Abstract
We review evidence for two distinct cognitive processes by which humans and animals represent the navigable environment. One process uses the shape of the extended 3D surface layout to specify the navigator’s position and orientation. A second process uses objects and patterns as beacons to specify the locations of significant objects. Although much of the evidence for these processes comes from neurophysiological studies of navigating animals and neuroimaging studies of human adults, behavioral studies of navigating children shed light both on the nature of these systems and on their interactions.
Introduction
An animal’s ability to find its way around the world is of utmost importance to its survival. Most animals navigate by keeping track of the direction and extent of their own movement with respect to the visual environment by updating their position as they move (Mittelstaedt & Mittelstaedt, 1980; Gallistel, 1990; Wang & Spelke, 2002; Burgess, 2006). This process of path integration requires no encoding or memory of environmental features, but it is limited both in precision and in the complexity of the navigation patterns it supports (Etienne, 2004; Wang et al., 2006). To overcome these limits, navigating animals and humans encode properties of the environment, including its objects and landscapes, and they use that information both to identify significant locations and to maintain or recover a sense of their own position and orientation. Thus, the spatial representations of the environment that guide navigating animals are best revealed when path integration is pushed beyond its limits or disabled altogether by disorientation.
The present review focuses on behavioral evidence, mainly from studies of disoriented preschool children, supporting the existence of two independent cognitive processes by which spatial properties of the environment are analyzed for purposes of navigation. One process performs a geometric analysis of the environment’s extended 3D surfaces, and it uses the distances and directions of surfaces to specify the navigator’s position. The other process performs geometric and featural analyses of objects and 2D patterns, and it uses their distinctive properties to indicate specific goal locations. These two processes normally work together, but they can be distinguished in four ways: they operate on different kinds of input, they perform different kinds of computations, they yield different kinds of information about the navigator and the environment, and they are associated with activity in different brain systems. We consider first the evidence for these two processes in adult non-human animals and humans. Then we turn to the evidence from studies of young animals and human children.
Mature systems of navigation
Cheng and Gallistel (Cheng & Gallistel, 1984; Cheng, 1986) were the first to discover that untrained rats rely primarily on the shape of the navigable space to specify a goal location. When rats were disoriented after observing the burying of food in a rectangular chamber with a variety of detectable landmarks such as odors, 2D contrast patterns, and walls of different brightness, they then dug for the food in two locations: its actual position and a geometrically congruent position on the opposite side of the chamber (see Figure 1). Because the room’s landmarks served to distinguish these positions, rats’ search patterns suggested that they were guided only by the shape of the room. With training, rats came to use objects positioned directly at the food’s location, through a curious behavioral pattern. Immediately after disorientation, trained rats headed either for the correct corner or for the geometrically equivalent opposite corner, as did their untrained counterparts. Before digging, however, the trained rats checked for patterns near the goal position, and they reversed direction if the correct pattern failed to appear.
Figure 1.
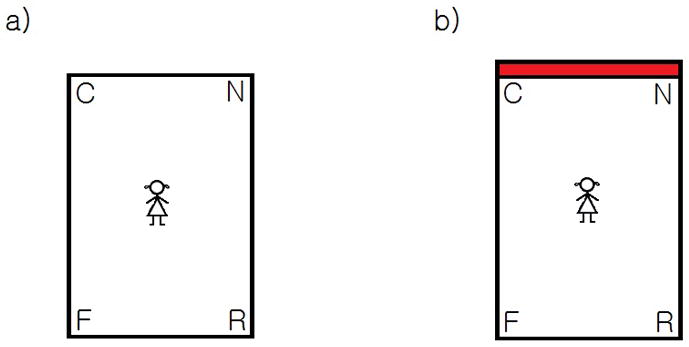
Schematic, overhead depiction of two testing environments with and without a featural cue (a distinctively colored wall). After an object is hidden at C and participants are disoriented in (a), humans and non-humans animals alike use the geometric shape of the rectangular room to search the correct (C) and rotationally symmetric (R) corners more often than the near (N) and far (F) geometrically incorrect corners. Under some conditions, moreover, children and non-human animals fail to use the potential landmark in (b) to break the room’s symmetry, and therefore search at R as often as at C.
Based on these observations, Cheng and Gallistel proposed that two distinct processes guided disoriented rats’ navigation: a computation of layout geometry operating automatically and without training, and a beacon-guidance process sensitive to objects or patterns near the goal location and subject to attention and learning. Gallistel (1990) argued for the adaptiveness of a geometric process applied only to a representation of 3D surface layouts. Objects in nature such as trees and rocks are poor guides to reorientation because they have many featural look-alikes that can only be distinguished through fine-grained, computationally inefficient comparisons. Moreover, such objects tend to be movable or to have features, such as leaves, that change over time. From an ecological standpoint, therefore, extended 3D surface landscapes are the most stable, reliable, distinctive cues in the natural environment that remain distinguishable even with a relatively coarse-grained representation.
In recent years, Gallistel’s argument has been supported by research in robotics. Early attempts to design autonomously navigating robots relied on raw images, either from artificial vision systems or from range-finding systems, that failed to segregate information about enduring, extended surfaces from information about objects. One difficulty faced by such systems, however, was the error caused by misrecognition of a location when the robot encountered similar or displaced objects in different parts of the environment (Thrun, 2002). A second difficulty was the combinatorial explosion that accrued as the robot moved into new territory and recorded all the spatial details in the cluttered environment. By eliminating objects and representing only extended surfaces, a navigating robot can form representations that are more economical, because surfaces tend to be smooth and therefore can be presented with just a few points each (Egerton, Callaghan, & Chernett, 2000; Gee, Chekhlov, Calway, & Mayol-Cuevas, 2008; Silveira, Malis, & Rives, 2008). Both ecological and computational considerations therefore favor a distinct process of navigation by surface layout geometry.
In the years since Cheng and Gallistel’s original studies with rats, research on reorientation was conducted with many species of animals, including pigeons, chicks, fish, monkeys, humans, and ants (for review, see Cheng & Newcombe, 2005). The experiments on ants are of special interest, because they reveal a pattern of behavior very close to that originally reported by Cheng (1986). In these experiments (Wystrach & Beugnon, 2009), oriented ants navigated from their nest to a testing space where they encountered food to be transported back to the nest, at the center of a rectangular arena. Before setting off for the nest, ants were disoriented within this arena. In the first experiments, the arena had holes in each of its four corners by which ants could return to the nest, and the surface layout beyond the arena was clearly visible, with geometric structure that uniquely specified the ant’s orientation. Ants quickly learned to locate a corner exit from the arena; thereafter, they moved directly to this single corner immediately after disorientation when the larger array was visible, and they moved with equal frequency to this single corner and to the geometrically equivalent opposite corner when the larger array was hidden. The latter performance indicated that the ants were indeed disoriented, and that they used the shape of the visible surface layout to reorient themselves.
In later experiments, ants were trained with distinctive patterns at each corner of the arena. Although the patterns were both visible and discriminable from the ant’s starting location at the arena’s center, the ants ignored them and reoriented only by the shape of the arena. The authors noted, however, that the minimal training did not strongly encourage beacon guidance, so they performed a further experiment with more extensive training, in which only one corner of the rectangular array led to the nest, with its position indicated by a unique pattern. Ants’ behavior was striking. Like Cheng’s rats, they learned to exit only through the corner marked by the unique pattern, but they found this corner through a circuitous route: They approached the correct corner and the rotationally opposite corner with high frequency, and then reversed direction if they found themselves in the corner with the wrong patterning. Like rats, disoriented ants’ search appeared to be guided by two distinct processes: a reorientation process sensitive to layout geometry, and a beacon-guidance process sensitive to a learned, visual pattern.
Although the hypothesis of separate systems for navigating by layout geometry vs. distinctive patterns or objects originally was suggested by studies of reorientation, the evolutionary and computational considerations described above suggest that separate systems would be computationally efficient for oriented animals as well. Consistent with this suggestion, there is evidence for independent representation of landmark objects vs. surface layout geometry in oriented animals. For example, rats in a water maze learn the location of a hidden platform faster with respect to geometric than featural information (Benhamou and Poucet, 1998). In many studies, moreover, animals encode layout geometry automatically but learn to use objects as landmarks only with heightened attention or training (Cheng & Newcombe, 2005; though cf. Cheng, 2008).
Consistent with these behavioral data, there is evidence that multiple systems of specialized neural structures underlie spatial behavior (Packard & McGaugh, 1996, White & McDonald, 2002). Research on the hippocampus and surrounding regions has resulted in identification of regions that process surface layout geometry but not landmark objects or surface colors and patterns. Single-cell recording studies of rats’ hippocampal place cells, which fire when an untrained, freely moving animal moves to a particular location in the environment, have shown that extended cues, particularly walls of the testing space, are crucial to the representation of location (O’Keefe & Burgess, 1996; O’Keefe & Nadel, 1978). Changes in surface boundaries affect place cell activity, but changes in surface texture and material often have no effect, unless these features change so radically that the animals represent the environment as completely novel (Lever, Wills, Cacucci, Burgess, & O’Keefe, 2002). Moreover, the firing fields of place cells and nearby head-direction cells (which fire when a rat is oriented a particular way with respect to the environment) are controlled by objects placed near the walls of the testing space, but not by objects placed well within the environment (Cressant, Muller, & Poucet, 1997; Zugaro, Berthoz, & Wiener, 2001). All these studies suggest that freely moving rodents spontaneously encode their location with respect to the distance and direction of extended surfaces in the environment, but not with respect to the colors or textures of surfaces or to the positions of freestanding objects.
The hippocampus has also been shown to encode view-independent representations of space, capturing the relative positions of multiple elements in the environment, rather than view-specific associations to individual landmarks (Burgess, Maguire, O’Keefe, 2002). Hippocampal lesions produce selective impairments in spatial tasks that require the encoding of room shape or relationships among multiple environmental features but not in tasks that require the use of an object for beacon guidance (e.g., Morris, Garrad, Rawlins, & O’Keefe, 1982; McGregor, Hayward, Pearce, & Good, 2004). However, the hippocampus is not the only brain region that informs spatial navigation using the 3D environmental surface layout. Representation of geometric borders has recently been found in the entorhinal cortex of rats; these cells are hypothesized to define the perimeter of the environment and serve as reference frames for encoding locations within that environment (Solstad, Boccara, Kropff, Moser, & Moser, 2008).
Spatial functional specialization has also been found in the avian brain. Studies manipulating brain regions in pigeons and chicks have revealed dissociable neural architecture for geometric analysis of room shape and the use of feature cues (Nardi & Bingman, 2007; Tommasi, Gagliardo, Andrew, & Vallortigara, 2003); for instance, left-eyed split-brained chicks reorient by both featural cues and room shape, whereas right-eyed chicks only reorient by featural cues (Vallortigara, Pagni, & Sovrano, 2004).
In humans, functional neuroimaging studies have shown preferential activation of the right posterior hippocampus for processing locations with respect to environmental boundaries and activation of the right dorsal striatum for landmark-related locations (Doeller, King, & Burgess, 2008). These researchers provided behavioral evidence, using the same visual displays and spatial memory task, that while object-related learning obeys associative reinforcement principles, boundary-related learning is incidental, consistent with the above findings from behavioral studies of animals (Doeller, & Burgess, 2008). Neuroimaging studies have also identified a parahippocampal region (Epstein & Kanwisher, 1998; Epstein, Harris, Stanley, & Kanwisher, 1999), as well as the transverse occipital sulcus (TOS) (Epstein, Higgins, & Thompson-Schill, 2005; Grill-Spector, 2003), as areas that specifically respond to visual scenes but not objects. Damage to the parahippocampal place area (PPA) impairs recognition of spatial scenes while preserving object recognition (Epstein, DeYoe, Press, Rosen, & Kanwisher, 2001; Mendez & Cherrier, 2003; Pitcher, Charles, Devlin, Walsh, & Duchaine, 2009). And abnormalities in the TOS, observed in adults with Williams Syndrome, are associated with impaired reorientation by geometry but preserved use of a color feature as a beacon (Lakusta, Dessalegn & Landau, 2010).
Many branches of neuroscience are dedicated to the study of neural systems underlying spatial cognition and navigation, and our brief review does not do them justice. There is considerable debate concerning the functions of the various neural systems activated during navigation (e.g., Burgess, 2006; Cheng, 2008), the frames of reference by which navigating animals represent both the positions of objects and the distances and directions of extended surfaces (e.g., Wang & Spelke, 2002; Burgess, 2006; Nardini, Thomas, Knowland, Braddick, & Atkinson, 2009), and the respective roles of local versus global processing in navigation (e.g., Sutton, 2009). Nevertheless, there is growing evidence for separate brain systems dedicated to the processing of geometric surface layouts, on one hand, and objects, colors and surface markings on the other.
Alternative accounts
Two main alternative theories to the present two-process view have been proposed to explain reorientation behavior. One theory claims that reorientation depends on a single, coarse-grained view-matching system based on 2D retinal snapshot representations (Cheng, 2008; Sheynikhovich, Chavarriaga, Strösslin, Arleo, & Gerstner, 2009) similar to those found in insects (Cartwright & Collett, 1982; Collett & Collett, 2002). According to this view, an animal moves to reduce the discrepancy between a stored snapshot representation of the scene and the scene it currently perceives. Consequently, the snapshot account predicts that retinal salience is the determining factor for what does and does not affect an animal’s reorientation. Computer simulation studies have demonstrated that “geometric search” using long and short wall-like surfaces can result from such a model of navigation (Cheng, 2008; Stürzl, Cheung, Cheng, & Zeil, 2008). In contrast to the two-process view, this theory makes no distinction between 2D surface features, object arrays, or surface layouts, and it gives no privileged role to 3D surface layouts apart from their retinal salience.
Retinal image matching processes can capture a rich array of behavioral and neurophysiological findings on navigating animals, especially if they are combined with other navigation processes. For example, one problem for image matching theories is that animals who have viewed an environment can continue to navigate effectively in the dark, while exhibiting very few neurophysiological differences between navigating in light and in darkness (Goodridge, Dudchenko, Worboys, Golob, & Taube, 1998; Lever, Burton, Jeewajee, O’Keefe, & Burgess, 2009; Quirk, Muller, & Kubie, 1990). A recent computational theory accounts for this phenomenon and others through a combination of path integration and image-matching processes (Sheynikhovich et al., 2009). By itself, moreover, a single image-matching process cannot readily explain why rats and ants engage separate processes for navigating by the shape of the layout and by landmark patterns. Wystrach and Beugnon (2009) suggest, however, that two distinct image-matching processes, operating at different spatial scales, could explain this behavior pattern. Thus, image-matching views present a serious challenge to Cheng and Gallistel’s thesis of a system for reorienting by layout geometry.
The second theory is the adaptive combination theory, which holds that both landmarks and geometry are processed by the same underlying mechanism, weighted according to their experienced validity and salience (Newcombe & Ratliff, 2007). On this view, individuals learn through experience that small, movable objects are unreliable as cues to reorientation. Large objects in a large room, on the other hand, are distal, stable, and salient; children learn that such potential landmarks are reliable cues for navigation and assign high weights for their use in the task of reorienting (Learmonth, Newcombe, & Huttenlocher, 2001). Like the snapshot view, the adaptive combination view grants no special advantage for 3D surface layouts other than the higher influence they have on behavior due to their salience, size, stability, and learned reliability.
In contrast to these two theories, we propose, following Cheng and Gallistel’s original theory, that humans and animals navigate by (a) an automatic process for analyzing the shape of the extended surface layout, and (b) an attention-dependent process for locating objects relative to perceptible, nearby beacons. This view makes three specific predictions that distinguish it from the image-matching or adaptive combination theories. First, animals will navigate by the geometry of the extended surface layout even if they are raised under conditions that give them no experience of the reliability of this information. Second, even subtle perturbations to the 3D surface layout will guide navigation automatically, whereas large and salient 2D image features will not. Third, objects, 2D patterns, and surface colors will be used by navigating animals through a separate, beacon-guidance process. We now review behavioral studies of young animals and children that test these predictions.
Controlled-rearing Studies
Studies of controlled-reared animals, conducted by two independent groups of researchers, provide evidence that the capacity to navigate by surface layout geometry develops in the absence of experience navigating in environments with informative shapes. Brown, Spetch, and Hurd (2007) reared separate groups of fish in environments with no informative geometrical structure (a circular tank) or environments with walls of distinctive lengths and directions (a rectangular tank). When both groups of fish subsequently were disoriented in a rectangular environment, those with prior experience in the rectangular environment performed no better than those with no such experience. In contrast, use of surface features (wall color) was influenced by these rearing conditions. When fish were trained to use a blue wall to reorient, those who were reared in a circular tank learned to use the feature faster and relied on it more heavily when geometric and featural cues were placed in conflict. These findings suggest that processing of featural cues depends in part on past experience, whereas processing of layout geometry does not.
In similar experiments, Chiandetti and Vallortigara (2008) reared chicks in circular, rectangular, or C-shaped environments and then tested their reorientation in a rectangular space. Chicks in all three rearing groups were equally good at reorienting by surface layout geometry, consistent with the findings with fish. Interestingly, chicks reared in the circular and rectangular environments also were equally good at learning to use the features of corner panels as beacons to the correct corner of the rectangular testing space, and all continued to rely on surface layout when the corner features were removed. While the feature conflict tested by Brown et al. (2007) may not be directly comparable to the feature removal tested by Chiandetti and Vallortigara (2008), both studies clearly show that reorientation by the 3D layout geometry occurs independently of past experience with those geometric properties, in contrast with the predictions of the adaptive combination view.
Developmental Studies of Reorientation
Young human children show navigational abilities and limits that are similar to those of nonhuman animals. In the first studies, children aged 18–24 months saw a toy hidden in one corner of a rectangular testing arena with one blue and three white walls and then were disoriented by slow turning at the center of the room with eyes covered. When the children were released and encouraged to locate the toy, they tended to search at both the correct corner and the geometrically equivalent opposite corner (see Figure 1; Hermer & Spelke, 1994, 1996). Children’s search at these two corners provided evidence that they, like rats, navigated in accord with the shape of the surrounding surface layout. Children’s failure to search the correct corner more often than the opposite corner suggested that children, like rats, encode surface layout geometry more automatically than non-geometric features like wall color (Cheng & Newcombe, 2005).
Further experiments extended these findings and revealed that disoriented children also use objects and 2D patterns as direct beacons to guide their search. When children were tested in a circular room with a rectangular or triangular array of identical freestanding objects, they searched only at the objects in the room, showing that they encoded the spatial relationship of the hidden toy to its container and remembered this relationship at test (Gouteux & Spelke, 2001). Interestingly, however, children searched the containers at random, suggesting that children failed to encode or use their geometric configuration.
Subsequent research revealed that toddlers do respond to the configural properties of a rectangular array of objects when the objects are placed at the periphery of a circular enclosure (Garrad-Cole, Lew, Bremner, & Whitaker, 2001; Lew, Gibbon, Murphy, & Bremner, 2010). Further experiments suggest that children respond to objects at the walls of the room as part of surface layout geometry. Although children failed to use a freestanding object on one side of a room to distinguish between geometrically identical corners (Hermer & Spelke, 1996), they succeeded when the object was replaced by a 3D bulge on one of the walls of the room (Wang, Hermer, & Spelke, 1999). Moreover, children failed to reorient by an arrangement of two objects offset from the walls of a circular room, but they successfully reoriented by the same two objects when they were placed flush against those walls (Lee & Spelke, in press). Like rats, children navigate primarily in relation to the positions of the borders of the array (O’Keefe & Burgess, 1996; Solstad et al., 2008).
These findings suggest that children are especially sensitive to 3D surface layouts, contrary to both the image-matching and adaptive combination theories and in accord with the prediction that the geometry of extended 3D surfaces, both subtle and small, guides navigation. We tested this prediction further by comparing children’s reorientation in rectangular environments varying in height, connectedness, and dimensionality (Lee & Spelke, 2008). Children reoriented by the geometric shape of an arena made of short walls that blocked neither their vision nor locomotion, but they failed to reorient by a rectangular array of large freestanding columns. Children also failed to reorient either by a 2D rectangular figure on the floor (Lee & Spelke, 2008) or by a pair of large, 2D rectangular patches on the walls (Lee & Spelke, in press; cf. Newcombe, Ratliff, Shallcross, & Twyman, 2009). Importantly, children confined their search to one of the columns, 2D corners, or 2D patches in these environments, showing that they encoded and remembered the hiding places. Nevertheless, they searched randomly at the various columns, corners, or patches. These findings suggest that children’s reorientation is guided neither by the immediate functional relevance of walls nor by their size or connectedness, but rather by a specific sensitivity to the shape of the 3D surface layout.
More recent work has shown even more striking differences in children’s use of featural cues and surface layout geometry (Lee & Spelke, in review). Children aged 3–4 years, tested in a white circular room, oriented themselves in accord with a tiny (2-cm-thick, 2-cm-high) light-colored frame placed on the floor, and also in accord with two smooth bumps on the floor, showing sensitivity to highly subtle perturbations in the 3D surface layout. In contrast, children failed to orient by the shape of a salient, dark, 2D rectangle or by an array of cylinders connected by a string that delimited the rectangular space as effectively as did the bumps and frame. These findings provide evidence that children’s reorientation depends not on the visual salience of layout features but on 3D surface layout geometry.
Children’s failure to navigate with respect to the relative distances and sense relations between large, salient objects and 2D forms, in contrast to their successful reorientation by subtle 3D surface layouts, provides compelling evidence that the cognitive processes involved in reorientation do not apply to an array of objects as they do to an array of extended 3D surfaces. Yet, in all experiments, children correctly remembered the relevant hiding locations according to the features, confirming the third contrasting prediction of the theories of reorientation, that objects and surface features are used as beacons or direct markers to location but not as relative position cues for reorientation.
Despite the privileged status of extended 3D surface layouts for reorientation, disoriented children and nonhuman animals are clearly able to use featural cues to remember locations (for review, Cheng & Newcombe, 2005). For instance, when the testing space is large, and contains a large colored wall, both human toddlers and animals are much better at using the wall color cue to guide their search (Learmonth et al., 2001; Learmonth, Nadel, & Newcombe, 2002; Sovrano, Bisazza, & Vallortigara, 2006). There are two possible interpretations of these data. First, a large colored wall, like room shape, may be used as a relative position cue (i.e., “the correct location is northwest when I am oriented to the blue wall”). Alternatively, a colored wall may serve as a beacon that indicates whether the goal location is toward it or away from it.
Research with nonhuman animals suggests that objects and surface features are used as direct markers or beacons to location but are not used spontaneously as indirect cues to direction and orientation. Fish (Sovrano, Bisazza, & Vallortigara, 2003) and chicks (Vallortigara, Zanforlin, & Pasti, 1990) use the features at the goal location to distinguish a corner of a rectangular space, but when the direct features are removed they fail to find the correct corner with respect to more distant featural cues. Similarly, children’s search is guided by a large colored wall when objects are hidden at the corners of a large room, but not when they are hidden near the room’s center, displaced from the cue (Learmonth, Newcombe, Sheridan, & Jones, 2008). Moreover, children search more effectively when objects are hidden at one of the two corners that are adjacent to the colored wall than when objects are hidden far from the wall (Shusterman, Lee, & Spelke, in review). Because these studies involved the use of features within a room with informative geometric shape, however, it is possible that the room shape cues dominated the reorientation behavior, decreasing the influence of distal landmark cues.
Lee, Shusterman, and Spelke (2006) tested whether disoriented children use landmarks to reorient or only as beacons. In a series of studies, children were tested in a circular room with with three equidistant containers forming a triangle at its center. In one study, the three containers differed in color and shape: when an object was hidden in one container and then children were disoriented, they readily retrieved it. In a second study, the array consisted of two identical containers and one distinctive container. Disoriented children searched correctly when an object was hidden in the featurally distinctive container, but they searched correctly only about half of the time when it was hidden in one of the two identical containers. This finding suggested that children used the containers as beacons but not as relative reorienting cues, but it was possible that children reoriented by the goal container but ignored the other containers, resulting in successful reorientation when the critical container was distinctive and misorientation on half of the trials when it was not. The authors addressed this possibility with two further experiments in which two objects were hidden in two different containers on each trial. When all three containers were featurally distinctive, disoriented children accurately retrieved both objects, showing that hiding two objects did not overly tax children’s memory or goal-directed action. When presented with one distinctive and two identical containers, however, disoriented children were only able to correctly locate the object in the distinctive container and searched randomly between the two featurally identical containers. These findings provide the clearest evidence that children used the features of the containers only as direct indicators of the hidden object’s location and not as guides to reorientation.
Huttenlocher and Lourenco (2007) investigated the effects of 2D surface features in a square-shaped room whose opposing pairs of walls differed in color or pattern. Contrary to the predictions of image-matching theories, children failed to reorient when adjacent walls differed in color1 or pattern. Interestingly, however, children succeeded when tested in a room whose opposite walls displayed circles that were either large or small. Similar results were found with mice (Twyman, Newcombe, & Gould, 2009), suggesting homologous systems of reorientation across distantly related species. The authors proposed that children’s reorientation was driven by a relational comparison along a common dimension, but this account fails to explain why variations along the size dimension were effective when variations along the color dimension were not.
An alternative interpretation of Huttenlocher and Lourenco’s findings rests on the evidence from studies of depth perception, that adjacent arrays of small and large circles present children with the visual depth cue of relative size (e.g., Yonas, Granrud, & Pettersen, 1985). By presenting large circles on two walls and small circles on the others, the investigators may have created an illusion of distance such that the room was perceived as slightly rectangular. But can young children reorient by only a slightly rectangular array? To address this question, we tested children’s reorientation in a slightly rectangular room (Lee, Winkler-Rhoades & Spelke, unpublished). Children confined their search to the two geometrically appropriate corners of a rectangular arena 132 cm by 122 cm (about 8:9 ratio), suggesting that children show considerable sensitivity to subtle differences in the positions and directions of walls. If the depth cue of relative size can induce a perception of differences of this magnitude, then the findings of Huttenlocher & Lourenco may be explained by a reorientation system that is highly sensitive to perceived layout geometry.
A final source of evidence for a distinctive process of navigating by layout geometry comes from a recent study comparing the navigation performance of young children to that of adults with Williams Syndrome (Lakusta et al., 2010). Typically developing young children and WS adults were given the same reorientation task in the same environment: a rectangular chamber with a single distinctively colored wall. As in past studies of children, the young children’s search after disorientation was guided by the shape of the room and not by the color of the wall. Adults with WS, however, showed the opposite pattern: their search benefitted from the colored wall but not from the distinctive shape of the room. This double dissociation provides the clearest evidence in humans for separate processes of navigating by layout geometry vs. surface features. Because Williams Syndrome results from a specific genetic deletion, moreover, the abject failure of adults with Williams Syndrome to reorient by layout geometry suggests that this reorientation system has quite a specific genetic basis.
Despite all these findings, there is robust evidence that human adults and older children can use purely nongeometric color cues to locate hidden objects. When human adults are disoriented in a rectangular room with one colored wall, they readily and consistently confine their search for a hidden object to its uniquely correct location, despite the room’s symmetry (Hermer & Spelke, 1994; Hermer-Vazquez, Spelke, & Katsnelson, 1999; Ratliff & Newcombe, 2008). Children begin to show this pattern at about 5–7 years of age (Hermer-Vazquez, Moffet, & Munkholm, 2001). This developmental change suggests the emergence of new navigation processes that may be unique to humans and dependent on capacities for language and symbolic representation (Landau & Lakusta, 2009; Shusterman & Spelke, 2005). Like the uniquely human system of natural number that is constructed from core systems shared with other animals (Carey, 2009), a uniquely human system of spatial representation may be constructed from independent parallel systems of navigation and object recognition (e.g., Spelke, Lee, & Izard, in press). Such processes are beyond the scope of the present article.
Summary
The findings reviewed above support three key predictions of Cheng and Gallistel’s original two-process account of reorientation against the predictions of both visual snapshot theories and adaptive combination theories. First, animals raised in environments that provide them with no experience with informative geometric shape nevertheless reorient by environmental shape just as well as those raised in geometrically informative environments. Second, children use even small and subtle properties of the 3D surface layout for reorientation, but they fail to use large and salient properties of objects or surface features such as color or 2D patterns. Thus, disoriented children’s selective use of 3D surface layouts fails to be explained by effects of cue salience, size, stability, distance, rectilinearity, or brightness contrast. Finally, children and animals use objects and 2D surface features as direct cues to a hidden object’s location, even as they fail to reorient by them, providing evidence for distinct processes of reorientation and beacon guidance.
It is important to note that evidence for the presence of a geometric computation of 3D surface layouts and a separate mechanism of beacon-use does not rule out the existence of visual snapshots or adaptive learning. Image-matching and adaptive learning are navigational strategies that are available to nonhuman animals (Cartwright & Collett, 1982) and to humans (Wang & Spelke, 2002). The evidence suggests, however, that these mechanisms cannot explain children’s reorientation by the shape of the 3D surface layout.
Conclusion
Characterizing core knowledge systems is important in understanding the organization of the mind, because they form the basis for later learning and conceptual change. The study of early-developing navigation abilities, and of the cognitive capacities which allow humans to combine the outputs of specialized mental computations, together promise to shed light on the process through which human children and adults come to build new, more powerful human knowledge systems, including systems of formal geometry (Spelke et al., in press).
Research using behavioral, neuroimaging, and neurophysiological measures, conducted on animals from ants to educated adult humans, provides evidence for at least one core system at the foundations of knowledge of geometry: a system for recording one’s position relative to the distances and directions of the extended surface borders in the layout. This evidence, however, leaves many questions unanswered. First, what are the geometric relationships that this system captures? In all of the above studies involving rectangular arrays, children and animals navigated in accord with the distances and directions of surface borders. In Euclidean geometry, however, shape is characterized by angle as well as distance and direction. Behavioral and neurophysiological studies raise questions concerning both rats’ and children’s abilities to navigate by angle information (Lever et al., 2002; Hupbach & Nadel, 2005; Lee & Spelke, in review; Lourenco & Huttenlocher, 2006). It is possible, therefore, that the system for navigating by the extended surface layout captures only part of the information at the heart of formal Euclidean geometry.
A second question concerns the accessibility of the geometric information by which children reorient: Can information about the shape of the surrounding surface layout be harnessed by other cognitive systems to permit uniquely human forms of navigation such as map use or abstract geometrical reasoning? When children first begin to navigate by purely geometric maps, their navigation appears to be guided by distance relations, but not angular relations, suggesting a connection between maps and representations of the extended surface layout (Shusterman, Lee, & Spelke, 2008). Moreover, when children’s intuitions about points and lines first are probed in purely abstract contexts, they reason about distance but not angle relations among paths through space (Izard, Pica, Spelke & Dehaene, in review). Both these findings suggest that children’s developing capacities for geometrical reasoning build, in part, on their sensitivity to geometry for navigation. Nevertheless, much more research needs to be conducted linking children’s navigation to their geometrical reasoning.
A final question concerns the development of abstract geometry. For adults, geometrical forms and relations apply not only to navigable environments but to everything of which we can conceive, including lines and planes extend infinitely in space. Moreover, humans use space to represent relations among colors (the color space), sounds (a high note), social partners (a close relationship), and time (a short trip). We use space to characterize entities that cannot be perceived either in practice (bacteria are larger than viruses) or in principle (justice is a distant goal). Core systems of navigation, in contrast, are highly limited in their application. If abstract geometry is founded upon them, then its development must pose a significant challenge for children, and understanding of that development poses a central challenge for students of developmental cognitive neuroscience. Studies of children’s navigation may help to meet this challenge.
Footnotes
In a similar test with toddlers, Nardini, Atkinson, & Burgess (2008) found success with two white walls and two blue walls, both plain and patterned. Lourenco, Addy, & Huttenlocher (2009), on the other hand, replicated their failure of alternating features using red or blue patterns, rather than solid walls. Because of these conflicting results, children’s ability to use of color cues for reorientation remains unclear.
References
- Benhamou S, Poucet B. Landmark use by navigating rats (Rattus norvegicus): Contrasting Geometric and featural information. Journal of Comparative Psychology. 1998;112:317–322. [Google Scholar]
- Brown AA, Spetch ML, Hurd PL. Growing in circles: Rearing environment alters spatial navigation in fish. Psychological Science. 2007;18:569–573. doi: 10.1111/j.1467-9280.2007.01941.x. [DOI] [PubMed] [Google Scholar]
- Burgess N. Spatial memory: How egocentric and allocentric combine. Trends in Cognitive Sciences. 2006;10:551–557. doi: 10.1016/j.tics.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire E, O’Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Carey S. The origin of concepts. New York, NY: Oxford University Press; 2009. [Google Scholar]
- Cartwright BA, Collett TS. How honey bees use landmarks to guide their return to a food source. Nature. 1982;295:560–564. [Google Scholar]
- Cheng K. A purely geometric module in the rats’ spatial representation. Cognition. 1986;23:149–178. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- Cheng K. Whither geometry? Troubles of the geometric module. Trends in Cognitive Sciences. 2008;12:355–361. doi: 10.1016/j.tics.2008.06.004. [DOI] [PubMed] [Google Scholar]
- Cheng K, Gallistel CR. Testing the geometric power of an animal’s spatial representation. In: Roitblat HL, Bever TG, Terrace HS, editors. Animal Cognition: Proceedings of the Harry Frank Guggenheim Conference. Hillsdale, NJ: Erlbaum; 1984. pp. 409–242. [Google Scholar]
- Cheng K, Newcombe NS. Is there a geometric module for spatial reorientation? Squaring theory and evidence. Psychonomic Bulletin and Review. 2005;12:1–23. doi: 10.3758/bf03196346. [DOI] [PubMed] [Google Scholar]
- Chiandetti C, Vallortigara G. Is there an innate geometric module? Effects of experience with angular geometric cues on spatial re-orientation based on the shape of the environment. Animal Cognition. 2008;11:139–146. doi: 10.1007/s10071-007-0099-y. [DOI] [PubMed] [Google Scholar]
- Collett TS, Collett M. Memory use in insect visual navigation. Nature Reviews Neuroscience. 2002;3:542–552. doi: 10.1038/nrn872. [DOI] [PubMed] [Google Scholar]
- Cressant A, Muller RU, Poucet B. Failure of centrally placed objects to control the firing fields of hippocampal place cells. Journal of Neuroscience. 1997;17:2531–2542. doi: 10.1523/JNEUROSCI.17-07-02531.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehaene S, Izard V, Pica P, Spelke ES. Core knowledge of geometry in an Amazonian indigene group. Science. 2006;311:381–384. doi: 10.1126/science.1121739. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Burgess N. Distinct error-correcting and incidental learning of location relative to landmarks and boundaries. Proceedings of the National Academy of Sciences. 2008;105:5909–5914. doi: 10.1073/pnas.0711433105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doeller CF, King JA, Burgess N. Parallel striatal and hippocampal systems for landmarks and boundaries in spatial memory. Proceedings of the National Academy of Sciences. 2008;105:5915–5920. doi: 10.1073/pnas.0801489105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodridge JP, Dudchenko PA, Worboys KA, Golob EJ, Taube JS. Cue control and head direction cells. Behavioral Neuroscience. 1998;112:749–761. doi: 10.1037//0735-7044.112.4.749. [DOI] [PubMed] [Google Scholar]
- Egerton S, Callaghan V, Chernett P. A biologically inspired mapping model for autonomous mobile robots. In: Mohammadin M, editor. New frontiers in computational intelligence and its applications. Amsterdam: IOS Press; 2000. pp. 20–29. [Google Scholar]
- Epstein R, DeYoe EA, Press DZ, Rosen AC, Kanwisher N. Neuropsychological evidence for a topographical learning mechanism in parahippocampal cortex. Cognitive Neuropsychology. 2001;18:481–508. doi: 10.1080/02643290125929. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–125. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Epstein RA, Higgins JS, Thompson-Schill SL. Learning places from views: variation in scene processing as a function of experience and navigational ability. Journal of Cognitive Neuroscience. 2005;17:73–83. doi: 10.1162/0898929052879987. [DOI] [PubMed] [Google Scholar]
- Etienne AS. Path integration in mammals. Hippocampus. 2004;14:180–192. doi: 10.1002/hipo.10173. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge, M.A: MIT Press; 1990. [Google Scholar]
- Garrad-Cole F, Lew AR, Bremner JG, Whitaker C. Use of cue configuration geometry for spatial orientation in human infants (Homo sapiens) Journal of Comparative Psychology. 2001;115:317–320. doi: 10.1037/0735-7036.115.3.317. [DOI] [PubMed] [Google Scholar]
- Gee AP, Chekhlov D, Calway A, Mayol-Cuevas W. Discovering higher level structure in visual SLAM. IEEE Transactions on Robotics. 2008;24:980–990. [Google Scholar]
- Gouteux S, Spelke ES. Children’s use of geometry and landmarks to reorient in an open space. Cognition. 2001;81:119–148. doi: 10.1016/s0010-0277(01)00128-7. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K. The neural basis of object perception. Current Opinions in Neurobiology. 2003;13:159–166. doi: 10.1016/s0959-4388(03)00040-0. [DOI] [PubMed] [Google Scholar]
- Hermer L, Spelke ES. A geometric process for spatial reorientation in young children. Nature. 1994;370:57–59. doi: 10.1038/370057a0. [DOI] [PubMed] [Google Scholar]
- Hermer L, Spelke E. Modularity and development: The case of spatial reorientation. Cognition. 1996;61:195–232. doi: 10.1016/s0010-0277(96)00714-7. [DOI] [PubMed] [Google Scholar]
- Hermer-Vazquez L, Moffet A, Munkholm P. Language, space, and the development of cognitive flexibility in humans: the case of two spatial memory tasks. Cognition. 2001;79:263–299. doi: 10.1016/s0010-0277(00)00120-7. [DOI] [PubMed] [Google Scholar]
- Hermer-Vazquez L, Spelke ES, Katsnelson AS. Sources of flexibility in human cognition: Dual-task studies of space and language. Cognitive Psychology. 1999;39:3–36. doi: 10.1006/cogp.1998.0713. [DOI] [PubMed] [Google Scholar]
- Hupbach A, Nadel L. Reorientation in a rhombic environment: no evidence for an encapsulated geometric module. Cognitive Development. 2005;20:279–302. [Google Scholar]
- Huttenlocher J, Lourenco SF. Coding location in enclosed spaces: Is geometry the principle? Developmental Science. 2007;10:741–746. doi: 10.1111/j.1467-7687.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- Izard V, Pica P, Spelke ES, Dehaene S. Euclidean intuitions of geometry in an Amazonian indigene group. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakusta L, Dessalegn B, Landau B. Impaired geometric reorientation caused by genetic defect. Proceedings of the National Academy of Sciences. 2010;107:2813–2817. doi: 10.1073/pnas.0909155107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landau B, Lakusta L. Spatial representations across species: geometry, language, and maps. Current Opinion in Neurobiology. 2009;19:1–8. doi: 10.1016/j.conb.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learmonth AE, Nadel L, Newcombe NS. Children’s use of landmarks: Implications for modularity theory. Psychological Science. 2002;13:337–341. doi: 10.1111/j.0956-7976.2002.00461.x. [DOI] [PubMed] [Google Scholar]
- Learmonth AE, Newcombe NS, Huttenlocher J. Toddlers’ use of metric information and landmarks to reorient. Journal of Experimental Child Psychology. 2001;80:225–244. doi: 10.1006/jecp.2001.2635. [DOI] [PubMed] [Google Scholar]
- Learmonth AE, Newcombe NS, Sheridan N, Jones M. Why size counts: Children’s spatial reorientation in large and small enclosures. Developmental Science. 2008;11:414–426. doi: 10.1111/j.1467-7687.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Shusterman S, Spelke ES. Reorientation and landmark-guided search by young children: Evidence for two systems. Psychological Science. 2006;17:577–582. doi: 10.1111/j.1467-9280.2006.01747.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Spelke ES. Children’s use of geometry for reorientation. Developmental Science. 2008;11:743–749. doi: 10.1111/j.1467-7687.2008.00724.x. [DOI] [PubMed] [Google Scholar]
- Lee SA, Spelke ES. Modular geometric mechanisms for navigation in disoriented children. Cognitive Psychology (in press) [Google Scholar]
- Lee SA, Spelke ES. Navigation as a source of geometric knowledge: Young children’s use of distance, direction, and angle in a disoriented search task. (under revision) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Spelke ES. Young children reorient by computing layout geometry, not by matching images of the environment. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Burton S, Jeewajee A, O’Keefe J, Burgess N. Boundary vector cells in the subiculum of the hippocampal formation. The Journal of Neuroscience. 2009;29:9771–9777. doi: 10.1523/JNEUROSCI.1319-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever C, Wills T, Cacucci F, Burgess N, O’Keefe J. Long-term plasticity in hippocampal place-cell representation of environmental geometry. Nature. 2002;416:90–94. doi: 10.1038/416090a. [DOI] [PubMed] [Google Scholar]
- Lew AR, Gibbon B, Murphy C, Bremner JG. Use of geometry for spatial reorientation in children only applies to symmetrical spaces. Developmental Science. 2010;13:490–498. doi: 10.1111/j.1467-7687.2009.00904.x. [DOI] [PubMed] [Google Scholar]
- Lourenco S, Addy D, Huttenlocher J. Location representation in enclosed spaces: What types of information afford young children an advantage? Journal of Experimental Child Psychology. 2009;104:313–325. doi: 10.1016/j.jecp.2009.05.007. [DOI] [PubMed] [Google Scholar]
- Lourenco SF, Huttenlocher J. How do young children determine location? Evidence from disorientation tasks. Cognition. 2006;100:511–529. doi: 10.1016/j.cognition.2005.07.004. [DOI] [PubMed] [Google Scholar]
- McGregor A, Hayward AJ, Pearce JM, Good MA. Hippocampal lesions disrupt navigation based on the shape of the environment. Behavioural Neuroscience. 2004;118:1011–1021. doi: 10.1037/0735-7044.118.5.1011. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Cherrier MM. Agnosia for scenes in topographagnosia. Neuropsychologia. 2003;41:1387–1395. doi: 10.1016/s0028-3932(03)00041-1. [DOI] [PubMed] [Google Scholar]
- Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaft. 1980;67:566. [Google Scholar]
- Morris RGM, Garrard P, Rawlins JNP, O’Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nardi D, Bingman VP. Asymmetrical participation of the left and right hippocampus for representing environmental geometry in homing pigeons. Behavioural Brain Research. 2007;178:160–171. doi: 10.1016/j.bbr.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Nardini M, Atkinson J, Burgess N. Children reorient using the left/right sense of coloured landmarks at 18–24 months. Cognition. 2008;106:519–527. doi: 10.1016/j.cognition.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Nardini M, Thomas R, Knowland V, Braddick O, Atkinson J. A viewpoint-independent process for spatial reorientation. Cognition. 2009;112:241–248. doi: 10.1016/j.cognition.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Newcombe NS, Ratliff KR. Explaining the development of spatial reorientation: Modularity-plus-language versus the emergence of adaptive combination. In: Plumert J, Spencer J, editors. The Emerging Spatial Mind. New York: Oxford University Press; 2007. pp. 53–76. [Google Scholar]
- Newcombe NS, Ratliff KR, Shallcross WL, Twyman AD. Young children’s use of features to reorient is more than just associative: Further evidence against a modular view of spatial processing. Developmental Science. 2009;12:1–8. doi: 10.1111/j.1467-7687.2009.00877.x. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Burgess N. Geometric determinants of the place fields of hippocampal neurons. Nature. 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- O’Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford, England: Clarendon Press; 1978. [Google Scholar]
- Pitcher D, Charles L, Devlin J, Walsh V, Duchaine B. Triple dissociation between faces, bodies, and objects in extrastriate cortex. Current Biology. 2009;19:319–324. doi: 10.1016/j.cub.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL. The firing of hippocampal place cells in the dark depends on the rat’s recent experience. Journal of Neuroscience. 1990;10:2008–2017. doi: 10.1523/JNEUROSCI.10-06-02008.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratliff KR, Newcombe NS. Reorienting when cues conflict: Evidence for an adaptive-combination view. Psychological Science. 2008;19:1303–1307. doi: 10.1111/j.1467-9280.2008.02239.x. [DOI] [PubMed] [Google Scholar]
- Sheynikhovich D, Chavarriaga R, Strösslin T, Arleo A, Gerstner W. Is there a geometric module for spatial orientation? Insights from a rodent navigation model. Psychological Review. 2009;116:540–566. doi: 10.1037/a0016170. [DOI] [PubMed] [Google Scholar]
- Shusterman A, Lee SA, Spelke ES. Young children’s spontaneous use of geometry in maps. Developmental Science. 2008;11:F1–F7. doi: 10.1111/j.1467-7687.2007.00670.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman A, Lee SA, Spelke ES. Cognitive effects of language on human navigation. Cognition. doi: 10.1016/j.cognition.2011.04.004. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shusterman A, Spelke E. Language and the development of spatial reasoning. In: Carruthers P, Laurence S, Stich S, editors. The structure of the innate mind. New York: Oxford University Press; 2005. pp. 89–106. [Google Scholar]
- Silveira G, Malis E, Rives P. An efficient direct approach to visual SLAM. IEEE Transactions on Robotics. 2008;24:969–979. [Google Scholar]
- Solstad T, Boccara CN, Kropff E, Moser M, Moser EI. Representation of geometric borders in the entorhinal cortex. Science. 2008;322:1865–1868. doi: 10.1126/science.1166466. [DOI] [PubMed] [Google Scholar]
- Sovrano V, Bisazza A, Vallortigara G. Modularity as a fish (Xenotoca eiseni) views it: Conjoining geometric and nongeometric information for spatial reorientation. Journal of Experimental Psychology: Animal Behavior Processes. 2003;29:199–210. doi: 10.1037/0097-7403.29.3.199. [DOI] [PubMed] [Google Scholar]
- Sovrano V, Bisazza A, Vallortigara G. How fish do geometry in large and small spaces. Animal cognition. 2006;10:47–54. doi: 10.1007/s10071-006-0029-4. [DOI] [PubMed] [Google Scholar]
- Spelke ES, Lee SA, Izard V. Beyond core knowledge: Natural geometry. Cognitive Science. doi: 10.1111/j.1551-6709.2010.01110.x. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürzl W, Cheung A, Cheng K, Zeil J. The information content of panoramic images I: The rotational errors and the similarity of views in rectangular experimental arenas. Journal of Experimental Psychology: Animal Behavior Processes. 2008;34:1–14. doi: 10.1037/0097-7403.34.1.1. [DOI] [PubMed] [Google Scholar]
- Sutton J. What is geometric information and how do animals use it? Behavioural Processes. 2009;80:339–343. doi: 10.1016/j.beproc.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Thrun S. Robotic mapping: A survey. In: Lakemeyer G, Nebel B, editors. Exploring artificial intelligence in the new millenium. Morgan Kaufmann; 2002. [Google Scholar]
- Tommasi L, Gagliardo A, Andrew RJ, Vallortigara G. Separate processing mechanisms for encoding geometric and landmark information in the avian hippocampus. European Journal of Neuroscience. 2003;17:1695–1702. doi: 10.1046/j.1460-9568.2003.02593.x. [DOI] [PubMed] [Google Scholar]
- Twyman AD, Newcombe NS, Gould TJ. Of mice (mus musculus) and toddlers (Homo sapiens): Evidence of species-general spatial reorientation. Journal of Comparative Psychology. 2009;123:342–345. doi: 10.1037/a0015400. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Pagni P, Sovrano V. Separate geometric and non-geometric modules for spatial reorientation: Evidence from a lopsided animal brain. Journal of Cognitive Neuroscience. 2004;16:390–400. doi: 10.1162/089892904322926737. [DOI] [PubMed] [Google Scholar]
- Vallortigara G, Zanforlin M, Pasti G. Geometric modules in animals’ spatial representation: A test with chicks. Journal of Comparative Psychology. 1990;104:248–254. doi: 10.1037/0735-7036.104.3.248. [DOI] [PubMed] [Google Scholar]
- Wang RF, Crowell JA, Simons DJ, Irwin DE, Kramer AF, Ambinder MS, Thomas LE, Gosney JL, Levinthal BR, Hsieh BB. Spatial updating relies on an egocentric representation of space: Effects of the number of objects. Psychonomic Bulletin & Review. 2006;13:281–286. doi: 10.3758/bf03193844. [DOI] [PubMed] [Google Scholar]
- Wang RF, Hermer L, Spelke ES. Mechanisms of reorientation and object localization by children: A comparison with rats. Behavioral Neuroscience. 1999;113:475–485. doi: 10.1037//0735-7044.113.3.475. [DOI] [PubMed] [Google Scholar]
- Wang RF, Spelke ES. Human spatial representation: Insights from animals. Trends in Cognitive Sciences. 2002;6:376–382. doi: 10.1016/s1364-6613(02)01961-7. [DOI] [PubMed] [Google Scholar]
- Wystrach A, Beugnon G. Ants learn geometry and features. Current Biology. 2009;19:61–66. doi: 10.1016/j.cub.2008.11.054. [DOI] [PubMed] [Google Scholar]
- Yonas A, Granrud CE, Pettersen L. Infants’ sensitivity to relative size information for distance. Developmental Psychology. 1985;21:161–167. [Google Scholar]
- Zugaro MB, Berthoz A, Wiener SI. Background, but not foreground, spatial cues are taken as references for head direction responses by rat anterodorsal thalamus neurons. Journal of Neuroscience. 2001;21:1–5. doi: 10.1523/JNEUROSCI.21-14-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]


