Abstract
In this prospective nested case-control study we analyzed the circumferential differences in estimated cortical thickness (Est CTh) of the mid femoral neck as a risk factor for osteoporotic hip fractures in elderly women and men. Segmental QCT analysis of the mid femoral neck was applied to assess cortical thickness in anatomical quadrants. The superior region of the femoral neck was a stronger predictor for hip fracture than the inferior region, particularly in men. There were significant gender differences in Est CTh measurements in the control group but not in the case group. In multivariable analysis for risk of femoral neck (FN) fracture, Est CTh in the supero-anterior (SA) quadrant was significant in both women and men, and remained a significant predictor after adjustment for FN areal BMD (aBMD, dimensions g/cm2, DXA-like), (p=0.05 and p<0.0001, respectively). In conclusion, Est CTh in the SA quadrant best discriminated cases (n=143) from controls (n=298), especially in men. Cortical thinning superiorly in the hip might be of importance in determining resistance to fracture.
Keywords: cortical thickness, hip fracture, quantitative computed tomography, proximal femur, BMD
1. Introduction
The number of hip fractures is increasing worldwide due to rapidly aging populations and an exponential relationship exists between hip fracture and age in both sexes [1, 2]. Hip fracture can lead to permanent disability or death [3]. One out of every 4 to 5 patients does not survive more than 1 year after the fracture [3, 4]. In 90% of all hip fractures cases, a fracture is sustained through a fall [5].
Current methods of evaluating the risk of proximal femur fracture are based on the idea that reduced bone strength as measured by (areal) bone mineral density (BMD) is related to fracture risk [6-8]. Dual-energy X-ray absorptiometry (DXA) and quantitative computed tomography (QCT) are used to estimate BMD. Yet BMD alone measured by DXA or QCT in femoral neck does not satisfactorily identify individual subjects at high risk of fracture [9].
The proximal femur is a complex structure comprised of cortical and trabecular bone. Marked thinning of the mid-femoral neck cortex with advancing age was first described ex-vivo in a small sample of male post mortem samples [10] and later in a larger and arguably representative sample of women and men [11] and is also evident in femoral neck fracture specimens [11-13]. In normal gait, the greatest stresses occur in the sub-capital and medial mid-femoral neck regions [14]. Bone is preferentially lost during ageing in the superior lateral femoral neck [11, 15] which is already thin in childhood where it coincides with the persistent intra-epiphyseal fibrocartilaginous region [16]. Ageing bone loss may be due to the stress-shielding effect of bipedalism [17], and such thinning contributes to the fragility of the elderly human femoral neck in a sideways fall on to the trochanter [9, 11, 12, 14, 18, 19], when a high compressive load is generated. The contribution of trabecular bone to bone strength in the femoral neck may be low during stance [20] but Manske et al. studying cadaveric proximal femora by QCT reported that both cortical and trabecular bone contribute similarly to failure load [21]. Trabecular bone is important in preventing buckling, but may be less important if the failure mechanism is crushing [22].
In this prospective nested case-control study, our primary aim was to investigate the predictive ability of regional cortical measurements at the mid femoral neck (using multi-slice computed tomography, CT) for femoral neck and trochanteric fracture in elderly men and women. Using baseline CT scans of femoral neck and trochanteric fracture cases and controls from the population-based AGES-REYKJAVIK study, cortical thickness estimates in anatomical quadrants were made within a cross sectional region of interest of the mid-femoral neck, (orthogonal to the femoral neck axis). We used a CT image analysis protocol that has been shown to be highly reproducible in location and not subject to anatomical drift with age [23].
2. Material and methods
2.1 Study participants
Individuals were participants in the Age Gene/Environment Susceptibility-Reykjavik Study (AGES-REYKJAVIK), a single-center prospective ongoing population study of Icelandic men and women. Design and recruitment have been described in detail [24, 25]. Medical records were checked biannually from all hospitals receiving hip fractures in Iceland. From these records, we identified all low trauma fractures, defined as a fracture resulting from a fall from a standing position or lower. All reported fractures were verified by an orthopedic surgeon (BM) who reviewed X-rays and categorized the fractures into femoral neck fracture and trochanteric fracture (using the ICD 10 classification). During an average 4.5 years of follow-up of 4831 participants in AGES-REYKJAVIK study (with baseline QCT scans between 2002 and 2006), 154 first low trauma incident hip fractures (S72.0, S72.1 and S72.2) were reported. Of those, eleven cases could not be analyzed due to suboptimal image quality (motion-, slice misregistration artifacts, etc). We designed a case-control study nested within this prospective cohort study. Using a random selection procedure, 2 controls were matched to each fracture case matched for calendar year of recruitment, sex, and age (n=308). Of those, 10 controls could not be analyzed due to suboptimal image quality. The final sample size resulted in 143 cases (88 women and 55 men) and 298 controls (187 women and 111 men). Differences in characteristics of the random samples and the entire cohort were compared using t-tests. Overall, the height, weight, BMI and integral femoral neck vBMD were similar in the control group as in the full AGES-REYKJAVIK cohort after adjustment for age (p>0.42), confirming the validity of this randomized selection.
At the baseline visit, height and weight were measured and participants were asked to bring all medications used in the previous 2 weeks to the clinic representing current usage. Medications known to affect bone density including estrogen replacement therapy, tibolone, antiepileptics, systemic glucocorticosteroids and agents for the treatment of osteoporosis (raloxifene, calcitonin or bisphosphonates) were identified. All participants provided written informed consent, and the study was approved (VSN 00-063) by the National Bioethics Committee in Iceland as well as the Institutional Review Board of the Intramural Research Program of the National Institute of Aging.
2.2 Quantitative computerized tomography (QCT) scanning
CT measurements were performed in the hip using a 4-row detector CT system (Sensation, Siemens Medical Systems, Erlangen, Germany). To calibrate CT Hounsfield units to equivalent bone mineral concentration, all subjects lay on a calibration phantom (Image Analysis, Columbia, KY, USA), which extended from a position superior to the L1 vertebral body to the mid-femoral shaft. The phantom contained calibration cells of 0, 75 and 150 mg/cm3 equivalent concentration of calcium hydroxyapatite. Scans were acquired using a standardized protocol and encompassed the proximal femur from a level 1 cm superior to the acetabulum to a level 3-5 mm inferior to the lesser trochanter at settings of 120 kVp, 140mAs, 1-mm slice thickness, pitch=1, pixel size of 0.977 mm and 512×512 matrix in spiral reconstruction mode using the standard kernel with a 50-cm reconstruction FOV. The imaging centre uses highly stringent and reproducible daily quality assurance tests to monitor scanner stability based on a phantom test including measurements of slice geometry, spatial uniformity, density linearity, spatial resolution and noise. In addition the imaging centre performs weekly measurements to monitor density linearity of the calibration phantom described above. The scanner is calibrated to water once every month.
2.3 QCT-derived femoral neck measures
The CT images were processed to extract measures of volumetric BMD (vBMD), areal BMD (aBMD, DXA equivalent) and bone architecture at the femoral neck using QCT PRO CTXA software (Mindways, Austin, Texas) according to a protocol described in detail recently by Poole et al. [23].
Bone segmentation
Automated segmentation of bone from soft-tissue voxels generated axial, coronal and sagittal reconstructions of the hip followed by 3D rendering and femoral neck axis placement. A region-growing algorithm classified each pixel as either ‘bone’ or ‘not bone’ using an adaptive classifier. A graphical user interface allowed the operator to optimize the reconstructed images in 3 planes of section; to properly position the hip for cortical thickness measurements, to trim excessive soft tissue pixels, fill artefactual bone holes and optimize the femoral neck axis on the 3D surface-rendered image.
Cortical thickness measurements
A scripting command, written to extract automatically six contiguous cross-sectional slices of 1 mm thickness of the mid-femoral neck from a reproducible location for measurements, was used with the QCT PRO Bone Investigational Toolkit (BIT2) software (Mindways, Austin, Texas). The mid-femoral neck cross-section placement was automatically directed perpendicular to the femoral neck axis where the approximate ratio of maximum to minimum diameters equaled to 1.4; slice 6 in Fig. 1. Studies have shown that where the ratio of the distances between the superior to inferior (maximum) and anterior to posterior diameters (minimum) is 1.4, this region of the femoral neck has the lowest cross-sectional area and can be considered the mid-neck [13]. Five further slices were extracted at 1 mm intervals medially. The extracted images had an interpolated pixel size of 0.488 mm. Estimated cortical thickness (Est CTh) at the mid-femoral neck was determined in anatomical quadrants. The quadrants were defined by using the centre of mass and equal angle to simulate anatomical stance. The centre of area was the internal reference point, with 16 equal sectors defined by equal angles (22.5°) and the first sector boundary defined by a vertical line; see figure 1. This resulted in four anatomical quadrants (fig. 1); supero-anterior (SA: from sectors 2,3,4,5), infero-anterior (IA: 6,7,8,9) and infero-posterior (IP: 10,11,12,13) supero-posterior (SP:14,15,16,1). The cortical bone threshold was chosen at 450 mg/cm3 because this minimized differences from cortical thickness estimates made by higher resolution CT and histological methods [23, 26]. Est CTh was calculated for each of the 16 sectors as follows; the cortical area was measured automatically (by pixel counting) on each cross-sectional image. In each sector, the cortical bone mass was assumed to be evenly distributed between the two surface boundaries (one periosteal and the other endosteal). The boundaries were approximated as concentric arcs of constant curvature, which enclosed the point of the cortical centre of mass (relative to the intersection point of the sector lines, as estimated by density-weighted pixel counting). The software automatically selected two radii of curvature (one periosteal, one endosteal) that matched the pixel counted cortical area. Est CTh by quadrants was averaged across corresponding sectors and the data from the 6 cross-sections were used to derive a single mean estimate for each quadrant. Average CTh (AvgCTh) was evaluated as the mean across the four quadrants. The intra-observer variability of the CTh measurements by quadrants was estimated using coefficient of variation (CV). The CVs were 4.6% for SP CTh; 6.5% for SA CTh; 3.9% for IP CTh; and 4.4% for IA CTh (Twenty measurements were repeated once by one observer (FJ)).
Figure 1.
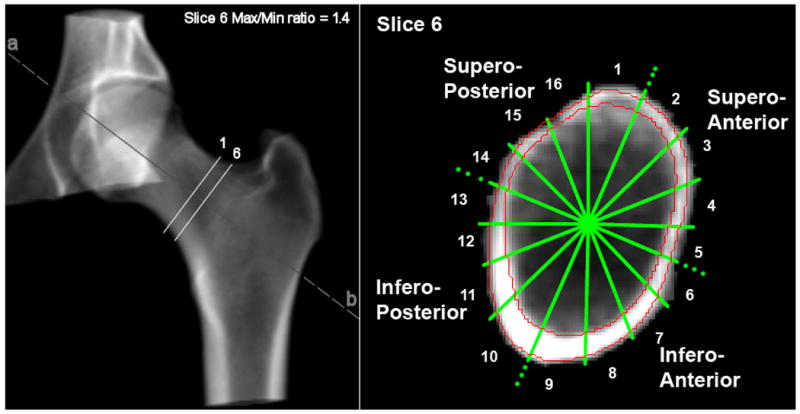
3D CT rendering (left) showing ROIs and Slice Position. Shape of FN at max/min ratio 1.4 (right) showing anatomical quadrants (note the clockwise shift of one sector due to sagittal positioning). The red contours show the cortex.
DXA-equivalent femoral neck aBMD, Hip axis length and vBMD
Single mean integral volumetric BMD (IntvBMD) estimate was made from the 6 cross-sections in the mid-femoral neck. DXA-equivalent for the total femoral neck aBMD (FNaBMD) was determined by the software and hip axis length (HAL) was measured from 2D projection screen images using the QCT PRO slice-pick measurement tool.
2.4 Statistical analyses
The variables were analyzed after natural log-transformation to normalize their distributions. The estimated cortical thickness measurements were log-transformed using log(Est CTh+1). One-way analysis of variance (ANOVA) was used to compare variables among controls, FN cases, and TR cases. Repeated measures MANOVA were used to determine the differences and parallelism between controls and cases across the quadrants. Student's t-test was used to test for female-male differences. The percent differences between cases and controls in Est CTh by quadrants and FNaBMD were estimated using linear regression on the log-transfromed data. General linear models were used to estimate percent changes over a 10-years age interval having the outcomes (CTh) on log-scale; then exponentiating the regression parameter (slope) for age generates the percent change in 1-year. The correlation coefficient between two quadrants was estimated assuming unstructured covariance structure using a mixed model.
The analysis of time to low trauma hip fracture was performed using the Cox proportional hazards regression model and results were expressed as hazard ratios (HRs; and 95%CIs) per 1 SD change in the parameters. All analyses were adjusted for age, weight and height. Stepwise selection methods were used in the multiple predictor proportional hazard models to examine interdependence of CTh by quadrants and total femoral neck aBMD. Women and men were analyzed separately, as were femoral neck and trochanteric fracture cases.
3. Results
3.1 Characteristics of the study group
Table 1 shows the distribution of hip fractures by age group and sex in our population. The proportions of FN and TR fractures were similar in both sexes (p=0.73). Table 2 shows baseline characteristics of the study subjects. Women who sustained TR fracture had lower body weight (p=0.006) and lower BMI (p=0.002) than controls. Male TR fracture cases had marginally lower BMI than male controls (p=0.08). Average age at fracture was 83 years, independent of sex and fracture type (p=0.62 and p=0.87, respectively).
Table 1. Fractures vs age in the study group.
| Women | |||
|---|---|---|---|
| Controls (n=187) | FN Cases (n=47) | TR Cases (n=41) | |
| Mean [SD] | |||
| Age (years) | 79.3 [5.2] | 79.4 [6.1] | 79.7 [4.8] |
| Age at fracture (years) | - | 83.4 [6.4] | 82.3 [5.3] |
| Age group (years) | n | n | n |
|
| |||
| -69 | 9 | 5 | 0 |
| 70-74 | 24 | 5 | 7 |
| 75-79 | 64 | 12 | 14 |
| 80-84 | 63 | 18 | 14 |
| 85+ | 27 | 7 | 6 |
|
| |||
| Men | |||
|
| |||
| Controls (n=111) | FN Cases (n=31) | TR Cases (n=24) | |
|
|
|||
| Mean [SD] | |||
|
| |||
| Age (years) | 79.6 [5.1] | 80.2 [5.8] | 80.1 [5.2] |
| Age at fracture (years) | - | 83.0 [6.1] | 83.0 [5.4] |
| Age group (years) | n | n | n |
|
| |||
| -69 | 1 | 0 | 0 |
| 70-74 | 22 | 8 | 4 |
| 75-79 | 28 | 5 | 7 |
| 80-84 | 41 | 10 | 8 |
| 85+ | 19 | 8 | 5 |
Table 2.
Baseline characteristics and QCT variables at femoral neck; controls vs. fracture cases (mean and SD).
| Women | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Controls | Femoral Neck Cases | Trochanteric Cases | Overall | Ctrl vs FN | Ctrl vs TR | % Reduction FN | % Reduction TR | FN vs TR | |
| Mean (SD) | Mean (SD) | Mean (SD) | p-value | p-value | p-value | Mean (95%CI) | Mean (95%CI) | p-value | |
| Height (cm) | 159.8 (5.4) | 161.3 (6.5) | 159.0 (5.8) | 0.15 | 0.13 | 0.38 | - | - | 0.09 |
| Weight (kg) | 67.4 (10.9) | 68.5 (15.3) | 62.3 (12.8) | 0.016 | 0.83 | 0.006 | - | - | 0.04 |
| BMI (kg/cm2) | 26.5 (4.5) | 26.2 (4.8) | 24.6 (4.4) | 0.008 | 0.59 | 0.002 | - | - | 0.10 |
| HAL (mm) | 114.0 (5.5) | 116.4 (5.8) | 114.5 (6.3) | 0.037 | 0.01 | 0.62 | - | - | 0.14 |
| Femoral neck aBMD (mg/cm2) | 662 (132) | 607 (93) | 573 (99) | <0.0001 | 0.01 | <0.0001 | 8 (1, 14) | 14 (7, 22) | 0.07 |
| IntvBMD* (mg/cm3) | 230 (47) | 204.8 (32) | 196 (32) | <0.0001 | 0.0005 | <0.0001 | - | - | 0.20 |
| Avg CTh* (mm) | 1.823 (0.568) | 1.595 (0.449) | 1.464 (0.353) | 0.0002 | 0.013 | 0.0002 | 12 (2, 22) | 20 (10, 30) | 0.17 |
| SP Est CTh (mm) | 0.771 (0.522) | 0.574 (0.381) | 0.489 (0.346) | 0.0008 | 0.017 | 0.0008 | 11 (2, 21) | 17 (7, 28) | 0.24 |
| SA Est CTh (mm) | 0.952 (0.632) | 0.614 (0.494) | 0.566 (0.417) | <0.0001 | 0.0003 | 0.0002 | 20 (9, 33) | 22 (10, 36) | 0.62 |
| IP Est CTh (mm) | 2.392 (0.613) | 2.272 (0.590) | 2.116 (0.435) | 0.025 | 0.20 | 0.01 | 5 (-4, 13) | 11 (2, 19) | 0.20 |
| IA Est CTh (mm) | 3.196 (0.813) | 2.922 (0.658) | 2.636 (0.552) | <0.0001 | 0.037 | <0.0001 | 8 (-1, 16) | 16 (7, 25) | 0.04 |
|
| |||||||||
| Men | |||||||||
|
| |||||||||
| Controls | Femoral Neck Cases | Trochanteric Cases | Ctrl vs Cases | Ctrl vs FN | Ctrl vs TR | % Reduction FN | % Reduction TR | FN vs TR | |
|
|
|||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | p-value | p-value | p-value | Mean (95%CI) | Mean (95%CI) | p-value | |
|
| |||||||||
| Height (cm) | 174.6 (6.5) | 175.7 (5.1) | 175.4 (6.7) | 0.66 | 0.82 | 0.56 | - | - | 0.86 |
| Weight (kg) | 82.1 (14.8) | 79.3 (9.2) | 77.3 (13.7) | 0.27 | 0.47 | 0.12 | - | - | 0.43 |
| BMI (kg/cm2) | 26.8 (4.0) | 26.5 (3.4) | 25.2 (3.5) | 0.22 | 0.78 | 0.08 | - | - | 0.16 |
| HAL (mm) | 130.6 (7.3) | 132.1 (5.7) | 131.4 (6.6) | 0.61 | 0.33 | 0.70 | - | - | 0.64 |
| Femoral neck aBMD (mg/cm2) | 772 (125) | 671 (98) | 622 (134) | <0.0001 | <0.0001 | <0.0001 | 15 (7, 23) | 25 (16, 36) | 0.08 |
| IntvBMD* (mg/cm3) | 242 (40) | 197.2 (25) | 190 (36) | <0.0001 | <0.0001 | <0.0001 | - | - | 0.30 |
| Avg CTh* (mm) | 2.133 (0.646) | 1.602 (0.329) | 1.544 (0.512) | <0.0001 | <0.0001 | <0.0001 | 26 (17, 35) | 33 (22, 45) | 0.35 |
| SP Est CTh (mm) | 1.11 (0.585) | 0.645 (0.289) | 0.858 (0.578) | 0.0001 | <0.0001 | 0.032 | 25 (13, 39) | 14 (1, 29) | 0.29 |
| SA Est CTh (mm) | 1.394 (0.609) | 0.731 (0.432) | 0.633 (0.335) | <0.0001 | <0.0001 | <0.0001 | 37 (24, 52) | 40 (25, 57) | 0.45 |
| IP Est CTh (mm) | 2.610 (0.539) | 2.259 (0.370) | 1.911 (0.635) | <0.0001 | 0.0027 | <0.0001 | 14 (6, 22) | 35 (24, 45) | 0.0007 |
| IA Est CTh (mm) | 3.232 (0.595) | 2.774 (0.689) | 2.707 (0.763) | <0.0001 | 0.0003 | 0.0001 | 17 (8, 25) | 21 (11, 31) | 0.54 |
IntvBMD: mean integral vBMD measured from mid-femoral neck; AvgCTh: average of the four quadrants.
3.2 Bone measurements in cases and controls
At baseline the female femoral neck fracture cases had longer mean hip axis length, thinner average cortex, lower total femoral neck aBMD and lower mean integral vBMD at mid-femoral neck compared to their control group. The women with trochanteric fractures had lower mean total femoral neck aBMD and integral vBMD at mid-femoral neck and thinner average cortex than controls. Both the femoral neck and trochanteric male fracture cases had thinner average cortex, lower mean total femoral neck aBMD and integral vBMD at mid-femoral neck compared to controls.
3.3 Cortical thickness results analyzed by quadrants
Table 2 shows that in women FN fracture cases had significantly thinner mean Est CTh in SP and SA quadrants and marginally significant thinner in IA Est CTh than controls. TR fracture cases had significantly thinner Est CTh in all quadrants. In men, both FN and TR fracture cases had thinner mean Est CTh in all quadrants compared to controls. The relative differences between FN cases and controls in Est CTh by quadrants were greatest in the superior quadrants for both men and women. Details are presented in Table 2.
The statistical effects of age on cortical thickness at mid femoral neck were estimated by quadrants in the controls (Fig. 2). There was a significant decrease in all quadrants for females but not males (p>0.6 for men). Among women, CTh was reduced by 11% (95%CI:-17,-4; p: 0.003) per 10 years in age in the SP quandrant, 18% (95%CI: -25,-11; p<0.0001) in SA quadrant, 8% (95%CI: -12,-3; p: 0.001) in IP quadrant and reduced by 9% (95%CI: -14,-5; p:0.0002) in IA quadrant.
Figure 2.
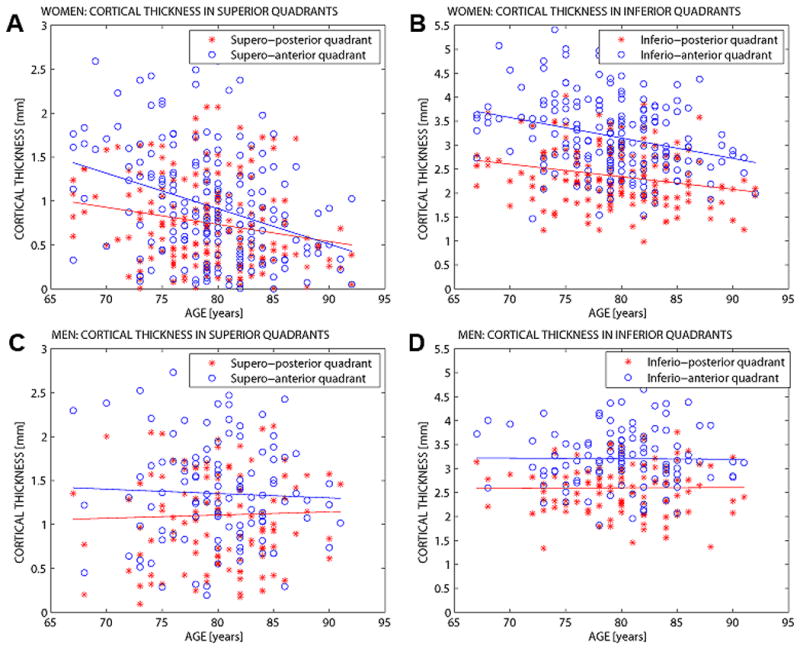
Cortical thickness by quadrants and regression lines with age in both female and male controls. A) Women – CTh in superior quadrants. B) Women – CTh in inferior quadrants. C) Men – CTh in superior quadrants. D) Men – CTh in posterior quadrants. (Posterior region in red and anterior in blue).
3.4 Gender comparison
Mean values for characteristics and QCT variables at femoral neck at baseline showed no significant difference in BMI between sexes when comparing controls and cases (p>0.45) (table 2). Female controls had 14% lower total femoral neck aBMD (p<0.0001), 5% lower mean integral vBMD at mid-femoral neck (p=0.01) and 15% thinner mean cortical thickness than male controls (p<0.001) although in the infero-anterior quadrant this was not significant (p=0.42). Male FN cases were not significantly different from female FN cases (p>0.27) except male FN cases had higher total femoral neck aBMD (p=0.004) which being two dimensional is not fully corrected for bone size. The male TR cases did not have significantly different mean integral vBMD or Est CTh compared to female TR cases, except that female TR cases had a thinner mean cortex in the supero-posterior quadrant (p=0.002).
In men, correlation coefficient between two quadrants was: SP∼SA: 0.68; SP∼IP: 0.49; SP∼IA: 0.50; SA∼IP: 0.36; SA∼IA: 0.52; IP∼IA: 0.52. In women, showing: SP-SA: 0.71; SP∼IP: 0.58; SP∼IA: 0.60; SA∼IP: 0.62; SA∼IA: 0.63; IP∼IA: 0.66.
3.5 Comparison between fracture sites
Male TR cases had a thinner mean cortex in the infero-posterior quadrant than male FN cases (p<0.001); (see table 2).
3.6 Cortical thickness distribution by quadrants
In Cox proportional hazards regression models, the quadrant that best predicted fracture was the supero-anterior (see later). Figure 3 shows the cumulative distribution of estimated cortical thickness in the supero-anterior quadrant and femoral neck aBMD in controls and cases in women and men. Eighty-two percent of female TR cases and 62% of FN cases had Est CTh less than or equal to 0.5 mm in SA quadrant compared with 44% of the controls. Differences in men were even greater, 65% of male TR cases and 58% of FN cases had Est CTh less than or equal to 0.5 mm compared with only 17% of the male controls.
Figure 3.
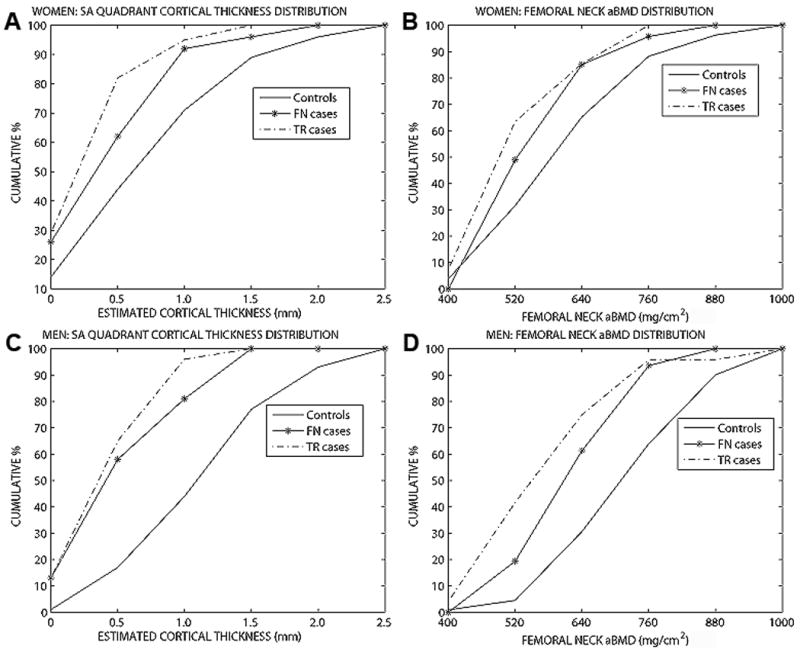
The cumulative distribution of estimated cortical thickness in the supero-anterior quadrant and femoral neck aBMD in controls and cases in women and men. A) Women – SA Est CTh B) Women – FN aBMD C) Men- SA Est CTh D) Men – FN aBMD
3.7 Association of QCT variables and risk of incident hip fracture
Table 3 shows height, weight and age adjusted HRs for incident hip fracture associated with 1 SD change in each QCT variables, bone density and geometry parameters, at the femoral neck and medication use in women. The other, structural variables were all significantly associated with hip fracture apart from the infero-anterior cortical thickness in women. The significance levels of the risk ratios as well as the risk ratios themselves tended to be higher in men (Table 3).
Table 3.
Hazard ratios for hip fracture per 1 SD of QCT variables at the femoral neck adjusted for height, weight and age (standardized for 1 SD decrease in all parameters except for hip axis length where it is standardized for increase).
| Women | |||
|---|---|---|---|
| Any hip fracture | Femoral Neck Fracture | Trochanteric Fracture | |
| HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | |
| Hip axis length (mm) | 1.3 (1.0-1.6), 0.06 | 1.4 (1.0-2.0), 0.037 | 1.2 (0.8-1.7), 0.32 |
| Femoral neck aBMD (gm/cm2) | 1.8 (1.4-2.5), 0.0001 | 1.7 (1.2-2.6), 0.006 | 2.1 (1.3-3.2), 0.001 |
| IntvBMD* (mg/cm3) | 1.9 (1.4-2.6), <0.0001 | 1.8 (1.2-2.6), 0.003 | 2.4 (1.5-3.8), 0.0002 |
| Avg CTh* (mm) | 1.7 (1.3-2.3), 0.0002 | 1.6 (1.1-2.3), 0.009 | 2.0 (1.3-3.1), 0.002 |
| SP Est CTh (mm) | 1.6 (1.2-2.1), 0.001 | 1.5 (1.1-2.2), 0.023 | 1.8 (1.2-2.8), 0.007 |
| SA Est CTh (mm) | 1.8 (1.4-2.4), <0.0001 | 1.8 (1.3-2.7), 0.0014 | 2.1 (1.3-3.2), 0.001 |
| IP Est CTh (mm) | 1.3 (1.0-1.6), 0.04 | 1.2 (0.9-1.7), 0.29 | 1.5 (1.0-2.1), 0.05 |
| IA Est CTh (mm) | 1.5 (1.2-2.0), 0.0008 | 1.5 (1.0-2.1), 0.023 | 1.7 (1.2-2.5), 0.005 |
| Medication | 0.8 (0.5-1.4), 0.47 | 1.0 (0.5-2.0), 0.92 | 0.6 (0.2-1.3), 0.19 |
|
| |||
| Men | |||
|
| |||
| Any hip fracture | Femoral Neck Fracture | Trochanteric Fracture | |
|
|
|||
| HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | |
|
| |||
| Hip axis length (mm) | 1.2 (0.8-1.6), 0.40 | 1.1 (0.6-1.4), 0.60 | 1.2 (0.7-1.9), 0.50 |
| Femoral neck aBMD (gm/cm2) | 3.1 (2.1-4.5), <0.0001 | 2.7 (1.7-4.5), <0.0001 | 4.4 (2.4-8.2), <0.0001 |
| IntvBMD* (mg/cm3) | 2.9 (2.1-4.0), <0.0001 | 2.9 (1.8-4.5), <0.0001 | 3.2 (1.9-5.3), <0.0001 |
| Avg CTh* (mm) | 3.1 (2.2-4.4), <0.0001 | 3.2 (2.0-5.3), <0.0001 | 3.6 (2.1-6.2), <0.0001 |
| SP Est CTh (mm) | 2.1 (1.5-3.0), <0.0001 | 2.8 (1.6-4.6), <0.0001 | 1.6 (1.0-2.6), 0.05 |
| SA Est CTh (mm) | 3.6 (2.4-5.3), <0.0001 | 3.5 (2.1-5.8), <0.0001 | 4.3 (2.3-8.0), <0.0001 |
| IP Est CTh (mm) | 2.5 (1.8-3.4), <0.0001 | 2.1 (1.4-3.2),0.0006 | 3.7 (2.2-6.1), <0.0001 |
| IA Est CTh (mm) | 2.1 (1.5-2.8), <0.0001 | 2.1 (1.4-3.3), 0.0003 | 2.3 (1.5-3.8), 0.0004 |
IntvBMD: mean integral vBMD measured from mid-femoral neck; AvgCTh: average of the four quadrants.
Controlling for medication known to affect bone parameters did not change the association amongst women but numbers were too small in men to be assessed. Medication usage known to affect bone density (as described in methods) was as follows: 25% of female controls, 21% of female FN cases, 17% of female TR cases, 7% of male controls, 3% of male FN cases and 25% of male TR cases.
3.8 Multivariable analysis: Independent predictors of hip fracture
In women the average cortical thickness, as well as the IP, IA and SP cortical thicknesses, were not significantly independent predictors when combined with total FN aBMD (table 4). SA Est CTh and total femoral neck aBMD were associated with hip fracture when analyzed alone with adjustment for height, weight and age. SA Est CTh remained as a borderline significant predictor (p=0.05) for FN fracture after adjustment for total femoral neck aBMD whereas in this model femoral neck aBMD was not independently significant (p=0.52) (table 4). SA CTh and total femoral neck aBMD were not significant predictors for TR fracture when included in the same model (p=0.13 and p=0.17, respectively). Nor were Est CTh in others quadrants significant predictors of TR fractures when combined with total FN aBMD.
Table 4.
Hazard ratios for hip fracture in multivariate models including estimated cortical thickness and total femoral neck aBMD adjusted for height, weight and age (standardized for 1 SD decrease in all parameters).
| Women | |||||
|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | |
| Any hip fracture | |||||
|
| |||||
| Avg CTh* (mm) | 1.2 (0.8-2.0), 0.35 | - | - | - | - |
| SP Est CTh (mm) | - | 1.2 (0.8-1.7), 0.35 | - | - | - |
| SA Est CTh (mm) | - | - | 1.5 (1.0-2.2), 0.04 | - | - |
| IP Est CTh (mm) | - | - | - | 0.7 (0.5-1.1), 0.11 | - |
| IA Est CTh (mm) | - | - | - | - | 1.2 (0.8-1.7), 0.35 |
| Femoral neck aBMD (gm/cm2) | 1.5 (0.9-2.5), 0.10 | 1.6 (1.1-2.4), 0.01 | 1.4 (0.9-2.0), 0.13 | 2.4 (1.5-3.7), 0.0001 | 1.6 (1.1-2.4), 0.01 |
|
| |||||
| Femoral Neck Fracture | |||||
|
| |||||
| Avg CTh* (mm) | 1.2 (0.6-2.2), 0.65 | - | - | - | - |
| SP Est CTh (mm) | - | 1.1 (0.7-1.8), 0.59 | - | - | - |
| SA Est CTh (mm) | - | - | 1.6 (1.0-2.8), 0.05 | - | - |
| IP Est CTh (mm) | - | - | - | 0.6 (0.3-1.1), 0.07 | - |
| IA Est CTh (mm) | - | - | - | - | 1.1 (0.7-1.8), 0.61 |
| Femoral neck aBMD (gm/cm2) | 1.5 (0.8-3.0), 0.22 | 1.6 (0.9-2.6), 0.09 | 1.2 (0.7-2.0), 0.52 | 2.6 (1.4-5.0), 0.002 | 1.6 (0.9-2.6), 0.08 |
|
| |||||
| Trochanteric Fracture | |||||
|
| |||||
| Avg CTh* (mm) | 1.4 (0.7-2.8), 0.33 | - | - | - | - |
| SP Est CTh (mm) | - | 1.3 (0.7-2.2), 0.37 | - | - | - |
| SA Est CTh (mm) | - | - | 1.6 (0.9-2.8), 0.13 | - | - |
| IP Est CTh (mm) | - | - | - | 0.8 (0.4-1.4), 0.45 | - |
| IA Est CTh (mm) | - | - | - | - | 1.3 (0.8-2.1), 0.37 |
| Femoral neck aBMD (gm/cm2) | 1.6 (0.8-3.2), 0.19 | 1.7 (1.0-3.1), 0.05 | 1.5 (0.8-2.7), 0.17 | 2.5 (1.3-4.8), 0.006 | 1.8 (1.0-3.1), 0.04 |
|
| |||||
| Men | |||||
|
| |||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
|
|
|||||
| HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | HR 95% CI, p | |
|
| |||||
| Any hip fracture | |||||
|
| |||||
| Avg CTh* (mm) | 2.2 (1.4-3.7), 0.001 | - | - | - | - |
| SP Est CTh (mm) | - | 1.2 (0.7-1.8), 0.50 | - | - | - |
| SA Est CTh (mm) | - | - | 2.8 (1.8-4.4), <0.0001 | - | - |
| IP Est CTh (mm) | - | - | - | 1.7 (1.1-2.5), 0.01 | - |
| IA Est CTh (mm) | - | - | - | - | 1.1 (0.7-1.7), 0.55 |
| Femoral neck aBMD (gm/cm2) | 1.6 (0.9-2.6), 0.09 | 2.8 (1.7-4.6), <0.0001 | 1.7 (1.1-2.5), 0.012 | 2.2 (1.4-3.4), 0.006 | 2.8 (1.6-4.7), 0.0001 |
|
| |||||
| Femoral Neck Fracture | |||||
|
| |||||
| Avg CTh* (mm) | 2.9 (1.5-5.5), 0.001 | - | - | - | - |
| SP Est CTh (mm) | - | 2.0 (1.1-3.6), 0.02 | - | - | - |
| SA Est CTh (mm) | - | - | 3.2 (1.8-5.7), <0.0001 | - | - |
| IP Est CTh (mm) | - | - | - | 1.4 (0.8-2.5), 0.20 | - |
| IA Est CTh (mm) | - | - | - | - | 1.4 (0.8-2.4), 0.24 |
| Femoral neck aBMD (gm/cm2) | 1.2 (0.7-2.2), 0.51 | 1.8 (1.0-3.2), 0.05 | 1.4 (0.8-2.4), 0.17 | 2.2 (1.2-4.0), 0.008 | 2.1 (1.1-4.1), 0.02 |
|
| |||||
| Trochanteric Fracture | |||||
|
| |||||
| Avg CTh* (mm) | 1.9 (0.8-4.4), 0.14 | - | - | - | - |
| SP Est CTh (mm) | - | 0.5 (0.2-1.1), 0.06 | - | - | - |
| SA Est CTh (mm) | - | - | 2.8 (1.3-5.9), 0.007 | - | - |
| IP Est CTh (mm) | - | - | - | 2.7 (1.4-5.0), 0.002 | - |
| IA Est CTh (mm) | - | - | - | - | 0.9 (0.4-1.9), 0.78 |
| Femoral neck aBMD (gm/cm2) | 2.7 (1.0-6.9), 0.04 | 8.7 (3.2-22.9), <0.0001 | 2.4 (1.2-4.9), 0.01 | 2.4 (1.2-5.0), 0.01 | 5.2 (2.1-13), 0.003 |
AvgCTh: average of the four quadrants.
In men, the average cortical thickness was a significant predictor when combined with total FN aBMD for FN cases (p=0.001) but not for TR cases (table 4). SA quadrant was the only CTh measurement that was significant in multivariable analysis including CTh in all quadrants when considering FN fracture. SA Est CTh remained as a significant predictor for FN fracture after adjustment for total femoral neck aBMD (p<0.0001) whereas total femoral neck aBMD was not significant in this model (p=0.17) (table 4). Among men, SA Est CTh and IP Est CTh remained as significant predictors for TR fracture when included in the same model (SA Est CTh - HR: 2.7, 95% CI: 1.4-5.4, p=0.002 and IP Est CTh - HR: 2.8, 95% CI: 1.6-5.1, p=0.0004) even after adjustment for total femoral neck aBMD (SA Est CTh - HR: 2.3, 95% CI: 1.1-4.6, p=0.03 and IP Est CTh - HR: 2.4, 95% CI: 1.3-4.5, p=0.007) while total femoral neck aBMD was not significant (p=0.24). Total femoral neck aBMD was however a significant predictor for TR fracture when adjusted for either SA CTh or IP CTh (p=0.01) (table 4).
4. Discussion
We have applied a segmental QCT analysis of the mid femoral neck to explore the utility of cortical thickness in different regions for predicting incident femoral neck and trochanteric fracture in elderly men and women. Our results suggest that, despite having worse measurement precision than areal (i.e. DXA-like) BMD, cortical thickness estimated in the supero-anterior quadrant was the best discriminator of cases from controls.
We wondered if thinning of the cortex could be a good imaging biomarker for hip fracture for several reasons. Previous studies have indicated that there is marked thinning in the superior region at the mid-femoral neck with advancing age [10, 11, 15, 23]. Maximal compressive strain from a sideways fall on to the greater trochanter occurs in the superior femoral neck [11, 14, 18, 19, 27] with maximal tensile strain in the inferior cortex [22]. In load-to-failure testing, cadaveric femurs often fractured at the thin superior cortex [28] in a sideways fall simulation as predicted [11, 18, 22]. The results presented in this paper support our hypothesis: in Cox proportional hazard models we found a greater risk of fracture with thinner superior cortices, although the effect was greatest in the SA quadrant. Furthermore, in both sexes SA Est CTh continued to make an independent contribution after adjustment for total femoral neck aBMD for FN fracture, whereas in men, SA and IP Est CTh were significant after adjustment for total femoral neck aBMD for TR fracture.
Others have also reported thinner cortex as a risk for hip fracture [9, 29, 30]. Black et al [9] found that percent cortical volume was associated with hip fracture risk in men after adjustment for aBMD. These findings support the hypothesis that femoral neck fracture is related to cortical strength and/or instability especially in the superior part, although in an analysis of 2D data from DXA, Kaptoge et al[31] could not demonstrate an independent effect, which suggests there may be an advantage to 3D analysis.
When the endosteal rate of resorption is greater than the periosteal rate of apposition, thinning of cortical bone [32] will occur. A critical situation might be reached when this cortical thinning is greater in one region of the femoral neck than in others causing high stresses and instability [12, 32]. The greatest difference in mean cortical thickness between cases and controls was in the SA quadrant and this difference was considerably greater in men than women. Both, percent differences in mean and cumulative distribution of femoral neck aBMD and SA Est CTh imply that the discrimination is sharper with cortical thickness. Our study suggests that, majority of elderly women and a minority of men have a substantially thinned cortex in the superior neck and therefore preventing falls in this population is of great importance in addition to strengthening the cortex if possible.
When comparing men and women in our study, there was no difference in Est CTh in infero-anterior quadrant in controls, which is highly loaded during walking contrasting with the other quadrants. Interestingly, there were no differences in CTh between male and female femoral neck fracture cases. The same results were observed when comparing male and female trochanteric fracture cases except men had significantly thicker cortex in SP quadrant (p=0.002). This suggests that women and men might sustain hip fracture at a similar cortical thickness threshold. Cross-sectional variances in cortical thickness per 10 years in age showed considerable gender difference. Female controls apparently lost CTh whereas male controls maintained their CTh. The bone decrement with age seems to be different according to locations within the femoral neck more so in the superior region which is concordant with published studies [11, 15, 23]. This needs to be confirmed in a longitudinal study.
The relative contribution of cortical and trabecular bone to fragility of the proximal femur has caused controversy, but much of this may be explained by different load configurations in mechanical testing. To investigate the load distribution between cortical and trabecular bone during a sideways fall, Lotz et al [14], used finite element analysis (FEA) with a coarse mesh, suggesting that cortical bone carried 50% of the load at the mid-neck during a fall. Verhulp et al [27] examined the load distribution in one healthy and one osteoporotic human proximal femur during a fall to the side with a much finer mesh. They suggested that the trabecular and cortical bone contributions to bone strength were similar but that the highest strains were located in the cortical shell. The trabecular compartment appeared minimally loaded in the osteoporotic femur viewed in an orthogonal plane to the neck axis. Manske et al [21] suggested that both cortical and trabecular bone compartments contribute to proximal femur strength during a sideways fall and when they used a high threshold to differentiate cortical from trabecular bone, cortical bone contributed more to the variance in failure load than trabecular bone.
The average age at fracture was 83 years, independent of both sex and fracture type. This contrasts with the results of Mautalen et al [33], where TR fracture cases were older than FN cases. Among women, hip axis length was an independent predictor of FN fracture but not of trochanteric fractures, consistent with findings of some other studies [19, 34, 35]. HAL was, however, not an independent predictor of hip fracture in men, in agreement with Pandel et al [36].
In our study the main contrast between fracture cases and controls was in the superior quadrants. Duboeuf et al [34] found that upper femoral neck aBMD measurement is a better predictor of FN fracture than other areas of the hip. They concluded that hip BMD (including the inferior cortex) seems to play a more important role in the trochanteric than in the FN hip fracture. Generally in our study the trochanteric fracture cases were thinner in the inferior neck compared to FN cases. In the TR fracture group the total femoral neck aBMD continued to make an independent contribution when combined with CTh.
A statistical association does not automatically imply direct causation, so our results should not be interpreted to imply that the initiating cortical crack that starts the fracture event is in the same supero-anterior quadrant that discriminates best between cases and controls. However for purposes of clinical prediction the SA quadrant best discriminated cases from controls. The recent paper by de Bakker et al [28] empirically confirmed the hypothesis that hip fracture initiate with a failure in the superior femoral neck where stresses are primarily compressive during a sideways fall. As predicted by Mayhew et al. [11], de Bakker's movies showed the initial crack rapidly accelerating infero-medially as complete fracture ensued.
Our cortical thickness estimates are sensitive to uncertainties in porosity and degree of mineralization. Porosity of human cortical bone increases with age [13] and cortical bone porosity was significantly greater in femoral neck specimens from fracture cases compared to age-matched controls [12]. Material properties of bone, particularly stiffness and strength are strongly dependent on the porosity [37-40]. Cortical porosity can vary from less than 5% to almost 30%. Both elastic modulus and ultimate stress can be reduced by 50% when porosity increased from 5% to 30%[39]. The density of cortical bone is a function of both its porosity and its degree of mineralization. It is generally accepted that in the absence of osteomalacia or very rapid turnover, variability in bone porosity has a bigger influence on cortical bone density than its degree of mineralization.
This nested case-control study has several important strengths. It included both women and men and the spatial resolution QCT-scans were made at a single-center. All measurements were performed on data acquired before the occurrence of hip fractures, and cases and controls are part of the same cohort, which ensure their comparability. The analysis of the scan was performed in a blinded fashion by a single reader. The case-cohort approach provided adequate power to detect meaningful associations between the proximal femur measures and fracture risk.
This study also has some limitations. A fracture begins in most structures at a point of weakness, otherwise known as a flaw which, as a result of applied stress, is caused to grow to macroscopic size, leading eventually to failure. These flaws can not be identified with QCT. We evaluated a limited number of QCT structural parameters because of the adverse trade off between multiple testing and the limited number of cases for inclusion at this stage in the AGES study. No trochanteric measurements were included in part because we had no way of validating their accuracy as was done in previous work for the femoral neck by Mayhew et al[11]. So we can not exclude that assessment of both femoral neck and trochanteric measurements might give different results. There are certain technical limitations to the measurement of cortical thickness in vivo. What we have measured with the BIT2 technique cannot be considered an accurate estimate of the thickness of the cortex in the thin superior zones, due to the partial volume effect and the tendency to underestimate the thin cortices to zero. Femoral neck histological cross sections [12] have demonstrated regions of supero-anterior and supero-posterior cortex in fracture cases that are typically about 0.3-0.7mm thick. Such thin cortical zones might have implications for risk of fracture initiation when not only compressive, but also torsional forces are applied. For simplicity in application, we used a single threshold to delineate cortex from trabecular bone. The difficulties of resolving thin bone cortices in the femur using a thresholding technique were documented recently [26]. The partial volume effect tends to make trabecular bone close to the endosteal boundary appear more dense and cortical bone less dense than they really are [41, 42]. These effects lead to cortical thickness overestimation particularly in the inferior region of the femoral neck cortex. Downward bias can also occur when true cortical thickness approaches the pixel size, especially for highly porous cortices [41] and this might affect our results more in women than men because of thinner cortexes among women. Higher apparent cortical density in the inferior regions compared to the superior regions appeared exaggerated compared to direct measurements made with a scanning SEM technique [43]. Nevertheless, by average adjacent CTh measurements along the femoral neck axis to increase the precision, the CVs are comparable to those obtained in 2D Hip Strength Analysis [44] and to CVs of the architectural parameters estimated by HR-pQCT [45, 46] while remaining larger than CVs for 2D BMD [47]. As discussed by Kaptoge et al [31], 2-D BMD's generally excellent coefficients of variation give it an advantage for diagnostic purposes over less precise outcome measures, even though it may be less directly related to the predisposing cause of hip fracture. While partial volume errors clearly affect true estimates, they are unlikely to mask large trends. One reason that the SP quadrant did not discriminate (compared with SA) may be the greater tendency for the SP quadrant to be made up of zero-value sectors, both in cases and controls.
In conclusion; an analysis of the spatial distribution of cortical bone within the femoral neck using computed tomography acquired with thin (1 mm) slices and sophisticated imaging analysis software may give additional information to DXA in discriminating patients destined to suffer a hip fracture. Our results need confirmation in a similar cohort or an independent case control study. They also raise the question of whether a practical intervention could be found that would strengthen the superior femoral neck. It is possible to gain access with a needle to the superior cortex and its underlying trabecular foundation as a minor procedure through the greater trochanter. Alternatively, anabolic therapies both current and in prospect might increase subcortical trabecular bone and, as Thomas et al [22] suggest, reduce the risk of cortical buckling.
Research Highlights.
Cortical thickness by QCT in mid femoral neck in hip fracture cases and controls.
Cortical thickness (CTh) in the super-anterior quadrant best predicted hip fracture.
There was no significant difference in CTh in the fracture group between sexes.
Majority of elderly women and minority of men have a critical thinned superior cortex.
Thin superior cortex is a stronger risk factor than the inferior region.
Acknowledgments
The following institutes provided support: the NIH, contract N01-AG-1-2100; the NIA Intramural Research Program; the Icelandic Heart association; the Icelandic Parliament and the Icelandic Centre of Research. Dr Sigurdsson acknowledges support from the University of Iceland Research Fund. Dr Poole acknowledges the support of the Arthritis Research UK and the Cambridge NIHR Biomedical Research Centre. The authors would like to thank Keenan Brown (Mindways software, Texas, USA) for his assistance.
Abbreviations
- Est CTh
estimated cortical thickness
- SA
supero-anterior
- SP
supero-posterior
- IA
inferio-anterior
- IP
inferio-posterior
- FN
femoral neck
- TR
trochanter
- QCT
quantitative computed tomography
- vBMD
volumetric bone mineral density
- aBMD
areal bone mineral density
- HR
hazard ratio
Footnotes
Conflict of Interest Page: All authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Fjola Johannesdottir, Email: fjolajo@hi.is.
Kenneth E.S. Poole, Email: kenpoole@doctors.org.uk.
Jonathan Reeve, Email: jonathan@srl.cam.ac.uk.
Kristin Siggeirsdottir, Email: kristin@hjarta.is.
Thor Aspelund, Email: aspelund@hjarta.is.
Brynjolfur Mogensen, Email: brynjolf@landspitali.is.
Brynjolfur Y. Jonsson, Email: Brynjolfur.Jonsson@skane.se.
Sigurdur Sigurdsson, Email: sigurdur@hjarta.is.
Tamara B. Harris, Email: harris99@nia.nih.gov.
Vilmundur G. Gudnason, Email: v.gudnason@hjarta.is.
References
- 1.Kanis JA. The incidence of hip fracture in Europe. Osteoporos Int. 1993;3:10–5. doi: 10.1007/BF01621853. [DOI] [PubMed] [Google Scholar]
- 2.Turner CH The biomechanics of hip fracture. Lancet. 2005;366:98–9. doi: 10.1016/S0140-6736(05)66842-0. [DOI] [PubMed] [Google Scholar]
- 3.Moyad MA. Osteoporosis: a rapid review of risk factors and screening methods. Urol Oncol. 2003;21:375–9. doi: 10.1016/s1078-1439(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 4.Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos Int. 2005;16:S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz AV, Kelsey JL, Maggi S, Tuttleman M, Ho SC, Jónsson PV, Poór G, Sisson de Castro JA, Xu L, Matkin CC, Nelson LM, Heyse SP. International variation in the incidence of hip fractures: cross-national project on osteoporosis for the World Health Organization Program for Research on Aging. Osteoporos Int. 1999;9:242–53. doi: 10.1007/s001980050144. [DOI] [PubMed] [Google Scholar]
- 6.Jergas M, Glüer C, Grampp S, Köster O. Radiologic diagnosis of osteoporosis. Current methods and outlook. Aktuelle Radiol. 1992;2:220–9. [PubMed] [Google Scholar]
- 7.Lang P, Steiger P, Faulkner K, Glüer C, Genant HK. Osteoporosi. Current techniques and recent developments in quantitative bone densitometry. Radiol Clin North Am. 1991;29:49–76. [PubMed] [Google Scholar]
- 8.Riggs BL, Wahner HW, Seeman E, Offord KP, Dunn WL, Mazess RB, Johnson KA, Melton LJ. Changes in bone mineral density of the proximal femur and spine with aging: Differences between the postmenopausal and senile osteoporosis syndromes. J Clin Invest. 1982;70:716–23. doi: 10.1172/JCI110667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res. 2008;23:1326–33. doi: 10.1359/JBMR.080316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyce TM, Bloebaum RD. Cortical aging differences and fracture implications for the human femoral neck. Bone. 1993;14:769–78. doi: 10.1016/8756-3282(93)90209-s. [DOI] [PubMed] [Google Scholar]
- 11.Mayhew PM, Thomas CD, Clement JG, Loveridge N, Beck TJ, Bonfield W, Burgoyne CJ, Reeve J. Relation between age, femoral neck cortical stability, and hip fracture risk. Lancet. 2005;366:129–35. doi: 10.1016/S0140-6736(05)66870-5. [DOI] [PubMed] [Google Scholar]
- 12.Bell KL, Loveridge N, Power J, Garrahan N, Stanton M, Lunt M, Meggitt BF, Reeve J. Structure of the femoral neck in hip fracture: cortical bone loss in the inferoanterior to superoposterior axis. J Bone Miner Res. 1999;14:111–9. doi: 10.1359/jbmr.1999.14.1.111. [DOI] [PubMed] [Google Scholar]
- 13.Crabtree N, Loveridge N, Parker M, Rushton N, Power J, Bell KL, Beck TJ, Reeve J. Intracapsular hip fracture and the region-specific loss of cortical bone: analysis by peripheral quantitative computed tomography. J Bone Miner Res. 2001;16:1318–28. doi: 10.1359/jbmr.2001.16.7.1318. [DOI] [PubMed] [Google Scholar]
- 14.Lotz JC, Cheal EJ, Hayes WC. Stress distributions within the proximal femur during gait and falls: Implications for osteoporotic fracture. Osteoporosis Int. 1995;5:252–61. doi: 10.1007/BF01774015. [DOI] [PubMed] [Google Scholar]
- 15.Crabtree N, Lunt M, Holt G, Kroger H, Burger H, Grazio S, Khaw KT, Lorenc RS, Nijs J, Stepan J, Falch JA, Miazgowski T, Raptou P, Pols HAP, Dequeker J, Havelka S, Hoszowski K, Jajic I, Czekalski S, Lyritis G, Silman AJ, Reeve J. Hip geometry, bone mineral distribution, and bone strength in European men and women: the EPOS study. Bone. 2000;27:151–9. doi: 10.1016/s8756-3282(00)00300-8. [DOI] [PubMed] [Google Scholar]
- 16.Serrat MA, Reno PL, McCollum MA, Meindl RS, Lovejoy CO. Variation in mammalian proximal femoral development: comparative analysis of two distinct ossification patterns. J Anat. 2007;210:249–58. doi: 10.1111/j.1469-7580.2007.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lovejoy CO. Evolution of human walking. Sci Am. 1988;259:118–25. doi: 10.1038/scientificamerican1188-118. [DOI] [PubMed] [Google Scholar]
- 18.Carpenter RD, Beaupré GS, Lang TF, Orwoll ES, Carter DR. New QCT analysis approach shows the importance of fall orientation on femoral neck strength. J Bone Miner Res. 2005;20:1533–42. doi: 10.1359/JBMR.050510. [DOI] [PubMed] [Google Scholar]
- 19.Pinilla TP, Boardman KC, Bouxsein ML, Myers ER, Hayes WC. Impact direction from a fall influences the failure load of the proximal femur as much as age-related bone loss. Calcif Tissue Int. 1996;58:231–5. doi: 10.1007/BF02508641. [DOI] [PubMed] [Google Scholar]
- 20.Holzer G, von Skrbensky G, Holzer LA, Pichl W. Hip fractures and the contribution of cortical versus trabecular bone to femoral neck strength. J Bone Miner Res. 2009;24:468–74. doi: 10.1359/jbmr.081108. [DOI] [PubMed] [Google Scholar]
- 21.Manske SL, Liu-Ambrose T, Cooper ML, Kontulainen S, Guy P, Forster BB, McKay HA. Cortical and trabecular bone in the femoral neck both contribute to proximal femur failure load prediction. Osteoporos Int. 2009;20:445–53. doi: 10.1007/s00198-008-0675-2. [DOI] [PubMed] [Google Scholar]
- 22.Thomas CD, Mayhew PM, Power J, Poole KE, Loveridge N, Clement JG, Burgoyne CJ, Reeve J. Femoral neck trabecular bone: loss with aging and role in preventing fracture. J Bone Miner Res. 2009;24:1808–18. doi: 10.1359/jbmr.090504. [DOI] [PubMed] [Google Scholar]
- 23.Poole KE, Mayhew PM, Rose CM, Brown JK, Bearcroft PJ, Loveridge N, Reeve J. Changing structure of the femoral neck across the adult female lifespan. J Bone Miner Res. 2010;25:482–91. doi: 10.1359/jbmr.090734. [DOI] [PubMed] [Google Scholar]
- 24.Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V. Age, Gene/Environment Susceptibility-Reykjavik Study: multidisciplinary applied phenomics. Am J Epidemiol. 2007;165:1076–87. doi: 10.1093/aje/kwk115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sigurdsson G, Aspelund T, Chang M, Jonsdottir B, Sigurdsson S, Eiriksdottir G, Gudmundsson A, Harris TB, Gudnason V, Lang TF. Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility-Reykjavik study (AGES-REYKJAVIK) Bone. 2006;39:644–51. doi: 10.1016/j.bone.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 26.Treece GM, Gee AH, Mayhew PM, Poole KE. High resolution cortical bone thickness measurement from clinical CT data. Med Image Anal. 2010;14:276–90. doi: 10.1016/j.media.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Verhulp E, van Rietbergen B, Huiskes R. Load distribution in the healthy and osteoporotic human proximal femur during a fall to the side. Bone. 2008;42:30–5. doi: 10.1016/j.bone.2007.08.039. [DOI] [PubMed] [Google Scholar]
- 28.de Bakker PM, Manske SL, Ebacher V, Oxland TR, Cripton PA, Guy P. During sideways falls proximal femur fractures initiate in the superolateral cortex: evidence from high-speed video of simulated fractures. J Biomech. 2009;42:1917–25. doi: 10.1016/j.jbiomech.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 29.Ito M, Wakao N, Hida T, Matsui Y, Abe Y, Aoyagi K, Uetani M, Harada A. Analysis of hip geometry by clinical CT for the assessment of hip fracture risk in elderly Japanese women. Bone. 2010;46:453–7. doi: 10.1016/j.bone.2009.08.059. [DOI] [PubMed] [Google Scholar]
- 30.Rivadeneira F, Zillikens MC, De Laet CE, Hofman A, Uitterlinden AG, Beck TJ, Pols HA. Femoral neck BMD is a strong predictor of hip fracture susceptibility in elderly men and women because it detects cortical bone instability: the Rotterdam Study. J Bone Miner Res. 2007;22:1781–90. doi: 10.1359/jbmr.070712. [DOI] [PubMed] [Google Scholar]
- 31.Kaptoge S, Beck TJ, Reeve J, Stone KL, Hillier TA, Cauley JA, Cummings SR. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23:1892–904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Power J, Loveridge N, Lyon A, Rusthon N, Parker M, Reeve J. Bone remodelling at the endocortical surface of the human femoral neck: A mechanism for regional cortical thinning in cases of hip fracture. J Bone Miner Res. 2003;18:1775–80. doi: 10.1359/jbmr.2003.18.10.1775. [DOI] [PubMed] [Google Scholar]
- 33.Mautalen C, Vega E, Einhorn T. Are the etiologies of cervical and trochanteric hip fractures different? Bone. 1996;18:133–7. doi: 10.1016/8756-3282(95)00490-4. [DOI] [PubMed] [Google Scholar]
- 34.Duboeuf F, Hans D, Schott AM, Kotzki PO, Favier F, Marcelli C, Meunier PJ, Delmas PD. Different morphometric and densitometric parameters predict cervical and trochanteric hip fracture: The EPIDOS study. J Bone Miner Res. 1997;12:1895–902. doi: 10.1359/jbmr.1997.12.11.1895. [DOI] [PubMed] [Google Scholar]
- 35.Faulkner KG, Wacker WK, Barden HS, Simonelli C, Burke PK, Ragi S, Del Rio L. Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int. 2006;17:593–9. doi: 10.1007/s00198-005-0019-4. [DOI] [PubMed] [Google Scholar]
- 36.Pande I, O'Neill TW, Pritchard C, Scott DL, Woolf AD. Bone mineral density, hip axis length and risk of hip fracture in men: results from the Cornwall Hip Fracture Study. Osteoporos Int. 2000;11:866–70. doi: 10.1007/s001980070046. [DOI] [PubMed] [Google Scholar]
- 37.Currey JD. The effect of porosity of mineral content on the Young's modulus of elasticity of compact bone. J Biomech. 1988;21:131–9. doi: 10.1016/0021-9290(88)90006-1. [DOI] [PubMed] [Google Scholar]
- 38.Dong XN, Guo XE. The dependence of transversely isotropic elasticity of human femoral cortical bone on porosity. J Biomech. 2004;37:1281–7. doi: 10.1016/j.jbiomech.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 39.McCalden RW, McGeough JA, Barker MB, Court-Brown CM. Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am. 1993;75:1193–205. doi: 10.2106/00004623-199308000-00009. [DOI] [PubMed] [Google Scholar]
- 40.Wachter NJ, Krischak GD, Mentzel M, Sarkar MR, Ebinger T, Kinzl L, Claes L, Augat P. Correlation of bone mineral density with strength and microstructural parameters of cortical bone in vitro. Bone. 2002;31:90–5. doi: 10.1016/s8756-3282(02)00779-2. [DOI] [PubMed] [Google Scholar]
- 41.Davis KA, Burghardt AJ, Link TM. The effects of geometric and threshold definitions on cortical bone metrics assessed by in vivo high-resolution peripheral quantitative computed tomography. Calcif Tissue Int. 2007;81:364–71. doi: 10.1007/s00223-007-9076-3. [DOI] [PubMed] [Google Scholar]
- 42.Prevrhal S, Fox JC, Shepherd JA, Genant HK. Accuracy of CT-based thickness measurement of thin structures: modeling of limited spatial resolution in all three dimensions. Med Phys. 2003;30:1–8. doi: 10.1118/1.1521940. [DOI] [PubMed] [Google Scholar]
- 43.Loveridge N, Power J, Reeve J, Boyde A. Bone mineralization density and femoral neck fragility. Bone. 2004;35:929–41. doi: 10.1016/j.bone.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 44.Khoo BC, Beck TJ, Qiao QH, Parakh P, Semanick L, Prince RL, Singer KP, Price RI. In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone. 2005;37:112–21. doi: 10.1016/j.bone.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Walker M, McMahon D, Udesky J, Liu G, Bilezikian J. Application of high resolution skeletal imaging to measurements of volumetric bone density and skeletalmicroarchitecture in Chinese American and white women: Explanation of a paradox. J Bone Miner Res. 2009;24:1953–9. doi: 10.1359/JBMR.090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Wang Q, Ghasem-Zadesh A, Evans A, McLeod C, Iuiano-Burns S, Seeman E. Differences in macroand micro-architecture of the appendicular skeleton in young Chinese and white women. J Bone Miner Res. 2009;24:1946–52. doi: 10.1359/jbmr.090529. [DOI] [PubMed] [Google Scholar]
- 47.Lorentzon M, Landin K, Mellström D, Ohlsson C. Leptin is a negative independent predictor of areal BMD and cortical bone size in young adult swedish men. J Bone Miner Res. 2006;21:1871–8. doi: 10.1359/jbmr.060814. [DOI] [PubMed] [Google Scholar]


