Abstract
Objective
Dopamine (DA) is critical for motor performance, motor learning, and corticostriatal plasticity. The relationship between motor performance and learning, and the role of DA in the mediation of them, however, remain unclear.
Methods
To examine this question, we took advantage of PITx3-deficient mice (aphakia mice), in which DA in the dorsal striatum is reduced by 90%. PITx3-deficient mice do not display obvious motor deficits in their home cage, but are impaired in motor tasks that require new motor skills. We used the accelerating rotarod as a motor learning task.
Results
We show that the deficiency in motor skill learning in PITx3(−/−) is dramatic and can be rescued with levodopa treatment. In addition, cessation of levodopa treatment after acquisition of the motor skill does not result in an immediate drop in performance. Instead, there is a gradual decline of performance that lasts for a few days, which is not related to levodopa pharmacokinetics. We show that this gradual decline is dependent on the retesting experience.
Interpretation
This observation resembles the long-duration response to levodopa therapy in its slow buildup of improvement after the initiation of therapy and gradual degradation. We hypothesize that motor learning may play a significant, underappreciated role in the symptomatology of Parkinson disease as well as in the therapeutic effects of levodopa. We suggest that the important, yet enigmatic long-duration response to chronic levodopa treatment is a manifestation of rescued motor learning.
Dopamine (DA) plays an important role in motor performance and motor learning. Loss of nigrostriatal DA innervation leads to Parkinson disease (PD). In rodent models of PD, injections of 6-hydroxydopamine1 or methylphenyltetrahydropyridine2 or genetic elimination of DA3 produce motor performance deficiencies similar to those in PD. Nigrostriatal DA is critical for motor learning as well,4 – 6 presumably through modulation of synaptic plasticity in the striatum.7 In vivo recordings during rotarod motor learning task indicates that activity in the dorsal striatum changes during different phases of learning.8 In addition, genetic disruption of dorsal striatal synaptic plasticity leads to impairments in motor learning.9
Despite considerable evidence that DA mediates both motor performance and learning, isolating these separate functions of DA, and the relationship between them, remains challenging as manipulations of DA, such as lesion models, often severely impair motor performance,1,3 obscuring potential effects on motor learning. The PITx3-deficient mouse line offers an alternative model of DA denervation that may allow for the investigation of the role of DA in motor learning. Also known as aphakia (ak), these mice display selective nigrostriatal (A9) neuron loss,10,11 resulting in a 90% reduction in dorsal striatal DA. Extensive behavioral testing10,12–16 has indicated that Pitx3-deficient mice show no gross motor impairments, and no abnormalities in reflexes or basic neurological function,10,13,17 but do show mild performance impairments in tasks that require sensorimotor integration and significant deficits in procedural learning.12,14 –16 Some of these deficits can be rescued with levodopa (L-dopa) treatment.14,15
In this study, we use the PITx3-deficient mouse line to investigate whether potential motor learning deficits arising from DA denervation may be dissociated from performance deficits. The line’s responsiveness to L-dopa rescue allows for transient manipulations of DA in the dorsal striatum during different stages of motor learning, permitting a closer examination of learning versus direct performance effects of DA. We show that PITX3-deficient mice exhibit profound impairments in motor learning on the rotarod that can be rescued with L-dopa. On cessation of treatment, however, acquired performance degrades gradually in an experience-dependent manner. This suggests that prior and ongoing learning contributes to observed motor performance, and that DA is critical for not only the expression but also the acquisition and maintenance of learned skills. These data are significant in understanding the long-duration response (LDR) to L-dopa treatment, an important but poorly understood component of L-dopa therapy in PD.
Materials and Methods
Animals
Mice were housed in standard conditions on a 06:00 to 18:00 light cycle with ad libitum food and water. Experiments were carried out during the light cycle. Animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Chicago.
PITx3-Deficient mice
PITx3-deficient (ak) mice are almost completely devoid of tyrosine hydroxylase-positive cells in the substantia nigra pars compacta, and have a 90% reduction of dorsal striatal DA at P0.10,11,13,18,19 The ventral tegmental area is not affected at birth, but exhibits gradual loss of DA neurons.19 No other brain regions are affected,10,18 and the overall morphological and molecular organization of the ak striatum is unaffected.11,18 The PITx3-deficient mice are blind, but blindness does not significantly impact their performance on the task used here. Heterozygote littermates were used as controls, as the mutation is recessive.
Behavior Tests
A computer-controlled rotarod apparatus (Rotamex-5, Columbus Instruments, Columbus, OH) with a rat rod (7cm diameter) was set to accelerate from 4 to 40 revolutions per minute over 300 seconds, and recorded time to fall. Mice received 5 consecutive trials per session, 1 session per day. Rest between trials was approximately 30 seconds. As an alternative motor task (see Results), mice were run on a horizontal treadmill (Digigait, Mouse Specifics, Quincy, MA) moving at a rate of 10cm/s and were provided 5 20-second trials in each session.
Drug Administration
All injections were intraperitoneal at 0.01ml/gram of body weight. L-dopa (3,4-dihydroxy-L-phenylalanine 25mg/kg with 12.5mg/kg benserazide) was administered 1 hour prior to the start of each session, unless otherwise noted. SCH 23390 at 0.1mg/kg and eticlopride at 0.16mg/kg was administered 30 minutes prior to sessions.
HPLC
Immediately after harvest, brains were cut into 1mm sections on an ice-cold dissection plate. Two samples from the dorsal striatum were collected from 2 sections per brain with a biopsy punch (2mm diameter). Samples were homogenized with 0.1M perchloric acid (containing 1 × 10−6M dihydroxybenzoic acid and 100μM ethylenediaminetetraacetic acid). DA content was analyzed by reverse-phase high-performance liquid chromatography (HPLC) with electrochemical detection and calculated using internal standards. Final concentrations of DA were expressed per protein amount. Protein levels were measured by bicinchoninic acid protein assay kit.
Data Analysis
All analysis of statistical significance was done using analysis of variance with a statistical analysis program (Statview, SAS Institute, Cary, NC).
Results
PITx3(−/−) Mice Exhibit Impaired Rotarod Performance That Is Rescued by L-dopa Administration
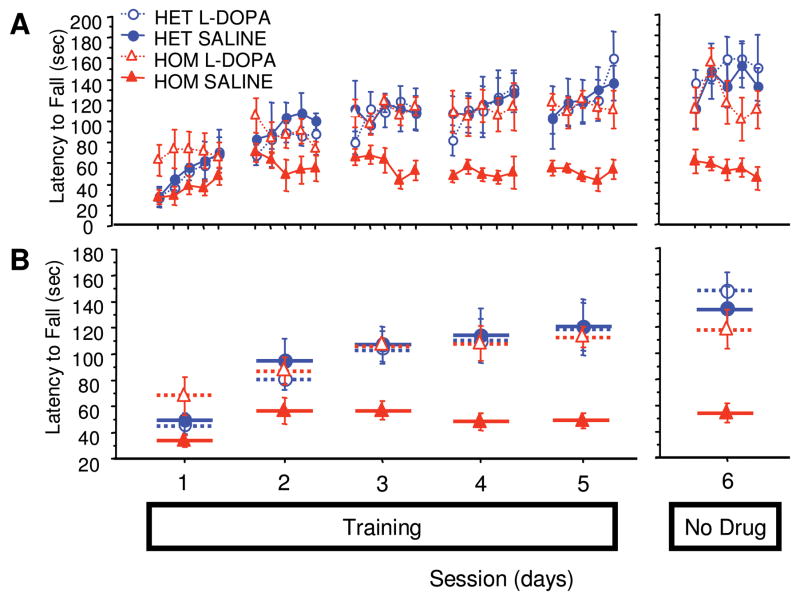
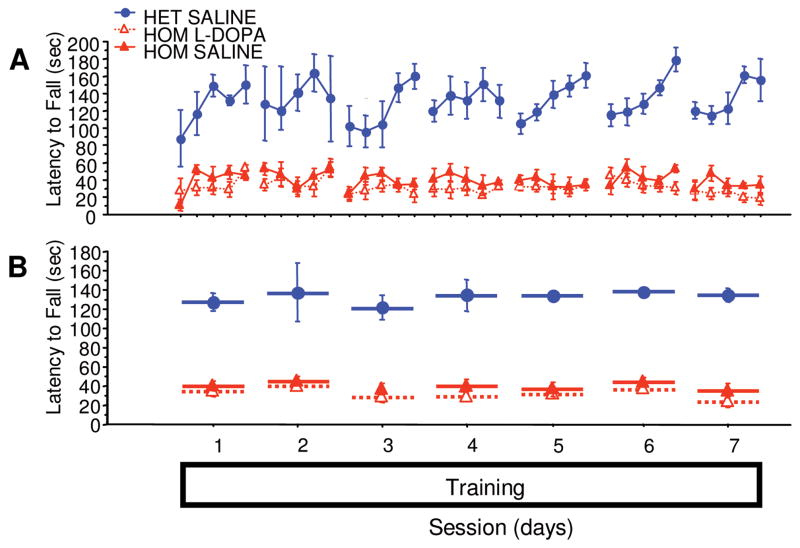
Compared with PITx3(+/−) littermates, PITx3(−/−) mice showed decreased asymptotic performance (Fig 1B, mean of sessions 3–5, F[1,10] = 11.6, p = 0.0067). Control mice exhibited clear between-session improvements, whereas PITx3(−/−) mice, after initial improvement following the first session, showed no between-session improvement (sessions 1–5, genotype × repeated measure, F[4,40] = 8.035, p < 0.0001). When administered L-dopa, PITx3(−/−) mice achieved the same level of asymptotic performance as control mice (mean of sessions 3–5, F[1,10] = 0.057, p = 0.8162) and showed identical between-session improvement (sessions 1–5; genotype/treatment × repeated measure, F[4,40] = 0.846, p = 0.5046). These data indicate that L-dopa can rescue rotarod performance in the PITx3-deficient mice, and more generally, that dorsal striatal DA is required to learn this task.
FIGURE 1.
Rotarod performance with and without L-dopa treatment. Mice were trained on the rotarod with either saline or L-dopa for 5 sessions (sessions 1–5). After a 3-day treatment discontinuation break, the mice were tested without treatment (session 6). (A) Latency to fall in each trial. (B) Average latency to fall during each session. n = 6 per genotype/treatment. HET = heterozygote; HOM = homozygote.
Rotarod Performance Initially Retained after Cessation of L-Dopa Treatment
After the 5th day of training and L-dopa administration, mice received 3 days of rest to eliminate potential residual L-dopa effects and were subsequently tested without treatment. PITX3(−/−) mice that had received training under a regimen of L-dopa performed comparably to their last training day with L-dopa (see Fig 1B, session 5 with L-dopa compared with session 6 without L-dopa, F[1,10] = 0.088, p = 0.7730). This suggests that L-dopa treatment rescues the learning component of this task, since in the absence of L-dopa treatment, performance did not immediately drop to levels comparable to PITX3(−/−) mice treated with saline during training.
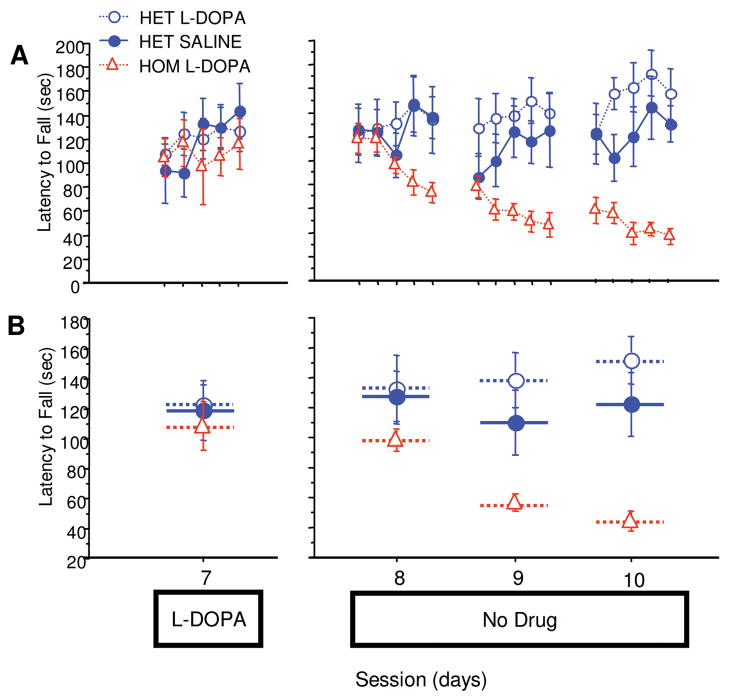
Performance Diminishes Gradually on Cessation of L-dopa Treatment
Animals were given 1 more training day with L-dopa, then a 5-day break, and then were run for 3 consecutive sessions without any treatment. The PITx3(−/−) group treated with L-dopa during training started these sessions at their asymptotic performance level (Fig 2A, session 7 with L-dopa compared with first trial of session 8 without L-dopa, F[1,10] = 0.318, p = 0.5855) and gradually declined in performance across sessions. By the third session, they exhibited the same level of performance as the saline-treated PITx3(−/−) group achieved during training (see Fig 1B, PITx3[−/−] saline-trained session 5 compared with Fig 2B, PITx3[−/−] L-dopa–trained after L-dopa cessation, F[1,10] = 0.350, p = 0.5670). These data suggest that DA is critical for the maintenance of learned motor skills.
FIGURE 2.
Performance after discontinuation of L-dopa. The same mice from Figure 1 were retrained on the rotarod with either saline or L-dopa for 1 session (session 7). After a 5-day treatment discontinuation break, mice were run for 3 sessions without any treatment (sessions 8–10). (A) Latency to fall in each trial. (B) Average latency to fall during each session. n = 6 per genotype/treatment. HET = heterozygote; HOM = homozygote.
Loss of Performance after Cessation of L-Dopa Treatment Is Not Dependent on L-Dopa Pharmacokinetics but Rather on Task-Specific Experience
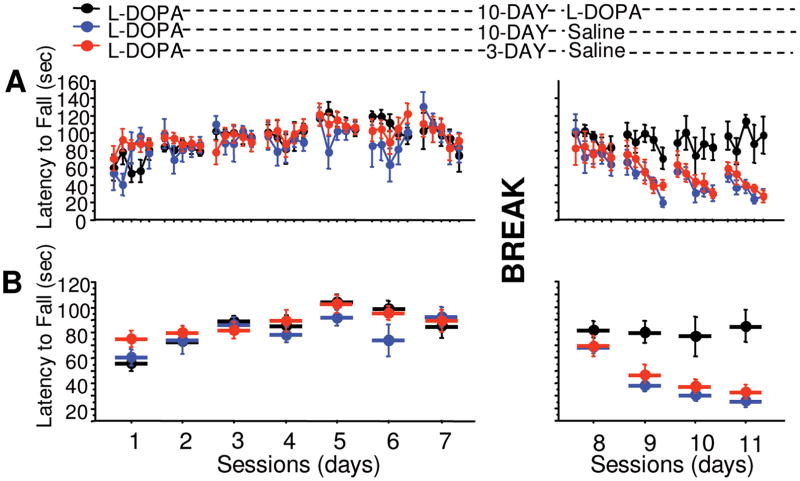
To determine whether this gradual decline in performance is dependent on the pharmacokinetics of L-dopa or experience with the task in the absence of L-dopa, we tested the effects of L-dopa discontinuation on learned performance after 2 different intervals, 3 or 10 days following the last administration. On the initial test trial, all groups retained performance comparable to those achieved during training with L-dopa (Fig 3A, 3 days treatment → no treatment group, session 7 compared with first trial of session 8, F[1,22] = 0.651, p = 0.4283; 10 days treatment → no treatment group, session 7 compared with first trial of session 8, F[1,10] = 2.159E–4, p = 0.9886; 10 days treatment → treatment group, session 7 compared with first trial of session 8, F[1,10] = 0.039, p = 0.8465). Both groups tested without L-dopa showed a gradual decline in performance with no significant difference arising from the interval between L-dopa discontinuation and testing (see Fig 3B, interval × repeated, F[3,48] = 0.111, p = 0.9535). Mice that continued L-dopa treatment during testing maintained their performance (see Fig 3B, session 7 compared with mean of sessions 8 –11 = 91 ± 7 seconds, F[1,10] = 0.127, p = 0.7285). These data suggest that the loss of performance is not dependent on the pharmacokinetics of L-dopa, but on experience with the rotarod in the absence of L-dopa.
FIGURE 3.
Effect of elapsed time after discontinuation of L-dopa on rotarod performance. PITx3(−/−) mice were trained with L-dopa for 7 sessions (sessions 1–7). One group was tested without treatment 3 days following discontinuation of L-dopa (red circles, sessions 8 –11), another group was tested 10 days after L-dopa discontinuation (blue circles, sessions 8 –11), and a final group was tested with L-dopa treatment after a 10-day suspension of L-dopa (black circles, sessions 8 –11). (A) Latency to fall in each trial. (B) Average latency to fall during each session. n = 12 for the 3-day interval group; n = 6 for 10-day interval groups.
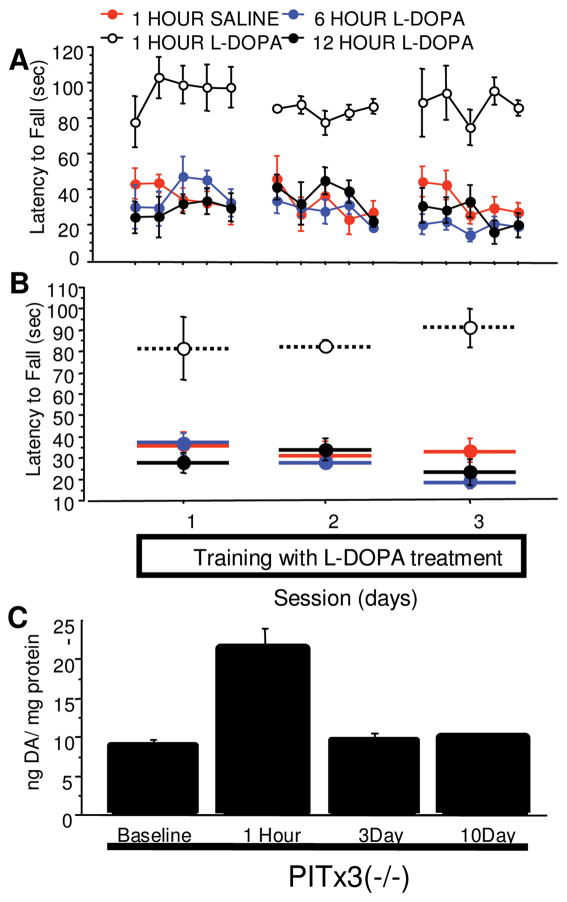
To further test the contribution of L-dopa pharmacokinetics to performance rescue, L-dopa was administered 6 and 12 hours prior to testing. Performance was indistinguishable from saline controls (Fig 4A and B, treatment main effect: 1-hour L-dopa vs 1-hour saline, F[1,20] = 34.434, p = 0.0002; 6-hour L-dopa vs 1-hour saline, F[1,20] = 0.993, p = 0.3426; 12-hour L-dopa vs 1-hour saline, F[1,20] = 0.512, p = 0.4905), demonstrating that the acute effects of L-dopa last <6 hours. It remains possible that repeated L-dopa administration results in a long-term accumulation of DA stores. Therefore, we measured DA content in tissue samples from the dorsal striatum using HPLC. Acute but not chronic administration significantly increased DA content (see Fig 4C, 1-hour group vs baseline, F[1,10] = 18.641, p = 0.0015; 3-day group vs baseline, F[1,10] = 0.464, p = 0.5111; 10-day group vs baseline, F[1,10] = 1.043, p = 0.3311). There were no observable differences from baseline DA content in mice chronically administered L-dopa followed by a 3-or 10-day cessation. This indicates that residual alterations in DA cannot account for the initially retained performance observed after L-dopa cessation, and that the phenomenon is not the result of L-dopa pharmacokinetics from acute or chronic administration.
FIGURE 4.
Time-course of L-dopa treatment effects. PITx3(−/−) mice were given L-dopa or saline injections at different time points (1 hour, 6 hours, or 12 hours) before training for 3 days. (A) Latency to fall in each trial. (B) Average latency to fall during each session (n = 6 per treatment). (C) Dopamine (DA) content in dorsal striatum of L-dopa–naive PITx3(−/−) animals, PITx3(−/−) animals receiving an acute L-dopa injection (1 hour prior to sample collection), and PITx3(−/−) animals receiving chronic L-dopa treatment for 7 days and treatment cessation for either 3 or 10 days. n = 6 per treatment.
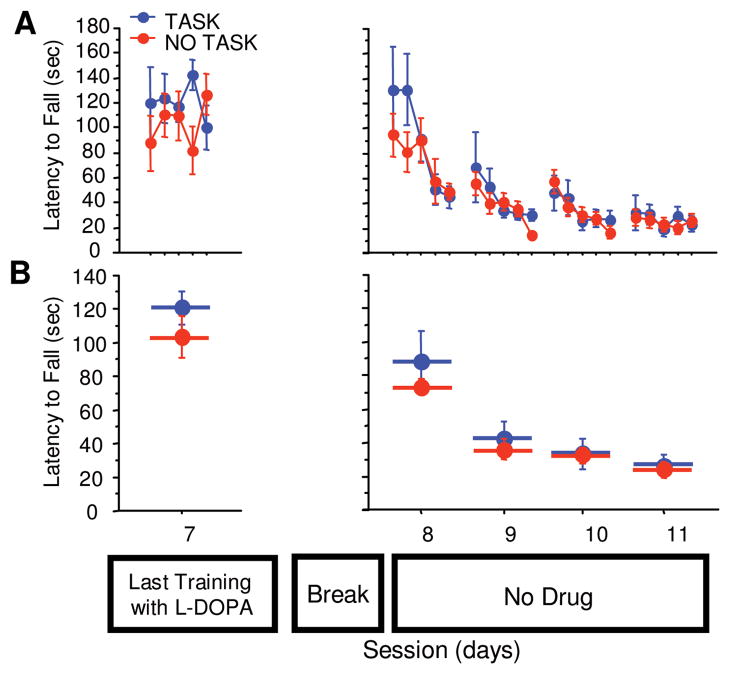
To test whether the experience-dependent loss of performance following L-dopa cessation is task specific, 2 groups of PITx3 homozygotes were trained with L-dopa as before and provided a 10-day break following discontinuation of L-dopa treatment. During the break, 1 group was provided 10 daily sessions of training on a similar motor task, running a treadmill. No difference was observed between the groups in the testing following L-dopa discontinuation (Fig 5B, training not shown, repeated measures × group, F[1,3] = 0.810, p = 0.4986), suggesting that the experience-dependent decline in performance is task specific.
FIGURE 5.
Task-specificity of loss of performance. PITx3(−/−) mice were trained for 7 session with L-dopa (last session of training, session 7, is shown). The No Task group was given a 10-day break without rotarod testing nor L-dopa injections. The Task group was also given a 10-day break without rotarod test nor L-dopa injections, but mice were allowed to run on a treadmill every day during those 10 days. Rotarod performance was tested after the 10-day break without L-dopa (sessions 8–11) for 4 consecutive days. (A) Latency to fall in each trial. (B) Average latency to fall during each session. n = 6 per treatment.
Rescue of Rotarod Learning Requires L-dopa during the Task Performance
To determine whether L-dopa is required during or following task performance to rescue learning, we administered treatments after the last trial of each session rather than before. Pitx3(+/−) animals treated with saline displayed a normal performance curve, whereas performance of PITx3(−/−) animals treated with saline was impaired (Fig 6A and B, F[1,7] = 109.902, p < 0.0001). PITx3(−/−) mice receiving L-dopa treatment after the trials showed no improvement in performance over time (see Fig 6B, repeated measure, F[6,24] = 1.676, p = 0.1702), and their performance resembled that of the saline-treated PITx3(−/−) group (see Fig 6B, treatment × repeated measure, F[6,48] = 0.295, p = 0.9365). These data indicate that the presence of DA while performing the rotarod task is essential for learning to be rescued.
FIGURE 6.
L-Dopa administration following training sessions. Mice were trained for 7 sessions with either saline or L-dopa administered following the last trial of each session. (A) Latency to fall in each trial. (B) Average latency to fall during each session. n = 5 per genotype/treatment. HET = heterozygote; HOM = homozygote.
Observed Learning Effects Are Attributable Specifically to Alterations in DA Signaling Mediated Primarily by D2 Receptors
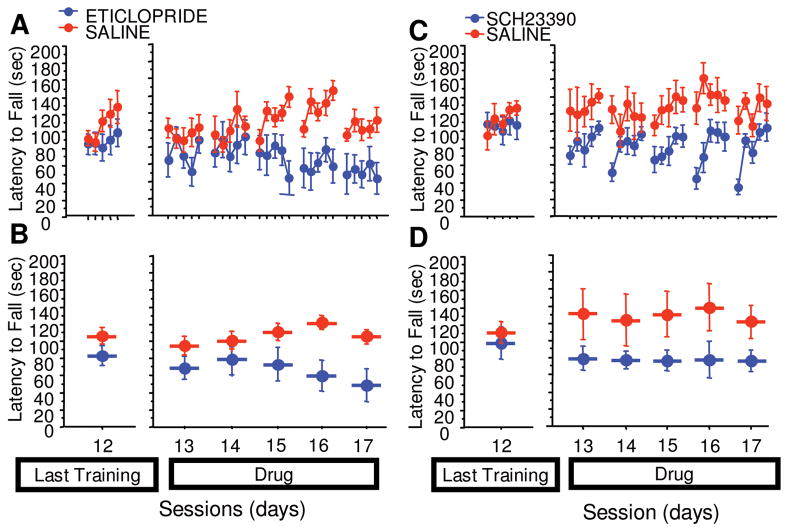
Because the PITx3 mutation is constitutive, it is important to demonstrate that the phenomena we observe arise as a specific consequence of alterations in DA signaling rather than as an aberrant response arising from developmental compensations in this mouse line. We asked if we could observe similar phenomena in wild-type mice using pharmacological manipulations. After training, mice administered eticlopride (Fig 7A and B, drug main effect F[1,40] = 7.944, p = 0.0182; repeated × group, F[1,4] = 5.014, p = 0.0023) exhibited a gradual decline in performance similar to that observed in the PITx3 homozygotes subsequent to cessation of L-dopa administration. In contrast, administration of SCH 23390 (see Fig 7C and D, drug main effect, F[1,40] = 11.451, p = 0.0070; repeated × group, F[1,4] = 0.644, p = 0.6346) resulted in an immediate decrement in performance. These data indicate that the gradual loss of performance we observed following L-dopa cessation can be attributed to altered DA signaling and can be pharmacologically replicated in wild-type mice through DA D2 receptor blockade.
FIGURE 7.
Effect of D1 and D2 antagonists on rotarod performance in wild-type animals. Animals were trained on the rotarod for 12 days without injections (the last training session, session 12, is shown). Animals were then given either a D1 blocker (SCH 23390) or a D2 blocker (eticlopride) and tested on the rotarod for 5 consecutive days (sessions 13–17). (A) Latency to fall in each trial (eticlopride). (B) Average latency to fall during each session (eticlopride). (C) Latency to fall in each trial (SCH 23390). (D) Average latency to fall during each session (SCH 23390).
Discussion
Parsing the function of DA in motor performance and learning—or the acquisition and expression of behavior more generally—has been controversial and difficult. Because learning can only be discerned by changes in performance, dopaminergic manipulations that directly impact motor performance often obscure and confound potential learning deficits. This difficulty is well illustrated in the study of PD. Most widely used animal models employ lesions of the nigrostriatal DA system, which result in abrupt and severe DA denervation. In PD, however, DA cell loss occurs gradually over decades,20 and is likely to be accompanied by subtle pathophysiology and compensatory changes prior to frank symptom onset later in life. Using partial DA lesions, Ogura and colleagues4 found that lesions that did not significantly impair motor performance nonetheless resulted in deficits in motor learning, suggesting that in the course of gradual denervation, learning deficits will precede frank performance deficits and may represent an important pathophysiology during the presymptomatic stage of PD. Partial lesions, however, tend to occur in particular anatomical regions within the striatum, which subserve different functions or somatotopic areas,21 and tend to be variable in degree, making them difficult models to use reliably.
The PITx3-deficient mouse line exhibits a 90% reduction in dorsal striatal DA, similar to advanced PD.10,11,13,18,19 Moreover, they show molecular changes similar to those found in adult lesion models.11,14,15 As a consequence, many have suggested that these mice might serve as a good model for PD.11,12,15 The question is, what aspect of PD can they provide insight into? Despite the dramatic loss of dopaminergic innervation, they show only subtle motor performance deficits. However, precisely because they have compensated and preserved gross motor function, pathologies related to DA denervation that would otherwise be obscured by severe motor performance deficits may be unmasked and available to investigation.
In the present study, the PITx3-deficient mice showed a severe impairment adapting to the accelerating rotarod task. When administered L-dopa, both performance and learning were rescued, enabling the mice to acquire and perform the task indistinguishably from control mice. After cessation of L-dopa treatment, the PITx3-deficient mice exhibited a gradual rather than abrupt decline in performance, which appeared to be dependent on experience with the task in the absence of L-dopa. This phenomenon cannot be attributed to L-dopa pharmacokinetics, as the interval (3 or 10 days) between discontinuation and testing made no difference, and the DA content 3 or 10 days after discontinuation was identical to that of mice never administered L-dopa. Moreover, the experience-dependent decline we observed is task specific, as mice given experience with a different motor task during the discontinuation interval performed identically when subsequently tested on the rotarod. The gradual rather than immediate decrement in performance following L-dopa cessation suggests an aberrant learning process rather than direct performance effects and demonstrates that DA is necessary for the acquisition and maintenance, in addition to the performance, of learned motor skills. The observations in PITx3-deficient mice can be replicated with pharmacological manipulation of D2 signaling in wild-type mice, indicating that the phenomenon we observe in PITx3-deficient mice following L-dopa cessation is specific to decreased DA and reflects a pathophysiology of normal DA function.
In PD, symptoms are believed to result primarily from overactivity of the inhibitory, D2-expressing indirect pathway.22 Our data suggest an aberrant learning process in parallel with an imbalance between inhibitory and facilitatory (ie, D1-expressing, direct pathway) motor control. Specifically, we hypothesize that increased activity in the D2-expressing inhibitory pathway results in inappropriate, learned inhibition of motor actions. Two recent reports provide support for this hypothesis. In a study of context-dependent sensitization of haloperidol-induced catalepsy, Wiecki et al23 presented a model of their experimental data that suggests that alterations in DA signaling that shift the balance between the direct, facilitatory and the indirect, inhibitory pathways also shift the relative probability of synaptic plasticity in these 2 pathways. Increased activity in the inhibitory pathway, such as arises from diminished DA or D2 blockade, results in increased synaptic plasticity and increased inhibitory learning. This hypothesis is further supported by the elegant work of Shen et al,24 who demonstrate that under hypodopaminergic conditions, the role of DA in regulating bidirectional plasticity is disrupted, resulting in abnormal long-term potentiation in the D2-expressing inhibitory pathway.
In the rotarod task, mice must learn to associate sensory states (proprioceptive, vestibular, position in space and on rod) with the appropriate motor response to facilitate remaining on the rod rather than falling (see supplemental material for video clips and discussion). We suggest the result of this learning is a repertoire of stimulus-responses comprised of (1) avoided actions, mediated by the inhibitory pathway; and (2) corrective actions, mediated by the facilitatory pathway. Overactivity in the inhibitory pathway results in increased inhibitory synaptic plasticity inducing inappropriate inhibitory learning. As a result, all motor responses, including appropriate, corrective actions, become dominated by the indirect, inhibitory pathway. One might say that poor performance (or akinesia in PD) becomes learned.
In PD, treatment with L-dopa results in a motor response with 2 main components: the short-duration response (SDR) and the LDR. The SDR is an acute response to L-dopa that lasts a few hours after a single dose of L-dopa treatment.25 The pharmacokinetics of L-dopa is the underlying mechanism of SDR, since it parallels plasma L-dopa concentrations and, presumably, striatal synaptic DA concentrations.26 The LDR, on the other hand, is a sustained motor improvement response that is acquired through chronic L-dopa treatment, lasts for hours, days, and even weeks after L-dopa treatment cessation, and represents an important component of therapeutic efficacy.27 The underlying mechanisms involved in LDR are still unknown, although it is clear that it is not due to continued peripheral circulation of L-dopa. One hypothesis suggests that LDR is supported by presynaptic mechanisms in which stored DA is released over a prolonged period.28 However, LDR can also be elicited after treatment with DA agonists such as apomorphine, lisuride, and ropinirole, suggesting a postsynaptic mechanism.29 There have been no animal models of LDR to investigate its mechanism.
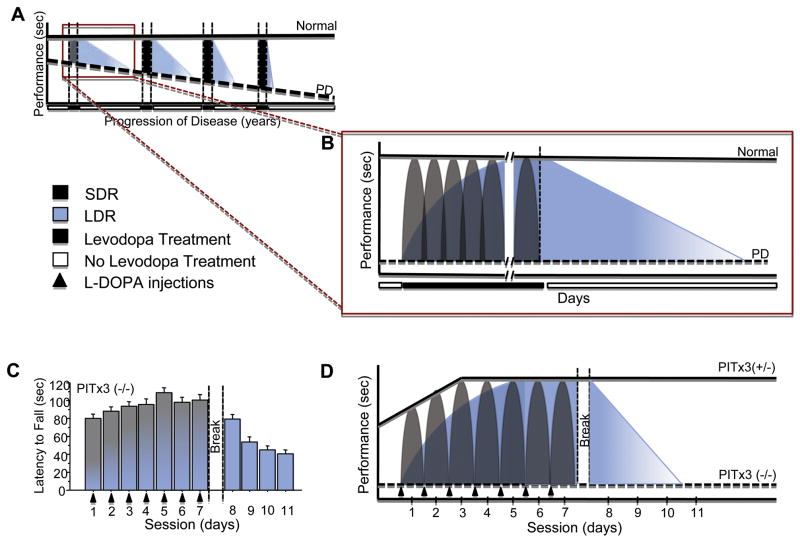
The present data mirror the LDR (Fig 8 schematic) and suggest a specific, alternative hypothesis to account for LDR: it arises from learning processes. L-Dopa, in addition to restoring the balance between the direct and indirect pathways, thus enabling movement, also restores appropriate synaptic plasticity and learning, giving rise to the sustained, gradual improvement seen over time with L-dopa treatment. When treatment is suspended, prior skill learning is initially retained and supports motor performance. Without L-dopa, however, aberrant learning resumes, and performance gradually declines. As the disease progresses and DA terminals become increasingly sparse, not only does the ability of L-dopa to rescue performance diminish, but the capacity for synaptic plasticity and learning also decreases. As a consequence, the LDR diminishes as the disease progresses.
FIGURE 8.
Schematic comparing LDR in L-dopa treatment of Parkinson disease (PD) and effects of L-dopa treatment on rotarod performance in PITx3-deficient mice. (A) Short-duration response (SDR) (gray) and long-duration response (LDR) (blue) during the progression of PD. As the disease progresses, baseline performance (dashed line) decreases. In addition, SDR increases in magnitude throughout the disease, although this is due to the progressive decline in baseline performance of patients.36 LDR, however, decreases in duration as the disease progresses.37–39 (B) SDR and LDR in a single treatment period in PD. Before L-dopa treatment, baseline performance (dashed line) is significantly lower in PD patients than in normal patients (solid line). With L-dopa treatment, SDR is observed after each L-dopa dose (gray shading). After L-dopa treatment discontinuation, performance is not immediately lost, but displays a gradual decline due to LDR (blue shading).25,27,28,39,40 (C) Performance on rotarod task of PITx3(−/−) mice during L-dopa treatment and following discontinuation. (D) Hypothesized SDR and LDR in PITx3-deficient mice. Before L-dopa treatment, baseline performance of PITx3(−/−) (dashed line) on the rotarod task is significantly lower than that of PITx3(+/−) (solid line). With each L-dopa injection, PITx3(−/−) display SDR (gray shading), which rescues performance on the rotarod. Multiple training sessions with L-dopa administration allow learning to occur, as observed in gradual improvement across sessions (blue shading). After L-dopa treatment is discontinued, performance gradually degrades, similarly to the decline in LDR observed in patients.
Problems with motor performance arising from bradykinesia and tremor have been the traditional focus of treatment in PD, and the clinical significance of motor learning in PD has remained controversial.30 However, impairments in procedural learning are being increasingly recognized.31–33 Moreover, there is evidence that motor learning (ie, practice) may improve treatment efficacy in restoring motor performance34 in L-dopa treatment of PD. The model proposed here would suggest that motor training/practice during L-dopa treatment may facilitate appropriate learning and mitigate previous aberrant learning, whereas training/practice when L-dopa is low or discontinued may actually accelerate aberrant learning, contributing to an overall worsening of symptoms. Such mechanisms may underlie recent observations of long-lasting enhancement of motor performances in patients treated with various dopaminergic agents such as levodopa or monoamine oxidase inhibitors compared with untreated patients.35 Although impairments in motor procedural learning may occur prior to frank onset of significant motor performance symptoms, the present data suggest that learning abnormalities may continue to play a role even during symptomatic stages of PD.
Our data represent the first animal model of LDR. Further investigation of specific mechanisms underlying the aberrant learning hypothesized here may provide targets for therapeutic strategies designed to maximize the LDR and perhaps, more generally, correct or block aberrant learning.
Supplementary Material
Acknowledgments
This work was supported by NIH NIDA grants DA025875 (J.A.B., X.Z.) and NIH NINDS NS064865 (U.J.K.), and the American Parkinson’s Disease Association Center for Advanced Research (X.Z., U.J.K.).
Footnotes
Potential Conflicts of Interest
Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Ungerstedt U. 6-Hydroxy-dopamine induced degeneration of central monoamine neurons. Eur J Pharmacol. 1968;5:107–110. doi: 10.1016/0014-2999(68)90164-7. [DOI] [PubMed] [Google Scholar]
- 2.Sedelis M, Schwarting RK, Huston JP. Behavioral phenotyping of the MPTP mouse model of Parkinson’s disease. Behav Brain Res. 2001;125:109–125. doi: 10.1016/s0166-4328(01)00309-6. [DOI] [PubMed] [Google Scholar]
- 3.Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. doi: 10.1016/0092-8674(95)90145-0. [DOI] [PubMed] [Google Scholar]
- 4.Ogura T, Ogata M, Akita H, et al. Impaired acquisition of skilled behavior in rotarod task by moderate depletion of striatal dopamine in a pre-symptomatic stage model of Parkinson’s disease. Neurosci Res. 2005;51:299–308. doi: 10.1016/j.neures.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annu Rev Neurosci. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- 6.Miyachi S, Hikosaka O, Lu X. Differential activation of monkey striatal neurons in the early and late stages of procedural learning. Exp Brain Res. 2002;146:122–126. doi: 10.1007/s00221-002-1213-7. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds JN, Wickens JR. Substantia nigra dopamine regulates synaptic plasticity and membrane potential fluctuations in the rat neostriatum, in vivo. Neuroscience. 2000;99:199–203. doi: 10.1016/s0306-4522(00)00273-6. [DOI] [PubMed] [Google Scholar]
- 8.Yin HH, Mulcare SP, Hilario MR, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12:333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang MT, Yokoi F, Yin HH, et al. Disrupted motor learning and long-term synaptic plasticity in mice lacking NMDAR1 in the striatum. Proc Natl Acad Sci U S A. 2006;103:15254–15259. doi: 10.1073/pnas.0601758103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang DY, Ardayfio P, Kang UJ, et al. Selective loss of dopaminergic neurons in the substantia nigra of Pitx3-deficient aphakia mice. Brain Res Mol Brain Res. 2003;114:123–131. doi: 10.1016/s0169-328x(03)00162-1. [DOI] [PubMed] [Google Scholar]
- 11.Smits SM, Mathon DS, Burbach JP, et al. Molecular and cellular alterations in the Pitx3-deficient midbrain dopaminergic system. Mol Cell Neurosci. 2005;30:352–363. doi: 10.1016/j.mcn.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 12.Ardayfio P, Moon J, Leung KK, et al. Impaired learning and memory in Pitx3 deficient aphakia mice: a genetic model for striatum-dependent cognitive symptoms in Parkinson’s disease. Neurobiol Dis. 2008;31:406–412. doi: 10.1016/j.nbd.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beeler JA, Cao ZF, Kheirbek MA, Zhuang X. Loss of cocaine locomotor response in Pitx3-deficient mice lacking a nigrostriatal pathway. Neuropsychopharmacology. 2009;34:1149–1161. doi: 10.1038/npp.2008.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding Y, Restrepo J, Won L, et al. Chronic 3,4-dihydroxyphenylalanine treatment induces dyskinesia in aphakia mice, a novel genetic model of Parkinson’s disease. Neurobiol Dis. 2007;27:11–23. doi: 10.1016/j.nbd.2007.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang DY, Fleming SM, Ardayfio P, et al. 3,4-Dihydroxyphenylalanine reverses the motor deficits in Pitx3-deficient aphakia mice: behavioral characterization of a novel genetic model of Parkinson’s disease. J Neurosci. 2005;25:2132–2137. doi: 10.1523/JNEUROSCI.3718-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singh B, Wilson JH, Vasavada HH, et al. Motor deficits and altered striatal gene expression in aphakia (ak) mice. Brain Res. 2007;1185:283–292. doi: 10.1016/j.brainres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kas MJ, van der Linden AJ, Oppelaar H, et al. Phenotypic segregation of aphakia and Pitx3-null mutants reveals that Pitx3 deficiency increases consolidation of specific movement components. Behav Brain Res. 2008;186:208–214. doi: 10.1016/j.bbr.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Nunes I, Tovmasian LT, Silva RM, et al. Pitx3 is required for development of substantia nigra dopaminergic neurons. Proc Natl Acad Sci U S A. 2003;100:4245–4250. doi: 10.1073/pnas.0230529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Munckhof P, Luk KC, Ste-Marie L, et al. Pitx3 is required for motor activity and for survival of a subset of midbrain dopaminergic neurons. Development. 2003;130:2535–2542. doi: 10.1242/dev.00464. [DOI] [PubMed] [Google Scholar]
- 20.Brooks DJ. The early diagnosis of Parkinson’s disease. Ann Neurol. 1998;44:S10–S18. doi: 10.1002/ana.410440704. [DOI] [PubMed] [Google Scholar]
- 21.Chang JW, Wachtel SR, Young D, Kang UJ. Biochemical and anatomical characterization of forepaw adjusting steps in rat models of Parkinson’s disease: studies on medial forebrain bundle and striatal lesions. Neuroscience. 1999;88:617–628. doi: 10.1016/s0306-4522(98)00217-6. [DOI] [PubMed] [Google Scholar]
- 22.Bergman H, Feingold A, Nini A, et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- 23.Wiecki TV, Riedinger K, von Ameln-Mayerhofer A, et al. A neurocomputational account of catalepsy sensitization induced by D2 receptor blockade in rats: context dependency, extinction, and renewal. Psychopharmacology (Berl) 2009;204:265–277. doi: 10.1007/s00213-008-1457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science. 2008;321:848–851. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muenter MD, Tyce GM. L-Dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc. 1971;46:231–239. [PubMed] [Google Scholar]
- 26.Tedroff J, Pedersen M, Aquilonius SM, et al. Levodopa-induced changes in synaptic dopamine in patients with Parkinson’s disease as measured by [11C]raclopride displacement and PET. Neurology. 1996;46:1430–1436. doi: 10.1212/wnl.46.5.1430. [DOI] [PubMed] [Google Scholar]
- 27.Nutt JG, Carter JH, Woodward WR. Long-duration response to levodopa. Neurology. 1995;45:1613–1616. doi: 10.1212/wnl.45.8.1613. [DOI] [PubMed] [Google Scholar]
- 28.Zappia M, Colao R, Montesanti R, et al. Long-duration response to levodopa influences the pharmacodynamics of short-duration response in Parkinson’s disease. Ann Neurol. 1997;42:245–248. doi: 10.1002/ana.410420217. [DOI] [PubMed] [Google Scholar]
- 29.Metman LV, Locatelli ER, Bravi D, et al. Apomorphine responses in Parkinson’s disease and the pathogenesis of motor complications. Neurology. 1997;48:369–372. doi: 10.1212/wnl.48.2.369. [DOI] [PubMed] [Google Scholar]
- 30.Siegert RJ, Taylor KD, Weatherall M, Abernethy DA. Is implicit sequence learning impaired in Parkinson’s disease? A metaanalysis Neuropsychology. 2006;20:490–495. doi: 10.1037/0894-4105.20.4.490. [DOI] [PubMed] [Google Scholar]
- 31.Ghilardi MF, Eidelberg D, Silvestri G, Ghez C. The differential effect of PD and normal aging on early explicit sequence learning. Neurology. 2003;60:1313–1319. doi: 10.1212/01.wnl.0000059545.69089.ee. [DOI] [PubMed] [Google Scholar]
- 32.Jackson GM, Jackson SR, Harrison J, et al. Serial reaction time learning and Parkinson’s disease: evidence for a procedural learning deficit. Neuropsychologia. 1995;33:577–593. doi: 10.1016/0028-3932(95)00010-z. [DOI] [PubMed] [Google Scholar]
- 33.Krebs HI, Hogan N, Hening W, et al. Procedural motor learning in Parkinson’s disease. Exp Brain Res. 2001;141:425–437. doi: 10.1007/s002210100871. [DOI] [PubMed] [Google Scholar]
- 34.Behrman AL, Cauraugh JH, Light KE. Practice as an intervention to improve speeded motor performance and motor learning in Parkinson’s disease. J Neurol Sci. 2000;174:127–136. doi: 10.1016/s0022-510x(00)00267-7. [DOI] [PubMed] [Google Scholar]
- 35.DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Parkinson Study Group. Arch Neurol. 1989;46:1052–1060. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- 36.Nutt JG, Carter JH, Van Houten L, Woodward WR. Short-and long-duration responses to levodopa during the first year of levodopa therapy. Ann Neurol. 1997;42:349–355. doi: 10.1002/ana.410420311. [DOI] [PubMed] [Google Scholar]
- 37.Madden DL, Morris JG, Graham SJ, et al. The long duration response to levodopa in Parkinson’s disease. J Clin Neurosci. 1995;2:48–54. doi: 10.1016/0967-5868(95)90030-6. [DOI] [PubMed] [Google Scholar]
- 38.Nutt JG, Carter JH, Lea ES, Sexton GJ. Evolution of the response to levodopa during the first 4 years of therapy. Ann Neurol. 2002;51:686–693. doi: 10.1002/ana.10189. [DOI] [PubMed] [Google Scholar]
- 39.Quattrone A, Zappia M, Aguglia U, et al. The subacute levodopa test for evaluating long-duration response in Parkinson’s disease. Ann Neurol. 1995;38:389–395. doi: 10.1002/ana.410380308. [DOI] [PubMed] [Google Scholar]
- 40.Fahn S, Oakes D, Shoulson I, et al. Levodopa and the progression of Parkinson’s disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.