Abstract
Context
The contribution of reproductive hormones to mood has been the focus of considerable research. Results from clinical and epidemiological studies have been inconsistent. It remains unclear whether alterations in serum hormone levels across the menopausal transition are linked to depressive symptoms.
Objectives
To evaluate the relationship between serum hormone levels and high depressive symptoms and whether hormone levels or their change might explain the association of menopausal status with depressive symptoms previously reported in a national sample of midlife women.
Design
A longitudinal, community-based, multisite study of menopause. Data were collected at baseline and annually from December 1995 to January 2008 on a range of factors. Early follicular phase serum samples were assayed for levels of estradiol, follicle-stimulating hormone, testosterone, and dehydroepiandrosterone sulfate.
Setting
Seven communities nationwide.
Participants
A community-based sample of 3302 multiethnic women, aged 42 to 52 years, still menstruating and not using exogenous reproductive hormones.
Main Outcome Measure
Depressive symptoms assessed with the Center for Epidemiological Studies Depression Scale (CES-D). The primary outcome was a CES-D score of 16 or higher.
Results
In multivariable random-effects logistic regression models, log-transformed testosterone level was significantly positively associated with higher odds of a CES-D score of 16 or higher (odds ratio=1.15; 95% confidence interval, 1.01–1.31) across 8 years, and a larger increase in log-transformed testosterone from baseline to each annual visit was significantly associated with increased odds of a CES-D score of 16 or higher (odds ratio=1.23; 95% confidence interval, 1.04–1.45). Less education, being Hispanic, and vasomotor symptoms, stressful life events, and low social support at each visit were each independently associated with a CES-D score of 16 or higher. No other hormones were associated with a CES-D score of 16 or higher. Being perimenopausal or post-menopausal compared with being premenopausal remained significantly associated with a CES-D score of 16 or higher in all analyses.
Conclusions
Higher testosterone levels may contribute to higher depressive symptoms during the menopausal transition. This association is independent of menopausal status, which remains an independent predictor of higher depressive symptoms.
The contribution of reproductive hormones to mood has been a focus of efforts to explain sex differences in depression. Recent longitudinal studies have found that women are more susceptible to higher levels of depressed mood during the menopausal transition than just prior to it,1–3 reinforcing the need to address the question about the role of reproductive hormones in the development of depression and negative mood.
Multiple theories have been proposed to identify and explain the hormonal dynamics that might be physiologically related to depressed mood. These range from early notions that the decreases in or ultimate low levels of estradiol (E2) induce depression postmenopausally to the more recent hypothesis that the unstable and irregular pattern of hormone production during the perimenopausal transition may increase vulnerability to mood disorders in susceptible women.4 Indeed, neurobiological data have indicated that gonadal steroids are capable of influencing all aspects of neuro-transmitter activity.4 Specific nuclear receptors for estrogen have been identified in areas of the brain such as the pituitary and hypothalamus,5 and estrogens, progestins, and androgens affect a wide range of neuromodulator processes, including the neuromodulators serotonin and norepinephrine, implicated in the development of depression.6,7
Yet, the numerous epidemiological and clinical studies that have examined associations between reproductive hormones and depression have yielded inconsistent results, showing both a positive and a negative association between depression and E2 or its variability,2,8 follicle-stimulating hormone (FSH),2,8 testosterone (T),9,10 dehydroepiandrosterone (DHEA), and DHEA sulfate (DHEA-S).11,12 The net result of this literature is the absence of a consistent or coherent hormonal explanation for depression particularly during the menopausal transition.
The research to date has also had methodological limitations, including cross-sectional designs, small sample sizes, primarily white participants, and, in the few longitudinal studies, limited periods of follow-up. The Study of Women’s Health Across the Nation (SWAN), a prospective study of the menopausal transition, has followed up a large, ethnically diverse sample for 8 years. Thus, SWAN has addressed many of the previous studies’ limitations. In the current analyses, we build on our earlier findings from analyses of the first 5 annual assessments in which we reported that high depressive symptoms as measured by a standard questionnaire, the Center for Epidemiological Studies Depression Scale (CES-D), were more likely to be reported when midlife women were perimenopausal and postmenopausal than when pre-menopausal,1 independent of multiple psychosocial factors and vasomotor symptoms.
The overall aim of the current analyses was to examine the relationship between serum hormone levels and high depressive symptoms. We evaluated the following: (1) whether our previously reported findings were replicated with 3 additional years of follow-up; (2) the concurrent relationships of depressive symptoms and serum levels of the reproductive hormones (FSH, E2, T, and the somatic adrenal hormone DHEA-S) over 8 years; (3) associations between changes in each hormone from baseline and high depressive symptoms; and (4) whether the hormones attenuated or nullified the association of menopausal status defined by bleeding patterns with elevated depressive symptoms.
METHODS
STUDY PARTICIPANTS
The Study of Women’s Health Across the Nation is a longitudinal, multiethnic, multisite, community-based study of menopause and aging among 3302 premenopausal and early peri-menopausal women.13 Eligibility for enrollment was assessed between December 1995 and October 1997 with a screening survey of health, reproductive, demographic, and lifestyle information. Of the 16 065 women screened, 3302 eligible women were enrolled in the longitudinal cohort. Each of 7 sites recruited white women and women from 1 specified minority group (African American women in Pittsburgh, Pennsylvania, in Boston, Massachusetts, in the area of Detroit, Michigan, and in Chicago, Illinois; Japanese women in Los Angeles, California; Chinese women in the region of Oakland, California; and Hispanic women in Newark, New Jersey). Eligibility included being aged between 42 and 52 years, having an intact uterus, having at least 1 menstrual period and no use of exogenous reproductive hormones in the previous 3 months, not being pregnant or lactating, and self-identification with 1 of the site’s designated racial/ethnic groups. Study retention at the end of the eighth follow-up visit was 74%, at which time two-thirds of the participants (66%) were postmenopausal and 11% were late peri-menopausal. The New Jersey site did not retain women beyond year 5 because of administrative reasons unrelated to the purpose of the study. The institutional review boards at all participating sites approved the study protocol. After excluding 2 women missing the CES-D data,14 1 woman missing covariate data, and 3 women missing serum hormone levels at all examinations, our sample consisted of 3296 women.
PROCEDURES
The SWAN participants were assessed at study entry (baseline) and once a year with a common standardized protocol. All study forms and materials were available in English, Spanish, Japanese, and Cantonese and bilingual staff was used as appropriate. Baseline and annual assessments included self- and interviewer-administered questionnaires about health, lifestyle, and psychosocial factors. Height and weight were measured using a common protocol, and a fasting blood sample was obtained in the early follicular phase of the menstrual cycle if possible. Participants provided signed, written informed consent prior to study entry.
MEASURES
Assessment of Depressive Symptoms
Depressive symptoms were assessed at baseline and annually with the CES-D Scale, a 20-item measure that asks about the frequency of being bothered by depressive symptoms during the previous week on a 4-point scale of 0 (rarely) to 3 (most or all of the time).14 A score of 16 or higher is generally used to identify potential clinical depression15 and was used to indicate clinically relevant depressive symptoms in this study. The CES-D has been shown to be valid and reliable in diverse ethnic populations.16–18 To examine more or less severe depressive symptoms, we also compared 3 groups of women: those with CES-D scores lower than 16, those with CES-D scores of 16 to lower than 22, and those with CES-D scores of 22 or higher (the median of the group with CES-D scores of ≥16).
Serum Hormone Measurements
The fasting blood draw was targeted to days 2 to 5 of the follicular phase of the menstrual cycle in menstruating women and within 90 days of the anniversary of the baseline examination date. If a timed sample could not be obtained after 2 attempts, a random fasting sample was taken within a 90-day window of the annual visit. Blood was refrigerated within 1 to 2 hours after phlebotomy. All samples were maintained at 4°C until separated; following centrifugation, the serum was aliquotted, frozen at −80°C, and shipped on dry ice to the central laboratory. All assays used a double-antibody chemiluminescent immunoassay with a solid-phase anti-IgG immunoglobulin conjugated to paramagnetic particles, antiligand antibody, and competitive ligand labeled with dimethylacridinium ester.
The E2 assay modified the rabbit anti-E2-6 ACS-180 immunoassay (Bayer Diagnostics Corp, Norwood, Massachusetts) to increase sensitivity, with a lower limit of detection of 1.0 pg/mL (to convert to picomoles per liter, multiply by 3.671). The assay for T modified the rabbit polyclonal anti-T ACS-180 immunoassay, with a lower limit of detection of 2.19 ng/dL (to convert to nanomoles per liter, multiply by 0.0347). Serum FSH concentrations were measured with a 2-site chemiluminometric immunoassay, with a lower limit of detection of 1.1m IU/mL(to convert to international units per liter, multiply by 1.0). The absolute concentrations of FSH were somewhat higher in this assay compared with values from many clinical laboratories based on differences in the standards selected. The de novo 2-site chemiluminescent assays for serum sex hormone–binding globulin(SHBG) and DHEA-S concentrations involved competitive binding of dimethylacridinium ester–labeled SHBG or DHEA-S to a commercially available rabbit anti-SHBG or anti–DHEA-S antibody, with lower limits of detection of 0.22 μg/mL (to convert to nanomoles per liter, multiply by 8.896) and 2 μg/dL (to convert to micro-moles per liter, multiply by 0.027), respectively. The respective intra-assay and interassay coefficients of variation were 8.5% and 13.8% for E2,16 9.7% and 11.3% for T,17 12.0% and 6.0% for FSH, 9.9% and 6.1% for SHBG, and 11.3% and 7.6%, for DHEA-S. Duplicate E2 assays were conducted with results reported as the arithmetic mean for each woman, with a coefficient of variation of 3% to 12%. All other assays were single determinations. Total T was indexed to SHBG to calculate the free T index (FTI) (FTI=[100× T in nanograms per deciliter]/[28.84×SHBG in nanomoles per liter]). Likewise, total E2 was indexed to SHBG to calculate the free E2 index (free E2 index = [100 ×E2 in picograms per milliliter]/[272.11× SHB Ginnanomoles per liter]). Hormoneas-says were conducted at the SWAN Endocrine Laboratory, University of Michigan, AnnArbor , using the ACS-180 automated analyzer (Bayer Diagnostics Corp).
Menopausal Status
Menopausal status was based on menstrual bleeding patterns in the previous 12 months and was categorized as the following: (1) premenopausal (menstrual period in the past 3 months with no change in regularity in the past 12 months); (2) early perimenopausal (menstrual period in the past 3 months with change in regularity over the previous 12 months); (3) late peri-menopausal (no menstrual period within the past 3 months but some menstrual bleeding within the past 12 months); and (4) postmenopausal (no menstrual period within the past 12 months). The classifications are similar to those recommended by the World Health Organization19 and the Stages of Reproductive Aging Workshop.20 Based on SWAN eligibility requirements, all women were premenopausal or early peri-menopausal at baseline.
Covariates
Age, race/ethnicity, and education were obtained at the baseline examination. The CES-D score and all other variables were obtained at each annual examination. Body mass index (BMI) was calculated as weight (obtained with a calibrated scale) in kilograms divided by height (obtained with a stadiometer) in meters squared. Smoking status was assessed as current vs not. Women self-reported medication use for nerves or depression at least twice per week in the month prior to interview; this was verified by examination of medication containers in the clinic by the interviewers or participants reading labels to the interviewer over the telephone.
Vasomotor symptoms were coded as presence or absence of hot flashes, cold sweats, or night sweats in the previous 2 weeks. Psychosocial variables included social support, 4 items from the Medical Outcomes Study Social Support Survey (score ranged from 0-16),21 and upsetting life events based on a checklist of 18 life events since the last study visit rated according to how upsetting they were (categorized as 0, 1, or ≥2 very upsetting events).
STATISTICAL ANALYSIS
For the longitudinal analyses, we excluded observations (ie, visits) at which a woman reported using exogenous hormones, being pregnant, or breastfeeding since the last study visit; we censored data from women who reported surgical menopause (bilateral oophorectomy or hysterectomy) at subsequent follow-up visits; and we did not include observations with missing covariate data. Minimally adjusted models examining concurrent serum hormone levels from baseline through follow-up visit 8 included 3296 women. Models examining change in hormone levels at the follow-up visits included 2882 women. The decrease in sample size came primarily from women who dropped out after the initial visit (n=293). Women who dropped out were more likely than those who enrolled to smoke, to have depressive symptoms, lower education, and higher BMI, and to be of Hispanic race/ethnicity and were less likely to be Chinese or Japanese. These women did not differ on menopausal status, age, or hormone levels at baseline.
Separate random-effects logistic regression analyses were conducted to determine associations between CES-D scores of 16 or higher and concurrent serum hormone levels and change in hormone levels through visit 8. We ran 3 sets of models: (1) a replication of previously reported associations between menopausal status and high depressive symptoms with 3 years of additional data; (2) an analysis of concurrent hormones; and (3) an analysis of change in hormones from baseline. Each set of analyses consisted of a minimally adjusted model and a fully adjusted model as described later. The inclusion of a (woman-specific) random intercept quantifies the correlation between all observations from a given woman to overall variation and enables us to ascribe a woman-specific interpretation to model parameters. Random-effects logistic regression models are relatively robust to missing data and make use of all available data across all visits. Natural logarithm transformation was used for serum hormone concentrations to reduce skewness. To examine more or less severe depressive symptoms, separate models were repeated comparing the middle CES-D score group (scores ≥16 and <22) and the highest CES-D score group (scores ≥22) with the lowest CES-D score group (scores <16).
We modeled T and DHEA-S as a function of aging because these hormones have been shown to decrease slowly with age and show little effect of the menopausal transition itself.22 In minimally adjusted models for each of the hormones predicting high depressive symptoms, we included race/ethnicity, study site, baseline age, and years since baseline (ie, aging). Although E2 and FSH manifest a clear inflection at the time of the final menstrual period, only 1272 women had observable final menstrual periods. Thus, we conducted and report analyses of E2 and FSH as a function of time. Results of these analyses were similar to those from analyses modeling E2 and FSH as a function of age at the final menstrual period. We also included a quadratic term for each hormone to account for potential nonlinear associations with depression scores across time. None of these analyses yielded significant results, and they are not discussed further.
Hormones showing significant associations with depressive symptoms in a minimally adjusted model were assessed further in analyses that included covariates. For the fully adjusted model, we selected potential confounders and covariates based on the literature and a priori hypotheses. Appropriateness of each variable was evaluated individually and via model-building techniques, including using a cutoff of P < .05. Final models also included baseline education and time-varying covariates of vasomotor symptoms, medication use, BMI, social support, life events, smoking, and menopausal status. When examining change in hormone levels, the minimally and fully adjusted models included baseline serum hormone values.
Because bleeding patterns became less predictable as women progressed through the transition, it became increasingly difficult to anchor a serum sample to a phase of the menstrual period. We previously found that the timing of the blood draw can be considered a surrogate for the transition.23 Therefore, given the larger portion of noncycling observations (ie, late peri-menopausal and postmenopausal) over the 8 years and observed links between status and depressive symptoms in SWAN,1 we included menopausal status (rather than cycle day) in multivariable analyses.
Additional analyses explored the association of the E2-T ratio with high CES-D scores and examined whether the relationship between hormones and depressive symptoms differed for women with certain characteristics, including low education, high financial strain, low social support, or very upsetting life events, using interaction terms between significant serum hormone levels and dichotomous variables of each characteristic. Neither the E2-T ratio nor any of the interactions were significant.
Analyses were run using SAS version 9.1 statistical software (SAS Institute, Inc, Cary, North Carolina) and Stata version 9 statistical software (StataCorp LP, College Station, Texas). P < .05 was considered statistically significant.
RESULTS
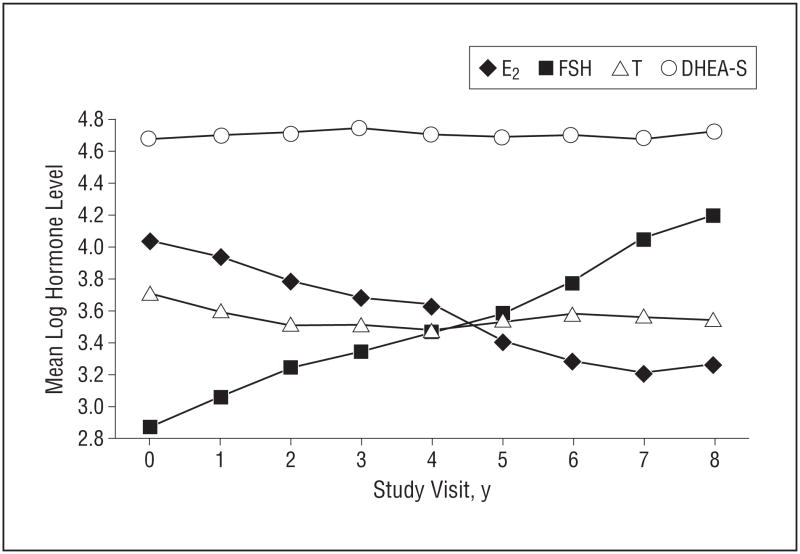
Participants are described in Table 1. At baseline, endogenous hormone levels did not differ between women with high CES-D scores (≥16) and those with low CES-D scores (<16). Women with depressive symptoms at baseline were more likely to be African American or His-panic, be current smokers, have a higher BMI, and be early perimenopausal. Over the 8 years of follow-up, mean logFSH values increased and mean logE2 values decreased, whereas there were minimal changes in mean logT and mean logDHEA-S values (Figure 1).
Table 1.
Baseline Characteristics of 3292 Women Without and With High Depressive Symptomsa
| Baseline Characteristic | CES-D Score <16 (n=2490) | CES-D Score ≥16 (n=802) | P Value |
|---|---|---|---|
| CES-D, median (IQR) | 6.0 (3.0–9.0) | 23.0 (18.0–30.0) | <.001 |
| Age, mean (SD), y | 46.5 (2.7) | 45.9 (2.6) | <.001 |
| Hormone level, median (IQR) | |||
| E2, pg/mL | 54.8 (33.0–87.9) | 58.1 (33.1–93.9) | .34 |
| FSH, mIU/mL | 16.1 (10.9–26.6) | 15.5 (10.4–25.6) | .16 |
| T, ng/dL | 41.6 (30.0–56.5) | 41.0 (29.4–55.5) | .28 |
| SHBG, μg/mL | 4.6 (3.2–6.5) | 4.6 (3.1–6.5) | .86 |
| DHEA-S, μg/dL | 114.8 (75.5–170.3) | 110.2 (70.4–165.5) | .11 |
| FTI, median (IQR)b | 3.6 (2.2–6.0) | 3.6 (2.1–6.0) | .44 |
| FEI, median (IQR)c | 0.5 (0.3–0.9) | 0.5 (0.3–0.9) | .60 |
| Race/ethnicity, No. (%) | <.001 | ||
| African American | 680 (27) | 253 (32) | |
| White | 1195 (48) | 350 (44) | |
| Chinese | 214 (9) | 35 (4) | |
| Hispanic | 161 (6) | 123 (15) | |
| Japanese | 240 (10) | 41 (5) | |
| BMI, mean (SD) | 27.8 (6.9) | 29.7 (7.9) | <.001 |
| Menopausal status, No. (%) | <.001 | ||
| Premenopausal | 1356 (56) | 366 (47) | |
| Early perimenopausal | 1075 (44) | 419 (53) | |
| Current smoker, No. (%) | 369 (15) | 199 (25) | <.001 |
| Education, No. (%) | <.001 | ||
| ≤High school | 526 (21) | 292 (37) | |
| Some college/technical school | 785 (32) | 262 (33) | |
| College graduate | 1155 (47) | 242 (30) | |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiological Studies Depression Scale; DHEA-S, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle-stimulating hormone; FEI, free estradiol index; FTI, free testosterone index; IQR, interquartile range; SHBG, sex hormone–binding globulin; T, testosterone.
SI conversion factors: To convert E2 to picomoles per liter, multiply by 3.671; to convert FSH to international units per liter, multiply by 1.0; to convert T to nanomoles per liter, multiply by 0.0347; to convert SHBG to nanomoles per liter, multiply by 8.896; and to convert DHEA-S to micromoles per liter, multiply by 0.027.
Although our sample consisted of 3296 women, 4 without baseline CES-D scores are not included; additional subjects are missing select baseline characteristics. High depressive symptoms are indicated by a CES-D score of 16 or higher.
Calculated as [100×T in nanograms per deciliter]/[28.84×SHBG in nanomoles per liter].
Calculated as [100×E2 in picograms per milliliter]/[272.11 × SHBG in nanomoles per liter].
Figure 1.
Mean log-transformed hormone level by visit. E2 indicates estradiol; FSH, follicle-stimulating hormone; T, testosterone; and DHEA-S, dehydroepiandrosterone sulfate. Study visit 0 indicates the baseline visit.
MENOPAUSAL STATUS AND DEPRESSIVE SYMPTOMS
Consistent with the previously reported first 5 years of follow-up, with an additional 3 years of follow-up, women continued to have monotonically increasing odds of a CES-D score of 16 or higher when they were early and late perimenopausal and postmenopausal compared with when they were premenopausal (adjusted models excluding hormone levels), with odds ratios (ORs) ranging from 1.31 (early perimenopause) to 1.79 (postmeno-pause) (P<.001).
SERUM HORMONE LEVELS AND DEPRESSIVE SYMPTOMS
In the minimally adjusted random-effects logistic models, no significant effects of logE2, logFSH, logDHEA-S, or logfree E2 index were observed in odds of a high CES-D score (Table 2). However, logT and logFTI each significantly increased the odds of a high CES-D score by 19% and 11%, respectively, for each 1-unit increase in the log-transformed value of each hormone. In the fully adjusted models, logFTI was no longer significant, whereas logT remained significant (Table 3). In the fully adjusted analyses of the 3 levels of CES-D scores, concurrent logT was associated with an OR of 1.20 (95% confidence interval, 1.00-1.44) for the group with the highest CES-D scores compared with the group with low CES-D scores and was associated with an OR of 1.08 (95% confidence interval, 0.93–1.25) for the group with middle CES-D scores compared with the group with low CES-D scores.
Table 2.
Minimally Adjusted Random-Effects Logistic Regression Models of the Odds of High Depressive Symptoms for Each Hormonea
| OR (95% CI)
|
||
|---|---|---|
| Hormoneb | Concurrent Hormone | Change in Hormone Levels From Baseline |
| logE2 | 1.00 (0.93–1.06) | 1.01 (0.94–1.08) |
| logFSH | 1.05 (0.97–1.13) | 1.04 (0.85–1.14) |
| logT | 1.19 (1.05–1.36)c | 1.24 (1.06–1.46)c |
| logDHEA-S | 0.96 (0.86–1.08) | 1.09 (0.92–1.29) |
| logFTI | 1.11 (1.02–1.20)d | 1.14 (1.03–1.27)d |
| logFEI | 1.00 (0.94–1.06) | 1.02 (0.96–1.10) |
Abbreviations: CES-D, Center for Epidemiological Studies Depression Scale; CI, confidence interval; DHEA-S, dehydroepiandrosterone sulfate; E2, estradiol; FSH, follicle-stimulating hormone; FEI, free estradiol index; FTI, free testosterone index; OR, odds ratio; T, testosterone.
High depressive symptoms are indicated by a CES-D score of 16 or higher. Each model is adjusted for race/ethnicity, study site, baseline age, and aging/time since baseline (n=3296); the change in hormone models also includes the baseline value of that particular hormone (n=2882). Concurrent hormone uses data from baseline through visit 8; change in hormone levels from baseline uses data from visits 1 through 8.
Log indicates natural logarithm transformation (ie, base e).
P<.01.
P<.05.
Table 3.
Fully Adjusted Random-Effects Logistic Regression Model Examining the Association of Concurrent Testosterone With the Odds of High Depressive Symptoms From Baseline Through Visit 8a
| Covariate/Estimated Parameter | OR (95% CI) | P Value |
|---|---|---|
| Concurrent logtestosterone | 1.15 (1.01–1.31) | .04 |
| Status, premenopause as reference | <.001 | |
| Early perimenopause | 1.35 (1.14–1.61) | |
| Late perimenopause | 1.68 (1.28–2.20) | |
| Postmenopause | 1.83 (1.40–2.42) | |
| Race/ethnicity, white as reference | .006 | |
| African American | 1.09 (0.86–1.38) | |
| Chinese | 1.01 (0.64–1.59) | |
| Hispanic | 2.12 (1.22–3.70) | |
| Japanese | 1.81 (1.17–2.80) | |
| Baseline age | 0.93 (0.90–0.97) | <.001 |
| Aging | 0.90 (0.87–0.93) | <.001 |
| Baseline education, ≥college as reference | <.001 | |
| ≤High school | 2.11 (1.66–2.68) | |
| >High school/some college | 1.48 (1.20–1.82) | |
| Current smoker | 1.43 (1.16–1.77) | .001 |
| Medication for nerves or depression 2 times/wk in past month | 2.43 (1.99–2.96) | <.001 |
| BMI | 1.01 (1.001–1.03) | .04 |
| Any vasomotor symptoms | 1.62 (1.43–1.84) | <.001 |
| Social support | 0.81 (0.79–0.83) | <.001 |
| Upsetting life events, 0 as reference | <.001 | |
| 1 | 2.47 (2.13–2.87) | |
| ≥2 | 5.13 (4.45–5.92) | |
| Study site | .009 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiological Studies Depression Scale; CI, confidence interval; OR, odds ratio.
High depressive symptoms are indicated by a CES-D score of 16 or higher. All covariates are time varying unless otherwise noted.
CHANGE IN SERUM HORMONE LEVELS AND DEPRESSIVE SYMPTOMS
In the minimally adjusted models, only change in levels of logT and logFTI over time were significantly associated with high CES-D scores (Table 2), while only change in logT remained significant in the fully adjusted analyses (Table 4). Overall, about 75% of the observed differences in logT over time were either decreases (−3.93 to 0.00 ng/dL) or very small increases (up to 0.16 ng/dL); 25% were increases (0.16 to 2.42 ng/dL). Results for the change in logT from baseline showed that women with high depressive symptoms had smaller decreases or greater increases in logT from baseline. For ease of interpretation Figure 2 illustrates the relationship of quartiles of change in all hormones with odds of a high CES-D score. It can be seen that as the change in logT positively increases, the greater the odds of a high CES-D score become. In the fully adjusted analyses of the 3 levels of CES-D scores, change in logT from baseline was associated with an OR of 1.38 (95% confidence interval, 1.09–1.75) for the group with the highest CES-D scores compared with the group with low CES-D scores and was associated with an OR of 1.10 (95% confidence interval, 0.91–1.32) for the group with middle CES-D scores compared with the group with low CES-D scores.
Table 4.
Fully Adjusted Random-Effects Logistic Regression Model Examining the Association of Change in Testosterone Levels With the Odds of High Depressive Symptoms From Visits 1 Through 8a
| Covariate/Estimated Parameter | OR (95% CI) | P Value |
|---|---|---|
| Change in logtestosterone level from baseline | 1.23 (1.04–1.45) | .01 |
| Baseline logtestosterone | 1.27 (1.03–1.57) | .03 |
| Status, premenopause as reference | .002 | |
| Early perimenopause | 1.51 (1.17–1.94) | |
| Late perimenopause | 1.82 (1.31–2.54) | |
| Postmenopause | 1.87 (1.33–2.63) | |
| Race/ethnicity, white as reference | .001 | |
| African American | 1.11 (0.85–1.45) | |
| Chinese | 1.20 (0.72–1.98) | |
| Hispanic | 2.69 (1.32–5.48) | |
| Japanese | 2.21 (1.36–3.59) | |
| Baseline age | 0.96 (0.92–0.996) | .03 |
| Aging | 0.93 (0.90–0.96) | <.001 |
| Baseline education, ≥college as reference | <.001 | |
| ≤High school | 1.99 (1.52–2.61) | |
| >High school/some college | 1.42 (1.12–1.79) | |
| Current smoker | 1.41 (1.09–1.81) | .008 |
| Medication for nerves or depression 2 times/wk in past month | 2.70 (2.15–3.40) | <.001 |
| BMI | 1.01 (0.999–1.03) | .06 |
| Any vasomotor symptoms | 1.61 (1.39–1.87) | <.001 |
| Social support | 0.81 (0.79–0.83) | <.001 |
| Upsetting life events, 0 as reference | <.001 | |
| 1 | 2.62 (2.20–3.11) | |
| ≥2 | 5.99 (5.07–7.08) | |
| Study site | .001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CES-D, Center for Epidemiological Studies Depression Scale; CI, confidence interval; OR, odds ratio.
High depressive symptoms are indicated by a CES-D score of 16 or higher. All covariates are time varying unless otherwise noted.
Figure 2.
Odds ratios and 95% confidence intervals comparing the quartiles of log-transformed estradiol (E2) (A), follicle-stimulating hormone (FSH) (B), testosterone (T) (C), and dehydroepiandrosterone sulfate (DHEA-S) (D) level difference from baseline, adjusted for race, site, time, baseline age, and log-transformed hormone level. A, Quartiles indicate the following: 1, reference group in which logE2 decreases the most from baseline (mean difference=−1.92; range, −5.99 to −1.14); 2, logE2 decreases slightly from baseline (mean difference=−0.74; range, −1.14 to −0.40); 3, logE2 does not change much from baseline (mean difference=−0.10; range, −0.40 to 0.23); and 4, logE2 increases from baseline (mean difference=0.96; range, 0.23 to 4.18). B, Quartiles indicate the following: 1, reference group in which logFSH decreases from baseline (mean difference=−0.47; range, −2.94 to 0.01); 2, logFSH does not change much from baseline (mean difference=0.29; range, 0.01 to 0.58); 3, logFSH increases slightly from baseline (mean difference=0.93; range, 0.58 to 1.32); and 4, logFSH increases the most from baseline (mean difference=1.96; range, 1.32 to 4.48). C, Quartiles indicate the following: 1, reference group in which logT decreases the most from baseline (mean difference=−0.84; range, −3.93 to −0.46); 2, logT decreases slightly from baseline (mean difference=−0.29; range, −0.46 to −0.14); 3, logT does not change much from baseline (mean difference=−0.00; range, −0.14 to 0.16); and 4, logT increases from baseline (mean difference=0.43; range, 0.16 to 2.42). D, Quartiles indicate the following: 1, reference group in which logDHEA-S decreases the most from baseline (mean difference=−0.56; range, −3.82 to −0.26); 2, logDHEA-S decreases slightly from baseline (mean difference=−0.13; range, −0.26 to −0.01); 3, logDHEA-S does not change much from baseline (mean difference=0.12; range, −0.01 to 0.25); and 4, logDHEA-S increases from baseline (mean difference=0.54; range, 0.25 to 3.66).
Finally, in all analyses, the odds of high depressive symptoms were significantly greater when women were perimenopausal and postmenopausal than when they were premenopausal (Table 3 and Table 4).
COVARIATES AND DEPRESSIVE SYMPTOMS
Associations among the covariates and between each covariate and logT were small (r ≤ 0.16). Although associations were significant, the covariates reduced the effect of logT by only a small amount (concurrent logT: OR=1.19 in the minimally adjusted model; OR=1.15 in the fully adjusted model). Of note, in models of both concurrent logT and change in logT over time, covariates including upsetting life events, lower education, and vasomotor symptoms showed strong associations with high depressive symptoms. Social support had a protective effect, being associated with lower odds of having high depressive symptoms (Table 3 and Table 4).
COMMENT
In this study, we assessed whether reproductive hormones were related to risk of high depressive symptoms (CES-D score ≤16) over 8 years of follow-up and whether they might explain the previously observed association between menopausal status and these symptoms in the early years of SWAN.1 Although we found no significant effect of the level of or change in E2 or FSH on risk of depressive symptoms, we did find that higher current total testosterone levels and an increase in testosterone levels from baseline were significantly associated with high CES-D scores whether defined as a score of 16 or greater or 22 or greater. This association was independent of multiple covariates and confounders and, most important, of menopausal status and vasomotor symptoms. Importantly, perimenopause and postmenopause continued to be significantly independently associated with odds of high depressive symptoms.
Neuroendocrine theories suggest that estrogens in particular have a role in the development of depression in women as they may modulate the activity of the serotonin neurotransmitter system that has been linked to depression.24,25 At one time, the biological link between menopause and mood was hypothesized to be that low levels of estrogen were associated with negative mood. However, epidemiological studies of menopause have shown either no relationship between negative mood symptoms and menopausal status26–28 or higher levels of symptoms during perimenopause29–33 and not during post-menopause when estrogen levels are low. Although several short-term randomized controlled trials have shown efficacy of exogenous estrogen in attenuating depression in perimenopausal women,34,35 no studies demonstrate direct associations between depression and se rum E2 levels. 8,9,36
More recently, it has been postulated that the fluctuating and irregular pattern of E2 and FSH secretion occurring during the menopausal transition37,38 may confer neuroendocrine susceptibility to depression in vulnerable women.36 However, by its very nature, the changing hormonal milieu during the transitional years is difficult to measure adequately. Two studies have supported this hypothesis.2,39 Freeman et al2 found that over 8 years of follow-up of 231 premenopausal women without high depressive symptoms at baseline, average variability in the means of FSH and E2 levels was significantly positively associated with odds of high depressive symptoms. Ryan et al39 reported that among postmenopausal women, declines in serum E2 levels and large increases in FSH levels over 2 years were associated with increased odds of high depressive symptoms, but absolute hormone levels were not. These studies included only select periods of the menopausal transition, including pre-menopause and early perimenopause in the study by Free-man and colleagues and postmenopause in the study by Ryan and colleagues. Neither study encompassed the entire menopausal transition through postmenopause. The use of 2 annual blood draws 1 month apart by Freeman et al2 may have increased the ability to detect effects of hormones during the transition. In the postmenopausal cohort,39 serum hormone levels of E2 and FSH are more stable than are those obtained in perimenopausal women; thus, the hormone measures may be more reliably informative.
Animal and clinical studies indicate that androgens, which are produced in women directly or indirectly from peripheral conversion of precursors from the adrenal glands and the ovaries, influence mood and behavior—particularly aggressive and sexual behavior.24,40 The literature indicating that DHEA and DHEA-S may modulate mood has yielded contradictory findings; depressive symptoms have been positively12 and negatively11,41,42 correlated with these androgen precursors.
Although T in women has typically been studied in the context of sexual behavior, the cross-sectional and longitudinal studies that have considered the association between T and mood have reported mixed results.8–10,42–45 In the Seattle Midlife Women’s Health Study, urinary T collected repeatedly over 10 years was not associated with high depressive symptoms, nor were any other reproductive hormones.8,43 Similar null findings were reported for serum T in a group of midlife women in Baltimore, Maryland,9 and Victoria, Australia,46 and in a group of women aged 49 to 65 years participating in the African American Health project in St Louis, Missouri.42 A study of a large sample of elderly women reported an inverse association between free T levels and depressive symptoms.47 Several studies found an improvement in mood and well-being with the use of exogenous T, but these produced supraphysiological levels.48–50
Studies of select samples of females, including early adolescents,45 women with severe premenstrual symptoms,51 peripartal women,52 those with major depression,10,53 and women with polycystic ovary syndrome,54,55 have reported findings similar to ours. Two small clinical trials reported a significant positive relationship between logT and major depression in women compared with age-matched controls.10,53 Eriksson et al51 in Sweden found that serum levels of T were significantly higher at 3 points during the menstrual cycle in 11 women with severe premenstrual irritability and dysphoria than in age-matched controls without menstrual symptoms. In a study of 193 pregnant women at term, serum logT levels were significantly correlated with depression scores prepartum and on the first and second postpartum days (r=0.15–0.18).52 Among a representative sample of 369 girls aged 9 to 13 years residing in western North Carolina, mean levels of logT were highly significantly associated with major depression. Finally, among women with polycystic ovary syndrome, a common endocrine disorder characterized by hyperandrogenism, logT and depression were positively associated.55
The inconsistency in the results of multiple studies of T and mood associations may be explained in part by the heterogeneity of the samples and designs. Further, the positive associations between logT and depression have been reported largely in select samples during periods of reproductive hormone fluctuations and extreme excursions as exemplified by puberty, by peripartum, and premenstrually or among women with an endocrine disorder, suggesting that the hormonal milieu at these times may alter the dynamics of the relationship between T and depressive symptoms in vulnerable women.
In women, the adrenal glands and the ovaries are the main sources of circulating T, derived either directly from these organs or indirectly through the peripheral conversion of androstenedione.22,56 Testosterone is also aromatized to estradiol and, similar to E2, T increases density of serotonin receptors in the brain.40 If this were the mechanism by which T influences mood, we would expect to find an inverse association between T and mood. On the other hand, perhaps the relative androgenicity of the hormonal milieu as women move through the menopausal transition influences neurotransmission and disrupts mood. However, we found no significant effects of a higher level of serum T relative to E2 (E2-T ratio) or change in this ratio on depressive symptoms, which may be owing to the limitations of annual serum samples to adequately measure the variability in E2 levels during the menopausal transition. As noted later, T is not subject to the same variability.
Noteworthy is that although we observed a significant association of logT with depressive symptoms, this was substantially smaller than those between other independent variables and symptoms, including menopausal status. Importantly, upsetting life events increased the odds of high depressive symptoms by 2.5- to 5-fold, and high social support decreased the odds by one-fifth.
The study had several limitations. Serum samples were collected annually, which underrepresents the variability of E2 and FSH levels and limits the capturing of the dynamic nature of the changes in these hormones during the early menopausal transition and the increasing menstrual irregularity of late perimenopause. This is somewhat less of a problem for the measurement of T because it changes more slowly with age and is not subject to the variability that E2 and FSH levels are during the menopausal transition.22,57 Commercially available T assays have limitations.58 Therefore, SWAN used a modified immunoassay that extends the low limit of detection and was cross validated with mass spectrometry.
Another limitation was that the CES-D is a measure of depressive symptoms, not major depression. Additionally, some studies in primary care settings show that the cutoff score of 16 or higher is also a useful screen for general anxiety and panic disorders59,60 and that the CES-Dis highly correlated with anxiety measures such as the Beck Anxiety Inventory (r=0.68),59 suggesting that the CES-D is not specific for depression. However, our analyses using a higher CES-D cutoff score (≥22) showed results consistent with those identified with the cutoff score of 16 or higher.
To our knowledge, the current study is the first longitudinal study of serum hormones, particularly T, and depressive symptoms to be conducted in a large and diverse sample of midlife women traversing the menopausal transition. The results are independent of multiple relevant and confounding covariates, including BMI and the presence of vasomotor symptoms. Notably, the association between menopausal status and high depressive symptoms remained strong and consistently independent of any of the specific hormones. Along with the modest association between T and high depressive symptoms, this suggests that high depressive symptoms during the menopausal transition are unlikely to be due only to levels of or marked changes in the reproductive hormone environment during this period.
Acknowledgments
Funding/Support: The Study of Women’s Health Across the Nation (SWAN) is supported by grants NR004061 from the National Institute of Nursing Research and AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, and AG012495 from the National Institute on Aging and by the Office of Research on Women’s Health, National Institutes of Health.
Study of Women’s Health Across the Nation (SWAN)
Clinical Centers
University of Michigan, Ann Arbor: MaryFran Sowers, PhD, principal investigator; Massachusetts General Hospital, Boston: Robert Neer, MD, principal investigator 1994 to 1999; Joel Finkelstein, MD, principal investigator 1999 to present; Rush University Medical Center, Chicago, Illinois: Lynda Powell, PhD, principal investigator 1994 to 2009, Howard Kravitz, DO, MPH, principal investigator 2009 to present; University of California, Davis/Kaiser: Ellen Gold, PhD, principal investigator; University of California, Los Angeles: Gail Greendale, MD, principal investigator; University of Medicine and Dentistry–New Jersey Medical School, Newark: Gerson Weiss, MD, principal investigator 1994 to 2004; Nanette Santoro, MD, principal investigator 2004 to present; and University of Pittsburgh, Pittsburgh, Pennsylvania: Karen Matthews, PhD, principal investigator.
National Institutes of Health Program Office
National Institute on Aging, Bethesda, Maryland: Marcia Ory, PhD, 1994 to 2001, Sherry Sherman, PhD, 1994 to present; National Institute of Nursing Research, Bethesda: program officers.
Central Laboratory
University of Michigan: Daniel McConnell, PhD (Central Ligand Assay Satellite Services).
Coordinating Center
New England Research Institutes, Watertown, Massachusetts: Sonja McKinlay, PhD, principal investigator 1995 to 2001; University of Pittsburgh: Kim Sutton-Tyrrell, PhD, principal investigator 2001 to present.
Steering Committee
Chris Gallagher, MD, chair; Susan Johnson, MD, chair.
Footnotes
Financial Disclosure: None reported.
Disclaimer: The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging, National Institute of Nursing Research, Office of Research on Women’s Health, or National Institutes of Health.
References
- 1.Bromberger JT, Matthews KA, Schott LL, Brockwell S, Avis NE, Kravitz HM, Everson-Rose SA, Gold EB, Sowers M, Randolph JF., Jr Depressive symptoms during the menopausal transition: the Study of Women’s Health Across the Nation (SWAN) J Affect Disord. 2007;103(1–3):267–272. doi: 10.1016/j.jad.2007.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 3.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 4.Rubinow D, Schmidt P. The neurobiology of menstrual cycle-related mood disorders. In: Charney D, Nestler E, Bunneyu B, editors. Neurobiology of Mental Illness. New York, NY: Oxford University Press; 1999. pp. 907–914. [Google Scholar]
- 5.McEwen BS, Alves SE. Estrogen actions in the central nervous system. Endocr Rev. 1999;20(3):279–307. doi: 10.1210/edrv.20.3.0365. [DOI] [PubMed] [Google Scholar]
- 6.Golden R, Gilmore J. Serotonin and mood disorders. Psychiatr Ann. 1990;20(10):580–586. [Google Scholar]
- 7.Janowsky H, Halbreich U, Rausch J. Association among ovarian hormones, other hormones, emotional disorders, and neurotransmitters. In: Jensvold M, Halbreich U, Hamilton J, editors. Psychopharmacology and Women: Sex, Gender, and Hormones. Washington, DC: American Psychiatric Press; 1996. pp. 85–106. [Google Scholar]
- 8.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Mariella A, Mitchell ES. Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause. 2008;15(2):223–232. doi: 10.1097/gme.0b013e3181450fc2. [DOI] [PubMed] [Google Scholar]
- 9.Gallicchio L, Schilling C, Miller SR, Zacur H, Flaws JA. Correlates of depressive symptoms among women undergoing the menopausal transition. J Psychosom Res. 2007;63(3):263–268. doi: 10.1016/j.jpsychores.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Weber B, Lewicka S, Deuschle M, Colla M, Heuser I. Testosterone, androstenedione and dihydrotestosterone concentrations are elevated in female patients with major depression. Psychoneuroendocrinology. 2000;25(8):765–771. doi: 10.1016/s0306-4530(00)00023-8. [DOI] [PubMed] [Google Scholar]
- 11.Barrett-Connor E, von Mühlen D, Laughlin GA, Kripke A. Endogenous levels of dehydroepiandrosterone sulfate, but not other sex hormones, are associated with depressed mood in older women: the Rancho Bernardo Study. J Am Geriatr Soc. 1999;47(6):685–691. doi: 10.1111/j.1532-5415.1999.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 12.Fabian TJ, Dew MA, Pollock BG, Reynolds CF, III, Mulsant BH, Butters MA, Zmuda MD, Linares AM, Trottini M, Kroboth PD. Endogenous concentrations of DHEA and DHEA-S decrease with remission of depression in older adults. Biol Psychiatry. 2001;50(10):767–774. doi: 10.1016/s0006-3223(01)01198-2. [DOI] [PubMed] [Google Scholar]
- 13.Sowers MF, Crawford SL, Sternfeld B, Morgenstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopause. In: Lobo R, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology. San Diego, CA: Academic Press; 2000. pp. 175–178. [Google Scholar]
- 14.Radloff L. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 15.Boyd JH, Weissman MM, Thompson WD, Myers JK. Screening for depression in a community sample: understanding the discrepancies between depression symptom and diagnostic scales. Arch Gen Psychiatry. 1982;39(10):1195–1200. doi: 10.1001/archpsyc.1982.04290100059010. [DOI] [PubMed] [Google Scholar]
- 16.Guarnaccia PJ, Angel R, Worobey JL. The factor structure of the CES-D in the Hispanic Health and Nutrition Examination Survey: the influences of ethnicity, gender, and language. Soc Sci Med. 1989;29(1):85–94. doi: 10.1016/0277-9536(89)90131-7. [DOI] [PubMed] [Google Scholar]
- 17.Jones-Webb RJ, Snowden LR. Symptoms of depression among blacks and whites. Am J Public Health. 1993;83(2):240–244. doi: 10.2105/ajph.83.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying YW. Depressive symptomatology among Chinese-Americans as measured by the CES-D. J Clin Psychol. 1988;44(5):739–746. doi: 10.1002/1097-4679(198809)44:5<739::aid-jclp2270440512>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization Scientific Group. Research on the Menopause in the 1990s. Geneva, Switzerland: World Health Organization; 1996. WHO Technical Report Series 866. [PubMed] [Google Scholar]
- 20.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Climacteric. 2001;4(4):267–272. [PubMed] [Google Scholar]
- 21.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 22.Burger HG, Dudley EC, Cui J, Dennerstein L, Hopper JL. A prospective longitudinal study of serum testosterone, dehydroepiandrosterone sulfate, and sex hormone-binding globulin levels through the menopause transition. J Clin Endocrinol Metab. 2000;85(8):2832–2838. doi: 10.1210/jcem.85.8.6740. [DOI] [PubMed] [Google Scholar]
- 23.Randolph JF, Jr, Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, Brockwell SE, Matthews KA. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90(11):6106–6112. doi: 10.1210/jc.2005-1374. [DOI] [PubMed] [Google Scholar]
- 24.Rubinow DR, Schmidt PJ, Roca CA. Estrogen-serotonin interactions: implications for affective regulation. Biol Psychiatry. 1998;44(9):839–850. doi: 10.1016/s0006-3223(98)00162-0. [DOI] [PubMed] [Google Scholar]
- 25.Joffe H, Cohen LS. Estrogen, serotonin, and mood disturbance: where is the therapeutic bridge? Biol Psychiatry. 1998;44(9):798–811. doi: 10.1016/s0006-3223(98)00169-3. [DOI] [PubMed] [Google Scholar]
- 26.Matthews KA, Bromberger J, Egland G. Behavioral antecedents and consequences of the menopause. In: Korenman SG, editor. The Menopause. Norwell, MA: Serono Symposia; 1990. [Google Scholar]
- 27.Hunter M. The south-east England longitudinal study of the climacteric and postmenopause. Maturitas. 1992;14(2):117–126. doi: 10.1016/0378-5122(92)90004-n. [DOI] [PubMed] [Google Scholar]
- 28.Kaufert PA, Gilbert P, Tate R. The Manitoba Project: a re-examination of the link between menopause and depression. Maturitas. 1992;14(2):143–155. doi: 10.1016/0378-5122(92)90006-p. [DOI] [PubMed] [Google Scholar]
- 29.Kuh DL, Wadsworth M, Hardy R. Women’s health in midlife: the influence of the menopause, social factors and health in earlier life. Br J Obstet Gynaecol. 1997;104(8):923–933. doi: 10.1111/j.1471-0528.1997.tb14352.x. [DOI] [PubMed] [Google Scholar]
- 30.Freeman EW, Sammel MD, Liu L, Gracia CR, Nelson DB, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61(1):62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 31.Maartens LW, Knottnerus JA, Pop VJ. Menopausal transition and increased depressive symptomatology: a community based prospective study. Maturitas. 2002;42(3):195–200. doi: 10.1016/s0378-5122(02)00038-5. [DOI] [PubMed] [Google Scholar]
- 32.Avis NE, Brambilla D, McKinlay SM, Vass K. A longitudinal analysis of the association between menopause and depression: results from the Massachusetts Women’s Health Study. Ann Epidemiol. 1994;4(3):214–220. doi: 10.1016/1047-2797(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor VM, Del Mar CB, Sheehan M, Siskind V, Fox-Young S, Cragg C. Do psycho-social factors contribute more to symptom reporting by middle-aged women than hormonal status? Maturitas. 1994;20(2–3):63–69. doi: 10.1016/0378-5122(94)90001-9. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, Rubinow DR. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183(2):414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 35.Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Arch Gen Psychiatry. 2001;58(6):529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 36.Schmidt PJ, Murphy JH, Haq N, Danaceau MA, St Clair LS. Basal plasma hormone levels in depressed perimenopausal women. Psychoneuroendocrinology. 2002;27(8):907–920. doi: 10.1016/s0306-4530(02)00004-5. [DOI] [PubMed] [Google Scholar]
- 37.Santoro N, Brown JR, Adel T, Skurnick JH. Characterization of reproductive hormonal dynamics in the perimenopause. J Clin Endocrinol Metab. 1996;81(4):1495–1501. doi: 10.1210/jcem.81.4.8636357. [DOI] [PubMed] [Google Scholar]
- 38.Hall JE. Neuroendocrine physiology of the early and late menopause. Endocrinol Metab Clin North Am. 2004;33(4):637–659. doi: 10.1016/j.ecl.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 39.Ryan J, Burger HG, Szoeke C, Lehert P, Ancelin ML, Henderson VW, Dennerstein L. A prospective study of the association between endogenous hormones and depressive symptoms in postmenopausal women. Menopause. 2009;16 (3):509–517. doi: 10.1097/gme.0b013e31818d635f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fink G, Sumner B, Rosie R, Wilson H, McQueen J. Androgen actions on central serotonin neurotransmission: relevance for mood, mental state and memory. Behav Brain Res. 1999;105(1):53–68. doi: 10.1016/s0166-4328(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 41.Cawood EH, Bancroft J. Steroid hormones, the menopause, sexuality and well-being of women. Psychol Med. 1996;26(5):925–936. doi: 10.1017/s0033291700035261. [DOI] [PubMed] [Google Scholar]
- 42.Haren MT, Malmstrom TK, Banks WA, Patrick P, Miller DK, Morley JE. Lower serum DHEAS levels are associated with a higher degree of physical disability and depressive symptoms in middle-aged to older African American women. Maturitas. 2007;57(4):347–360. doi: 10.1016/j.maturitas.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woods NF, Smith-Dijulio K, Percival DB, Tao EY, Taylor HJ, Mitchell ES. Symptoms during the menopausal transition and early postmenopause and their relation to endocrine levels over time: observations from the Seattle Midlife Women’s Health Study. J Womens Health (Larchmt) 2007;16(5):667–677. doi: 10.1089/jwh.2006.0138. [DOI] [PubMed] [Google Scholar]
- 44.Markianos M, Tripodianakis J, Sarantidis D, Hatzimanolis J. Plasma testosterone and dehydroepiandrosterone sulfate in male and female patients with dysthymic disorder. J Affect Disord. 2007;101(1–3):255–258. doi: 10.1016/j.jad.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 45.Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29(5):1043–1053. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- 46.Bell RJ, Donath S, Davison SL, Davis SR. Endogenous androgen levels and well-being: differences between premenopausal and postmenopausal women. Menopause. 2006;13(1):65–71. doi: 10.1097/01.gme.0000191212.58856.96. [DOI] [PubMed] [Google Scholar]
- 47.Morsink LF, Vogelzangs N, Nicklas BJ, Beekman AT, Satterfield S, Rubin SM, Yaffe K, Simonsick E, Newman AB, Kritchevsky SB, Penninx BW Health ABC Study. Associations between sex steroid hormone levels and depressive symptoms in elderly men and women: results from the Health ABC Study. Psychoneuroendocrinology. 2007;32(8–10):874–883. doi: 10.1016/j.psyneuen.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Davis SR, McCloud P, Strauss BJ, Burger H. Testosterone enhances estradiol’s effects on postmenopausal bone density and sexuality. Maturitas. 1995;21 (3):227–236. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- 49.Sherwin BB, Gelfand MM, Brender W. Androgen enhances sexual motivation in females: a prospective, crossover study of sex steroid administration in the surgical menopause. Psychosom Med. 1985;47(4):339–351. doi: 10.1097/00006842-198507000-00004. [DOI] [PubMed] [Google Scholar]
- 50.Sherwin BB, Gelfand MM. Sex steroids and affect in the surgical menopause: a double-blind, cross-over study. Psychoneuroendocrinology. 1985;10(3):325–335. doi: 10.1016/0306-4530(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 51.Eriksson E, Sundblad C, Lisjo P, Modigh K, Andersch B. Serum levels of androgens are higher in women with premenstrual irritability and dysphoria than in controls. Psychoneuroendocrinology. 1992;17(2–3):195–204. doi: 10.1016/0306-4530(92)90058-f. [DOI] [PubMed] [Google Scholar]
- 52.Hohlagschwandtner M, Husslein P, Klier C, Ulm B. Correlation between serum testosterone levels and peripartal mood states. Acta Obstet Gynecol Scand. 2001;80(4):326–330. doi: 10.1034/j.1600-0412.2001.080004326.x. [DOI] [PubMed] [Google Scholar]
- 53.Baischer W, Koinig G, Hartmann B, Huber J, Langer G. Hypothalamic-pituitary-gonadal axis in depressed premenopausal women: elevated blood testosterone concentrations compared to normal controls. Psychoneuroendocrinology. 1995;20(5):553–559. doi: 10.1016/0306-4530(94)00081-k. [DOI] [PubMed] [Google Scholar]
- 54.Himelein MJ, Thatcher SS. Polycystic ovary syndrome and mental health: a review. Obstet Gynecol Surv. 2006;61(11):723–732. doi: 10.1097/01.ogx.0000243772.33357.84. [DOI] [PubMed] [Google Scholar]
- 55.Weiner CL, Primeau M, Ehrmann DA. Androgens and mood dysfunction in women: comparison of women with polycystic ovarian syndrome to healthy controls. Psychosom Med. 2004;66(3):356–362. doi: 10.1097/01.psy.0000127871.46309.fe. [DOI] [PubMed] [Google Scholar]
- 56.Arlt W. Androgen therapy in women. Eur J Endocrinol. 2006;154(1):1–11. doi: 10.1530/eje.1.02062. [DOI] [PubMed] [Google Scholar]
- 57.Sowers MF, Zheng H, McConnell D, Nan B, Karvonen-Gutierrez CA, Randolf JF., Jr Testosterone, sex hormone-binding globulin and free androgen index among adult women: chronological and ovarian aging. Hum Reprod. 2009;24(9):2276–2285. doi: 10.1093/humrep/dep209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rosner W, Auchus RJ, Azziz R, Sluss PM, Raff H. Position statement: utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab. 2007;92(2):405–413. doi: 10.1210/jc.2006-1864. [DOI] [PubMed] [Google Scholar]
- 59.McQuaid JR, Stein MB, McCahill M, Laffaye C, Ramel W. Use of brief psychiatric screening measures in a primary care sample. Depress Anxiety. 2000;12(1):21–29. doi: 10.1002/1520-6394(2000)12:1<21::AID-DA3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 60.Fechner-Bates S, Coyne JC, Schwenk TL. The relationship of self-reported distress to depressive disorders and other psychopathology. J Consult Clin Psychol. 1994;62(3):550–559. doi: 10.1037//0022-006x.62.3.550. [DOI] [PubMed] [Google Scholar]