Abstract
To determine the efficacy, safety and tolerability of an alternative short-course, artemisinin-based combination therapy for acute uncomplicated Plasmodium falciparum malaria, we compared Artequick®–a fixed-dosed combination of artemisinin (80 mg), piperaquine (400 mg), and primaquine (4 mg), per tablet–with a standard regimen of artesunate-mefloquine. A total of 130 patients were randomly assigned to treatment with an orally administered, once-daily, 3-day regimen of either Artequick® (Group A: 3.2 mg/kg/day of artemisinin, 16 mg/kg/day of piperaquine, and 0.16 mg/kg/day of primaquine) or artesunate-mefloquine (Group B: artesunate, 4 mg/kg/day, with mefloquine, 8 mg/kg/day). Patients receiving each regimen had a rapid clinical and parasitological response. All treatments were well tolerated, and no serious adverse effects occurred. No significant differences were found in fever- and parasite-clearance times between the two study groups. The 28-day cure rates were similarly high, at 98.5% and 100%, in groups A and B, respectively. We conclude that Artequick® was as effective and well tolerated as artesunate-mefloquine and could be used as an alternative treatment for multidrug-resistant Plasmodium falciparum malaria in Southeast Asia.
INTRODUCTION
The incidence of malaria has increased two- to three-fold over the past four decades, and nearly half the world’s population now lives in regions endemic for malaria: in Asia, Africa, and South America (Snow et al, 2005). Mortality is rising and approaching three million malarial deaths each year, in large part because of increasing resistance to antimalarial drugs (Breman et al, 2004). The World Health Organization (WHO) currently recommends artemisinin-based combination therapies (ACTs) as the best first-line treatment for uncomplicated falciparum malaria (WHO, 2006), but studies to ensure that current regimens are optimal are incomplete. In Southeast Asia, where P. falciparum is the most drug-resistant in the world, three-day artesunate-mefloquine treatment is generally the preferred treatment for uncomplicated malarial infection (Nosten et al, 1994; Silachamroon et al, 2005). Despite this recommendation, adoption of artesunate-mefloquine has been limited by the high cost (≈ US$ 3 for a single adult treatment), the frequency of adverse effects associated with mefloquine, and the lack of a formulation combining both antimalarials in a single tablet (Smithuis et al, 2006). In addition, reduced efficacy of artesunate-mefloquine has been reported recently from the southeastern border of Thailand (Vijaykadga et al, 2006).
Piperaquine is a bisquinoline antimalarial, first synthesized a half century ago and subsequently used extensively in China and Southeast Asia as both prophylaxis and treatment (Davis et al, 2005). Piperaquine is a highly lipid-soluble drug with large-volume distribution at a steady state, and a long terminal elimination half-life (t1/2 = 33 days) (Tarninget al, 2005). These properties, together with its tolerability, efficacy, and low cost, potentially make piperaquine an excellent partner drug in ACT (Ashley and White, 2005).
Artequick® is a fixed-dosed combination of artemisinin (80 mg), piperaquine (400 mg) and primaquine (4 mg) per tablet (Artepharm, China). Primaquine is included in the formulation with the intent of eliminating the gametocytes of P. falciparum. This fixed-dose combination, administered as a single daily dose for three days, has undergone clinical trials in China, Vietnam, Cambodia, and Indonesia and has provided some evidence for potential usefulness (Song J, personal communication; Tropical Medicine Institute, 2004). The objective of our study was to compare the efficacy, safety, and tolerability of orally administered, once daily, three-day regimens of Artequick®, with that of artesunate-mefloquine at the Hospital for Tropical Diseases, Faculty of Tropical Medicine, Mahidol University.
MATERIALS AND METHODS
Study site and recruitment procedures
This prospective, randomized study was reviewed and approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. Written informed consent was obtained from all patients or their legal representatives before enrollment. The study was conducted during May 2006-March 2007 at the Hospital for Tropical Diseases. The study cohort comprised all patients who fulfilled the inclusion criteria: male or female (if female, pregnancy-test-negative before enrollment) with acute uncomplicated falciparum malaria, with microscopically confirmed P. falciparum mono-infection, weight ≥ 40 kg and age ≥ 15 years, ability to take oral medication, and willingness and ability to comply with the study protocol for the duration of the trial. The patients were admitted to the Hospital for Tropical Diseases for at least 7 days, to assess the safety and efficacy of Artequick®, and requested to return to the Hospital for scheduled follow-up visits weekly until day 28 post-dosing. Excluded from the study were patients with severe malaria as defined by WHO criteria (WHO, 2000), lactation, glucose-6-phosphate dehydrogenase (G6PD) deficiency as tested by fluorescent-spot method, significant concomitant systemic diseases or diseases other than malaria requiring therapy, ingestion of other antimalarials in the past 14 days, or the presence of urine sulfonamides (lignin test) or 4-aminoquinolones (Wilson-Edeson test). Clinical evaluation and parasite counts were performed 12-hourly until negative, then daily and at every scheduled return visit.
Drug administration
Upon admission to the ward, patients who met the inclusion criteria and provided informed consent were randomly assigned at a ratio of 1:1 into groups A and B, as follows:
-
Group A
Artequick®(3.2 mg/kg/day of artemisinin, 16 mg/kg/day of piperaquine and 0.16 mg/kg/day of primaquine) was given once a day orally for 3 days. Each tablet of Artequick® contains artemisinin (80 mg) + piperaquine (400 mg) + primaquine (4 mg); supplied free of charge from Batch No. 20050901 by Artepharm Guangzhou, China.
-
Group B
ASMQ Artesunate (4 mg/kg/day) and mefloquine (8 mg/kg/day) were given orally once a day for 3 days.
The investigators provided the study drugs as directly observed therapy (DOT). Patients who vomited within one hour post-drug administration were re-dosed. The randomization list was computer-generated (Excel for Windows 2003; Microsoft USA). The treatment codes (group A or B) were sealed in individual envelopes labeled with the study code and patient number only. All patients were treated symptomatically (with intravenous fluids and antipyretics, as indicated) according to standard practice in the hospital. For any treatment failures (WHO, 2003a), such as, early treatment failure (ETF), late clinical failure (LCF), or late parasitological failure (LPF), the other standard antimalarial regimen for uncomplicated falciparum malaria [quinine 600 mg salt (2 tablets) every 8 hours and doxy-cycline 100 mg (1 capsule) twice a day for 7 days] and intravenous artesunate for severe malaria [artesunate 2.4 mg/kg at 0, 12, 24 hours, then 2.4 mg/kg daily] until the patient was able to tolerate oral medication, were given. The treatment would be complete with oral artesunate, 2.0 mg/kg daily, to complete 7 days of treatment. Doxycycline, 4 mg/kg/day for 7 days, would be added to artesunate.
Treatment outcomes
Malaria parasite counts per microliter were obtained by counting the number of asexual parasite forms per 200 white blood cells on thick smears and multiplying by the white blood cell count, or by counting the number of asexual parasite forms per 1,000 erythrocytes on thin smears and multiplying by the red blood cell counts. Parasite clearance time (PCT) was defined as the time from the start of treatment until the blood film was negative and remained negative for the next 24 hours. We also calculated the PCT at 90% and 50% reductions from initial parasitemia. Fever clearance time (FCT) was taken as the period from the start of treatment until the body temperature decreased to 37.5°C and remained at or below this temperature for the next 48 hours. Adverse effects were defined as signs and symptoms that occurred or became more severe after treatment started. Cure rate at Day28 (cured patients/evaluable patients × 100%) was defined as the absence of asexual parasite recrudescence during 28 days of follow-up. The classification of treatment outcomes was based on WHO criteria (WHO, 2003a).
Monitoring for safety
Patients were assessed daily during the scheduled follow-up. Adverse effects were assessed based on non-suggestive questioning by the investigators. Routine blood investigations (hematology and biochemistry) were performed prior to (Day0) and weekly for the 4 weeks of the study period. Toxicity grading scales of adverse effects were classified using the WHO toxicity grading scale for determining the severity of adverse events (WHO, 2003b).
Statistical analysis
Statistical analysis was performed using the Analyze It Add-Ins for Excel for Windows. All p-values reported were from 2-tailed testing, and statistical significance was set at 0.05. If the distribution of data did not show normality, as assessed by the Kolmogorov-Smirnov test, the results were expressed as geometric means (min-max) or medians (95%CI). The chi-square and Fisher exact tests were used to test the association between two groups of qualitative variables, as appropriate, and the Mann-Whitney U test was used to examine the difference between two groups of quantitative variables.
RESULTS
A total of 130 patients (119 male and 11 female, aged 15–54 years) were enrolled into this trial, with half (65) randomly assigned to each of two groups (Groups A and B). Most of the patients had contracted malaria on the Thai-Myanmar border. Patient demographic, clinical, and pretreatment laboratory characteristics are shown in Table 1. No significant differences in the demographic, clinical or initial laboratory data were found between the two treatment groups.
Table 1.
Clinical and laboratory characteristics of study groups before treatment.
| Group A (n = 65) | Group B (n = 65) | p-values | |
|---|---|---|---|
| Gender | |||
| Male/Female | 57/8 | 62/3 | 0.207 |
| Age | |||
| Median (95% CI) | 22.0 (21.0–25.5) | 24.0 (21.5–28.5) | 0.226 |
| Min-Max | 15–50 | 15–54 | |
| Body weight (kg) | |||
| Median (95% CI) | 50.0 (48.7–52.0) | 51.8 (49.1–53.7) | 0.103 |
| Min-Max | 40.5–70.0 | 40.1–74.0 | |
| Fever [Median (95% CI)] | |||
| Duration before admission (days) | 3.0 (2.0–4.5) | 3.0 (2.5–4.0) | 0.270 |
| Highest fever before treatment (°C) | 37.8 (37.6–37.9) | 37.8 (37.5–37.9) | 0.587 |
| Number of patients with: | |||
| Splenomegaly | 12 | 14 | 0.661 |
| Hepatomegaly | 43 | 38 | 0.365 |
| Geometric mean initial parasites count (/μl) | 12,585 | 10,902 | 0.208 |
| Min-Max | 120–147,730 | 104–134,580 | |
| Laboratory data [median (95% CI)] | |||
| Hematocrit (%) | 37.1 (34.8–.9) | 38.3 (34.4–39.2) | 0.224 |
| White blood cell count (103/μl) | 5.15 (4.88–5.70) | 5.40 (5.04–5.89) | 0.332 |
| Platelet count (103/μl) | 101.0 (97.8–130.0) | 112.0 (95.3–139.6) | 0.142 |
| Total bilirubin (mg %) | 0.80 (0.65–1.14) | 0.70 (0.66–1.15) | 0.192 |
| Aspartate aminotransferase (U/l) | 25.0 (21.7–43.2) | 22.0 (21.3–39.6) | 0.227 |
| Alanine aminotransferase (U/l) | 23.0 (21.7–41.4) | 24.0 (22.8 –43.5) | 0.697 |
| Blood urea nitrogen (mg %) | 13.0 (12.1–14.0) | 13.4 (12.9–14.7) | 0.306 |
| Creatinine (mg %) | 0.78 (0.73–0.87) | 0.80 (0.70–0.85) | 0.124 |
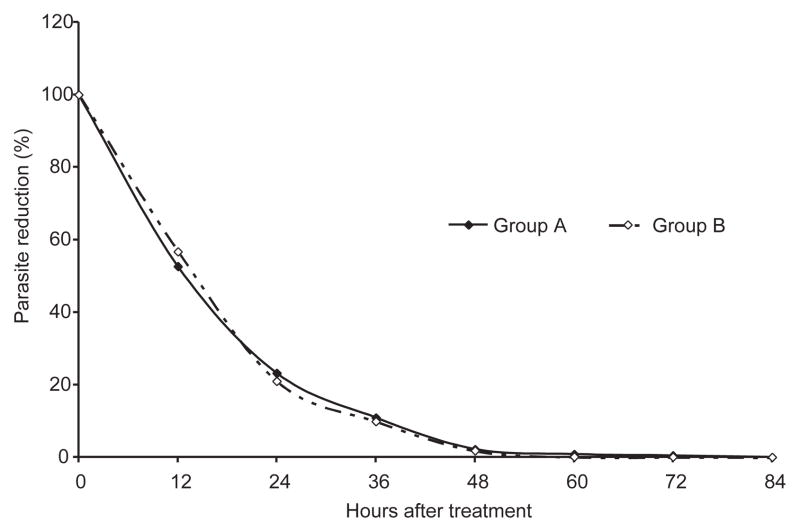
Only one patient in group A did not complete the 28-day follow-up due to social reasons unrelated to adverse effects. Thus, 129 of 130 patients (99.2%) completed the 28-day study. Parasitological and clinical responses are shown in Table 2. All patients in this study showed prompt responses to both antimalarial regimens (Fig 1). The median times for 100, 90, and 50% parasite clearances are shown in Table 2. Parasite clearances of 100% in each treatment group were rapid, with no significant differences between the two groups (group A and B, p-value = 0.098), median and min-max were 35.0 hours (24.0–82.0) and 33.0 hours (22.0–84.0), respectively. Parasites cleared from peripheral blood smears by 84 hours in all patients. In addition, the fever clearance times of the two treatment groups did not differ significantly (p-value = 0.580). The cure rates at 28-day follow-up of group A and B were 98.5 and 100%, respectively (p-value = 0.496). No patient had ETF or LCF. Only one treatment failure was found in group A during the trial: LPF, with reappearance of the asexual parasite form on Day21. This patient was successfully treated with the hospital’s standard regimen. Thus, all patients were parasitologically negative on discharge from hospital. No mixed P. vivax infection occurred during the study period.
Table 2.
Therapeutic responses.
| Group A (n = 65) | Group B (n = 65) | p-values | |
|---|---|---|---|
| N (%) of patients lost to follow-up | 1 (1.5) | 0 | NAa |
| N (%) of patients with 28-day follow-up | 64 (98.5) | 65 (100) | NAa |
| N (%) cured at Day28 | 63 (98.5)b | 65 (100) | 0.496 |
| Fever clearance time (hrs) | |||
| Median | 20 | 24 | 0.58 |
| Min-Max | 4.0–88.0 | 4.0–96.0 | |
| 50% Parasite clearance time (hrs) | |||
| Median | 8.7 | 9.0 | 0.361 |
| Min-Max | 4.0–26.0 | 4.0–28.0 | |
| 90% Parasite clearance time (hrs) | |||
| Median | 21.8 | 21.7 | 0.127 |
| Min-Max | 12.0–68.0 | 12.0–64.0 | |
| 100% Parasite clearance time (hrs) | |||
| Median | 35 | 33 | 0.098 |
| Min-Max | 24.0–82.0 | 22.0–84.0 | |
NA = not available
Reappearance of P. falciparum on Day21
Fig 1.
Reduction in the percentage of malaria parasites during treatment.
No patient died and no serious adverse effects occurred during treatment or during the 28-day follow-up period. No patient had vomiting related to the study drugs. Other adverse events, listed in Table 3, occurred during the first 3 days of administration of the study drugs, but these signs and symptoms could not be differentiated from those of malaria and disappeared within the first 5 days post-treatment.
Table 3.
Adverse effects in patients after treatment.
| Group Aa (n = 65) | Group Ba (n = 65) | |
|---|---|---|
| Headache | 5 (7.7) | 4 (6.1) |
| Weakness | 3 (4.6) | 1 (1.5) |
| Dizziness | 3 (4.6) | 2 (3.0) |
| Anorexia | 3 (4.6) | 2 (3.0) |
| Nausea | 2 (3.0) | 1 (1.5) |
| Insomnia | 1 (1.5) | 0 |
| Abdominal pain | 2 (3.0) | 3 (4.6) |
| Diarrhea | 1 (1.5) | 2 (1.5) |
Data are number of patients (%)
DISCUSSION
The use of artemisinin derivatives has been central to successful malaria control efforts in Thailand, Vietnam, and Cambodia (Looareesuwan et al, 1997; Denis et al, 2002; Hien et al, 2004). The artemisinin derivatives, the most rapidly acting and efficacious antimalarial drugs, have favorable pharmacodynamic properties, such as rapid absorption and activity against many stages of the malaria life cycle, including young asexual forms (rings) and early sexual forms (gametocytes) (Kumar and Zheng, 1990). Their short elimination half-lives (eg, <1 hour for artesunate) helped protect them from resistance. The tolerability of these drugs is excellent (Ashley and White, 2005). Because artemisinin derivatives are now the first-line treatment for multidrug-resistant falciparum malaria in many tropical countries, the appearance of artemisinin-resistant P. falciparum would be potentially disastrous. Thus, the development of suitable combinations of an artemisinin compound with other drugs to prevent the emergence of resistance is a major public health priority (WHO, 2006).
In this study, artemisinin was combined with piperaquine and primaquine with the goals of improving efficacy and compliance, and of reducing treatment cost. We report that a fixed dose of artemisinin-piperaquine-primaquine (Artequick®) once daily for three days was highly efficacious for treating acute uncomplicated P. falciparum malaria (cure rate 98.5%). Only one case, in group A, experienced a reappearance of P. falciparum during the scheduled follow-up period. This patient was successfully treated with Artequick®, with parasite clearance from peripheral blood at hour 36 post-treatment. The patient was discharged from hospital in the morning of Day8, as indicated in the protocol, and followed up weekly thereafter. On follow-up Day21, he came to the hospital with no malaria signs. He underwent a physical examination, with vital signs and blood-film testing, and showed a reappearance of falciparum malaria (parasite density = 675/μl). However, PCR was not run to determine reinfection or recrudescence since this patient had returned to the endemic area after treatment and reinfection could not be excluded. Based on our results, Artequick® showed a cure rate similar to the present standard regimen in Thailand (artesunate-mefloquine), and the fever-clearance and parasite-clearance times of the two treatments did not differ significantly. Regarding PCT, most patients in both groups were parasite-free and afebrile within 48 hours. However, a few cases (3 and 2 cases in groups A and B, respectively) did not clear parasites until > 48 hours; this was similar to the recent study in Indonesia (Ratcliff et al, 2007), where artemisinin derivatives in artemisinin combination therapies achieved their antimalarial effect through an initial rapid, short-term reduction in parasite biomass, with subsequent removal of the remaining parasites by the intrinsically less active, but more slowly eliminated, piperaquine or mefloquine.
P. vivax co-infection was not observed in any patient during the study period, probably because of the long-lasting action of the drugs administered with artemisinin. Piperaquine and mefloquine suppress vivax co-infection during a 28-day follow-up period because of their long elimination half-lives of 2–3.5 and 3–4 weeks, respectively (Ashley and White, 2005). With follow-up beyond 28 days, P. vivax malaria co-infection may become evident. As 28-day follow-up was a limitation of this study, a different study outcome might occur with a longer follow up period as discussed in a recent paper by Stepniewska and White (2006).
Artemisinin has a gametocytocidal effect on young gametocytes, whereas primaquine in the formulation of Artequick® should be useful for eradicating mature P. falciparum gametocytes (WHO, 2006). Thus, the combination of artemisinin and primaquine in Artequick® should kill both young and mature P. falciparum gametocytes, respectively. However, this was not examined in the present study. In Thailand, primaquine is routinely added to artesunate-mefloquine as prescribed by the national therapeutic regimen for uncomplicated falciparum malaria.
Regarding molecular marker testing, other factors contributing to drug resistance and/or recrudescence were not measured in this study, such as immune status, pharmacokinetic evidence, and available genetic data. It is well known that P. falciparum in some areas of the Mekong Region, particularly at the Thai-Cambodia border, is already resistant to mefloquine (a quinoline drug); this should be considered in any further study because the piperaquine in Artequick® is also a quinoline. Thus, there may be a risk of quinoline resistance developing, particularly in the Mekong Region.
Our hospital-based study showed that the artemisinin-piperaquine-primaquine combination is effective and well tolerated among Thai adults with acute uncomplicated P. falciparum malaria. Most patients treated with Artequick® in this study improved clinically and were parasitemia-negative by blood-smear at treatment Day3. The total cure rate was high (98–100%) in both groups. These results suggested that a 3-day course of Artequick® may be an alternative regimen for uncomplicated P. falciparum malaria. A shorter regimen of Artequick® is now being examined in clinical studies, with the goal of further improving compliance and the likelihood of completing a full treatment course. Artequick® may also be available at a lower cost than artesunate-mefloquine.
In summary, the results of this study indicated that a fixed-dose combination of artemisinin-piperaquine-primaquine (Artequick®) was as effective and well tolerated as the standard combination of artesunate-mefloquine and could be used as an alternative treatment for multidrug-resistant P. falciparum malaria in Southeast Asia. Despite these encouraging results, more studies with special groups, including children, and field-trials with long-term follow-up, pharmacokinetic, and genetic molecular characterization are needed to provide rational dosing regimens and guide the optimal use of Artequick®.
Acknowledgments
We thank our patients for their participation in this study and the nurses and laboratory technicians of the Hospital for Tropical Diseases for their help. The English of this manuscript was improved by Mr Paul R Adams, Specialist, Research and Academic Affairs Unit, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand. We also thank the Faculty of Tropical Medicine for page-charge support. This study was supported partly by a Mahidol University Research Grant and a grant from the Japanese Society for the Promotion of Science (B 19406013).
References
- Ashley EA, White NJ. Artemisinin-based combinations. Curr Opin Infect Dis. 2005;18:531–6. doi: 10.1097/01.qco.0000186848.46417.6c. [DOI] [PubMed] [Google Scholar]
- Breman JG, Alilio MS, Mills A. Conquering the intolerable burden of malaria: what’s new, what’s needed: a summary. Am J Trop Med Hyg. 2004;71:1–15. [PubMed] [Google Scholar]
- Davis TM, Hung TY, Sim IK, Karunajeewa HA, Ilett KF. Piperaquine: a resurgent antimalarial drug. Drugs. 2005;65:75–87. doi: 10.2165/00003495-200565010-00004. [DOI] [PubMed] [Google Scholar]
- Denis MB, Davis TM, Hewitt S, et al. Efficacy and safety of dihydroartemisinin-piperaquine (Artekin) in Cambodian children and adults with uncomplicated falciparum malaria. Clin Infect Dis. 2002;35:1469–76. doi: 10.1086/344647. [DOI] [PubMed] [Google Scholar]
- Hien TT, Dolecek C, Mai PP. Dihydroartemisinin-piperaquine against multidrug-resistant Plasmodium falciparum malaria in Vietnam: randomized clinical trial. Lancet. 2004;363:18–22. doi: 10.1016/s0140-6736(03)15163-x. [DOI] [PubMed] [Google Scholar]
- Kumar N, Zheng H. Stage-specific gametocytocidal effect in vitro of the antimalaria drug qinghaosu on Plasmodium falciparum. Parasitol Res. 1990;76:214–8. doi: 10.1007/BF00930817. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Wilairatana P, Vanijanonta S, Pitisuttithum P, Ratanapong Y, Andrial M. Monotherapy with artesunate for uncomplicated falciparum malaria: a comparison of 5-day and 7-day regimens. Acta Trop. 1997;67:197–205. doi: 10.1016/s0001-706x(97)00063-6. [DOI] [PubMed] [Google Scholar]
- Nosten F, Luxemburger C, ter Kuile FO, et al. Treatment of multidrug-resistant P. falciparum malaria with 3-day artesunate-mefloquine combination. J Infect Dis. 1994;170:917–27. doi: 10.1093/infdis/170.4.971. [DOI] [PubMed] [Google Scholar]
- Ratcliff A, Siswantoro H, Kenangalem E, et al. Two fixed dose artemisinin combinations for drug resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomized comparison. Lancet. 2007;369:757–65. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silachamroon U, Krudsood S, Thanachartwet W, et al. An open, randomized trial of three-day treatment with artesunate combined with a standard dose of mefloquine divided over either two or three days, for acute, uncomplicated falciparum malaria. Southeast Asian J Trop Med Public Health. 2005;36:591–6. [PubMed] [Google Scholar]
- Smithuis F, Kyaw MK, Phe O, et al. Efficacy and effectiveness of dihydroartemisinin-piperaquine versus artesunate-mefloquine in falciparum malaria: an open-label randomized comparison. Lancet. 2006;367:2075–85. doi: 10.1016/S0140-6736(06)68931-9. [DOI] [PubMed] [Google Scholar]
- Snow RW, Guerra CA, Noor AM, Myint HY, Hay SI. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature. 2005;434:214–7. doi: 10.1038/nature03342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepniewska K, White NJ. Some considerations in the design and interpretation of antimalarial drug trials in uncomplicated falciparum malaria. Malaria J. 2006;5:127. doi: 10.1186/1475-2875-5-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarning J, Lindegardh N, Annerberg A, et al. Pitfalls in estimating piperaquine elimination. Antimicrob Agents Chemother. 2005;49:5127–8. doi: 10.1128/AAC.49.12.5127-5128.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tropical Medicine Institute. Documentation of Artequick® for new drug registration. Guangzhou, China: Guangzhou University of Traditional Chinese Medicine; 2004. [Google Scholar]
- Vijaykadga S, Rojanawatsirivej C, Cholpol S, Phoungmanee D, Nakavej A, Wongsrichanalai C. In vivo sensitivity monitoring of mefloquine monotherapy and artesunate-mefloquine combinations for the treatment of uncomplicated falciparum malaria in Thailand in 2003. Trop Med Int Health. 2006;11:211–9. doi: 10.1111/j.1365-3156.2005.01557.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Antimalarial drug combination therapy: report of a WHO technical consultation. 2001 [Cited 2005 Dec 7]. Available from: URL: http://ww.whoglibdoc.who.int/hq/2001/WHO_CDS_RBM_2001.35pdf.
- World Health Organization. Assessment and monitoring of antimalarial drug efficacy for the treatment of uncomplicated falciparum malaria. Geneva: WHO; 2003a. [Google Scholar]
- World Health Organization. Toxicity grading scale for determining the severity of adverse events. 2003b [Cited 2006 Mar 14]. Available from: URL: http://www.icsssc.org/documents/20final.pdf.
- World Health Organization. Guidelines for the treatment of malaria. 1. Geneva: WHO; 2006. [Google Scholar]
- World Health Organization. Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94 (suppl 1):1–90. [PubMed] [Google Scholar]