Narrative abstract
Nuclear and cytoplasmic endometrial expression of Indian Hedgehog (IHH) increased from the late proliferative to mid- and late secretory phases in 26 healthy volunteers compared to 30 women with endometriosis. The abnormal expression of IHH protein in women with endometriosis suggests a resistance to progesterone action.
The cause(s) of infertility in women with endometriosis is not well understood. Endometriosis has been associated with increased levels of cytokines and growth factors in the peritoneal fluid, reduced fertilization, tubal obstruction or anatomic distortion, and impaired oocyte maturation (1,2).
Recent findings suggest that impaired endometrial receptivity may also contribute to infertility in endometriosis. Implantation and decidualization are abnormal in a baboon model (3) and women with endometriosis have altered endometrial gene expression in the mid-secretory phase (4, 5). Many of these genes produce progesterone-responsive proteins that may be critical for implantation. For example, the expression of N-acetylglucosamine-6-O-sulfotransferase, which glycosylates the L-selectin surface protein needed for blastocyst recognition of the endometrium, is down-regulated. Other endometrial peptides thought to be involved in implantation also are decreased, including aromatase, hepatocyte growth factor, HOXA10, HOXA11, αVβ3 integrin, 17-beta-hydroxysteroid dehydrogenase, leukemia inhibitory factor, endometrial bleeding factor, matrix metalloproteinases, osteoponin, lysophosphatidic acid receptor 3, HOXA10, and glycodelin A (5–7).
Indian hedgehog (Ihh) is an important mediator of implantation in rodents, as shown by failure of attachment and decidualization in Ihh null mice. While the full scope of its function is still under investigation, one role may be to facilitate inter-cellular connections at the maternal-fetal interface (8).
In rodents, progesterone appears to regulate Ihh production (8–11). IHH, and its downstream target GLI1, increase in healthy women during the secretory phase in both the glandular and stromal nuclei and cytoplasm (12). In this preliminary study we postulated that women with endometriosis may demonstrate lower secretory phase endometrial expression of IHH, as is seen with other putative implantation factors.
Women participated in protocols approved by the NICHD Investigational Review Board for the treatment of endometriosis (NT00001848) or for biopsy procurement of endometrium from normally cycling women (NCT0001454), and gave informed consent. No healthy volunteer had a history suggestive of endocrinological or gynecological disorders or subfertility. Each had normal menstrual cycles, with a cycle length of 24 to 35 days and a secretory phase of at least 12 days based on in-home detection of an LH surge (OvuQUICK™, Quidel, San Diego, CA). Samples were obtained from women with endometriosis during diagnostic laparoscopy. All samples were placed in 4% paraformaldehyde before paraffin embedding and sectioning. Representative slides were dated according to the criteria of Noyes et al. (13). Endometriosis was verified histologically. A standard immunohistochemistry (IHC) protocol was performed to detect and quantify IHH expression, as previously reported (12).
Based on the Noyes’ criteria, biopsies were categorized as late proliferative or early (days 15–18), mid (days 19–23) or late (days 24–28) secretory. Two independent examiners scored the IHC staining intensity (i) of at least 200 glandular epithelial cells and 200 stromal cells on each slide from 0 (none) to 3 (strong). A separate score was given for the staining of the nuclei and the cytoplasm of each cell. For nuclear staining, a histologic score (H-score) = ΣPi(i+1) was calculated, where Pi is the percentage of cells for each intensity, varying from 0 to 100%. Because cytoplasmic staining was consistent, the relative staining intensity in each field was scored by the same observers on a scale of 0 – 3 with 0.5 increments, and the scores for all fields were averaged.
Data are presented as mean ± standard deviation, unless otherwise specified. Statistical analysis was performed with the Tukey-Kramer test, the Wilcoxon/Kruskal-Willis test or unpaired t-test, as appropriate. Differences across the menstrual cycle phases in the H-scores and cytoplasmic staining were evaluated within the control and patient groups. Differences between the two groups at each cycle phase also were evaluated. When the Wilcoxon/Kruskal-Willis test yielded a significant p value (<0.05), Dunnett’s post-hoc test was performed, with the healthy volunteer late secretory phase used as a control.
The distribution of self-reported race was similar in the two groups. Patients were Asian (n=1), multiple races (n=1), Black (n=6), and White (n=22) while volunteers were Black (n=7), White (n=18) or did not report race (n=1). The groups had similar age (patients 30.4 years, volunteers 31.1 years, p = 0.67). The distribution of r-AFS staging was I (n = 9), II (n=11), III (n = 4) and IV (n=6), and was relatively evenly distributed across the menstrual cycle except for only stages I and II in the late proliferative phase (Supplemental Data Table 1).
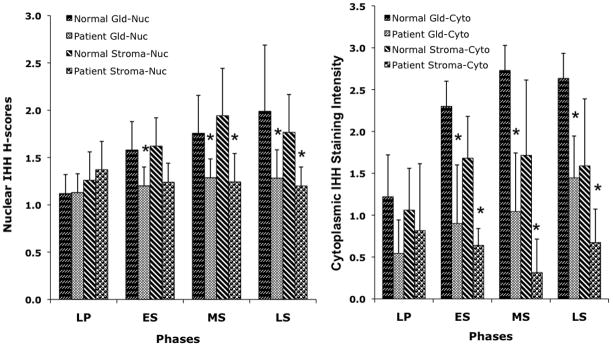
H-scores for nuclear staining and cytoplasmic staining intensities for Indian hedgehog are shown in the figure (and Supplemental data Table 2). In the healthy volunteers, IHH expression increased in both tissue type and sub-cellular compartments from the late proliferative to the mid- and late secretory phase. However, the early and mid-luteal phase values did not differ from the late luteal results. In patients with endometriosis, an upward trend was present only with comparison of the late proliferative and late secretory glandular cytoplasm, and values were significantly lower than in controls. Supplemental data figure 1 shows representative photographs. Cervix showed no staining (data not shown).
Figure 1.
A. Expression of IHH in endometrium of women with endometriosis (patient) or healthy volunteers (normal) in the nuclei (Nuc) of the glandular (Gld) or stromal (Stroma) compartments. Except for the early secretory phase stroma, all secretory phase patient values were significantly different from the corresponding menstrual phase and tissue result in healthy volunteers. LP = late proliferative, ES = early secretory, MS = mid-secretory, LS = late secretory
B. Expression of IHH in endometrium of women with endometriosis (patient) or healthy volunteers (normal) in the cytoplasm (Cyto) of the glandular (Gld) or stromal (Stroma) compartments. All secretory phase patient values were significantly different from the corresponding menstrual phase and tissue result in healthy volunteers. ES = early secretory, MS = mid-secretory, LS = late secretory.
Compared to the healthy volunteer result for the late secretory phase, the late proliferative stromal staining was similar for patients and volunteers, while the glands had decreased staining in both. In the same comparison, the patients showed decreased early and mid- secretory phase staining while the volunteers had similar staining at these times (Supplemental data Table 2).
Within the endometriosis group, compared to the late luteal phase, only the late proliferative glandular cytoplasm was different (p=0.0095). Among the healthy volunteers, in comparison to the late secretory phase, less staining was present in the late proliferative glandular nuclei (p=0.0119) and cytoplasm (p < 0.0001).
Compared to the healthy volunteers for each corresponding time interval, all cell compartments of endometriosis patients showed decreased Indian hedgehog expression for all secretory phase-paired samples (p = 0.0001 to 0.0459) except early secretory stromal nuclei.
Mounting evidence suggests that patients with endometriosis have abnormal expression of peptides that influence endometrial receptivity. In this study we show reduced luteal phase endometrial expression of Indian hedgehog in women with endometriosis. Indian hedgehog appears to be critical for implantation in the mouse, and our recent report of its luteal phase presence and apparent progesterone-responsiveness in women, suggests a possible role in the human (8, 12).
In this study, as expected, the expression of the Indian hedgehog in the volunteers increased from the late proliferative phase to the late secretory phase of the menstrual cycle in all tissue compartments (glandular and stromal nuclei and cytoplasm). In addition to the decreased expression of the protein in the patients with endometriosis as compared to the normal volunteers, the patients exhibited an increase only in the glandular cytoplasm. One limitation of this report is the lack of in situ hybridization data to validate the cellular source of the peptide. However, in a previous report we showed that localization of RNA by in situ hybridization corresponded to the results shown by immunohistochemistry (12).
Because progesterone levels are known to be normal in patients with endometriosis (14), the failure of IHH to increase during the luteal phase in these women suggests tissue resistance to progesterone. Bulun’s group has shown that conversion of 17beta-estradiol to estrone is impaired in endometriotic tissues due to deficient expression of 17betaHSD-2. This enzyme is normally expressed in eutopic endometrium in response to progesterone (15). This defect is apparently caused by an inability of the stromal cells to generate a progesterone-dependent paracrine factor that induces 17betaHSD-2 in epithelial cells. These findings support the notion of a resistance to progesterone action in endometriotic tissue.
We previously extended the concept of progesterone resistance of eutopic endometrium of women with endometriosis (7). The expression of four biomarkers of implantation, glycodelin A, osteopontin, lysophosphatidic acid receptor 3, and HOXA10, were reduced in the mid-secretory phase of women with endometriosis. As all of these markers have a progesterone-responsive element(s) in their promoter region, reduced expression of these genes provides additional evidence for progesterone resistance.
A report that progesterone receptor (PR) transcripts are lower in endometriotic lesions than in eutopic endometrium, and that PR A is the predominant PR in endometriosis, suggests a mechanism for progesterone resistance (16, 17). In women with endometriosis, epithelial cells in endometriotic implants (and eutopic endometrium) show hypermethylation of PRB, presumably leading to gene silencing and accounting for the suppressed PR levels (18). The increased expression in women with endometriosis of deoxyribonucleic acid methyltransferases responsible for methylation suggests a plausible mechanism by which multiple genes might be downregulated in this disorder (6). However, studies of stromal cells from eutopic endometrium show no difference in PR ratios between women with and without endometriosis, and there is no cyclic variability in the expression of the receptors (19). Thus, this mechanism would not explain the findings of abnormal stromal protein expression.
The importance of the IHH protein may be in its downstream regulation of BMP2, GLI1, GLI2, and COUP-TFII, all of which have been found to have a role in murine implantation (8, 20). Although the role of IHH in women is still being elucidated, the reduced expression of IHH in patients with endometriosis provides a potential additional mechanism for the infertility seen in these women.
Supplementary Material
Acknowledgments
Financial Support: This research was supported by the Intramural Program on Reproductive and Adult Endocrinology, NICHD, NIH.
Footnotes
Where the work was done:
Program on Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD
Conflict of interest: None
Presented at a meeting: Presented in part at the 66th Annual meeting of the American Society for Reproductive Medicine, Denver 2010
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arici A. Local cytokines in endometrial tissue: the role of interleukin-8 in the pathogenesis of endometriosis. Ann N Y Acad Sci. 2002;955:101–9. doi: 10.1111/j.1749-6632.2002.tb02770.x. discussion 18, 396–406. [DOI] [PubMed] [Google Scholar]
- 2.Halis G, Arici A. Endometriosis and inflammation in infertility. Ann N Y Acad Sci. 2004;1034:300–15. doi: 10.1196/annals.1335.032. [DOI] [PubMed] [Google Scholar]
- 3.Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med. 2010;28:75–80. doi: 10.1055/s-0029-1242997. [DOI] [PubMed] [Google Scholar]
- 4.Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: evidence for progesterone resistance. Semin Reprod Med. 2010;28:51–8. doi: 10.1055/s-0029-1242994. [DOI] [PubMed] [Google Scholar]
- 5.Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology. 2003;144:2870–81. doi: 10.1210/en.2003-0043. [DOI] [PubMed] [Google Scholar]
- 6.Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, et al. Altered expression of HOXA10 in endometriosis: potential role in decidualization. Mol Hum Reprod. 2007;13:323–32. doi: 10.1093/molehr/gam005. [DOI] [PubMed] [Google Scholar]
- 7.Wei Q, St Clair JB, Fu T, Stratton P, Nieman LK. Reduced expression of biomarkers associated with the implantation window in women with endometriosis. Fertil Steril. 2009;91:1686–91. doi: 10.1016/j.fertnstert.2008.02.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee K, Jeong J, Kwak I, Yu CT, Lanske B, Soegiarto DW, et al. Indian hedgehog is a major mediator of progesterone signaling in the mouse uterus. Nat Genet. 2006;38:1204–9. doi: 10.1038/ng1874. [DOI] [PubMed] [Google Scholar]
- 9.Kurihara I, Lee DK, Petit FG, Jeong J, Lee K, Lydon JP, et al. COUP-TFII mediates progesterone regulation of uterine implantation by controlling ER activity. PLoS Genet. 2007;3:e102. doi: 10.1371/journal.pgen.0030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simon L, Spiewak KA, Ekman GC, Kim J, Lydon JP, Bagchi MK, et al. Stromal progesterone receptors mediate induction of Indian Hedgehog (IHH) in uterine epithelium and its downstream targets in uterine stroma. Endocrinology. 2009;150:3871–6. doi: 10.1210/en.2008-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto H, Zhao X, Das SK, Hogan BL, Dey SK. Indian hedgehog as a progesterone-responsive factor mediating epithelial-mesenchymal interactions in the mouse uterus. Dev Biol. 2002;245:280–90. doi: 10.1006/dbio.2002.0645. [DOI] [PubMed] [Google Scholar]
- 12.Wei Q, Levens ED, Stefansson L, Nieman LK. Indian Hedgehog and its targets in human endometrium: menstrual cycle expression and response to CDB-2914. J Clin Endocrinol Metab. 2010;95:5330–7. doi: 10.1210/jc.2010-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyes RW, Hertig AT, Rock J. Dating the endometrial biopsy. Fertil Steril. 1950;1:3–25. doi: 10.1016/j.fertnstert.2019.08.079. [DOI] [PubMed] [Google Scholar]
- 14.Kusuhara K. Luteal function in infertile patients with endometriosis. Am J Obstet Gynecol. 1992;167:274–7. doi: 10.1016/s0002-9378(11)91674-3. [DOI] [PubMed] [Google Scholar]
- 15.Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, et al. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J Clin Endocrinol Metab. 1998;83:4474–80. doi: 10.1210/jcem.83.12.5301. [DOI] [PubMed] [Google Scholar]
- 16.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 17.Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, et al. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril. 2005;84:67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- 18.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1:106–11. doi: 10.4161/epi.1.2.2766. [DOI] [PubMed] [Google Scholar]
- 19.Gentilini D, Vigano P, Vignali M, Busacca M, Panina-Bordignon P, Caporizzo E, et al. Endometrial stromal progesterone receptor-A/progesterone receptor-B ratio: no difference between women with and without endometriosis. Fertil Steril. 2010;94:1538–40. doi: 10.1016/j.fertnstert.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Achache H, Revel A. Endometrial receptivity markers, the journey to successful embryo implantation. Hum Reprod Update. 2006;12:731–46. doi: 10.1093/humupd/dml004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.