Abstract
Median survival times (STs) for doxorubicin-treated canine lymphoma range from 5.7 to 9 months. Because dogs treated with multi-agent protocols have longer STs, we sought to evaluate whether adding cyclophosphamide would improve outcome in canine lymphoma patients while maintaining an acceptable level of toxicity. Thirty-two dogs with stage III–V multicentric lymphoma were treated with doxorubicin every 3 weeks for five total cycles and prednisone at a tapering dose for the first 4 weeks. Dogs were randomized to receive either cyclophosphamide or placebo concurrently. Seventeen dogs received doxorubicin and placebo, while 15 dogs received doxorubicin and cyclophosphamide. Response, toxicity, progression-free interval (PFI) and ST were evaluated. The combination of doxorubicin and cyclophosphamide was well tolerated, causing no increase in adverse events over doxorubicin alone. Despite a numeric improvement in outcome in cyclophosphamide treated dogs, the addition of cyclophosphamide did not result in statistically improved response rate, PFI or ST.
Keywords: adriamycin, cancer, chemotherapy, cytoxan, dog
Introduction
Lymphoma is the most common haematopoietic neoplasm in dogs. Standard of care treatment involves multi-agent chemotherapy protocols that incorporate doxorubicin. Most combination protocols are so-called CHOP-based, which use cyclophosphamide, doxorubicin, vincristine and prednisone, with 80–90% complete response (CR) rates and median survival times (STs) of approximately 12 months reported1,2; however, the use of multi-agent protocols is not always possible because of cost or time constraints on the part of owners.
Doxorubicin is an anthracycline derived from the Streptomyces yeast. It has multiple mechanisms of action. These include intercalation of DNA, which leads to inhibition of protein synthesis and free radical formation, and inhibition of topoisomerase enzymes. Major toxicities associated with doxorubicin are bone marrow suppression, gastrointestinal upset, including nausea, vomiting and diarrhoea, and myocardial toxicity, which is cumulative and dose limiting.3–6 Single-agent therapy with doxorubicin results in STs greater than those of prednisone alone for the treatment of canine lymphoma. Reported remission durations range from 4.3 to 6.8 months, STs from 5.7 to 9 months, and reported response rates of 59–85%.7–11
Cyclophosphamide is an alkylating agent that can be given orally in dogs, with relatively little toxicity, including bone marrow suppression and sterile haemorrhagic cystitis.3,12–15 Although doxorubicin has been evaluated as a single agent for lymphoma in dogs, cyclophosphamide has not. The ability to administer cyclophosphamide and prednisone orally allows for these drugs to be given concurrently with doxorubicin with minimal time or effort on the part of the owner, and with little added expense. Previously, cyclophosphamide was evaluated in combination with doxorubicin as a maintenance protocol following induction with vincristine and L-asparaginase for 28 dogs with stage III–V lymphoma. In this study, the median remission duration was 173 days (5.7 months), which appeared similar to those in single-agent doxorubicin protocols.16 Data regarding first-line use of doxorubicin/cyclophosphamide combination chemotherapy have not been reported to our knowledge.
The purpose of this prospective study was to evaluate whether the addition of oral cyclophosphamide to five doses of doxorubicin and oral prednisone would increase median progression-free interval (PFI), response rate, ST or toxicity in dogs with treatment-naïve multicentric lymphoma.
Materials and methods
Patient population
Thirty-two dogs with multicentric lymphoma that were presented to the Animal Cancer Center at Colorado State University or the University of Wisconsin-Madison School of Veterinary Medicine between September of 2007 and October of 2008 were included in the study. The study design was prospective in nature. Dogs were eligible for the study if they were stage II–V, substage a or b and the owners elected to treat with single-agent doxorubicin. Breed, sex and age at diagnosis were recorded for each dog. All dogs were naïve to chemotherapy including corticosteroids. The staging system of the World Health Organization for canine lymphoma was used to determine stage and substage. A complete blood count (CBC), serum chemistry and urinalysis were required for entry into the study. Thoracic radiographs, abdominal ultrasound and bone marrow aspirate were documented when performed for staging. Immunophenotype, as assessed by Polymerase Chain Reaction for antigen receptor rearrangement, immunohistochemistry, immunocytochemistry or flow cytometry, was recorded when available.
Treatment
If owners chose single-agent doxorubicin as treatment, and elected to enroll in the study, dogs were randomized to receive either cyclophosphamide or placebo. The randomization scheme was generated by using the web site Randomization.com (http://www.randomization.com). Patients were treated with doxorubicin (30 mg m−2) IV every 3 weeks for a total of five cycles and prednisone at a tapering dose for the first 4 weeks (Table 1). Based on randomization to treatment or placebo group, patients received either cyclophosphamide (target dose 50 mg m−2 daily for three days) or placebo concurrently, starting on the same day as the doxorubicin dosing.
Table 1.
Chemotherapy protocol dogs were scheduled to receive
| Weeks |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug and dosage | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Doxorubicin (30 mg m−2) | X | X | X | X | X | ||||||||
| Cyclophosphamide (50 mg m−2 daily × 3days)or Placebo |
X | X | X | X | X | ||||||||
| Prednisone (mg kg−1 day−1) | 2 | 1.5 | 1 | 0.5 | |||||||||
Response and toxicity
CR (complete resolution of disease), partial response (at least 30% or greater reduction in sums of the longest diameters of measurable peripheral nodes), PFI, ST and number of grade 3/4 adverse events were compared between groups. Response was determined using the Response Evaluation Criteria in Solid Tumors (RECIST) criteria.17 Stable disease was defined as neither a 30% decrease or 20% increase in the sums of the longest diameters of measurable peripheral lymph nodes, while progressive disease (PD) was defined as a greater than 20% increase in the sums of the longest diameters. The PFI was defined as the time from first treatment to the date of PD. The ST was calculated as the time from the date of the first treatment to the date of death. Toxicity was graded 1–4, and based on the Veterinary Co-operative Oncology Group common terminology criteria for adverse events.18 Using this grading scheme, Grade 1 neutropenia was defined as 1500 cells μL−1 to the lower limit of normal, which was 2000 cells μL−1 for both institutions. Haematological toxicity was evaluated 7 days after the first treatment, and subsequently at the time of each treatment, if dosage adjustments were not made.
Upon completion of the five treatments, it was recommended that animals be seen once monthly for rechecks involving a physical examination. Blood work was performed at the discretion of the clinician. If lymph node enlargement was palpated, cytology was used to confirm relapse. Information regarding rescue therapy pursued following relapse was collected, and outcome information collected following relapse via recheck examinations and telephone conversations with owners and referring veterinarians.
Statistical analysis
Power analysis was performed prospectively and prior to enrollment of patients. With a planned total of 32 dogs to enroll, this study was powered to detect a 3.1-fold increase in PFI or ST with 80% power and a P value of 0.05. CR versus partial or no response and the presence of grade 3/4 adverse events were compared between groups for significance using a two-tailed Fisher–s exact test. This test was also used to evaluate for differences between groups for substage, hypercalcaemia and T-cell immunophenotype, all of which have been associated with prognosis in previous studies. Stage was not evaluated as a result of inconsistencies in staging tests performed between patients. A Student–s two-tailed unpaired t-test was used to compare age between groups. The PFI and ST curves were generated by the Kaplan–Meier product limit method. A log rank (Mantel–Cox) test was used to compare the curves. In all analyses, a P value of <0.05 was considered statistically significant. Statistical analyses were performed using Prism 5 software (GraphPad, San Diego, CA, USA).
Results
Patients
Thirty-two dogs with lymphoma were included in the study. Patient characteristics by treatment group are listed in Table 2. All patients received full blood work as part of staging, while some patients received thoracic radiographs, abdominal ultrasound and/or bone marrow aspirates. There were no significant differences between the two groups with regard to age, weight, sex, substage, immunophenotype or the presence of hypercalcaemia.
Table 2.
Patient characteristics by treatment group
| Doxorubicin + cyclophos- phamide (n = 15) |
Doxorubicin + placebo (n = 17) |
P value | |
|---|---|---|---|
| Age (years) | 0.83 | ||
| Mean | 8.25 ± 2.57 | 8.47 ± 3.07 | |
| Median | 8 | 9 | |
| Range | 5–13 | 2–14 | |
| Body weight (mean in kg) |
31.7 ± 4.1 | 33.3 ± 3.2 | 0.76 |
| Sex | 1.0 | ||
| Male | 10 (66.7%) | 12 (64.7%) | |
| Female | 5 (33.3%) | 5 (35.2%) | |
| Substage | 0.32 | ||
| a | 12 (80.0%) | 16 (94.1%) | |
| b | 3 (20.0%) | 1 (5.9%) | |
| Immuno- phenotype |
0.49 | ||
| B | 6 (40.0%) | 5 (29.4%) | |
| T | 2 (13.3%) | ||
| Null | 2 (11.8%) | ||
| Hypercalcaemia | 2 (13.3%) | 1 (5.9%) | 0.58 |
Treatment and toxicity
The overall number of doses of doxorubicin and cyclophosphamide given ranged from 1 to 5 (median 5) for both groups. In the doxorubicin and placebo group, the mean starting dose of doxorubicin was 28.1 mg m−2 (range 19.7–30.3 mg m−2). In the doxorubicin and cyclophosphamide group, the mean starting dose of doxorubicin was 27.7 mg m−2 (range 18.1–30.3 mg m−2), while the mean starting dose of cyclophosphamide was 159 mg m−2 divided over 3 days (range 123–192 mg m−2). The distribution of adverse events is outlined in Table 3. In the cyclophosphamide group, there were three animals that did not have a follow-up CBC 1 week after the first treatment. There were six animals in the cyclophosphamide group that had grade 3 or 4 haematological toxicity, and no animals with grade 3/4 gastrointestinal toxicity. Of these patients, there were two patients that had dose reductions, one in the patient that had grade 3 thrombocytopenia and grade 4 neutropenia, and the other in a patient with grade 4 neutropenia. When dose reductions were made, the doxorubicin was reduced, as it was unknown whether patients were receiving cyclophosphamide or placebo.
Table 3.
Grade 3/4 adverse events by number of patients
| Cyclophosphamide | Placebo | |
|---|---|---|
| Grade 3/4 toxicitiesa | 6 (8 events) | 5 (5 events) |
| Grade 3/4 haematological toxicities |
6 (8 events) | 3 (3 events) |
| Grade 3 anaemia | 1 | 0 |
| Grade 3 neutropaenia | 1 | 0 |
| Grade 4 neutropaenia | 2 | 1 |
| Grade 3 thrombocy- topenia |
2 | 2 |
| Grade 4 thrombocy- topenia |
2 | 0 |
| Grade 3/4 gastrointestinal toxicity |
0 | 2 |
| Grade 3 vomiting | 0 | 2 |
P = 0.71.
In the placebo group, there were also three animals that did not have a follow-up CBC 1 week after the first treatment. There were three patients in the placebo group that had grade 3 or 4 haematological toxicity, and two with grade 3/4 gastrointestinal toxicity. Two patients had dose reductions in the placebo group. There was no significant difference in the number of patients with grade 3/4 toxicities between groups (P = 0.71) or the number of dose reductions between groups (P = 1.0). There were two patients in the study that died or were euthanized as a result of presumed cardiac disease. Both of these patients were in the cyclophosphamide group. There was no significant difference in cardiac disease between the groups (P = 0.21). There were two patients that died 6 and 7 days after the first treatment in the placebo group of unknown causes. Postmortem examinations were not performed on either patient. There were no other reported toxicities.
Outcome
Overall, there were 11/15 (73.3%) CRs in the cyclophosphamide group and 13/17 (76.4%) in the placebo group (P = 0.65). Most dogs experienced a CR by the time they were presented for their second treatment, although a few dogs did not achieve CR until after the second treatment. The median PFI for the cyclophosphamide group was 246 days (range of 7–337 days), while the PFI for the placebo group was 169 days (range 6–428; Fig. 1). This difference was not statistically significant (P = 0.58).
Figure 1.

Kaplan Meier curve of progression free interval comparing cyclophosphamide and placebo groups.
The median ST for the cyclophosphamide group was 423 days (range 7–564), while the median ST for the placebo group was 295 days (range 6–545; Fig. 2). This difference was also not statistically significant (P = 0.11). When evaluating rescue protocols received, 10 of the 10 dogs in the cyclophosphamide group eligible to receive rescue therapy were treated. Four dogs received CCNU, L-asparaginase and prednisone, three dogs received the investigational drug GS-9219 (Gilead Sciences, Foster City, CA, USA),19,20 one dog received an additional dose of doxorubicin and cyclophosphamide, and two dogs received multiple rescue protocols consisting of idarubicin, vinblastine, L-asparaginase/vincristine/melphalan, Cyclophosphamide/vincristine/prednisone (COP), CCNU or bleomycin/DTIC. Of the eight dogs eligible to receive rescue therapy in the placebo group, five received rescue therapy. Two dogs received CCNU, L-asparaginase and prednisone, one dog received cyclophosphamide and prednisone, and two dogs received multiple rescue protocols consisting of GS-9219, CCNU, L-asparaginase, vincristine or mitoxantrone. The difference in the percentage of dogs receiving rescue therapy at relapse between groups approached significance (P = 0.06). When only the dogs of each group that received rescue chemotherapy were compared for survival (Fig. 3), the median ST of cyclophosphamide dogs was 423 days, while that of placebo dogs was 318 days (P = 0.11). When the dogs receiving rescue therapy were separated from the eligible dogs who did not receive rescue, regardless of whether they received placebo or cyclophosphamide, dogs that received rescue therapy had a median ST of 352 days, which was significantly longer than those that did not receive rescue therapy (295 days; P = 0.01).
Figure 2.
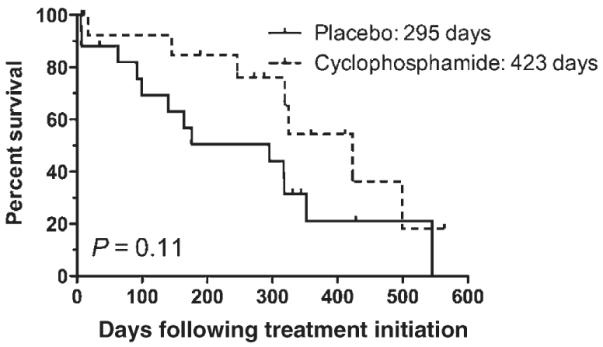
Kaplan Meier curve of survival time comparing cyclophosphamide and placebo groups.
Figure 3.
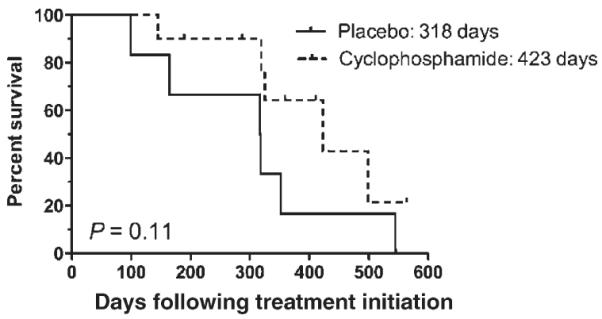
Kaplan Meier curve of survival time of dogs receiving rescue therapy comparing cyclophosphamide and placebo groups.
Discussion
This study compared outcome in dogs treated for multicentric lymphoma with doxorubicin, cyclophosphamide and prednisone to outcome in dogs treated with doxorubicin, placebo and prednisone. Results of the present study suggest that the combination of doxorubicin and cyclophosphamide for treatment of canine lymphoma was well tolerated, causing no significant increase in adverse events over doxorubicin alone. However, the addition of cyclophosphamide in this study did not result in significantly improved response, PFI or ST.
Although there was no statistical difference in the PFI or ST between the two groups, there was a longer median PFI and ST for the dogs treated with doxorubicin and cyclophosphamide. The most noticeable difference was in the ST between the two groups, with a ST of 423 days for the cyclophosphamide group versus 295 days for the placebo group. Given the differences in rescue therapy elected, we speculated that this difference could be explained in part by the difference in rescue protocols between groups. The cyclophosphamide group had a larger number of patients receiving rescue therapy than the placebo group, which statistically approached significance. When only patients that received rescue therapy were compared for survival between the two groups, the curves were similar to the initial survival curves (423 days versus 318 days for cyclophosphamide and placebo groups, respectively), with an equivalent P value, suggesting a minimal contribution of rescue therapy to patient outcome.
With a total of 32 dogs, this study was powered to detect a 3.1-fold increase in PFI or ST with 80% power and a P value of 0.05. In order to detect the 1.45-fold improvement in outcome observed in this study with 80% power, a total of 255 patients would have been required. Because of the minimal added expense, ease of administration, and lack of additional toxicity, it may be reasonable to add cyclophosphamide to doxorubicin and prednisone in this population. Given the limited power in this study, it may be dangerous to interpret that cyclophosphamide is not useful in addition to doxorubicin because of type II error, failing to accept the null hypothesis when it is in fact true.21
It has been previously shown that lymphoma dogs treated with single-agent doxorubicin are more responsive to rescue protocols than are dogs treated with COP.8 It seems that the addition of cyclophosphamide does not negatively affect the ST of dogs receiving rescue therapy, given that the ST of cyclophosphamide treated dogs remained greater when patients not receiving rescue therapy were removed from the survival curve. Thus, the addition of cyclophosphamide remains more convenient and less expensive than CHOP-based protocols, and likely does not influence the response to rescue therapy negatively.
The two populations of dogs in this study were comparable in terms of age, sex, weight and potential prognostic factors. The randomization scheme avoided potential biases between groups. One limitation was that most of the dogs were not staged with a bone marrow aspirate and many were not immunophenotyped, owing to a lack of financial support for these aspects of the trial. This makes it difficult to compare the groups for these two important prognostic factors, and although statistical differences did not exist between the groups, this could have contributed to the differences in PFI and ST.
Ultimately, most dogs that achieve remission are likely to experience a relapse of disease, possibly representing the emergence of resistant tumour clones. It is somewhat intuitive that dogs receiving rescue therapy after relapsing following induction would have a longer ST than those receiving prednisone alone or no rescue therapy, although this has never been evaluated systematically in dogs with lymphoma. This study demonstrates that patients receiving some form of chemotherapy following relapse had a statistically longer ST than those that received palliative therapy (prednisone) or no treatment. This seems logical and again could have contributed to the longer numerical ST in the cyclophosphamide group, although the difference among the groups in those patients that received rescue therapy was not statistically significant.
Prednisone was administered in addition to the doxorubicin in this study, as the authors felt that it may improve quality of life during the induction period and could also increase response and survival. The results for the placebo group, with a median ST of 295 days (9.8 months) is similar to those reported historically for doxorubicin alone (median ST 5.7–9 months).7–11 Similarly, there appears to be no difference in percentage of patients responding to treatment with the addition of prednisone, although comparison with historical controls does not allow meaningful statistical evaluation.
Doxorubicin-associated cardiomyopathy did not seem to be a significant occurrence in this study. There were two patients in this study that died because of suspected cardiac disease, one euthanized because of heart failure and one that died of suspected heart failure. Neither of these patients’ disease was confirmed to be a result of therapy with doxorubicin. Neither patient had a prescreening echocardiogram and one patient was a Doberman Pinscher, a breed known to be predisposed to dilated cardiomyopathy. Both of these patients were in the cyclophosphamide group, but there was no statistical difference in the incidence of cardiac disease between the two groups, although this study was not powered to detect such a small difference. It has been shown in people that cyclophosphamide given concurrently with doxorubicin may lower the cumulative dose necessary for the development of cardiac toxicity.22 A cumulative dose of 180 mg m−2 was the maximum dose given in the cyclophosphamide group in one dog, the remainder receiving 150 mg m−2. The addition of concurrent cyclophosphamide, although dosed over 3 days following the doxorubicin, did not seem to increase the development of cardiomyopathy.
In summary, we found no significant differences in the response rate, PFI, ST or prevalence of toxicity in dogs treated with doxorubicin, placebo and prednisone versus doxorubicin, cyclophosphamide and prednisone. This suggests that although well tolerated and given with little added expense, there was no statistical improvement in outcome with the addition of cyclophosphamide in the present population. The authors strongly feel that this question may be better answered in a larger population of dogs with lymphoma.
Acknowledgements
We would like to acknowledge Dr Jens Eickhoff for helpful discussions, as well as Drs Ruthanne Chun and David Vail for case management and helpful discussions. This study was funded in part by grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources (NCRR) National Institutes of Health (NIH) to T. J. S.
Footnotes
This data was presented in part at the meeting of the Veterinary Cancer Society on October 17, 2009 in Austin, Texas.
References
- 1.Garrett LD, Thamm DH, Chun R, Dudley R, Vail DM. Evaluation of a 6-month chemotherapy protocol with no maintenance therapy for dogs with lymphoma. Journal of Veterinary Internal Medicine. 2002;16:704–709. doi: 10.1892/0891-6640(2002)016<0704:eoacpw>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Keller E, MacEwen E, Rosenthal R. Evaluation of prognostic factors and sequential combination chemotherapy with doxorubicin for canine lymphoma. Journal of Veterinary Internal Medicine. 1993;7:289–295. doi: 10.1111/j.1939-1676.1993.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 3.Chun R, Garrett LD, Vail DM. Withrow and MacEwen–s Small Animal Clinical Oncology. 4th edn Saunders; St Louis: 2007. Cancer chemotherapy; pp. 163–191. [Google Scholar]
- 4.Langer S, Sehested M, Jensen P. Treatment of anthracycline extravasation with dexrazoxane. Clinical Cancer Research. 2000;6:3680–3686. [PubMed] [Google Scholar]
- 5.Cvetkovic R, Scott L. Dexrazoxane: a review of its use for cardioprotection during anthracycline chemotherapy. Drugs. 2005;65:1005–1024. doi: 10.2165/00003495-200565070-00008. [DOI] [PubMed] [Google Scholar]
- 6.Mauldin GE, Fox PR, Patnaik AK, Bond BR, Mooney SC, Matus RE. Doxorubicin-induced cardiotoxicosis. Journal of Veterinary Internal Medicine. 1992;6:82–88. doi: 10.1111/j.1939-1676.1992.tb03156.x. [DOI] [PubMed] [Google Scholar]
- 7.Vail DM, Young KM. Withrow & MacEwen’s Small Animal Clinical Oncology. 4th edn Saunders; St Louis: 2007. Canine lymphoma and lymphoid leukemia; pp. 699–733. [Google Scholar]
- 8.Carter R, Harris C, Withrow SJ. Chemotherapy of canine lymphoma with histopathological correlation: doxorubicin alone compared to COP as first treatment regimen. Journal of the American Animal Hospital Association. 1987;23:587–596. [Google Scholar]
- 9.Postorino NC, Susaneck SJ, Withrow SJ, Macy DW, Harris C. Single agent therapy with adriamycin for canine lymphosarcoma. Journal of the American Animal Hospital Association. 1989;25:221–225. [Google Scholar]
- 10.Mutsaers AJ, Glickman NW, DeNicola DB, Widmer WR, Bonney PL, Hahn KA, Knapp DW. Evaluation of treatment with doxorubicin and piroxicam or doxorubicin alone for multicentric lymphoma in dogs. Journal of the American Veterinary Medical Association. 2002;220:1813–1817. doi: 10.2460/javma.2002.220.1813. [DOI] [PubMed] [Google Scholar]
- 11.Valerius K, Ogilvie G, Mallinckrodt C, Getzy D. Doxorubicin alone or in combination with asparaginase, followed by cyclophosphamide, vincristine, and prednisone for treatment of multicentric lymphoma in dogs: 121 cases (1987–1995) Journal of the American Veterinary Medical Association. 1997;210:512–516. [PubMed] [Google Scholar]
- 12.Charney S, Bergman P, Hohenhaus A, Mcknight J. Risk factors for sterile hemorrhagic cystits in dogs with lymphoma receiving cyclophosphamide with or without concurrent administration of furosemide: 216 cases (1990-1996) Journal of the American Veterinary Medical Association. 2003;222:1388–1393. doi: 10.2460/javma.2003.222.1388. [DOI] [PubMed] [Google Scholar]
- 13.Crow S, Theilen G, Madewell B, Weller RE, Henness AM. Cyclophosphamide induced cystitis in the dog and cat. Journal of the American Veterinary Medical Association. 1977;171:259–262. [PubMed] [Google Scholar]
- 14.Stanton M, Legendre A. Effects of cyclophosphamide in dogs and cats. Journal of the American Veterinary Medical Association. 1986;188:1319–1322. [PubMed] [Google Scholar]
- 15.Peterson J, Couto C, Hammer A, Ayl AD. Acute sterile hemorrhagic cystitis after single intravenous administration of cyclophosphamide in three dogs. Journal of the American Veterinary Medical Association. 1992;201:1572–1573. [PubMed] [Google Scholar]
- 16.Price GS, Page RL, Fischer BM, Levine JF, Gerig TM. Efficacy and toxicity of doxorubicin/cyclophosphamide maintenance therapy in dogs with multicentric lymphosarcoma. Journal of Veterinary Internal Medicine. 1991;5:259–262. doi: 10.1111/j.1939-1676.1991.tb03131.x. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. Journal of the National Cancer Institute. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Veterinary co-operative oncology group – common terminology criteria for adverse events (VCOG-CTCAE) following chemotherapy or biological antineoplastic therapy in dogs and cats. Veterinary and Comparative Oncology. 2004;2:195–213. doi: 10.1111/j.1476-5810.2004.0053b.x. [DOI] [PubMed] [Google Scholar]
- 19.Reiser H, Wang J, Chong L, Watkins WJ, Ray AS, Shibata R, Birkus B, Cihlar T, Wu S, Li B, Liu X, Henne IN, Wolfgang GH, Desai M, Rhodes GR, Fridland A, Lee WA, Plunkett W, Vail D, Thamm DH, Jeraj R, Tumas DB. GS-9219 – a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin–s lymphoma. Clinical Cancer Research. 2008;14:2824–2832. doi: 10.1158/1078-0432.CCR-07-2061. [DOI] [PubMed] [Google Scholar]
- 20.Vail DM, Thamm DH, Reiser H, Ray AS, Wolfgang GH, Watkins WJ, Babusis D, Henne IN, Hawkins MJ, Kurzman ID, Jeraj R, Vanderhoek M, Plaza S, Anderson C, Wessel MA, Robat C, Lawrence J, Tumas DB. Assessment of GS-9219 in a pet dog model of non-Hodgkin–s lymphoma. Clinical Cancer Research. 2009;15:3503–3510. doi: 10.1158/1078-0432.CCR-08-3113. [DOI] [PubMed] [Google Scholar]
- 21.Baldi B, Moore DS. Inference in practice: the power of a statistical test. In: Bleyer C, editor. The Practice of Statistics in the Life Sciences. W. H. Freeman and Company; New York City: 2009. pp. 398–405. [Google Scholar]
- 22.Minow R, Benjamin R, Lee E, Gottlieb J. Adriamycin cardiomyopathy – risk factors. Cancer. 1977;39:1397–1402. doi: 10.1002/1097-0142(197704)39:4<1397::aid-cncr2820390407>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]


