Abstract
Duchenne muscular dystrophy (DMD) is an inherited severe muscle wasting disorder with, thus far, no effective therapy. DMD causes respiratory and cardiac failure as well as muscle wastage. Among the various symptoms, respiratory insufficiency is a major cause of death in DMD patients at about 20 years of age. So, naturally, the improvement of respiratory function will extend the patient's life. We report here, for the first time, a sensitive procedure using whole-body plethysmography to monitor respiratory parameters detected in the utrophin/dystrophin double knockout mouse (dko mouse), showing quite similar systemic symptoms to human DMD including restrictive ventilatory impairment. Furthermore, we show that a highly efficient dystrophin-transduction to the dko's diaphragm—achieved by simple intraperitoneal injection of a helper-dependent adenovirus vector (HDAdv) containing the full-length dystrophin expression cassette—provided beneficial results. In spite of dystrophin expression only in the diaphragm, this focal gene transfer could result in the rescue from ventilatory impairment (increased tidal volume (TV) and improvement of compensatory hyperpnea). Our result suggests that a DMD patient's mortal ventilatory impairment may be improved via technically easy means through the intraperitoneal injection of HDAdv.
Introduction
Duchenne muscular dystrophy (DMD) and the milder allelic Becker muscular dystrophy are X-linked genetic disorders. DMD is caused by the absence of dystrophin,1,2 a 427-kd protein encoded on the short arm of the X chromosome.3
DMD patients suffer from serious, progressive muscle wasting and weakness, become confined to wheelchairs in their early teens, and die at around age 20, usually of respiratory or cardiac failure.4 In particular, respiratory malfunction is a practical problem faced by a DMD patient. However, at this present time, there is no effective radical remedy for many genetic diseases including DMD. Mortal respiratory malfunction in DMD patients has been slightly palliated mainly thanks to mechanical ventilation. Therefore it is highly crucial to improve the function of respiration with an easy and effective method. However, in experiments using an animal model, what kind of assessment is directly correlated with whole-body respiratory function? Speaking of the physiological assessment of therapeutic efficacy by gene transfer to a myopathic model mouse, the improvement of muscle force was the principal indicator. This evaluation, though, is carried out under quite peculiar condition using an isolated muscle from the body. Although the muscle force measurement is very helpful for us to speculate the therapeutic efficacy, it is needless to say that the measurement of therapeutic efficacy by using the whole-body animal is ideal for us to better predict this efficacy. From a clinical point of view, we designed this study as follows: use of a symptomatic model mouse—a utrophin/dystrophin double knockout (dko) mouse—with easy gene-transfer protocol (viral vector inoculation into peritoneal cavity) in the clinical evaluation, and whole-body respiratory function by means of whole-body plethysmography.
In this study, we used a dko mouse as our animal model. The mdx mouse, which lacks dystrophin, has been employed most frequently as a DMD model mouse, but exhibits a more benign pathological phenotype than a human DMD patient.5 The diaphragm muscle of the mdx mouse exhibits progressive structural and functional deterioration consistent with DMD, whereas the limb muscles show relatively mild pathology.6 Utrophin, which is quite similar in molecular structure to dystrophin, is differently expressed in special and temporal pattern from dystophin in physiological condition.7,8 But the compensational expression of utrophin, in the mdx mouse, makes symptoms relatively milder compared to those of a DMD patient.9,10 So, the dko mouse acts very well as a DMD patient for purposes of our study. The dko mouse shows slack posture, reduced weight, affected mobility, abnormal breathing patterns, and abnormal field behavior—waddling gait with joint contractures—and early death.5
As we particularly wanted to evaluate the therapeutic efficacy using the whole-body animal, we chose this dko mouse. As regards our gene-transfer protocol in relation to the diaphragm we chose the trans-peritoneal cavity protocol. Recent advances have identified many possible therapeutic approaches including pharmacological treatments such as the use of myostatin antibodies,11 gene therapies, and cell therapy. In particular, the virus-mediated gene delivery of the dystrophin gene could be one of the more effective therapeutic approaches for DMD.
Among the various virus vectors, recombinant adeno-associated virus vector has been most popularly applied as a gene-transduction vector.12,13,14 Surprisingly, this viral vector containing micro-size dystrophin—lacking two thirds of the original dystrophin—but preserving partial function, made systemic delivery possible through the tail vein (due to its small packaging capacity, this dystrophin truncation is necessary).15 Although large-scale production methods have been under development, it is easily speculated that systemic therapeutic gene-transduction in humans would be quite difficult. On the other hand, we have reported the successful transduction of the full-length dystrophin gene into an mdx mouse using the helper-dependent adenovirus vector (HDAdv).16,17 Moreover, the dko mouse—injected by multiple intramuscular administrations of HDAdv carrying full-length dystrophin cDNA—also showed an increase in body weight, an improvement in motor performance, and a prolongation of life span.18 Therefore, we chose trans-peritoneal gene-transfer protocol.
Finally, with respect to the evaluation of therapeutic efficacy, we used whole-body plethysmography. In a previous report, we first showed that sensitive and systemic assay method, using whole-body plethysmography, could detect a significant decrease of tidal volume (TV) in an adult mdx mouse compared with an age-matched wild-type control mouse.19 Meanwhile, in various reports, specific force and histopathological analysis of the dko's diaphragm and limb muscles have been measured as therapeutic efficacy after gene transfer.15,20 But, as yet, no analysis addressing the respiratory function after gene transfer to the dko's diaphragm has been performed. As mentioned earlier, we would need to be prudent when dealing with the force of diaphragm detached from the body, and the respiratory parameters clinically adhered to [e.g., TV and respiratory rate (RR)].19 Therefore, the measurement of TV and RR after gene transfer to this specific mouse makes more clinical assessment possible. With whole-body plethysmography we can easily monitor some parameters of respiratory function without any restriction or anesthesia. We are convinced that the evaluation by this plethysmography is better suited for the evaluation of respiratory function than any kind of evaluation done previously.
We show here, for the first time, that the induction of full-length dystrophin to the diaphragm in the DMD model mouse can improve, not only the histological abnormalities, but also respiratory dysfunction.
Results
Easy and efficient gene transfer to the diaphragm by HDAdv via peritoneal cavity
First of all, by way of trial, in order to transfer a gene to a diaphragm we inoculated the HDAdv-LacZ-dys (Figure 1a) into the mdx mouse's peritoneal cavity.
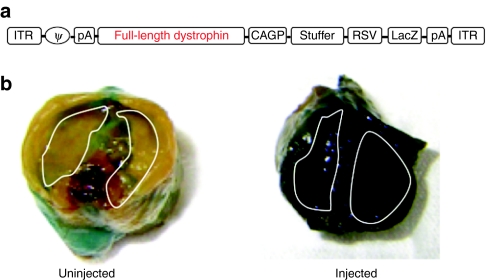
Figure 1.
Efficient gene transfer to the diaphragm by HDAdv. (a) The structure of HDAdv-LacZ-mFLdys. CAGP, the CAG promoter; stuffer, a part of the murine Emx2 gene; full-length dystrophin, the murine full-length dystrophin complementary DNA (cDNA); ITR, inverted terminal repeat of adenovirus; LacZ, β-galactosidase of E.coli; PA, simian virus 40 polyadenylation signal; RSV, Raus sarcoma virus promoter; φ, packaging signal. (b) β-galactosidase expression in diaphragm (white line) of injected 9-week-old mdx mice (left; uninjected, right; injected) by using HDAdv-LacZ-dys. A high expression of LacZ was shown in diaphragm of injected mdx mice after 8 weeks of intraperitoneal injection.
An aliquot of 100 µl of diluent of purified viral stock was aseptically injected into the peritoneal cavity of 1-week-old mice. Eight weeks later, following the injection, we confirmed that β-galactosidase was strongly expressed throughout the entire diaphragm without obvious harmful effects (Figure 1b). Therefore, high efficient gene transfer to the diaphragm was achieved without needing any particular skill.
Dystrophin expression improves the dystrophic pathology in the dko diaphragm
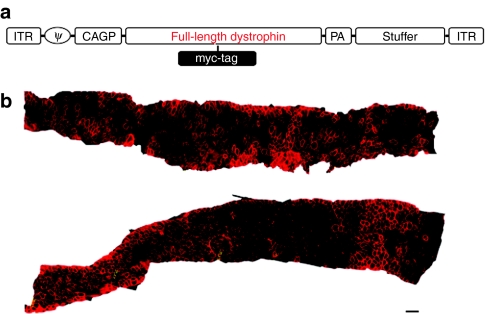
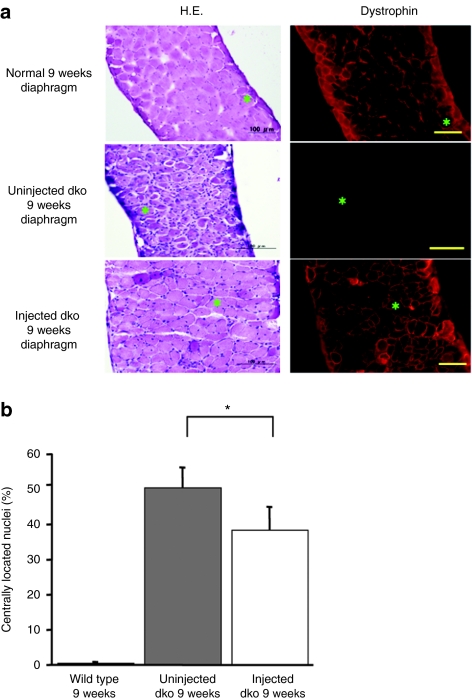
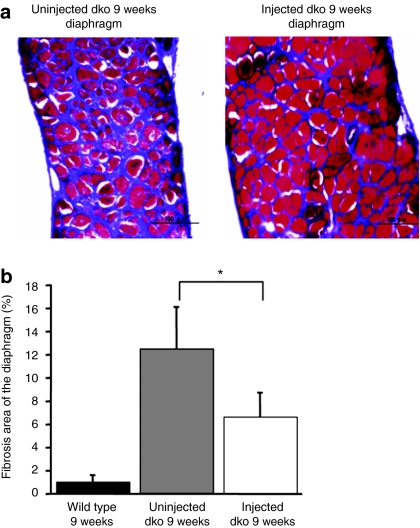
Next, we inoculated an HDAdv containing the murine full-length dystrophin expression cassette (HDAdv-mFLmyc-dys) (Figure 2a). Each 7-day-old dko mouse was injected with this vector into the peritoneal cavity. Eight weeks later the expressed dystrophin was detected by immuno-fluorescent staining. In the injected dko mouse's diaphragm, dystrophin was widely expressed (Figure 2b). Quantitative analyses of the dystrophin-positive fibers in the diaphragm strips of injected dko mice showed an average of transduction efficiency were 37.9 ± 15% (n = 6). As shown in Figure 2b, the dystrophin expressed from the introduced viral vector was clearly located along the sarcolemma, as in wild-type muscle. The area containing dystrophin-positive fibers were prevented from showing the dystrophic changes (severe muscle degeneration, central nucleation, extensive myofiber loss, and cell infiltrations increased fibrotic area) which were apparently observed in naive dko mice (Figure 3a). We then calculated the ratio of fibers with central nuclei, reflecting the abnormal cycles of fiber degeneration and regeneration. In dystrophin-positive fibers in the group of injected dko, we observed a marked reduction in the number of centrally nucleated myofibers compared with that of the uninjected dko group (Figure 3b, P < 0.05). Furthermore, in order to evaluate fibrotic changes, Masson trichrome staining for serial sections of the dko's diaphragm (9 weeks) was carried out. The area stained in blue, which represents fibrotic change consisting of a bunch of collagens, was quantified by WinROOF software (Figure 4a). Comparing with the wild-type mice the severe fibrotic diaphragm was observed in the uninjected diaphragm dko mice at the age of 9 weeks (12.5 ± 2.3%, n = 4). The fibrosis areas of the diaphragms in the injected dko decreased to 6.9 ± 1.1% (Figure 4b, n = 3, P < 0.05).
Figure 2.
Efficient dystrophin transduction to dko mouse's diaphragm. (a) The structure of HDAdv-myc-mFLdys. CAGP, the CAG promoter; stuffer, a part of the murine Emx2 gene; full-length dystrophin, the murine full-length dystrophin complementary DNA (cDNA); ITR, inverted terminal repeat of adenovirus; PA, simian virus 40 polyadenylation signal; RSV, Raus sarcoma virus promoter; φ, packaging signal. Myc-tag was inserted into the dystrophin cDNA between 6,886 and 6,887 nucleotides. (b) Mosaic image of an entire diaphragm showing widespread full-length dystrophin. The bilateral sections from the diaphragms of injected 9-week-old dko mice were immunostained for dystrophin (dystrophinH-300; 1:100). Wide spread full-length dystrophin in the diaphragm. Bar = 100 µm.
Figure 3.
Physiological expression of exogenous dystrophin and amelioration of dystrophic pathology. (a) Improvement of dystrophic change in the 9-week-old dko diaphragm (top row; wild type, middle row; uninjected dko, bottom row; injected dko). Dystrophin staining (right) and hematoxylin and eosin (H&E) staining (left). Bar = 100 µm. Dystrophin was expressed in wild-type mice and injected dko mice beneath the sarcolemma. (b) Reduction in the number of centrally nucleated fibers. Injected dko mice showed a significant reduction in the number of centrally nucleated fibers when compared with uninjected dko mice (n = 5–6 mice/group, wild type: 0.43 ± 0.40%, uninjected dko: 50.4 ± 4.8%, injected dko: 38.3 ± 6.3%, uninjected dko versus injected dko: *P < 0.05). dko, double knockout mouse.
Figure 4.
Reduction of secondary degeneration by exogenous dystrophin. (a) Decrease in fibrotic area in the injected dko diaphragm. The Masson trichrome staining reveals interstitial fibrosis (aniline blue stained). Masson trichrome-stained transverse sections of 9-week-old dko mice (left; uninjected, right; injected) were shown. Bar = 100 µm. (b) Fibrotic area of injected dko mice showed a significant decrease compared with uninjected dko mice (n = 3–4 mice/group wild type: 1.03 ± 0.26%, uninjected dko: 12.5 ± 2.3%, injected dko: 6.7 ± 0.81%, uninjected dko versus injected dko: *P < 0.05).
Improvement of respiratory function in the injected dko mice
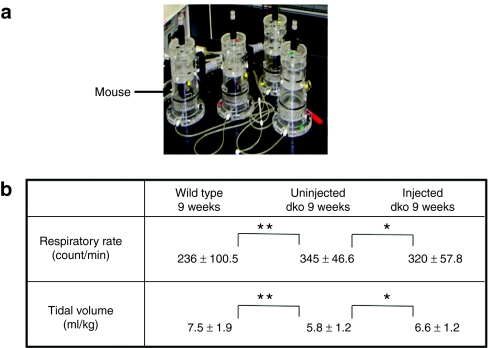
In order to evaluate the progression of respiratory dysfunction in dko mice, sensitive respiratory parameters of mdx mice and control animals were monitored by the latest version of whole-body plethysmography, which provides robust pulmonary analysis with minimal artifacts from animal movement or invasive techniques.19 The respiratory flow displayed on the computer of injected dko was improved, compared with very little flow within the uninjected dko mice. RR in uninjected 9-week-old dko mice (345 ± 46.6 counts/min) was significantly higher than that of age-matched control mice (236 ± 100.5 counts/min) (P < 0.0001) (Figure 5b). The average adjusted TV was 5.8 ± 1.2 ml/kg in uninjected 9-week-old dko mice, which was significantly lower than that of 9-week-old control mice (7.5 ± 1.9 ml/kg, P < 0.0001 versus uninjected dko mice) (Figure 5b). Hyperpnea in 9-week-old dko mice has compensated for their reduced TV. At the same time, the average RR and TV of the injected dko mice were 320 ± 57.8 counts/min and 6.6 ± 1.2 ml/kg, respectively. The injected dko mice showed increased TV (P < 0.01) and improvement in their tachypnea rate (P < 0.01). We measured the muscle force of the diaphragm when the mice were 9-weeks old. The mean maximal tetanic force of the injected dko mice's diaphragm strips was 2.65 ± 0.79 N/cm2 (n = 6), but the diaphragm strips of the uninjected dko mice was 1.59 ± 0.24 N/cm2 (n = 6). That is, the maximal tetanic force of the injected dko mouse's diaphragm became significantly stronger than that of the uninjected dko mouse (data not shown, P < 0.05).
Figure 5.
Improvement of respiratory function by dystrophin transduction. (a) The respiratory function of dko and age-matched wild-type mice were monitored by whole-body plethysmography, in which the animal was free to move around within an enclosed space. (b) Improvement of respiratory function in the 9-week-old injected dko mice. Uninjected 9-week-old dko mouse showed significantly decreased tidal volume (TV) and tachypnea compared with the age-matched (WT) wild-type mice. The injected dko mice showed increased TV and improvement of tachypnea (n = 6–7 mice/group, *P < 0.01, **P < 0.0001). TV was normalized by dividing by body weight (BW).
Discussion
DMD is a serious mortal systemic disease affecting cardiac and respiratory functions, as well as attacking skeletal muscles. Respiratory malfunction, especially, directly leads to a patient's death. Therefore it is crucial to improve respiratory function through means of an easy and effective method. This improvement would greatly impact on patient longevity.
To evaluate the therapeutic effect of gene transfer approach to diaphragm in dko mice, we examined not only the pathology and the electrophysiology on the diaphragm—by measuring specific force—but also the respiratory physiology by measuring the parameters of respiratory functions (TV and RR) using whole-body plethysmography. We could pathologically confirm that in the greater part of the diaphragms of the injected dko mice dystrophin was expressed and its expression prevented the diaphragms from dystrophic alteration. Furthermore, we showed that the respiratory function in the injected dko mice recovered from the obstructive respiratory function. Clinically, the severely aggravated DMD patient is able to breathe with shallow tachypnea in compensation for their small breathing capacity.21 The same symptom can be observed in two kinds of muscular dystrophy model mice: the mdx mouse and the dko mouse. Earlier, using whole-body plethysmography, we have developed a sensitive procedure to measure respiratory parameters in the early stage of respiratory dysfunction in the mdx mouse.19 Furthermore, in this report, we first showed that 9-week-old dko mice were already in respiratory distress. This noninvasive method makes serial measurement possible without killing the mice, which is useful to assess other animal models of neuro-muscular disorders such as motor neuron disease models, Guillain-Barre syndrome, and myasthenia gravis, and so on.
We observed the expression of dystrophin in diaphragms of dko mice injected with HDAdv-myc-mFLdys by intraperitoneal injection. The average of transduction efficiency reached 37% after only one injection into the dko mice's peritoneal cavity. Matecki et al. reported that expression of full-length dystrophin (at 23.6% level) in the diaphragms of 12-week-old mdx mice could achieve partial improvements in histopathology by injection directly into the diaphragm under a microscope. Moreover, maximal tetanic force of the diaphragm in the injected mdx mice was not improved.22 In spite of intraperitoneal administration, we could achieve high transduction efficiency. Moreover, the maximal tetanic force of the injected dko diaphragm was stronger than that of the uninjected dko mouse. We make the conjecture that the age of the mice at time of administration; the titer of viral vector; and the injection volume, all must be important elements that greatly influence the transduction efficiency. And there must be an appropriate range in each element. With respect to the injection volume, we got better transduction efficiency from the fourfold diluted viral vector with phosphate-buffered saline (for the increase of injection volume) than the nondiluted one, even though both contained the same viral particles. Gregorevic et al. reported that intravascular administration of recombinant adeno-associated viral vectors carrying a microdystrophin gene restored expression of truncated dystrophin in the respiratory, cardiac and limb musculature of dko mice, considerably reducing the pathologic changes of skeletal muscle tissue and extending lifespan. In this report, the percentage of the dystrophin-positive fibers in the diaphragms of injected dko mice showed over 60 % and its maximal tetanic force was improved, but analysis of respiratory function was not performed.15 In our study, cardiac muscle and other skeletal muscles (apart from the diaphragm) were rarely transduced (data not shown). In other words, though full-length dystrophin expression only within the diaphragm restored respiratory function in dko mice, it is apparent that full-length dystrophin-transduction in the diaphragm has a therapeutic effect. In 2008, we reported that injected dko mice with multiple intramuscular administrations of HDAdv carrying full-length dystrophin cDNA also showed an increase in body weight, improvement in motor performance, and a prolongation of life span.18 Following this, in the present study, it is clinically significant that we successfully transferred the full-length dystrophin cDNA into the diaphragm and confirmed the efficacy of this therapy by histological and physical observations. In this study, we couldn't analyze precise survival duration because all experimental mice were killed at 9 weeks of age. In our previous data, the body mass of the injected dko mice into multiple proximal skeletal muscles was larger than that of the uninjected dko mice in accordance with the infected dko's improvement in motor performance.18 Meanwhile, other skeletal muscles except diaphragm were rarely transduced in this study. So, the motor performance for dietary intake, the body mass, and survival duration of injected dko mice were same to those of the uninjected dko.
We hope various approaches toward establishing effective therapies for DMD patients will continue, and that the results from this study will contribute to them. Especially, before applying a candidate therapy for a human patient, various data—including the therapeutic efficacy, administration route, and safety—must be accumulated from the animal disease models. Therefore the overall assessment system, including whole-body plethysmography, may be useful to evaluate this data from the neuro-muscular disease models. Furthermore, in future, therapeutic gene transfer with HDAdv may ameliorate respiratory insufficiency in DMD patients.
Materials and Methods
Cell cultures. Cell lines used in this study were COS7 (monkey kidney cells; American Type Culture Collection, Manassas, VA), HEK293 (human embryonic kidney cells), and Cre-293 (HEK293 stably expressing the Cre recombinase).23 All cells were grown in Dulbecco's modified Eagle's medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (JRH Biosciences, Lenexa, KS), 50 U/ml penicillin and 50 U/ml streptomycin (Sigma, St Louis, MO). The cultures were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
Construction and preparation of HDAdv-LacZ-dys and HDAdv-myc-mFLdys. The constructs, HDAdv-LacZ-dys was made as previously reported.17 The construction and preparation method of the helper adenovirus (AdAsw) used in this study was described previously.17 We inserted a myc-tag oligonucleotide, derived from the myc protein and consisting of 10 amino-acids (EQKLISEEDL),24 into the dystrophin cDNA at a unique SwaI site and named pPN-myc-mFLdys. After construction by this method, the pPN-myc-mFLdys was digested at the NotI site, and transfected to Cre-293 cells using the calcium phosphate precipitation. The transfected Cre-293 cells were infected with the helper virus AdASw at a multiplicity of infection of three, and then propagated repeatedly. The virus was purified using CsCl density gradient, and the particle titer of the purified HDAdv (particles/ml) was measured in terms of optical density at 260 nm.25 Finally, we obtained a titer of 1.4 × 1012 virus particles/ml of HDAdv-myc-mFLdys. Helper virus contamination was <1%. In our previous report,18 we confirmed full-length dystrophin expression by western blotting in the skeletal muscles of dko mice by injection of HDAdv-myc-mFLdys.
Animal models. Mdx mice, dko mice, and C57BL/10 strains of mice (Central Institute for Experimental Animals, Kawasaki, Japan) were used in this study. All animal experiments were approved by the Kumamoto University Committee on Animal Research. The mice were housed in the Center for Animal Resources and Development (CARD) of Kumamoto University. The dko mice used in this study were originally generated by Deconinck et al.5 Experimental dko mice were obtained by crossing heterozygous mice in utrophin locus on an mdx background. The pre-6-day-old mice were genotyped by PCR using three primers as described by Deconinck et al.5 All surviving uninjected and injected dko mice were killed to evaluate respiratory function and muscle force at 9 weeks of age. We obtained one or two diaphragm muscle strips from one mouse and evaluated for histopathological analysis and the diaphragm force assay.
The injection protocol. Seven-day-old dko mice were injected with 100 µl of diluted viral vector containing 25 µl of purified the of HDAdv-myc-mFLdys by the intraperitoneal injection, using a 30-gauge half needle on a Hamilton syringe. Before the injection, the mice were anesthetized by placing them on ice. Eight weeks after the injection, mice were under the analysis of respiratory function and histology.
Histopathological analysis and immunostaining. The removed diaphragm after euthanasia by cervical dislocation was mounted into Temperature compound (OCT; Sakura Finetechnical, Tokyo, Japan) and frozen in isopentane precooled with liquid nitrogen. The frozen diaphragm was sectioned at 10 µm thickness and stained with the hematoxylin and eosin and Masson trichrome methods or for immunostaining. The following parameters were evaluated, as previously described14,19,26 (i) percentage of centrally nucleated fibers and (ii) the level degree of fibrosis was evaluated for dystrophic change with Masson trichrome staining. The area of fibrosis was calculated from the entire muscle cross-sectional area (%). Immunostaining for dystrophin was performed as previously described in detail.17 The primary antibody for dystrophin was rabbit polyclonal anti-dystrophin antibody, dystrophinH-300 (1:500 dilution; Santa Cruz Biotechnology, Santa Cruz, CA) and the secondary antibody was Alexa546-labeled goat anti-rabbit IgG (H+L) (1:1,000 dilution; Molecular Probes, Eugene, OR). Stained sections were observed using a confocal laser scanning microscope (LSM410; Carl Zeiss Microscopy, Sena, Germany) and an optical microscope (DP70-WPCXP; Olympus, Tokyo, Japan). The quantitative analyses of dystrophin-positive myofibers on the entire diaphragm muscle strip cross-section was then performed using image analysis WinROOF software (Ver 5.6, Mitani, Fukui, Japan).
Assessment of respiratory function. Mouse respiratory function was evaluated by whole-body plethysmography, as described previously.27,28 This latest version of the noninvasive monitoring system provides robust pulmonary analysis that minimizes artifact from animal movement. Briefly, each unrestrained conscious mouse, 9-week-old dko (uninjected and injected) and C57BL/10, was placed in a “free moving” chamber (450 ml, PLY3211, Buxco Electronics, Wilmington, NC), then monitored and analyzed by BioSystem XA software (Buxco Electronics) (Figure 5a). Each parameter RR and TV were recorded and analyzed in real time, then average values were calculated one per minute for each serial 10 minutes.
Muscle force measurements. The diaphragm was isolated from the mouse at 9 weeks of age, then carefully mounted in a chamber filled with oxygenated Ringer's solution (95% O2, 5% CO2) and maintained at 30 °C. One of the two tendons encompassing the isolated diaphragm was attached to a steel hook in the chamber, and the other was tied to the lever arm of a dual-mode servomotor system (Electronic Stimulator; Nihon Kohden, Tokyo, Japan) via 5-0 surgical silk. The muscle was stretched to the length at which a single twitch showed the highest amplitude (optimal length; Lo). The corresponding tetanic force was then measured at 20, 50, 100, and 150 Hz, 500 ms in duration, with a rest period of 120 seconds between tetani. The specific force (N/cm2) was calculated with muscle density assumed as 1.06 g/cm3.14,29
Statistical studies. Data were expressed as means ± SE of the mean. Statistical analysis was performed using a commercially available software package (StatView, Ver 5, SAS Institute, Cary, NC). Statistical comparisons were performed using one-factor or two-factor analysis of variance. The Mann–Whitney U test was applied to detect differences between the two groups. A P value <0.05 was considered statistically significant.
Acknowledgments
We thank Anne E Deconinck, Kay E Davies (Department of Biochemistry, Genetics Unit, University of Oxford, UK), Ikuya Nonaka (Central Institute for Experimental Animals, Japan), and Shinichi Takeda (National Institute of Neuroscience, National Center of Neurology and Psychiatry, Japan) for kindly providing dko mice. We thank Dr Shinsuke Tsumura (Department of Respitory laboratory, Kumamoto University Graduate School of Medical Sciences, Kumamoto, Japan) for useful technical advice of using whole-body plethysmography. We acknowledge the support of the Center for Animal Resources and Development of Kumamoto University. This work was supported by a Grant-in Aid for Scientific Research from the Japanese Ministry of Education, Science, Sports and Culture to Y.M. (20200133), and M.U. (18590951) and also by a Research Grant (19A-7) for Nervous and Mental Disorders from the Ministry of Health, Labor and Welfare, and partly by the Nakabayashi Trust For ALS Research.
REFERENCES
- Hoffman EP, Brown RH., Jr, and, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Zubrzycka-Gaarn EE, Bulman DE, Karpati G, Burghes AH, Belfall B, Klamut HJ.et al. (1988The Duchenne muscular dystrophy gene product is localized in sarcolemma of human skeletal muscle Nature 333466–469. [DOI] [PubMed] [Google Scholar]
- Koenig M, Monaco AP., and, Kunkel LM. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988;53:219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Bushby KM, Hill A., and, Steele JG. Failure of early diagnosis in symptomatic Duchenne muscular dystrophy. Lancet. 1999;353:557–558. doi: 10.1016/s0140-6736(98)05279-9. [DOI] [PubMed] [Google Scholar]
- Deconinck AE, Rafael JA, Skinner JA, Brown SC, Potter AC, Metzinger L.et al. (1997Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy Cell 90717–727. [DOI] [PubMed] [Google Scholar]
- Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B.et al. (1991The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy Nature 352536–539. [DOI] [PubMed] [Google Scholar]
- Blake DJ, Weir A, Newey SE., and, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev. 2002;82:291–329. doi: 10.1152/physrev.00028.2001. [DOI] [PubMed] [Google Scholar]
- Perkins KJ., and, Davies KE. The role of utrophin in the potential therapy of Duchenne muscular dystrophy. Neuromuscul Disord. 2002;12 Suppl 1:S78–S89. doi: 10.1016/s0960-8966(02)00087-1. [DOI] [PubMed] [Google Scholar]
- Hoffman EP. Dystrophin associated proteins fail in filling dystrophin's shoes. Nat Genet. 1994;8:311–312. doi: 10.1038/ng1294-311. [DOI] [PubMed] [Google Scholar]
- Tinsley JM., and, Davies KE. Utrophin: a potential replacement for dystrophin. Neuromuscul Disord. 1993;3:537–539. doi: 10.1016/0960-8966(93)90111-v. [DOI] [PubMed] [Google Scholar]
- Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS.et al. (2002Functional improvement of dystrophic muscle by myostatin blockade Nature 420418–421. [DOI] [PubMed] [Google Scholar]
- Blankinship MJ, Gregorevic P, Allen JM, Harper SQ, Harper H, Halbert CL.et al. (2004Efficient transduction of skeletal muscle using vectors based on adeno-associated virus serotype 6 Mol Ther 10671–678. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Blankinship MJ, Allen JM, Crawford RW, Meuse L, Miller DG.et al. (2004Systemic delivery of genes to striated muscles using adeno-associated viral vectors Nat Med 10828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Sakamoto M, Ikemoto M, Mochizuki Y, Yuasa K, Miyagoe-Suzuki Y.et al. (2004AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype Mol Ther 10821–828. [DOI] [PubMed] [Google Scholar]
- Gregorevic P, Allen JM, Minami E, Blankinship MJ, Haraguchi M, Meuse L.et al. (2006rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice Nat Med 12787–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura E, Maeda Y, Arima T, Nishida Y, Yamashita S, Hara A.et al. (2001Efficient repetitive gene delivery to skeletal muscle using recombinant adenovirus vector containing the Coxsackievirus and adenovirus receptor cDNA Gene Ther 820–27. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Maeda Y, Kimura E, Yamashita S, Nishida Y, Arima T.et al. (2005Effective repetitive dystrophin gene transfer into skeletal muscle of adult mdx mice using a helper-dependent adenovirus vector expressing the coxsackievirus and adenovirus receptor (CAR) and dystrophin J Gene Med 71010–1022. [DOI] [PubMed] [Google Scholar]
- Kawano R, Ishizaki M, Maeda Y, Uchida Y, Kimura E., and, Uchino M. Transduction of full-length dystrophin to multiple skeletal muscles improves motor performance and life span in utrophin/dystrophin double knockout mice. Mol Ther. 2008;16:825–831. doi: 10.1038/mt.2008.23. [DOI] [PubMed] [Google Scholar]
- Ishizaki M, Suga T, Kimura E, Shiota T, Kawano R, Uchida Y.et al. (2008Mdx respiratory impairment following fibrosis of the diaphragm Neuromuscul Disord 18342–348. [DOI] [PubMed] [Google Scholar]
- Yue Y, Liu M., and, Duan D. C-terminal-truncated microdystrophin recruits dystrobrevin and syntrophin to the dystrophin-associated glycoprotein complex and reduces muscular dystrophy in symptomatic utrophin/dystrophin double-knockout mice. Mol Ther. 2006;14:79–87. doi: 10.1016/j.ymthe.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misuri G, Lanini B, Gigliotti F, Iandelli I, Pizzi A, Bertolini MG.et al. (2000Mechanism of CO2 retention in patients with neuromuscular disease Chest 117447–453. [DOI] [PubMed] [Google Scholar]
- Matecki S, Dudley RW, Divangahi M, Gilbert R, Nalbantoglu J, Karpati G.et al. (2004Therapeutic gene transfer to dystrophic diaphragm by an adenoviral vector deleted of all viral genes Am J Physiol Lung Cell Mol Physiol 287L569–L576. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Kimura E, Uchida Y, Nishida Y, Yamashita S, Arima T.et al. (2003Cre/loxP-mediated adenovirus type 5 packaging signal excision demonstrates that core element VI is sufficient for virus packaging Virology 309330–338. [DOI] [PubMed] [Google Scholar]
- Campbell AM, Kessler PD., and, Fambrough DM. The alternative carboxyl termini of avian cardiac and brain sarcoplasmic reticulum/endoplasmic reticulum Ca(2+)-ATPases are on opposite sides of the membrane. J Biol Chem. 1992;267:9321–9325. [PubMed] [Google Scholar]
- Mittereder N, March KL., and, Trapnell BC. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J Virol. 1996;70:7498–7509. doi: 10.1128/jvi.70.11.7498-7509.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan JG, Hahn HS, Wong BL, Lorenz JN, Wenisch AS., and, Levin LS. Evolution of the mdx mouse cardiomyopathy: physiological and morphological findings. Neuromuscul Disord. 2004;14:491–496. doi: 10.1016/j.nmd.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Lefort J, Singer M, Leduc D, Renesto P, Nahori MA., and, Huerre M.et al. (1998Systemic administration of endotoxin induces bronchopulmonary hyperreactivity dissociated from TNF-α formation and neutrophil sequestration into the murine lungs J Immunol 161474–480. [PubMed] [Google Scholar]
- Schnyder-Candrian S, Quesniaux VF, Di Padova F, Maillet I, Noulin N, Couillin I.et al. (2005Dual effects of p38 MAPK on TNF-dependent bronchoconstriction and TNF-independent neutrophil recruitment in lipopolysaccharide-induced acute respiratory distress syndrome J Immunol 175262–269. [DOI] [PubMed] [Google Scholar]
- Gilbert R, Dudley RW, Liu AB, Petrof BJ, Nalbantoglu J., and, Karpati G. Prolonged dystrophin expression and functional correction of mdx mouse muscle following gene transfer with a helper-dependent (gutted) adenovirus-encoding murine dystrophin. Hum Mol Genet. 2003;12:1287–1299. doi: 10.1093/hmg/ddg141. [DOI] [PubMed] [Google Scholar]