter gene to the rat retina Mol Ther 35 Pt 1688–696. [DOI] [PubMed] [Google Scholar]
- Gong J, Chen D, Kashiwaba M., and, Kufe D. Induction of antitumor activity by immunization with fusions of dendritic and carcinoma cells. Nat Med. 1997;3:558–561. doi: 10.1038/nm0597-558. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Madoz JR, Prieto J., and, Smerdou C. Semliki forest virus vectors engineered to express higher IL-12 levels induce efficient elimination of murine colon adenocarcinomas. Mol Ther. 2005;12:153–163. doi: 10.1016/j.ymthe.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Tirapu I, Arina A, Mazzolini G, Duarte M, Alfaro C, Feijoo E.et al. (2004Improving efficacy of interleukin-12-transfected dendritic cells injected into murine colon cancer with anti-CD137 monoclonal antibodies and alloantigens Int J Cancer 11051–60. [DOI] [PubMed] [Google Scholar]
- Gambotto A, Tüting T, McVey DL, Kovesdi I, Tahara H, Lotze MT.et al. (1999Induction of antitumor immunity by direct intratumoral injection of a recombinant adenovirus vector expressing interleukin-12 Cancer Gene Ther 645–53. [DOI] [PubMed] [Google Scholar]
- Moullier P., and, Snyder RO. International efforts for recombinant adeno-associated viral vector reference standards. Mol Ther. 2008;16:1185–1188. doi: 10.1038/mt.2008.125. [DOI] [PubMed] [Google Scholar]
- Durocher Y, Perret S., and, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Potter M, Chesnut K.et al. (1999Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield Gene Ther 6973–985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In vivo determination of the optimal dose of Dox to induce Luc expression and region of interest (ROI) drawn to analyze Luc expression.
Viral genome copies were quantified by Q-PCR in the liver of mice injected with AAV8-Tetbidir-CMVm-luc, AAV8-Tetbidir-Alb-luc, and AAV8-Tetbidir-Alb-CMVm-luc.
Ex vivo biodistribution analysis of luciferase expression after AAV8-Tetbidir-pAlb-luc or AAV8-Tetbidir-CMV-luc injection in female and male BALB/c mice.
Analysis of serum AST levels in animals treated with 5 × 1012, 1.5 × 1012, and 1.5 × 1011 vg/kg AAV8-Tetbidir-Alb-IL-12 vector before and 1 and 7 days after Dox administration.