Abstract
Introduction
Dexmedetomidine is a highly selective α2-adrenoceptor agonist with sedative, anxiolytic and analgesic properties that has minimal effects on respiratory drive. Its sedative and hypotensive effects are mediated via central α2A and imidazoline type 1 receptors while activation of peripheral α2B–adrenoceptors result in an increase in arterial blood pressure and systemic vascular resistance (SVR). In this randomized, prospective, clinical study we attempted to quantify the short-term hemodynamic effects resulting from a rapid IV bolus administration of dexmedetomidine in pediatric cardiac transplant patients.
Methods
Twelve patients, aged ≤10 years of age, weighing ≤40kg, presenting for routine surveillance of right and left heart cardiac catheterization after cardiac transplantation were enrolled. After an inhaled or IV induction, the tracheas were intubated and anesthesia was maintained with 1 minimum alveolar concentration of isoflurane in room air, fentanyl (1mcg/kg) and rocuronium (1mg/kg). At the completion of the planned cardiac catheterization, 100% oxygen was administered. After recording a set of baseline values that included heart rate (HR), systolic blood pressure, diastolic blood pressure, central venous pressure, systolic pulmonary artery pressure, diastolic pulmonary artery pressure, pulmonary artery wedge pressure and thermodilution-based cardiac output, a rapid IV dexmedetomidine bolus of either 0.25mcg/kg or 0.5mcg/kg was administered over 5 seconds. The hemodynamic measurements were repeated at 1 min and 5 mins.
Results
There were 6 patients in each group. Investigation suggested that systolic blood pressure, diastolic blood pressure, systolic pulmonary artery pressure, diastolic pulmonary artery pressure, pulmonary artery wedge pressure and systemic vascular resistance all increased at 1 minute after rapid IV bolus for both doses, and decreased significantly to near baseline for both doses by 5 minutes. The transient increase in pressures was more pronounced in the systemic system than in the pulmonary system. In the systemic system there was a larger percent increase in the diastolic pressures than the systolic pressures. Cardiac output, CVP and pulmonary vascular resistance did not change significantly. HR decreased at 1 min for both doses and was, within the 0.5 mcg/kg group, the only hemodynamic variable still changed from baseline at the 5 min time point
Conclusion
Rapid IV bolus administration of dexmedetomidine in this small sample of children having undergone heart transplants was clinically well tolerated, although it resulted in a transient but significant increase in systemic and pulmonary pressure and a decrease in HR. In the systemic system there is a larger percent increase in the diastolic pressures than the systolic pressures, and furthermore these transient increases in pressures were more pronounced in the systemic system than in the pulmonary system.
Introduction
Dexmedetomidine is a highly selective α2-adrenoceptor agonist with sedative, anxiolytic and analgesic properties1–3. Because it has minimal effects on respiratory drive, dexmedetomidine’s use has been reported as part of a balanced anesthetic4–6, for the prevention and treatment of emergence delirium7, as a primary sedative drug in the intensive care unit (ICU)8–10, and as a sole drug for sedation during diagnostic procedures like magnetic resonance imaging11 and invasive procedures performed in the ICU12.
The sedative, anxiolytic and hypotensive effects of dexmedetomidine are mediated via stimulation of central α2A and imidazoline type 1 (I1) receptors13–16. The activation of these central receptors results in a decreased catecholamine release and an overall reduction in the sympathetic outflow from the locus ceruleus of the brainstem. The analgesic effects of dexmedetomidine are thought to be the result of activation of α2B-adrenoceptors at the level of the dorsal horn of the spinal cord and the inhibition of substance P release. Dexmedetomidine’s peripheral vascular effects result from stimulation of α2B–adrenoceptor in the peripheral vasculature and cause an initial increase in systemic vascular resistance (SVR) and decrease in the cardiac output (CO)15,17.
The hemodynamic effects of a slow bolus administration of dexmedetomidine over 10 minutes has been well described with regards to heart rate (HR) and arterial blood pressure in children18–21. In these studies, dexmedetomidine administration resulted in a decrease in blood pressure and HR. In adults, dexmedetomidine has been administered over 2 minutes, resulting in a biphasic hemodynamic response with an initial increase in blood pressure and reflex bradycardia followed by a stabilization of blood pressure and HR below baseline values22,23. However there are no data in the pediatric or adult literature concerning the hemodynamic effects after a rapid bolus.
Dexmedetomidine is used frequently to prevent and treat postoperative agitation7,24–26 in doses of 0.25–1mcg/kg. While preemptive administration is ideal in the prevention of postoperative agitation, a child who emerges from anesthesia with acute delirium requires prompt treatment because they can pose a significant risk to themselves and those around them. The use of dexmedetomidine as an infusion over 10 mins in such a situation is impractical and while in the above studies dexmedetomidine was administered over 2–5 minutes, it is now common practice at our institution to administer dexmedetomidine as a rapid (less than 5 seconds) IV bolus. We therefore designed a descriptive study in pediatric heart transplant patients to invasively measure systemic and pulmonary hemodynamics as well as CO during cardiac catheterization.
Methods
The study was approved by the IRB of the University of Pittsburgh and parental informed consent was obtained before the procedure. All patients were undergoing routine surveillance cardiac catheterization and endomyocardial biopsies as a part of their post-heart transplant medical management. All patients younger than or equal to 10 years of age and weighing 40 kg or less were eligible for enrollment. Patients were excluded if they had received dexmedetomidine in the preceding week or had an allergy to dexmedetomidine. Patients were allowed clear fluids up to 2 hours before surgery and received IV or oral premedication of midazolam as needed. After standard ASA monitors were placed, induction of general anesthesia was achieved either by inhaled (sevoflurane, 40% oxygen, 60% nitrous oxide) or IV (propofol, 100% oxygen) methods. After induction, tracheal intubation was facilitated with rocuronium (1mg/kg). Anesthesia was maintained with approximately 1 minimum alveolar concentration of isoflurane in room air and fentanyl (1mcg/kg) was administered just before the arterial and venous catheter (internal jugular vein and femoral artery) insertions. At the completion of the planned cardiac catheterization (approximately 1 hour after induction of anesthesia), and with the end-tidal isoflurane concentration of 1.2 and the Fi02 at 1.0, baseline values of HR, systolic blood pressure (SBP), diastolic blood pressure (DBP), central venous pressure (CVP), systolic pulmonary artery pressure, diastolic pulmonary artery pressure (dPAP), pulmonary artery wedge pressure (wPAP) and three thermodilution COs were recorded using indwelling catheters. The SVR and pulmonary vascular resistance (PVR) were calculated from these values. After these baseline measurements, a rapid IV bolus dose of either 0.25 mcg/kg or 0.5 mcg/kg dexmedetomidine was administered over 5 seconds. The dose administered to each patient was determined by alternating the dose to sequentially enrolled patients, i.e., the first patient enrolled received 0.25 mcg/kg, the second patient received 0.5 mcg/kg, the third 0.25 mcg/kg, etc. We successfully enrolled every available patient. The HR and systolic and pulmonary blood pressures were recorded continuously but only their 1 and 5 minute values were recorded for analysis while the wPAP and CO measurements were only taken at the 1 minute (peak effect) and at the 5 minute time point after the IV administration of dexmedetomidine. The 5 minute time point was chosen from preliminary studies that showed that all arterial blood pressure measurements had returned to baseline by this time. Because the hemodynamic effects were so transient, only one thermodilution CO was done at the 1 minute time point. All measurements were taken using the indwelling catheters and recorded on the Phillips Witt Hemodynamic System. At the end of the procedure the groin catheters were removed and the patient was awakened and admitted to the postanesthesia care unit for recovery.
Statistical analysis was performed using SPSS 16.0 for Windows (SPSS Inc., Chicago, Illinois). Values are presented as mean ± standard deviation (SD). For demographic data, a t-test was used with a p-value ≤ 0.05 indicating significance. For physiologic data, the graphs are presented with independent variables of either time (time 0, 1 minute and 5 minutes after intervention) or dose (0.25 mcg/kg and 0.5 mcg/kg). Because this study was purely descriptive, no other statistical tests were performed and the complete dataset for each patient is presented in the results.
Results
Twelve children were enrolled in the study. Six received 0.25 mcg/kg and 6 received 0.5 mcg/kg. No significant differences were found in baseline demographic characteristics between the two dosage groups (Table 1). The average values of the hemodynamic variables as functions of both dose and time are presented in Table 2.
Table 1.
Demographic data of all study patients.
| 0.25mcg/kg | 0.5mcg/kg | |
|---|---|---|
| Total number | 6 | 6 |
| Age (mo) | 66 ± 30 | 82 ± 23 |
| Weight (kg) | 20 ± 5 | 22 ± 1 |
| BSA (m2) | 0.79 ± 0.15 | 0.86 ± 0.10 |
| Time post tranplant (mo) | 42 ± 20 | 57 ± 38 |
| Gender - Male | 3 | 4 |
| - Female | 3 | 2 |
Data represented as an average ± SD. p=NS for all comparisons
BSA = body surface area
Table 2.
Hemodynamic responses to dexmedetomidine.
| 0.25mcg/kg | 0.5mcg/kg | |||||
|---|---|---|---|---|---|---|
| Baseline | 1 min | 5 min | Baseline | 1 min | 5 min | |
| SBP (mmHg) | 93 ± 4 | 110 ± 5 | 89 ± 2 | 96 ± 7 | 122 ± 6 | 93 ± 4 |
| DBP (mmHg) | 52 ± 3 | 67 ± 4 | 50 ± 2 | 56 ± 8 | 80 ± 9 | 54 ± 5 |
| HR (beats/min) | 92 ± 7 | 81 ± 5 | 86 ± 6 | 91 ± 5 | 80 ± 3 | 75 ± 4 |
| sPAP (mmHg) | 20 ± 1 | 22 ± 1 | 19 ± 2 | 21 ± 0.3 | 25 ± 1 | 21 ± 1 |
| dPAP (mmHg) | 11 ± 1 | 12 ± 2 | 10 ± 1 | 12 ± 1 | 13 ± 1 | 11 ± 1 |
| wPAP (mmHg) | 10 ± 1.0 | 12 ± 0.7 | 10 ± 0.8 | 12 ± 0.8 | 14 ± 0.9 | 12 ± 0.8 |
| CVP (mmHg) | 6 ± 0.8 | 6 ± 0.7 | 6 ± 0.7 | 5 ± 0.8 | 6 ± 1 | 6 ± 1 |
| CO (L/min) | 3.2 ± 0.1 | 3.0 ± 0.2 | 3.1 ± 0.2 | 3.3 ± 0.2 | 2.8 ± 0.3 | 3.2 ± 0.7 |
| SVR (dynes*sec/cm^5) | 1528 ± 92 | 2186 ± 238 | 1531 ± 125 | 1703 ± 287 | 2624 ± 272 | 1643 ± 228 |
| PVR (dynes*sec/cm^5) | 129 ± 8.8 | 141 ± 18 | 124 ±15 | 95 ± 19 | 125 ± 29 | 78 ± 10 |
SBP = systolic blood pressure
DBP = diastolic blood pressure
HR = heart rate
sPAP = systolic pulmonary artery pressure
dPAP = diastolic pulmonary artery pressure
CVP = central venous pressure
CO = cardiac output
SVR = systemic vascular resistance
PVR = pulmonary vascular resistance
Data represented as an average ± SD.
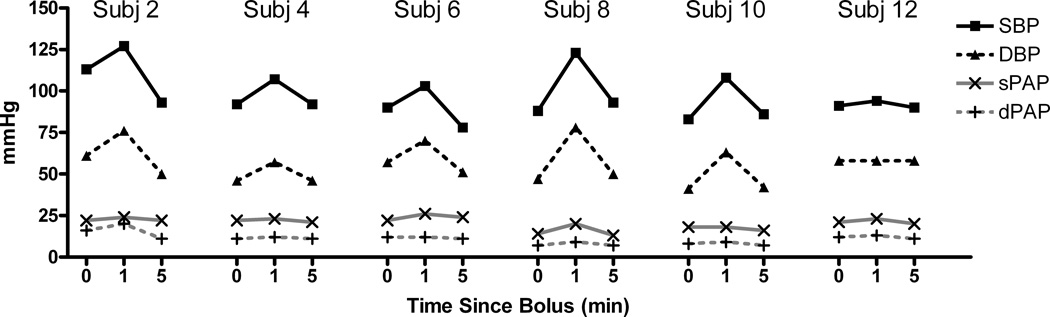
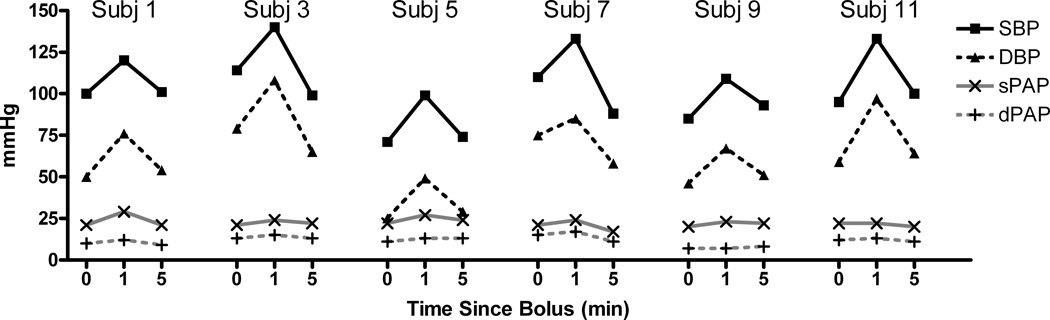
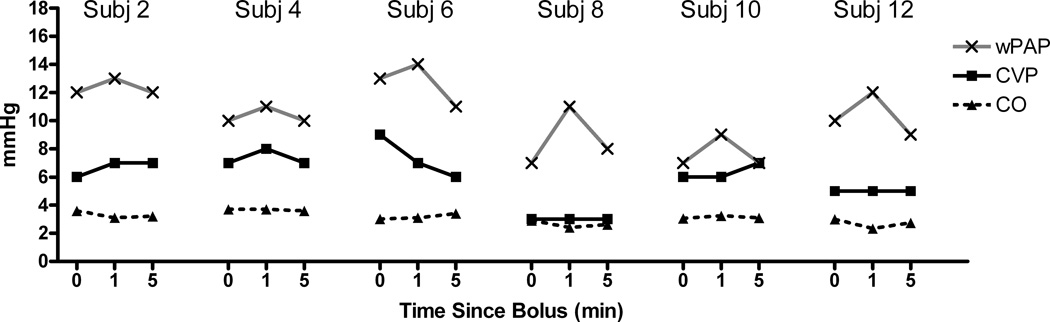
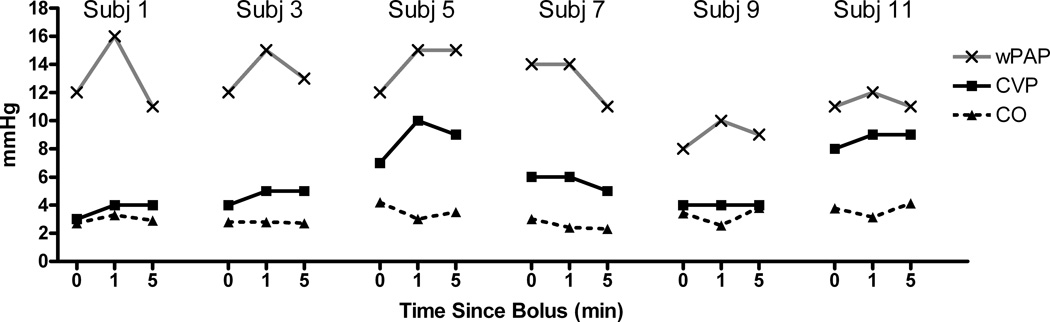
In every child SBP and DBP increased at 1 minute and then decreased to approximately the baseline value at 5 minutes for both the 0.25 mcg/kg (Figure 1) and the 0.5 mcg/kg (Figure 2) doses, although it was nearly imperceptible in subject 12 (0.25 mcg/kg). There was less change in pulmonary arterial pressures; although in those children in whom there was any appreciable change (e.g., subject 2), the small increase at 1 minute was transient and not observed at 5 minutes. There was a transient increase in wPAP at 1 minute, which returned to baseline at 5 minutes (Figures 3 and 4 for the 0.25 and 0.5 mcg/kg doses, respectively). There was no clear pattern to the very modest changes in CVP and CO (Figures 3 and 4), and none of the changes were of clinical concern.
Figure 1.
Systemic pressures and pulmonary pressures over time for dexmedetomidine dosed at 0.25 mcg/kg, showing substantial changes at 1 min that seem to dissipate at the 5 min time point.
Figure 2.
Systemic pressures and pulmonary pressures over time for dexmedetomidine dosed at 0.5 mcg/kg, again showing substantial changes at 1 min that seem to dissipate at the 5 min time point.
Figure 3.
Central venous pressure (CVP), cardiac output (CO), and pulmonary artery wedge pressure (wPAP) changes over time for dexmedetomidine dosed at 0.25 mcg/kg.
Figure 4.
Central venous pressure (CVP), cardiac output (CO), and pulmonary artery wedge pressure (wPAP) changes over time for dexmedetomidine dosed at 0.5 mcg/kg.
Table 3 shows the percent change from baseline at one and five minutes for the various hemodynamic variables measured. Consistent with the graphs, the hemodynamic changes were transient and did not appear to be of any clinical concern. However the percent increase in DBP appeared to be larger than the percent increase in SBP for both doses and furthermore, the percent increase in DBP was larger than the percent increase in diastolic pulmonary artery pressure for both doses at the 1 minute time point.
Table 3.
Hemodynamic changes represented by the percent change from baseline at 1 min and 5 min for the two different concentrations of dexmedetomidine administered.
| 1 min | 5 min | |||
|---|---|---|---|---|
| 0.25 mcg/kg | 0.5 mcg/kg |
0.25 mcg/kg |
0.5 mcg/kg | |
| SBP (%) | 19 ± 13 | 29 ± 9 | −4 ± 9 | −2 ± 12 |
| DBP (%) | 32 ± 24 | 51 ± 28 | −3 ± 9 | 0 ± 16 |
| HR (%) | −12 ± 4 | −12 ± 7 | −6 ± 7 | −17 ± 7 |
| sPAP (%) | 14 ± 15 | 17 ± 13 | −3 ± 7 | −1 ± 11 |
| dPAP (%) | 14 ± 11 | 10 ± 7 | −10 ± 12 | −4 ± 15 |
| wPAP (%) | 22 ± 19 | 19 ± 12 | −2 ± 10 | 2 ± 16 |
| CVP (%) | 1 ± 14 | 18 ± 18 | 0 ± 18 | 13 ± 19 |
| CO (%) | −8 ± 12 | −11 ± 19 | −3 ± 9 | −3 ± 14 |
| SVR (%) | 42 ± 26 | 69 ± 46 | 0 ± 14 | 0 ± 14 |
| PVR (%) | 9 ± 24 | 41 ± 61 | −4 ± 25 | −4 ± 44 |
SBP = systolic blood pressure
DBP = diastolic blood pressure
HR = heart rate
sPAP = systolic pulmonary artery pressure
dPAP diastolic pulmonary artery pressure
CVP = central venous pressure
CO = cardiac output
SVR = systemic vascular resistance
PVR = pulmonary vascular resistance
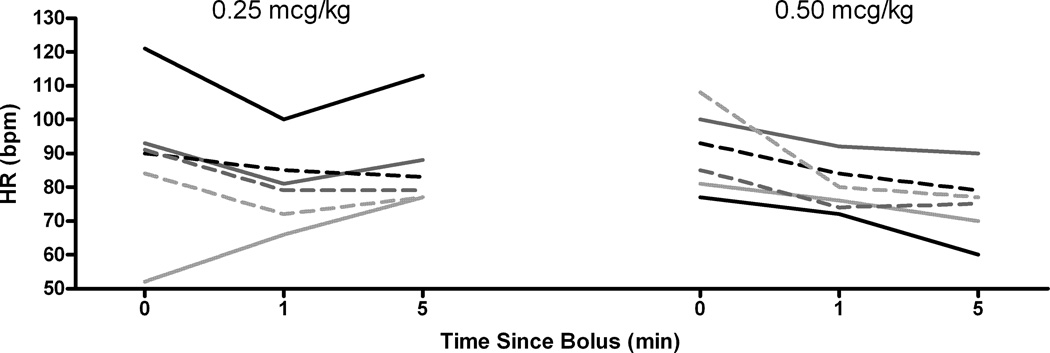
HR was the only hemodynamic variable for which visual inspection of the data suggested a different pattern of response for the 0.25 and 0.5 mcg/kg doses. One minute after the bolus HR decreased in every child except for subject 6, who was inexplicably bradycardic at baseline. The HR response at 1 minute was indistinguishable between the two groups (Figure 5 and Table 3). However, at 5 minutes the HR in children receiving 0.25 mcg/kg returned towards baseline, other than for subject 6, in whom the baseline bradycardia simply appeared to be resolving. A different pattern was seen in subjects receiving 0.5 mcg/kg. In these children there was further slowing of the HR 5 minutes after the dose.
Figure 5.
Heart rate (HR) changes over time for dexmedetomidine at both doses, highlighting the increase back to baseline in the 0.25 mcg/kg dose, while the HR remains slow for the 0.5 mcg/kg dose.
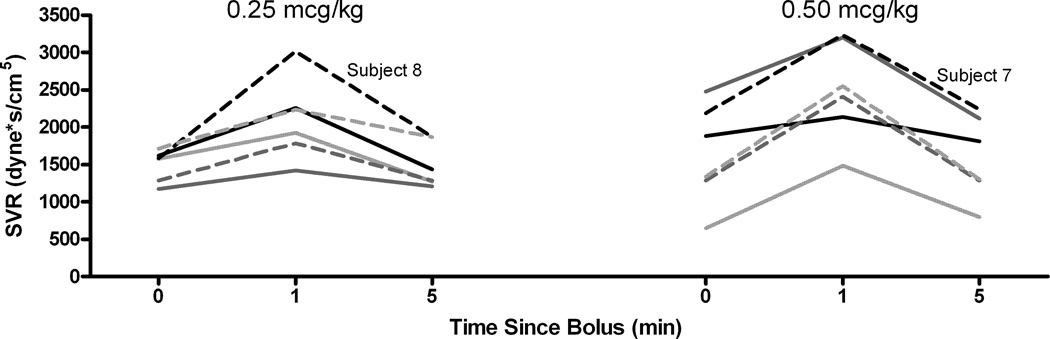
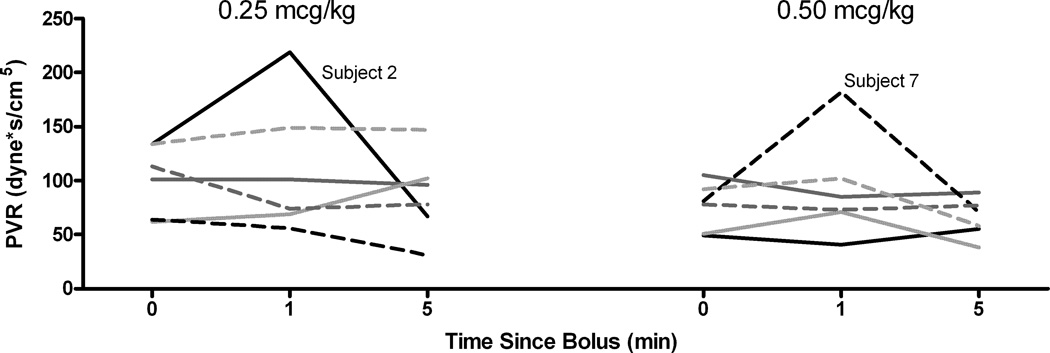
SVR followed the same pattern as arterial blood pressure. In every child SVR increased above baseline at 1 minute and returned to approximately the baseline value at 5 minutes (Figure 6). PVR, with the exception of one patient in each group, did not show an overall change from baseline (Figure 7) at any time point or dose.
Figure 6.
Systemic vascular resistance over time for both dexmedetomidine doses, again showing the increase at 1 min with a decrease back to baseline at 5 minutes.
Figure 7.
Pulmonary resistance over time for both dexmedetomidine doses. Little change is seen across time with either dose, except for one patient in each group.
The pulmonary to systemic blood pressure ratios were investigated as described by Lazol et al 27 and were not different from baseline in either of the 2 groups at any of the time points (Table 4).
Table 4.
Pulmonary/Systemic Pressure Ratios After Dexmedetomidine Bolus
| 0.25mcg/kg | 0.5mcg/kg | |||||
|---|---|---|---|---|---|---|
| Baseline | 1 min | 5 min | Baseline | 1 min | 5 min | |
| sPAP/ SBP ratio (%) | 21 ± 1 | 21 ± 1 | 22 ± 2 | 23 ± 2 | 21 ± 2 | 23 ± 2 |
| dPAP/DBP ratio (%) | 21 ± 2 | 19 ± 2 | 20 ± 2 | 23 ± 5 | 17 ± 2 | 22 ± 5 |
P=NS for all comparisons
SBP = systolic blood pressure
DBP = diastolic blood pressure
sPAP = systolic pulmonary artery pressure
dPAP = diastolic pulmonary artery pressure
Discussion
The rapid bolus administration of dexmedetomidine results in a transient but significant increase in systemic and pulmonary pressure. All hemodynamic measurements, except for the HR in the 0.5 mcg/kg group, returned to baseline by 5 minutes. In the systemic circulation, diastolic pressures increase more than systolic pressures and the increase in pressures and resistance are greater in the systemic circulation than in the pulmonary system.
Dexmedetomidine, a highly selective α2-adrenergic agonist, has both sedative and analgesic properties. Currently dexmedetomidine has Food and Drug Administration approval for use in intensive care unit sedation and procedural sedation in adult populations. Despite this relatively limited Food and Drug Administration approval, dexmedetomidine has been used widely and with relatively few adverse reactions in pediatric anesthesia and critical care medicine. Although infusions of the drug have been well reported, the acute hemodynamic effects after a rapid bolus administration have not been described. At our institution, rapid IV bolus administration occurs frequently when treating emergence delirium in children. The post-heart transplant pediatric patient undergoing routine surveillance cardiac catheterization provided a unique opportunity to be able to invasively measure CO and both systemic and pulmonary pressures and resistances both before and after IV bolus dexmedetomidine administration.
In both human and animal models, dexmedetomidine produces a biphasic blood pressure effect: an initial but transient increase in blood pressure followed by a long-lasting hypotensive effect23,28. The initial increase in SBP is thought to be from vasoconstriction secondary to stimulation of peripheral postsynaptic α-2B adrenergic receptors in the vascular smooth muscle. The long-lasting stabilization of blood pressure and HR at values slightly below the baseline is most likely the result of activation of central presynaptic α-2A adrenergic receptors resulting in sympatholysis.
In a study by Ebert et al., 10 healthy volunteers were subject to increasing concentrations of a dexmedetomidine infusion22. Initially, at the low infusion rates, the classic decrease in blood pressure and HR was documented; however, as the infusions increased to supra-clinical doses, Ebert et al. observed significant incremental increases in the systemic and pulmonary pressures, SVR, PVR, wPAP and CVP. The initial increases in systemic blood pressure, pulmonary blood pressures, SVR and PVR were most likely caused by peripheral vasoconstriction resulting from stimulation of postsynaptic α2B adrenergic receptors in the vascular smooth muscle 29–32. From our data it appears that DBP is affected more than SBP (30–50% vs. 20–30% respectively) in post-cardiac transplant patients and that these transient increases in pressures and vascular resistances are greater in the systemic system than in the pulmonary system. Although there are theoretical concerns regarding the safety of dexmedetomidine use in patients with pulmonary hypertension22,29,33, the pulmonary to systemic blood pressure ratio (often used to describe the severity of pulmonary hypertension, with pulmonary pressures around 20 – 25% of the systemic pressure being normal) suggests that dexmedetomidine has less of an effect on PVR when compared with SVR (Table 4). The apparent plateau effect observed in the pulmonary vasculature suggests that the density of α2B adrenergic receptors in the pulmonary bed may be reduced compared to the systemic vasculature or the increase in pulmonary pressure reflects the increase in systemic blood pressure rather than receptor-mediated vasoconstriction.
It should be noted that in our patient population with their denervated transplanted hearts, the loss of reflex control of HR may actually enhance the increased blood pressure effects of vasoconstrictive agents, such as norepinephrine or in this case dexmedetomidine 34. Therefore, the increases in blood pressures resulting from the rapid IV bolus administration of dexmedetomidine might be somewhat mitigated in patients with an intact autonomic reflex.
There are α-2 adrenergic and imidazoline I1 receptors in human heart tissue 35,36. While the central effects on these receptors are known, the effects that dexmedetomidine might have on these myocardial receptors is unclear. Flacke et al. 37 showed in a isolated dog heart model that pharmacologic doses of dexmedetomidine resulted in a direct release of catecholamines from cardiac stores. This phenomenon may occur with rapid IV bolus administration which might account for the increase in blood pressure, SVR and PVR.
In our study the acute decrease in HR after dexmedetomidine administration in the transplant patient with a denervated heart was unexpected. In denervated dog model experiments by Flacke et al. 37,38 no changes in HR after the administration of dexmedetomidine were reported. Xu et al., in a rabbit model, defined the role of the autonomic nervous system in the hemodynamic effects of dexmedetomidine30. They concluded that the initial HR decrease after a dexmedetomidine bolus is the result of activation of the baroreceptor reflex and that the sustained HR decrease is mediated via the central sympathetic depression, i.e., inhibition of the sympathetic and activation of the parasympathetic nervous system. The role of the myocardial α2-adrenergic and imidazoline I1 receptors is unclear as one would expect the HR to increase if the stimulation of these myocardial receptors resulted in a direct release of catecholamines from cardiac stores. Hammer et al. studied healthy children undergoing radiofrequency ablation for supraventricular tachycardia using propofol and ketamine as their anesthetic 39. Though none of the patients in the study by Hammer et al. developed clinically significant bradycardia, dexmedetomidine significantly depressed sinus and atrioventricular nodal function. Our findings of a sustained decrease in HR in these patients with denervated hearts suggest that further investigation is needed to clarify the potential direct interaction of dexmedetomidine on sinus node activity.
A limitation of our study is our small sample size; larger patient numbers may have shown a dose effect, particularly in the systemic circulation, and would have allowed statistical testing. Nonetheless, we feel these data provide the background for a proper power analysis and large scale study. Because of the very transient nature of these hemodynamic changes, a further potential limitation was the use of a thermodilution CO measurement technique instead of a continuous technique which limited us to one CO measurement at the 1 minute time interval. A further possible limitation of the study might be that no data were recorded while the subjects’ lungs were being ventilated with room air and the pulmonary vasodilator effect of 100% oxygen may have influenced the results.
This rapid bolus administration method of dexmedetomidine should not be extrapolated to either adults or healthy children because there have been reports of significant hypotension, bradycardia and sinus arrests with its administration1,5,40,41. It should be further noted that there have been case reports of significant bradycardia in pediatric patients with congenital heart disease and on cardiac medication such as digoxin 42. It may be that children with their relatively more rapid HRs, decreased incidence of sinus node dysfunction, and decreased use of HR-decreasing medications such as beta blockers and digoxin allow for more liberal use of dexmedetomidine.
In conclusion, the rapid IV bolus administration of 0.25 and 0.5mcg/kg of dexmedetomidine in this small sample of children having undergone heart transplants was clinically well tolerated, although it resulted in a transient but significant increase in systemic and pulmonary pressure and a decrease in HR. In the systemic system there is a larger percent increase in the diastolic pressures than the systolic pressures and furthermore these transient increases in pressures were more pronounced in the systemic system than in the pulmonary system.
Acknowledgements
This publication was made possible by Grant Number UL1 RR024153 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/.
Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp."
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: None except for Dr Chrysostomou (Consultant for Hospira)
References
- 1.Venn RM, Bradshaw CJ, Spencer R, Brealey D, Caudwell E, Naughton C, Vedio A, Singer M, Feneck R, Treacher D, Willatts SM, Grounds RM. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–1142. doi: 10.1046/j.1365-2044.1999.01114.x. [DOI] [PubMed] [Google Scholar]
- 2.Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988;150:9–14. doi: 10.1016/0014-2999(88)90744-3. [DOI] [PubMed] [Google Scholar]
- 3.McDonald T, Hoffman WE, Berkowitz R, Cunningham F, Cooke B. Heart rate variability and plasma catecholamines in patients during opioid detoxification. J Neurosurg Anesthesiol. 1999;11:195–199. doi: 10.1097/00008506-199907000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Belleville JP, Ward DS, Bloor BC, Maze M. Effects of intravenous dexmedetomidine in humans. I. Sedation, ventilation, and metabolic rate. Anesthesiology. 1992;77:1125–1133. doi: 10.1097/00000542-199212000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Peden CJ, Cloote AH, Stratford N, Prys-Roberts C. The effect of intravenous dexmedetomidine premedication on the dose requirement of propofol to induce loss of consciousness in patients receiving alfentanil. Anaesthesia. 2001;56:408–413. doi: 10.1046/j.1365-2044.2001.01553.x. [DOI] [PubMed] [Google Scholar]
- 6.Munro HM, Tirotta CF, Felix DE, Lagueruela RG, Madril DR, Zahn EM, Nykanen DG. Initial experience with dexmedetomidine for diagnostic and interventional cardiac catheterization in children. Paediatr Anaesth. 2007;17:109–112. doi: 10.1111/j.1460-9592.2006.02031.x. [DOI] [PubMed] [Google Scholar]
- 7.Isik B, Arslan M, Tunga AD, Kurtipek O. Dexmedetomidine decreases emergence agitation in pediatric patients after sevoflurane anesthesia without surgery. Paediatr Anaesth. 2006;16:748–753. doi: 10.1111/j.1460-9592.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- 8.Carroll CL, Krieger D, Campbell M, Fisher DG, Comeau LL, Zucker AR. Use of dexmedetomidine for sedation of children hospitalized in the intensive care unit. J Hosp Med. 2008;3:142–147. doi: 10.1002/jhm.282. [DOI] [PubMed] [Google Scholar]
- 9.Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:813–820. doi: 10.1097/00000542-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Triltsch AE, Welte M, von Homeyer P, Grosse J, Genahr A, Moshirzadeh M, Sidiropoulos A, Konertz W, Kox WJ, Spies CD. Bispectral index-guided sedation with dexmedetomidine in intensive care: a prospective, randomized, double blind, placebo-controlled phase II study. Crit Care Med. 2002;30:1007–1014. doi: 10.1097/00003246-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Rosen DA, Daume JT. Short duration large dose dexmedetomidine in a pediatric patient during procedural sedation. Anesth Analg. 2006;103:68–69. doi: 10.1213/01.ane.0000216289.52261.5e. table of contents. [DOI] [PubMed] [Google Scholar]
- 12.Barton KP, Munoz R, Morell VO, Chrysostomou C. Dexmedetomidine as the primary sedative during invasive procedures in infants and toddlers with congenital heart disease. Pediatr Crit Care Med. 2008;9:612–615. doi: 10.1097/PCC.0b013e31818d320d. [DOI] [PubMed] [Google Scholar]
- 13.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–952. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 14.Nelson LE, Lu J, Guo T, Saper CB, Franks NP, Maze M. The alpha2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology. 2003;98:428–436. doi: 10.1097/00000542-200302000-00024. [DOI] [PubMed] [Google Scholar]
- 15.Khan ZP, Ferguson CN, Jones RM. alpha-2 and imidazoline receptor agonists. Their pharmacology and therapeutic role. Anaesthesia. 1999;54:146–165. doi: 10.1046/j.1365-2044.1999.00659.x. [DOI] [PubMed] [Google Scholar]
- 16.Kamisaki Y, Ishikawa T, Takao Y, Omodani H, Kuno N, Itoh T. Binding of [3H]p-aminoclonidine to two sites, alpha 2-adrenoceptors and imidazoline binding sites: distribution of imidazoline binding sites in rat brain. Brain Res. 1990;514:15–21. doi: 10.1016/0006-8993(90)90430-j. [DOI] [PubMed] [Google Scholar]
- 17.Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283:R287–R295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- 18.Mason KP, Zgleszewski SE, Prescilla R, Fontaine PJ, Zurakowski D. Hemodynamic effects of dexmedetomidine sedation for CT imaging studies. Paediatr Anaesth. 2008;18:393–402. doi: 10.1111/j.1460-9592.2008.02451.x. [DOI] [PubMed] [Google Scholar]
- 19.Koroglu A, Teksan H, Sagir O, Yucel A, Toprak HI, Ersoy OM. A comparison of the sedative, hemodynamic, and respiratory effects of dexmedetomidine and propofol in children undergoing magnetic resonance imaging. Anesth Analg. 2006;103:63–67. doi: 10.1213/01.ANE.0000219592.82598.AA. table of contents. [DOI] [PubMed] [Google Scholar]
- 20.Koroglu A, Demirbilek S, Teksan H, Sagir O, But AK, Ersoy MO. Sedative, haemodynamic and respiratory effects of dexmedetomidine in children undergoing magnetic resonance imaging examination: preliminary results. Br J Anaesth. 2005;94:821–824. doi: 10.1093/bja/aei119. [DOI] [PubMed] [Google Scholar]
- 21.Mason KP, Zurakowski D, Zgleszewski SE, Robson CD, Carrier M, Hickey PR, Dinardo JA. High dose dexmedetomidine as the sole sedative for pediatric MRI. Paediatr Anaesth. 2008;18:403–411. doi: 10.1111/j.1460-9592.2008.02468.x. [DOI] [PubMed] [Google Scholar]
- 22.Ebert TJ, Hall JE, Barney JA, Uhrich TD, Colinco MD. The effects of increasing plasma concentrations of dexmedetomidine in humans. Anesthesiology. 2000;93:382–394. doi: 10.1097/00000542-200008000-00016. [DOI] [PubMed] [Google Scholar]
- 23.Bloor BC, Ward DS, Belleville JP, Maze M. Effects of intravenous dexmedetomidine in humans. II. Hemodynamic changes. Anesthesiology. 1992;77:1134–1142. doi: 10.1097/00000542-199212000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Guler G, Akin A, Tosun Z, Ors S, Esmaoglu A, Boyaci A. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15:762–766. doi: 10.1111/j.1460-9592.2004.01541.x. [DOI] [PubMed] [Google Scholar]
- 25.Ibacache ME, Munoz HR, Brandes V, Morales AL. Single-dose dexmedetomidine reduces agitation after sevoflurane anesthesia in children. Anesth Analg. 2004;98:60–63. doi: 10.1213/01.ANE.0000094947.20838.8E. table of contents. [DOI] [PubMed] [Google Scholar]
- 26.Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. 2005;15:1098–1104. doi: 10.1111/j.1460-9592.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 27.Lazol JP, Lichtenstein SE, Jooste EH, Shiderly D, Kudchadker NA, Tatum GH, Orr RA, Wearden PD, Morell VO, Munoz RA, Chrysostomou C. Effect of dexmedetomidine on pulmonary artery pressure after congenital cardiac surgery: A pilot study. Pediatr Crit Care Med. doi: 10.1097/PCC.0b013e3181ceae7d. [DOI] [PubMed] [Google Scholar]
- 28.Kallio A, Scheinin M, Koulu M, Ponkilainen R, Ruskoaho H, Viinamaki O, Scheinin H. Effects of dexmedetomidine, a selective alpha 2-adrenoceptor agonist, on hemodynamic control mechanisms. Clin Pharmacol Ther. 1989;46:33–42. doi: 10.1038/clpt.1989.103. [DOI] [PubMed] [Google Scholar]
- 29.Tobias JD. Dexmedetomidine: applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–131. doi: 10.1097/01.PCC.0000257100.31779.41. [DOI] [PubMed] [Google Scholar]
- 30.Xu H, Aibiki M, Seki K, Ogura S, Ogli K. Effects of dexmedetomidine, an alpha2-adrenoceptor agonist, on renal sympathetic nerve activity, blood pressure, heart rate and central venous pressure in urethane-anesthetized rabbits. J Auton Nerv Syst. 1998;71:48–54. doi: 10.1016/s0165-1838(98)00061-7. [DOI] [PubMed] [Google Scholar]
- 31.Drew GM, Whiting SB. Evidence for two distinct types of postsynaptic alpha-adrenoceptor in vascular smooth muscle in vivo. Br J Pharmacol. 1979;67:207–215. doi: 10.1111/j.1476-5381.1979.tb08668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nichols AJ, Motley ED, Ruffolo RR., Jr Effect of pertussis toxin treatment on postjunctional alpha-1 and alpha-2 adrenoceptor function in the cardiovascular system of the pithed rat. J Pharmacol Exp Ther. 1989;249:203–209. [PubMed] [Google Scholar]
- 33.Kastner SB, Kull S, Kutter AP, Boller J, Bettschart-Wolfensberger R, Huhtinen MK. Cardiopulmonary effects of dexmedetomidine in sevoflurane-anesthetized sheep with and without nitric oxide inhalation. Am J Vet Res. 2005;66:1496–1502. doi: 10.2460/ajvr.2005.66.1496. [DOI] [PubMed] [Google Scholar]
- 34.Regitz V, Bossaller C, Strasser R, Schuler S, Hetzer R, Fleck E. Myocardial catecholamine content after heart transplantation. Circulation. 1990;82:620–623. doi: 10.1161/01.cir.82.2.620. [DOI] [PubMed] [Google Scholar]
- 35.El-Ayoubi R, Gutkowska J, Regunathan S, Mukaddam-Daher S. Imidazoline receptors in the heart: characterization, distribution, and regulation. J Cardiovasc Pharmacol. 2002;39:875–883. doi: 10.1097/00005344-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 36.Mukaddam-Daher S, Gutkowska J. Imidazoline receptors in the heart: a novel target and a novel mechanism of action that involves atrial natriuretic peptides. Braz J Med Biol Res. 2004;37:1239–1245. doi: 10.1590/s0100-879x2004000800015. [DOI] [PubMed] [Google Scholar]
- 37.Flacke WE, Flacke JW, Blow KD, McIntee DF, Bloor BC. Effect of dexmedetomidine, an alpha 2-adrenergic agonist, in the isolated heart. J Cardiothorac Vasc Anesth. 1992;6:418–423. doi: 10.1016/1053-0770(92)90006-s. [DOI] [PubMed] [Google Scholar]
- 38.Flacke JW, Flacke WE, Bloor BC, McIntee DF. Hemodynamic effects of dexmedetomidine, an alpha 2-adrenergic agonist, in autonomically denervated dogs. J Cardiovasc Pharmacol. 1990;16:616–623. doi: 10.1097/00005344-199010000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Hammer GB, Drover DR, Cao H, Jackson E, Williams GD, Ramamoorthy C, Van Hare GF, Niksch A, Dubin AM. The effects of dexmedetomidine on cardiac electrophysiology in children. Anesth Analg. 2008;106:79–83. doi: 10.1213/01.ane.0000297421.92857.4e. table of contents. [DOI] [PubMed] [Google Scholar]
- 40.Ingersoll-Weng E, Manecke GR, Jr, Thistlethwaite PA. Dexmedetomidine and cardiac arrest. Anesthesiology. 2004;100:738–739. doi: 10.1097/00000542-200403000-00040. [DOI] [PubMed] [Google Scholar]
- 41.Khan ZP, Munday IT, Jones RM, Thornton C, Mant TG, Amin D. Effects of dexmedetomidine on isoflurane requirements in healthy volunteers. 1: Pharmacodynamic and pharmacokinetic interactions. Br J Anaesth. 1999;83:372–380. doi: 10.1093/bja/83.3.372. [DOI] [PubMed] [Google Scholar]
- 42.Berkenbosch JW, Tobias JD. Development of bradycardia during sedation with dexmedetomidine in an infant concurrently receiving digoxin. Pediatr Crit Care Med. 2003;4:203–205. doi: 10.1097/01.PCC.0000059737.86673.28. [DOI] [PubMed] [Google Scholar]