Abstract
Circulating angiogenic cells (CACs), represent a potential new therapeutic tool for the treatment of cardiovascular diseases, but their regenerative function is impaired in patients with coronary artery disease (CAD) and cardiac risk factors. The objective of this study is to assess the effect of lentiviral overexpression of endothelial nitric oxide synthase (eNOS) on the activity of CACs from patients with CAD and cardiac risk factors. In vitro and in vivo assays were employed to evaluate the regenerative capacity of the cells compared to CACs derived from healthy volunteers. Lentiviral eNOS transduction of cells from CAD patients significantly improved chemotactic migration compared with sham transduction, and increased the ability of CACs to induce angiogenic tube formation when cocultured with human umbilical vein endothelial cells (HUVECs) on Matrigel. In addition, eNOS transduction restored the ability of patient-derived CACs to enhance neovascularization and improve ischemic hind limb perfusion, approaching the efficacy of cells from healthy donors. These data indicate that CAC dysfunction seen in high-risk patients can be partially reversed by eNOS overexpression, suggesting that ex vivo gene delivery may improve the efficacy of autologous cell therapy for cardiovascular disease.
Introduction
Endothelial progenitor cells (EPCs), originally described by Asahara et al.,1 have generated considerable interest as important mediators of endogenous vascular repair and regeneration, as well as potential therapeutic agents. EPCs have been characterized by various means, initially by surface markers such as CD34, and vascular endothelial growth factor receptor 2 (VEGFR-2) and the ability to take up DiI-labeled acetylated low-density lipoprotein.1 Subsequently, other determinants such as CD133, have been used in an effort to enhance the specificity of their detection, however, there is still no consensus regarding the most reliable method for the identification of circulating EPCs.2 An alternative approach involves selection of highly angiogenic cells from the mononuclear cell fraction based on their ability to adhere and survive under defined endothelial culture conditions.3 These so-called “early growth” EPCs or circulating angiogenic cells (CACs)4 have been shown to potently stimulate neovascularization and endothelial repair in preclinical models5,6 and improve cardiac function after a myocardial infarction (MI) in otherwise healthy animals.7,8
A number of randomized controlled trials and meta analyses have demonstrated a significant improvement in myocardial function post acute MI following intracoronary injection of unselected, autologous bone marrow mononuclear cells or blood-derived CACs.9,10,11,12 While the degree of improvement in left ventricular ejection fraction is generally quite small (2–3%),9 host factors, in addition to optimal cell selection, culture and timing of delivery are implicated in the degree of benefit. In particular, cardiac risk factors, including advanced age, hypertension, hypercholesterolemia, cigarette smoking and diabetes, which are generally present in patients suffering an acute MI, have been shown to reduce the number and function of CACs,13,14,15,16,17,18 and may have greatly reduced the efficacy of autologous cell therapy trials.
The regenerative activity of CACs has been linked to the bioavailability of nitric oxide (NO), which is primarily produced by endothelial NO synthase (eNOS) in the vasculature. In eNOS−/− mice, progenitor cell mobilization was reduced and CACs exhibited impaired ability to stimulate angiogenesis in vivo.19 eNOS activity has been shown to be impaired in CACs from diabetic patients, with reduced NO production and consequent impaired function.20 Therefore, we hypothesized that increasing NO bioavailability in human CACs, by direct overexpression of eNOS, would improve their therapeutic potential.
We now show that lentiviral eNOS transduction of CACs derived from subjects with coronary artery disease (CAD) and cardiac risk factors significantly enhanced their regenerative activity in vitro, as well as in a xenotransplantation hind limb ischemia model in vivo. Therefore, overexpression of eNOS may represent an effective strategy to overcome the deleterious influence of host factors and enhance the efficacy of autologous cell therapy for patients with cardiovascular diseases.
Results
Subject characteristics
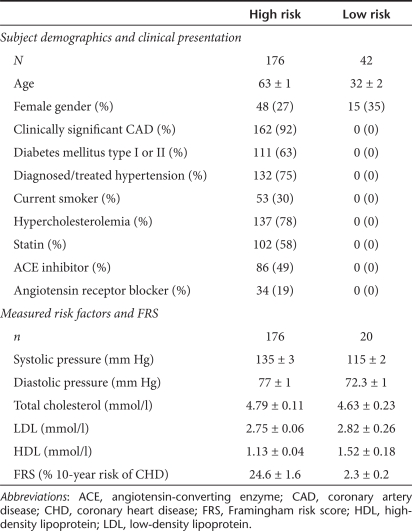
Demographic and medical information is summarized in Table 1. The high-risk group (n = 176) had a mean age of 63 ± 1 years compared to 32 ± 2 years in the low-risk group (n = 42). The age of our patient population closely reflects the mean age typically reported in post-MI trials.9 We obtained data on measurable risk factors in a subset20 of the low-risk subjects. By design, most high-risk subjects with a history of hypercholesterolemia and hypertension were also on appropriate medical management and therefore their mean cholesterol levels and blood pressures were only slightly elevated compared with healthy controls. Again this is consistent with the demographics of recent post-MI trials,9 confirming the relevance of this study population.
Table 1. Demographic and medical information.
CAC characterization
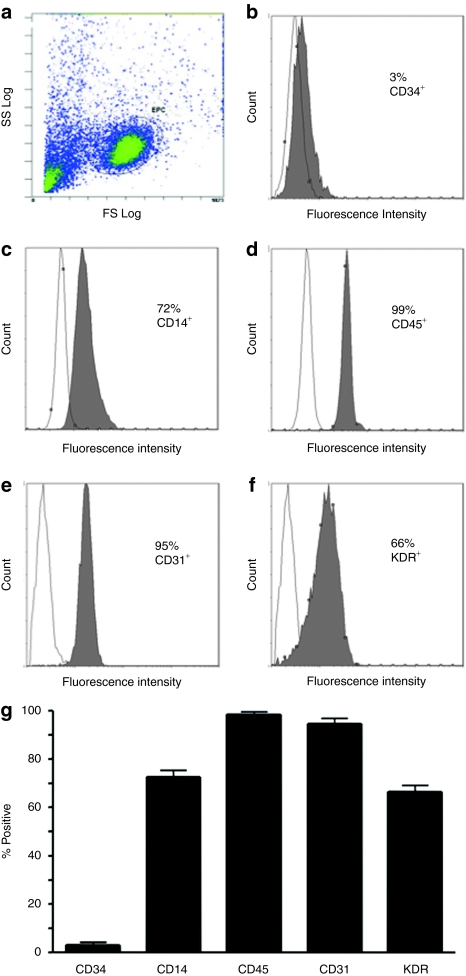
More than 90% of cultured cells were positive for both incorporation of DiI-labeled acetylated low-density lipoprotein and fluorescein isothiocyanate-conjugated ulex europaeus agglutinin-1 lectin staining (Supplementary Figure S1). Flow cytometric analysis determined that most of these cells were positive for a variety of endothelial markers such as VEGFR-2 (66%), and CD31 (95%), and the mononuclear cell markers, CD14 (72%) and CD45 (99%) (Figure 1), whereas only a small proportion were positive for CD34 (3%), which is typical for CACs (or “early EPCs” in earlier literature).21
Figure 1.
Flow cytometric characterization of day 7 circulating angiogenic cells (CACs). (a) Flow cytometry was used to characterize the expression of various surface markers of day 7 cells, gated as shown. Monoclonal antibodies to human (b) CD34, (c) CD14, (d) CD45, (e) CD31, and (f) KDR conjugated to either fluorescein isothiocyanate or phycoerythrin were used; isotype-matched antibodies were used as a negative control. (g) Summary data are also shown (n = 3).
eNOS overexpression
The expression of various endothelial growth factors, some of which are associated with eNOS production such as VEGF and PDGF, were reduced in high-risk CAC's (see Supplementary Data, Supplementary Table S1 and Supplementary Figure S1). Lentiviral transduction of CACs led to consistent overexpression of eNOS mRNA relative to β-actin (0.578 ± 0.217 versus 0.020 ± 0.006; P < 0.05; Supplementary Figure S3a,b), although still significantly lower than human umbilical vein endothelial cell (HUVEC) eNOS expression (25.01 ± 10.51). There was an approximately sixfold increase in eNOS protein compared to green fluorescent protein (GFP) (sham) transduction, as assessed by Western Blot and densitometry (Supplementary Figure S3c). There was no difference in cell survival between eNOS and sham (GFP)-transduced cells (1.4 ± 0.3 versus 1.5 ± 0.3 × 104 cells/cm2, respectively).
NO production and cGMP
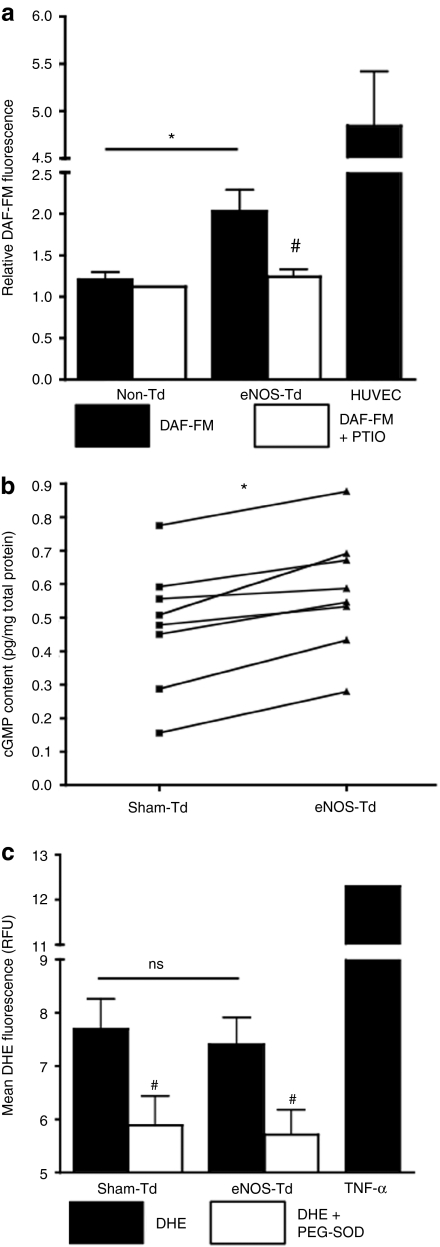
NO production was increased in eNOS-transduced cells, as assessed by 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) fluorescence (2.28 ± 0.29 versus 1.81 ± 0.17 fluorescence units; n = 8, P < 0.05; Figure 2a). The addition of 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (PTIO) reduced the DAF-FM signal to comparable levels in both cell groups, confirming the specificity of DAF-FM. In addition, eNOS transduction increased the intracellular cyclic guanosine monophosphate (cGMP) content compared with sham-transduced CACs (0.578 ± 0.063 versus 0.475 ± 0.066 pg/mg, respectively; n = 8, P < 0.001; Figure 2b). However, there was no difference in dihydroethidium (DHE) fluorescence, an indicator of superoxide (SO) production, between eNOS- and sham-transduced cells (Figure 2c).
Figure 2.
Production of nitric oxide (NO) or superoxide (SO). (a) We quantified NO and SO 5 days following transduction in high-risk subject cells. 4-Amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) was shown to be higher in endothelial NO synthase (eNOS)-transduced cells compared to control (n = 8). Human umbilical vein endothelial cells (HUVECs) were used as positive controls for NO production and a NO scavenger, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide, was used to show specificity of the reaction. (b) By enzyme-linked immunosorbent assay, we found that cyclic guanosine monophosphate (cGMP) levels were consistently elevated in eNOS-transduced circulating angiogenic cells (CACs) compared to sham-transduced cells from the same subject (n = 8). (c) Dihydroethidium (DHE), which indicates intracellular SO was not different between cell groups (n = 8) but largely elevated following 24-hour exposure to 1 ng/ml tumor necrosis factor-α (TNF-α) (positive control; n = 2). *P < 0.05 between cell groups (eNOS- versus sham/non-Td); #P < 0.05 ± scavenger. PEG-SOD, polyethylene glycol-SO dismutase; PTIO, 2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide; RFU, relative fluorescence unit.
Chemotactic migration
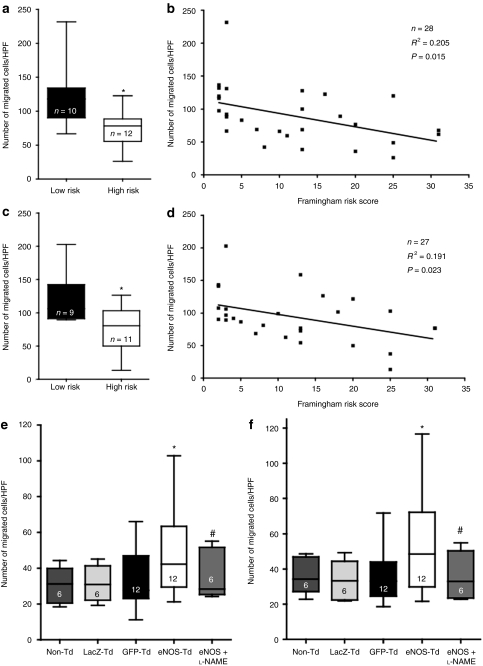
CACs from high-risk subjects exhibited reduced migration to both VEGF-A compared with cells from healthy controls (74 ± 9 versus 121 ± 15 cells/high power field; P < 0.05, Figure 3a) and stromal cell–derived factor-1 (80 ± 11 versus 119 ± 13 cells/high power field, respectively; P < 0.05, Figure 3c), and there was a significant inverse relationship between the Framingham risk score (FRS) and the migratory capacity toward both chemotactic agents (Figure 3b,d). Transduction of high-risk CACs with eNOS improved migration compared to non-, LacZ- and GFP-transduced cells to both VEGF (48.1 ± 7.0 versus 30.6 ± 3.8, 31.5 ± 3.8, and 33.0 ± 5.0 cells/high-power field, respectively; P < 0.05, Figure 3e) and stromal cell–derived factor-1 (55.5 ± 9.3 versus 36.1 ± 3.9, 33.3 ± 4.2 and 35.6 ± 4.4 cells/high-power field, respectively; P < 0.05, Figure 3f). There was no significant difference between non-, LacZ- and GFP-transduced CAC groups.
Figure 3.
Chemotactic migration. A modified Boyden chamber assay was used to quantify the ability of circulating angiogenic cells (CACs) migration toward (a,b) vascular endothelial growth factor (VEGF) or (c,d) stromal cell–derived factor-1 (SDF-1). (a,c) CACs from high-risk subjects had reduced migration compared to cells from low-risk subjects. (b,d) Using regression analysis, we also observed a significant correlation between subjects' Framingham risk score and migratory ability toward both chemotactic agents. Compared to sham transduction [LacZ and green fluorescent protein (GFP)], endothelial nitric oxide synthase (eNOS) transduction led to increased migration toward both (e) VEGF and (f) SDF-1, which was abrogated by pretreatment with NG-nitro--arginine methyl ester (-NAME). *P < 0.05 compared to sham-transduced. #P < 0.05 compared to eNOS-transduced only. HPF, high-power field.
Angiogenic tube formation
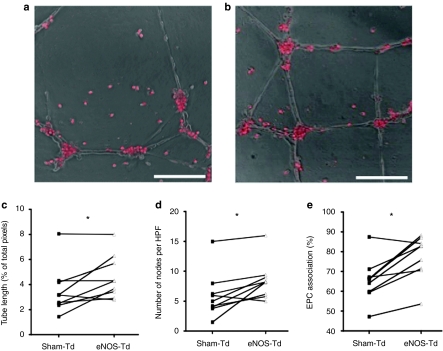
eNOS overexpression improved angiogenic activity of CACs in coculture with HUVECs in the Matrigel assay (Figure 4a,b), resulting in increased overall endothelial tube length compared with sham-transduced cells (4.6 ± 0.6 versus 3.5 ± 0.6% of total pixels per high power field, respectively; P < 0.05, Figure 4c) and a greater number of branch points (8.5 ± 1.1 versus 6.0 ± 1.3 nodes per high power field, respectively; P < 0.05, Figure 4d). Also, there was a higher degree of association of eNOS versus sham-transduced CACs with the endothelial tube-like networks, which occurred mainly at the nodal junctions (77.4 ± 3.7 versus 65.5 ± 3.6 % of total CACs per high power field, respectively; P < 0.01, Figure 4e). This result was found despite no difference in the adhesion of eNOS- or sham-transduced to activated endothelial cells (Supplementary Data and Supplementary Figure S4).
Figure 4.
Circulating angiogenic cell (CAC) stimulation of tube formation with a human endothelial vein endothelial cell monolayer. The ability of CACs (stained with CMTMR, red) to stimulate angiogenic tube formation was assessed when cocultured with human umbilical vein endothelial cells on Matrigel. Compared to (a) sham-transduced CACs, (b) endothelial nitric oxide synthase (eNOS)-transduced cells had an improved ability to stimulate tube formation, as measured by several parameters: (c) tube length, (d) the number of nodes, and (e) the rate of CAC association with endothelial cell tubes. *P < 0.05. Bar = 150 µm. EPC, endothelial progenitor cell.
Nude mouse hind limb ischemia model
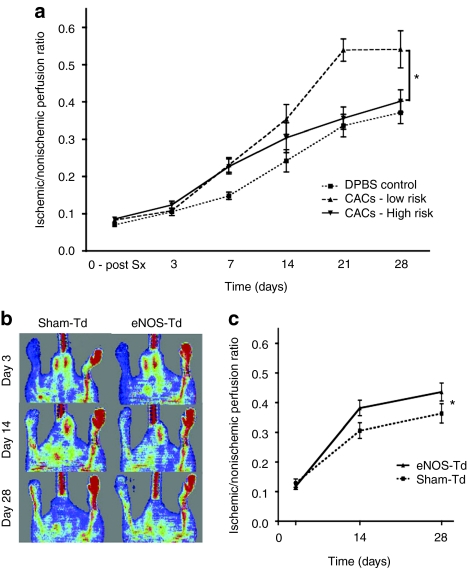
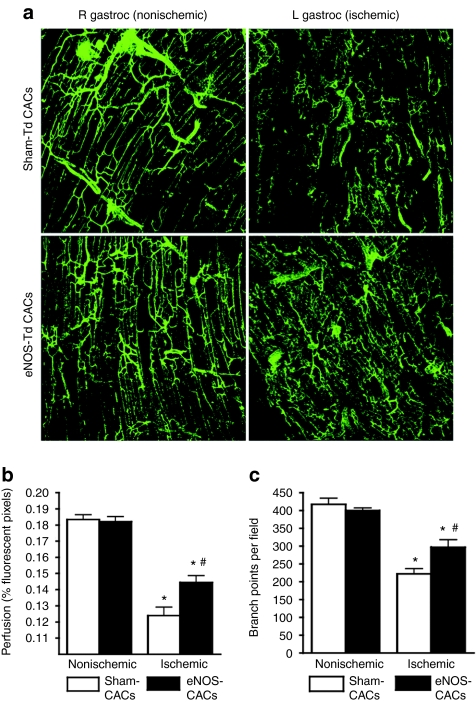
CACs from healthy subjects resulted in substantial overall improvement in perfusion recovery in the ischemic hindlimb from day 3 to day 28 postsurgery in the xenotransplantation, immune-deficient mouse model. In contrast, cells from the high-risk group failed to enhance ischemic limb perfusion compared to the injection of Dulbecco's phosphate buffered saline alone (P < 0.05, Figure 5a). In a separate experimental series, transduction with eNOS partially restored angiogenic activity of CACs from high-risk patients, leading to a significant increase in perfusion resolution from 3 to 28 days postsurgery, compared to sham-transduced cells (P < 0.05, Figure 5b,c). Fluorescent microangiography confirmed that the ischemic limb gastrocnemius muscle had dramatically reduced perfusion and microvessel branching compared to the nonischemic limb (P < 0.01, Figure 6). Furthermore, animals receiving eNOS-transduced CACs had improved muscle perfusion (P < 0.05) and vessel branching (P < 0.05) compared to those receiving sham-transduced cells.
Figure 5.
Nude mouse model of hindlimb ischemia. (a) Using laser Doppler perfusion imaging, we observed a significant reduction in the ability of circulating angiogenic cells (CACs) from high Framingham risk score (FRS) subjects (n = 9) to stimulate neovascularization and reperfusion compared to cells from low FRS subjects (n = 8). (b,c) Endothelial nitric oxide synthase (eNOS) overexpression increased the function of CACs isolated from the same patient population (n = 12). Data are expressed as the ischemic/nonischemic limb perfusion ratio. *P < 0.05 between (a) high and low FRS groups and (c) eNOS- and sham-Td.
Figure 6.
Fluorescent microangiography of gastrocnemius muscles. (a) Confocal microscopy stacked sections (b) show a highly significant reduction in gastrocnemius muscle perfusion and (c) microvessel branching in ischemic muscles compared to contralateral nonischemic muscles (n = 5). Mice receiving endothelial nitric oxide synthase (eNOS)-transduced circulating angiogenic cells (CACs) showed significant improvement in both parameters compared to those receiving sham-transduced CACs (n = 5). *P < 0.01 compared to nonischemic limb. #P < 0.05 compared to sham-transduced cells.
Discussion
In this study, we showed that eNOS gene transfer could restore the in vitro and in vivo angiogenic activity of circulating cells obtained from patients with established CAD or multiple coronary risk factors; a population that is at high risk for cardiac events and thus potentially eligible for post-MI cell therapy, yet one that exhibits a profound progenitor cell dysfunction. It has been previously well demonstrated that patients with individual cardiac risk factors exhibited reductions in the number and function of EPCs (the term employed in earlier literature)13,14,15,16 defined by a variety of criteria including quantification of surface-marker positive cells and colony forming units in vitro. However, this is the first to include in vitro functional assays and quantification of paracrine growth factors, as well as in vivo assessments of neovascularization, to more fully characterize the function of CACs derived from patients with risk profiles similar to those that suffer MIs. Therefore, these findings may have direct clinical relevance for autologous cell therapy for cardiovascular disease.
Unlike cells from healthy subjects, CACs from high-risk subjects did not enhance the recovery of perfusion in the immune-deficient mouse ischemic hindlimb over the 28-day study period. The lack of efficacy is consistent with reports that showed reduced in vivo angiogenic capacity of CACs from patients with diabetes,17 ischemic cardiomyopathy22 or advanced age.14 In our study, reduced angiogenic activity was seen even though the patients were generally well treated for their hypertension and hypercholesterolemia, two important modifiable risk factors. Therefore, it is likely that unmodifiable, or difficult to control factors such as older age or diabetes may be largely responsible for the observed dysfunction of angiogenic cells in our study. Moreover, the persistence of progenitor cell dysfunction despite good secondary prevention further underscores the importance of identifying specific manipulations to restore the angiogenic activity of cells from older patients in order to maximize the potential benefits of cell therapy.
NO is a potent vasodilator which also plays a critical role in EC repair and survival.23 NO has been shown to be involved in downstream VEGF signaling, and promotes EC proliferation, migration,24 and angiogenesis.25 In addition, NO has been reported to increase VEGF expression in vascular smooth muscle cells whether administered exogenously or endogenously.26 Moreover, eNOS knockout mice exhibit defects in neovascularization and functional blood flow reserve,19,27 despite having similar levels of ischemia and VEGF induction following femoral artery ligation. NO production has also been shown to be critical to the activity of EPCs, and cells derived from eNOS-deficient mice exhibit poor migratory activity in vitro, impaired ability to stimulate hindlimb neovascularization, and inadequate retinal neovessel formation.19,28 Also, it was recently reported that NO was required for EPC mobilization and that impaired reperfusion of hindlimb ischemia in eNOS−/− mice could be rescued by wild-type EPC delivery.19
Our results with eNOS gene transfer are consistent with a previous study that used a small molecule enhancer of eNOS transcription, AVE9488 (Sanofi Aventis, Paris, France), to improve the function of bone marrow cells from patients with ischemic cardiomyopathy.29 Lentiviral gene transfer led to an apparently greater degree of eNOS mRNA overexpression compared to AVE9488 treatment (40-fold versus 2-fold), and may thus represent a more robust method of enhancing eNOS activity. Also, an added advantage of this approach is stable transduction, and unlike small molecule conditioning, would be expected to produce more durable effects on eNOS expression following cell transplantation. Moreover, the specificity for AVE9488 for enhancing eNOS expression has not been clearly established. In contrast, gene delivery methods provide specific and robust levels of gene overexpression, and many have already been approved for clinical use.
Treatment of EPCs from elderly individuals with insulin growth factor 1 has also been shown to increase eNOS expression, and improve functional activity in a PI3K/Akt dependent manner.30 Similarly, pretreatment with statins resulted in upregulation of GTP cyclohydrolase I, a key enzyme in the production of tetrahydrobiopterin, thereby improving eNOS activity and EPC function in a model of diabetes.31 Kruppel-like factor-2 overexpression was also shown to improve NO production and improve neovascularization.32 Statin pretreatment was able to reverse eNOS uncoupling, which has been shown to reduce NO bioavailability in the context of diabetes.20 Recently, NO has also been implicated in CXCR4 signaling in CD34+ progenitors, most likely by the oxidation of intracellular protein thiols.33 Reduced NO bioavailability has also been implicated in the reduction in migration of CD34+ cells from diabetic patients compared to cells from healthy donors;34 diabetic cells were more rigid and less motile and this was reversible with administration of exogenous NO. The same group has recently suggested that NO is critical for elongation of actin filaments by vasodilator-stimulated phosphoproteins at the leading edge of migrating cells.35 Therefore, these reports suggest several mechanisms whereby enhancing eNOS expression and activity of autologous progenitor cells from patients with CAD or its risk factors can improve their regenerative activity.
In recent clinical trials, the administration of autologous bone marrow cells appears to result in modest improvement in myocardial function post-MI,9,10 despite the evidence that the activity of these cells is profoundly impaired by the effect of host factors. Therefore, strategies to overcome the deleterious effects of age, as well as other cardiac risk factors, on the regenerative activity of angiogenic cells from patients suffering cardiovascular disease will be critical in order to achieve more robust clinical benefit than currently seen in the trials to date. Given that eNOS gene transfer is already being used in a clinical trial for pulmonary arterial hypertension patients (http://www.clinicaltrials.gov/), the present results suggest that overexpression of eNOS by direct gene transfer could be used to enhance the efficacy of progenitor cell therapy for acute MI and other cardiovascular disorders.
Materials and Methods
Patient selection and recruitment. Healthy volunteers and patients with risk factors (advanced age, diabetes, hypertension, hypercholesterolemia, and smoking) with or without diagnosed CAD were enrolled. The FRS was used to confirm that volunteers were assigned to the “low-risk” group (healthy control, <5% FRS, n = 42). The “high-risk” group refers to enrolled patients (n = 176). The FRS was used to estimate the results of additive risk factors in patients for certain analyses. All human studies were approved by the research ethics board of St Michael's Hospital, Toronto, Ontario, Canada, and conform to the principles outlined in the Declaration of Helsinki.
Isolation and characterization of CACs. Blood samples were obtained by venipuncture or through the arterial sheath at the time of cardiac catheterization. Peripheral blood mononuclear cells (PB-MNCs) were isolated from 80 ml of blood by Ficoll gradient centrifugation (CPT Vacutainer Tubes; BD, Franklin Lakes, NJ) and plated at a density of 0.75 × 106 cells/cm2 on human fibronectin-coated dishes. Adherent cells were maintained in endothelial cell basal medium supplemented with 20% fetal bovine serum and several endothelial growth factors (EGM-2MV SingleQuots; Lonza, Basel, Switzerland), which was replaced every 48 hours.36 These CACs were characterized by staining with DiI-labeled acetylated low-density lipoprotein and UEA-1 lectin and by flow cytometry using monoclonal conjugated antibodies: anti-CD31 (Immunotech, Vaudreuil-Dorion, Canada), anti-CD14 (Beckman Coulter, Wilmington, DE), pooled anti-CD34 (Beckman Coulter), anti-CD45 (Beckman Coulter, Brea, CA), and anti-VEGF-R2/KDR (R&D Systems, Minneapolis, MN). Corresponding isotype control antibodies were used to establish negative control gating parameters.
eNOS overexpression. Following 3 days in culture, cells were exposed to lentivirus (LentiMax system; multiplicity of infection = 3; Lentigen, Gaithersburg, MD) containing the coding sequence of eNOS, GFP (sham) or LacZ (for migration assay). The LentiMax system includes a cytomegalovirus promoter and vesicular stomatitis virus G, which lead to constitutive and robust transgene expression as well as very high transduction efficiency (>98%).37 The CACs were incubated with the lentivirus for 5 hours in regular medium supplemented with 8 µg/ml polybrene. Cells were maintained in full medium for an additional 5 days prior to use in experiments (8 days after isolation).
Quantification of intracellular NO and SO. The intracellular content of NO and O2− were quantified using DAF-FM diacetate (Invitrogen) and DHE (Invitrogen, Burlington, Canada), respectively. A subset of the cells were exposed to NO and O2− scavengers 2-(4-Carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (carboxy-PTIO; 1 µmol/l; Cayman Chemical, Ann Arbor, MI) or polyethylene glycol-SO dismutase (100 U/l; Sigma, St Louis, MO) for 30 minutes prior to exposure to DAF-FM or DHE and maintained in the presence of the scavenger for the whole course of the experiment. In phenol red-free medium (with 1% fetal bovine serum), cells were exposed to DAF-FM (5 µmol/l) for 45 minutes or DHE (25 µmol/l) for 20 minutes, and the regular medium replaced for an additional hour prior to processing for flow cytometry. FL1 and FL2 channels were used for DAF-FM and DHE, respectively, during flow cytometry (Beckman Coulter FC500). HUVECs and HeLa cells were used as positive and negative controls of eNOS-producing cells respectively, and tumor necrosis factor-α (1 ng/ml for 24 hours) was used to induce SO generation (positive control) in DHE experiments.
Quantification of intracellular cGMP. An E1A cGMP ELISA kit (Cayman Chemical) was used to measure intracellular cGMP 5 days following transduction. Briefly, pelleted CACs were frozen at −80 °C until needed, then lysed using 0.1 mol/l HCl and processed according to the manufacturer's instructions. The total protein content was measured using the Bradford Reagent (Sigma).
Chemotactic migration assay. CAC migration was measured using a standard modified Boyden chamber assay, with VEGF165 (50 ng/ml) or human stromal cell–derived factor-1(100 ng/ml) as chemotactic agents, as previously described (Supplementary Materials and Methods).36 NG-nitro--arginine methyl ester (-NAME) pretreatment (30 minutes; 100 mmol/l) was used to competitively inhibit eNOS activity immediately prior to the migration assay.
CAC and HUVEC coculture tube formation assay. The ability of sham- or eNOS-transduced CACs to stimulate EC angiogenic tube formation was quantified by coculture with HUVECs on Matrigel (BD Biosciences, Franklin Lakes, NJ). CACs were prelabeled using CMTMR fluorescent dye (Invitrogen; 1 µmol/l). In 48-well plates coated with Matrigel (200 µl per well), 4 × 104 HUVECs and 2 × 104 CACs were cultured together (in EGM-2MV + 5% fetal bovine serum) for 16 hours at 37 °C. Confocal microscopy (Leica, Wetzlar, Germany) was used to capture five random fields per well, using both bright field and fluorescence. Endothelial tubes were skeletonized and various parameters quantified in a blinded manner in silico: total tube length (based on the total pixel % taken by skeletonized tubes), number of nodes (branch points), total number of CACs, and number of CACs associated with tubes (directly adjacent to or superimposed over tubes).
Nude mouse hind limb ischemia model. For xenotransplantation experiments using human cells, 8–10-week-old male Balb/c nude mice were used (BALB/cAnNCrl-nuBR, Charles River, Wilmington, MA). Following anesthesia with ketamine/xylazine (200/10 mg/kg), the proximal and distal ends of the left femoral artery, as well as all side branches, were ligated and the femoral artery cut at two points to induce limb ischemia. Analgesia was maintained for 48 hours using Ketoprofen (subcutaneous injections). On day 3, 5 × 105 human CACs were injected into the medial thigh and gastrocnemius muscles of the ischemic limb (total volume of 0.1 ml). At day 3 (prior to cell injection) and at regular intervals up to day 28, limb flow measurements were made using laser Doppler perfusion imaging (LDPI, Moor Instruments, Wilmington, DE). The ischemic/nonischemic limb perfusion ratio was used for analysis. All investigators were blinded as to animal allocation to each treatment group. The investigations were approved by the Animal Care Committee of St Michael's Hospital, and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
Fluorescent microangiography. Prior to killing, mice were anesthetized and the abdominal aorta cannulated distal to the renal arteries. A 1% agarose solution containing 10% fluorescent microbeads (Sigma; 0.2 µm diameter) was infused at 45 °C and allowed to cool following euthanization. Hindlimb gastrocnemius muscles were removed and placed in 10% formalin and sectioned (200 µm). Using confocal microscopy, a series of stacked images (4 µm slices) was taken and the middle 25 slices (100 µm total thickness) were projected to quantify the amount of perfusion in the muscle and the vessel architecture, as described previously.38
Statistics. Results of in vitro assays comparing high- and low-risk groups were analyzed using the Mann–Whitney nonparametric test. Results of in vitro assays comparing sham- and eNOS-transduced CACs were analyzed using the Students' paired t-test. Linear regression analysis was used to correlate FRS to in vitro migration. In vivo hind limb perfusion data were analyzed using repeated measures analysis of variance. All data are represented as mean ± SEM. Data were considered statistically significant if P < 0.05.
SUPPLEMENTARY MATERIAL Figure S1. Double staining for DiI-AcLDL and FITC-conjugated ulex uropaeus agglutinin-1 lectin. Figure S2. Comparison of angiogenic gene expression and protein secretion. Figure S3. Overexpression of eNOS. Figure S4. Adhesion to Activated HUVECs. Table S1. Primers used for quantitative PCR. Materials and Methods. Data.
Acknowledgments
The authors sincerely thank Rosemary Dunne, Nancy Camack, Mary Keith and Lawrence Leiter, and Danielle Bedard for their help in subject recruitment and REB applications. In addition, Liana Zucco, James Kowalewski, Qiuwang Zhang and Ivana Kandic were helpful in optimizing protocols. This work was supported by the Canadian Institutes of Health Research (CIHR) operating grant (Grant # MOP_81307 to MJBK). All of the work included was conducted in Toronto, Ontario, Canada. The authors declared no conflict of interest.
Supplementary Material
Double staining for DiI-AcLDL and FITC-conjugated ulex uropaeus agglutinin-1 lectin.
Comparison of angiogenic gene expression and protein secretion.
Overexpression of eNOS.
Adhesion to Activated HUVECs.
Primers used for quantitative PCR.
REFERENCES
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T.et al. (1997Isolation of putative progenitor endothelial cells for angiogenesis Science 275964–967. [DOI] [PubMed] [Google Scholar]
- Ward MR, Stewart DJ., and, Kutryk MJ. Endothelial progenitor cell therapy for the treatment of coronary disease, acute MI, and pulmonary arterial hypertension: current perspectives. Catheter Cardiovasc Interv. 2007;70:983–998. doi: 10.1002/ccd.21302. [DOI] [PubMed] [Google Scholar]
- Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J.et al. (2005Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease Circ Res 971142–1151. [DOI] [PubMed] [Google Scholar]
- Asosingh K, Aldred MA, Vasanji A, Drazba J, Sharp J, Farver C.et al. (2008Circulating angiogenic precursors in idiopathic pulmonary arterial hypertension Am J Pathol 172615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döbert N, Britten M, Assmus B, Berner U, Menzel C, Lehmann R.et al. (2004Transplantation of progenitor cells after reperfused acute myocardial infarction: evaluation of perfusion and myocardial viability with FDG-PET and thallium SPECT Eur J Nucl Med Mol Imaging 311146–1151. [DOI] [PubMed] [Google Scholar]
- Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK.et al. (2004Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis Arterioscler Thromb Vasc Biol 24288–293. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C.et al. (2003Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia Circulation 107461–468. [DOI] [PubMed] [Google Scholar]
- Fernández-Avilés F, San Román JA, García-Frade J, Fernández ME, Peñarrubia MJ, de la Fuente L.et al. (2004Experimental and clinical regenerative capability of human bone marrow cells after myocardial infarction Circ Res 95742–748. [DOI] [PubMed] [Google Scholar]
- Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H.et al. (2006Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction N Engl J Med 3551210–1221. [DOI] [PubMed] [Google Scholar]
- Li ZQ, Zhang M, Jing YZ, Zhang WW, Liu Y, Cui LJ.et al. (2007The clinical study of autologous peripheral blood stem cell transplantation by intracoronary infusion in patients with acute myocardial infarction (AMI) Int J Cardiol 11552–56. [DOI] [PubMed] [Google Scholar]
- Abdel-Latif A, Bolli R, Tleyjeh IM, Montori VM, Perin EC, Hornung CA.et al. (2007Adult bone marrow-derived cells for cardiac repair: a systematic review and meta-analysis Arch Intern Med 167989–997. [DOI] [PubMed] [Google Scholar]
- Jiang M, He B, Zhang Q, Ge H, Zang MH, Han ZH.et al. (2010Randomized controlled trials on the therapeutic effects of adult progenitor cells for myocardial infarction: meta-analysis Expert Opin Biol Ther 10667–680. [DOI] [PubMed] [Google Scholar]
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H.et al. (2001Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease Circ Res 89E1–E7. [DOI] [PubMed] [Google Scholar]
- Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M., and, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC.et al. (2004Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes Diabetes 53195–199. [DOI] [PubMed] [Google Scholar]
- Michaud SE, Dussault S, Haddad P, Groleau J., and, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–432. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR.et al. (2002Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures Circulation 1062781–2786. [DOI] [PubMed] [Google Scholar]
- Yue WS, Wang M, Yan GH, Yiu KH, Yin L, Lee SW.et al. (2010Smoking is associated with depletion of circulating endothelial progenitor cells and elevated pulmonary artery systolic pressure in patients with coronary artery disease Am J Cardiol 1061248–1254. [DOI] [PubMed] [Google Scholar]
- Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K.et al. (2003Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells Nat Med 91370–1376. [DOI] [PubMed] [Google Scholar]
- Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD.et al. (2007Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes Diabetes 56666–674. [DOI] [PubMed] [Google Scholar]
- Gulati R, Jevremovic D, Peterson TE, Chatterjee S, Shah V, Vile RG.et al. (2003Diverse origin and function of cells with endothelial phenotype obtained from adult human blood Circ Res 931023–1025. [DOI] [PubMed] [Google Scholar]
- Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH.et al. (2004Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease Circulation 1091615–1622. [DOI] [PubMed] [Google Scholar]
- Dimmeler S., and, Zeiher AM. Nitric oxide-an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- Ziche M, Morbidelli L, Choudhuri R, Zhang HT, Donnini S, Granger HJ.et al. (1997Nitric oxide synthase lies downstream from vascular endothelial growth factor-induced but not basic fibroblast growth factor-induced angiogenesis J Clin Invest 992625–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N.et al. (2003Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model Circulation 1082250–2257. [DOI] [PubMed] [Google Scholar]
- Józkowicz A, Dulak J, Nigisch A, Funovics P, Weigel G, Polterauer P.et al. (2004Involvement of nitric oxide in angiogenic activities of vascular endothelial growth factor isoforms Growth Factors 2219–28. [DOI] [PubMed] [Google Scholar]
- Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM.et al. (2005Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve Proc Natl Acad Sci USA 10210999–11004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie SM, Curtis LM, Mames RN, Simon GG, Grant MB., and, Scott EW. The nitric oxide pathway modulates hemangioblast activity of adult hematopoietic stem cells. Blood. 2005;105:1916–1922. doi: 10.1182/blood-2004-09-3415. [DOI] [PubMed] [Google Scholar]
- Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C.et al. (2006Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy Proc Natl Acad Sci USA 10314537–14541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P.et al. (2007Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1 Circ Res 100434–443. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Daiber A, Oelze M, Brandt M, Closs E, Xu J.et al. (2008Mechanisms underlying recoupling of eNOS by HMG-CoA reductase inhibition in a rat model of streptozotocin-induced diabetes mellitus Atherosclerosis 19865–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon RA, Urbich C, Fischer A, Fontijn RD, Seeger FH, Koyanagi M.et al. (2011Kruppel-like factor 2 improves neovascularization capacity of aged proangiogenic cells Eur Heart J 32371–377. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wittner M, Bouamar H, Jarrier P, Vainchenker W., and, Louache F. Identification of CXCR4 as a new nitric oxide-regulated gene in human CD34+ cells. Stem Cells. 2007;25:211–219. doi: 10.1634/stemcells.2006-0468. [DOI] [PubMed] [Google Scholar]
- Segal MS, Shah R, Afzal A, Perrault CM, Chang K, Schuler A.et al. (2006Nitric oxide cytoskeletal-induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes Diabetes 55102–109. [PubMed] [Google Scholar]
- Li Calzi S, Purich DL, Chang KH, Afzal A, Nakagawa T, Busik JV.et al. (2008Carbon monoxide and nitric oxide mediate cytoskeletal reorganization in microvascular cells via vasodilator-stimulated phosphoprotein phosphorylation: evidence for blunted responsiveness in diabetes Diabetes 572488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFabio JM, Thomas GR, Zucco L, Kuliszewski MA, Bennett BM, Kutryk MJ.et al. (2006Nitroglycerin attenuates human endothelial progenitor cell differentiation, function, and survival J Pharmacol Exp Ther 318117–123. [DOI] [PubMed] [Google Scholar]
- Liu JW, Pernod G, Dunoyer-Geindre S, Fish RJ, Yang H, Bounameaux H.et al. (2006Promoter dependence of transgene expression by lentivirus-transduced human blood-derived endothelial progenitor cells Stem Cells 24199–208. [DOI] [PubMed] [Google Scholar]
- Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL.et al. (2007Therapeutic arteriogenesis by ultrasound-mediated VEGF165 plasmid gene delivery to chronically ischemic skeletal muscle Circ Res 101295–303. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Double staining for DiI-AcLDL and FITC-conjugated ulex uropaeus agglutinin-1 lectin.
Comparison of angiogenic gene expression and protein secretion.
Overexpression of eNOS.
Adhesion to Activated HUVECs.
Primers used for quantitative PCR.