Abstract
Cyclophostin, a structurally unique and potent naturally occurring acetyl cholinesterase (AChE) inhibitor, and its unnatural diastereomer were prepared in 6 steps and 15% overall yield from hydroxymethyl butyrolactone. The unnatural diastereomer of cyclophostin was converted into cyclipostin P, a potent naturally occurring hormone sensitive lipase (HSL) inhibitor, using a one pot dealkylation-alkylation process. The inhibition [IC50] of human AChE by cyclophostin and its diastereomer are reported, as well as constituent binding (KI) and reactivity (k2) constants.
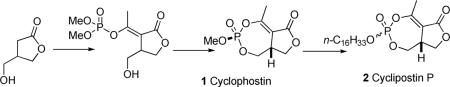
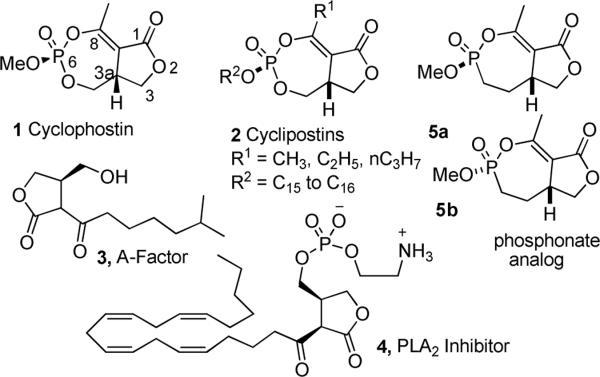
Cyclophostin 1 (Figure 1) is a novel bicyclic organophosphate isolated from a fermentation solution of Streptomyces lavendulae (strain NK901093).1 The natural product 1 showed potent inhibition of acetyl cholinesterase (AChE) from the housefly (CSMA strain) and the brown plant hopper with reported IC50 of 7.6 ×10−10 M. The structure of cyclophostin was first assigned by spectroscopic methods and then confirmed by single crystal X-ray diffraction studies as a bicyclic structure with a seven-membered cyclic enol-phosphate triester fused to a butyrolactone ring. There are chirality centers at both C3a and the phosphorus atom (6). The absolute configurations were determined to be 3aR, 6S.2]
Figure 1.
Cyclophostin and Related Structures.
The unusual bicyclic enolphosphate is also found in the family of structurally related natural products, named the cyclipostins 2.3 The cyclipostins 2 possess a core structure similar to that of cyclophostin, but differ in the phosphate ester. The cyclipostins 2 are phosphate esters of long chain lypophilic alcohols of various lengths and structures and all are potent inhibitors of hormone sensitive lipase (HSL). In addition, cyclophostin and the cyclipostins are probably biosynthetically related to A-factor 3 and the virginiae butanolides,4 and the PLA2 inhibitor 4.5
The significance of cyclophostin and the cyclipostins is due in part to their unique structures, but also to the enzymes they inhibit. Interest in AChE has re-emerged due its role as a therapeutic target for Alzheimer's disease,6 myasthenia gravis,7 and glaucoma,8 whereas HSL is a therapeutic target for type II diabetes.9
We recently reported the synthesis of a phosphonate analog 5a of cyclophostin.10 The activity of the phosphonates was ≥100 fold less than the value reported for cyclophostin. The trans isomer (H and OMe) 5b was 10 fold more active (IC50 of 3 μM human AChE) than the cis isomer 5a (IC50 of 30 μM human AChE). Since the natural product has the cis (H and OMe) configuration, the unnatural isomer may well prove more potent. In order to accurately compare the activity of cyclophostin, the phosphonate analog and their diastereomers with a detailed kinetic analysis, we needed reasonable quantities of the natural product. Furthermore, we proposed that cyclophostin would be an excellent precursor for the synthesis of the family of cyclipostins. Herein, we report the first synthesis of (±) cyclophostin and conversion into (±) cyclipostin P.
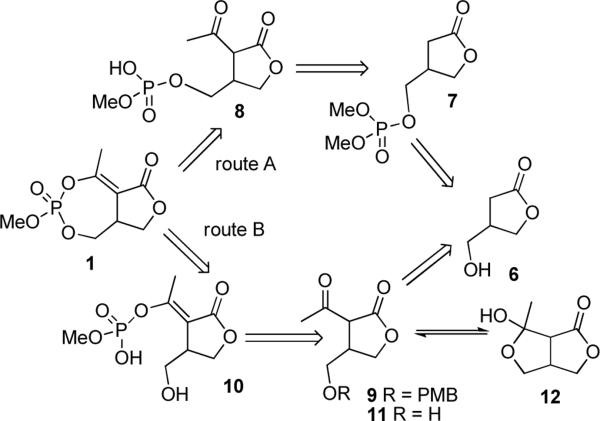
A retrosynthetic analysis (Scheme 1) of the bicyclic phosphate 1 suggested that the cyclic enolphosphate could be formed either by condensation of the acetyl group (as the enol) with a phosphoric acid via intermediate 8 (route A) or conversely condensation of the primary alcohol with an enolphosphoric acid via intermediate 10 (route B). Both intermediates can be formed by C-acylation of derivatives of hydroxymethyl lactone 6. The lactone 6 and various derivatives are available in the racemic modification11 and either enantiomer.12 It was thought necessary to protect the hydroxyl of lactone 6 prior to C-acetylation to avoid complications arising from cyclization of the acetyl lactone 11 to the hemiketal 12.12b The most expedient route would be to introduce the phosphate early in the synthesis, simultaneously protecting the hydroxyl group.
Scheme 1.
Cyclophostin Retrosynthetic Analysis.
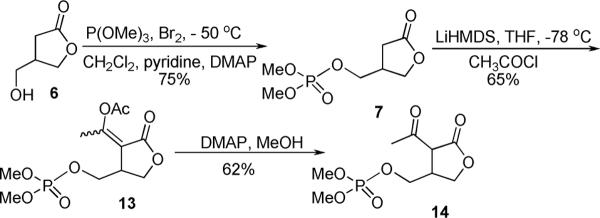
The racemic hydroxy lactone 6 was prepared using published methods.11 The hydroxyl was phosphorylated using dimethyl bromophosphate, prepared in situ by reaction of trimethyl phosphite with bromine, to give the phosphate 7 (Scheme 2). The phosphorylated butyrolactone 7 was deprotonated with one equivalent of LiHMDS in THF and the resulting enolate was acylated with acetyl chloride.4a,5 Initially, mixtures of the acetyl lactone 14 and the enolacetate 13 were observed, so an excess of acetyl chloride was added to ensure the complete acylation giving enolacetate 13 in 65% yield as a mixture of two geometrical isomers. The geometrical isomers could be separated, but were generally carried through to the next step as a mixture. Deacetylation of enolacetates 13 was achieved using a catalytic amount of DMAP in MeOH to give the acetyl lactone 14 in 62% yield.
Scheme 2.
Synthesis of Primary Phosphate.
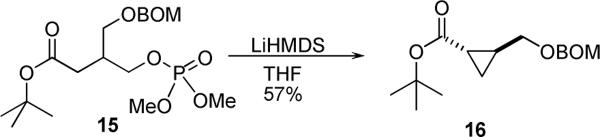
The successful C-acylation of the lactone 7 was both gratifying and somewhat surprising. It is well known that the enolates derived from γ-phosphoryloxy carboxylates cyclize to form cyclopropanes.13 Indeed, when the acyclic γ-phosphoryloxy carboxylate 15 was treated with LiHMDS and acetyl chloride (scheme 3), the only isolable product was the cyclopropane 16. In contrast, a solution the enolate of butyrolactone 7 was stable in the absence of an external electrophile up to −20 °C for one hour. At higher temperatures decomposition to intractable products was observed.
Scheme 3.
Formation of Cyclopropanes.
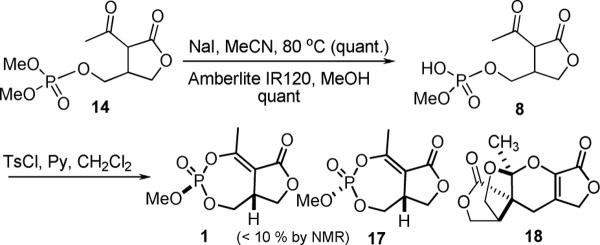
With the desired 2-acetyl butyrolactone intermediate 14 in hand, the final step of the cyclophostin synthesis was explored (Scheme 4). The intermediate 14 was demethylated using one equivalent of sodium iodide in refluxing acetonitrile solution to give the corresponding sodium salt in quantitative yield.14 The sodium salt was protonated using amberlite® (sulfonic acid) resin to yield the phosphoric acid 8. Attempted cyclization via intramolecular condensation of the monophosphoric acid moiety with the enol form of the acetyl group using EDC, HOBt and the Hunig's base and related reactions were not successful.15 These conditions had been used previously in the synthesis of the phosphonate analog.10 Other reaction conditions were examined. Reaction with TsCl and pyridine gave a crude product with signals at −7.6, −8,0 and −11.7 ppm in the 31P NMR spectrum (amongst others). After aqueous workup, a significant amount of mass was lost in the organic soluble crude product. The peak at −8.0 had disappeared and only the peaks at −7.6 and −11.7 ppm remained in the crude product. Chromatographic separation of the product mixture using preparative TLC yielded cyclophostin 1 and its unnatural diastereomer, 17 in very low yield (< 10% combined yield). The identity of cyclophostin was confirmed by comparing the 1H and 31P NMR data with those reported for the the natural product.1 A crystalline tetracyclic byproduct, 18 was also isolated and the structure was confirmed by X-ray diffraction study.16
Scheme 4.
Attempted Cyclization of the Primary Phosphate.
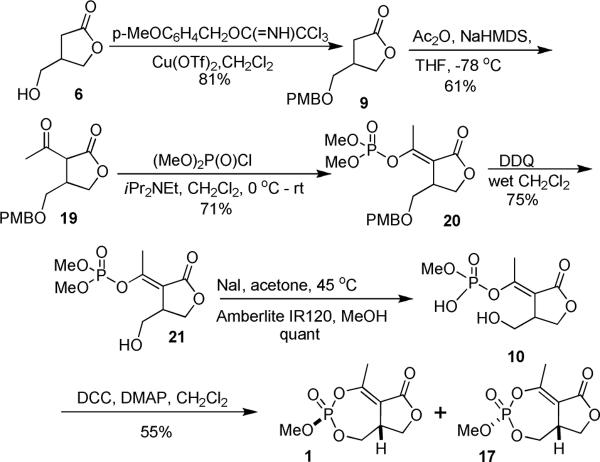
The lack of success in finding reaction conditions for the cyclization of 8 led us to examine an alternate route B (Scheme 5). The hydroxyl of lactone 6 was protected as a p-methoxybenzyl (pmb) ether 9 by the copper (II) triflate catalyzed reaction with p-methoxybenzyloxy trichloroacetimidate.17 Deprotonation with NaHMDS and acylation with acetic anhydride furnished the acetyl lactone 19 directly. Selective phosphorylation of 2-acetyl butyrolactone 19 was accomplished by reaction with dimethyl chlorophosphate using a procedure reported to give the E-enol phosphate.18,19
Scheme 5.
Synthesis and Cyclization of the Enolphosphate.
The pmb ether was removed using DDQ in wet CH2Cl2 and the enolphosphate 21 was selectively monodemethylated using one equivalent of sodium iodide in acetone at 45 °C. The sodium salt was protonated with Amberlite IR 120® resin to generate the corresponding phosphoric acid 10. Reaction of the phosphonic acid 10 with DCC and DMAP in CH2Cl2 successfully formed the cyclic enolphosphates 1 and 17 as a 1:1 mixture. The diastereomers were separated using silica gel chromatography to give natural cyclophostin 1 and its diastereomer 17 in 55% combined yield.
Cyclophostin and its diastereomer are potential precursors for the synthesis of the family of cyclipostins by ester exchange. We opted to explore a novel one pot process for this conversion (Scheme 6). The unnatural diastereomer 17 was treated with hexadecyl bromide (10 equivalents) and catalytic tetrabutylammonium iodide (TBAI) in refluxing dioxane to give cyclipostin P 2 and its diastereomer 22 with 95% conversion and 1:1 ratio. The diastereomers were separated by column chromatography to give a 77% combined yield. The reaction of either 2 or 22 with hexadecyl bromide and catalytic TBAI in refluxing dioxane resulted in a mixture of both 2 and 22 in a 1:1 ratio. There was no reaction in the absence of TBAI.
Scheme 6.
Conversion of Cyclophostin into Cyclipostin P.
It is proposed that the iodide cleaves the methyl (or alkyl) phosphate ester bond to give a phosphate anion which is then alkylated by the hexadecyl bromide. The formation of the anion would account for the scrambling of the stereochemistry at phosphorus.
(±) Cyclophostin and its diastereomer were examined for inhibitory activity against human AChE using a modified Ellman assay.20 Compounds 1 and 17 are potent inhibitors of human AChE: both have IC50s of around 40 nM (Table 1). This is only slightly weaker than the previously reported values for the natural product isolated from insect.1 This could be a reflection of either the difference in AChe source species or the selectivity of the single (natural) enantiomer versus the raemic (synthetic) muxture. There are two additional comparisons of interest. One is that although they are diastereomers, the IC50s for 1 and 17 are very similar. The other is that these IC50s represent 103–104 improvement in potency relative to the corresponding (±) phosphonate analogs 5.10,18
Table 1.
Inhibition of AChE by Cyclophostin and Related Compounds.
![]()
| no. | IC50 | KI | k2, min−1 | k−2, min−1 |
|---|---|---|---|---|
| 5a | 30 µM | 140 ± 60 µM | 0.4 ± 0.1 | 4e-3 |
| 5b | 3 µM | 24 ± 6.0 µM | 0.3 ± 0.06 | 0.02 |
| 1 | 45 nM | 140 ± 72 nM | 0.7 ± 0.02 | 0 |
| 17 | 40 nM | 210 ± 140 nM | 0.9 ± 0.5 | 0 |
To dissect this behavior, residual enzyme activities were obtained as a function of inhibitor concentration and incubation time for 1 and 17 and analyzed using equation 1 as described previously.18 Interestingly, the chemical (rate) constants k2 and k−2 vary little among the four compounds, but the KI which represents the noncovalent binding event, reflects 100–1000-fold stronger binding by the phosphates than the phosphonates. Thus while chemical intuition would suggest that the carbon replacement of the endocyclic oxygen would cause a difference in reactivity, these data indicate that this substitution instead has profoundly adverse affects on binding. We speculate that this is due to a combination of additional hydrogen bonds (to the O) and subtle conformational differences in the bicyclic ring system. This observation points to the exquisite ability of enzymes to discriminate compounds in their active sites.
In conclusion, cyclophostin and its diastereomer have been prepared in 6 steps and 15% overall yield from lactone 6. The diastereomer of cyclophostin was converted into cyclipostin P using a one pot process. Syntheses of additional examples of the cyclipostins and corresponding biological studies are ongoing and will be reported in due course.
Supplementary Material
Acknowledgment
This work was supported by grant number R01-GM076192 from National Institute of General Medical studies. We thank Prof. R. K. Winter and Mr. Joe Kramer of the Department of Chemistry and Biochemistry, University of Missouri-St. Louis for mass spectra. We also thank Prof. Paul Hanson, University of Kansas, for bringing cyclophostin and the cyclipostins to our attention.
Footnotes
Supporting Information Available Detailed experimental procedures and full spectroscopic data for all new compounds. The material is available free of charge via the Internet at http://pubs.acs.org.
References
- (1).Kurokawa T, Suzuki K, Hayaoka T, Nakagawa T, Izawa T, Kobayashi M, Harada N. J. Antibiot. 1993;46:1315. doi: 10.7164/antibiotics.46.1315. [DOI] [PubMed] [Google Scholar]
- (2).The configuration for cyclophostin named in reference 1, 3aR,6S, is actually incorrect. A common error is to treat P+–O− as P=O and hence assign the priority incorrectly. Natural cyclophostin is actually 3aR,6R. See Cahn RS, Ingold C, Prelog V. Angew. Chem., Int. Ed. 1966;5:385.. The configuration is correctly named chemical abstracts.
- (3).(a) Wink J, Schmidt F-R, Seibert G, Aretz W. J. Antibiot. 2002;55:472. doi: 10.7164/antibiotics.55.472. [DOI] [PubMed] [Google Scholar]; (b) Věrtesy L, Beck B, Brönstrup M, Ehrlich K, Kurz M, Műller G, Schummer D, Seibert G. J. Antibiot. 2002;55:480. doi: 10.7164/antibiotics.55.480. [DOI] [PubMed] [Google Scholar]
- (4).(a) Sakuda S, Tanaka S, Mizuno K, Sukcharoen O, Nihira T, Yamada Y. J. Chem. Soc., Perkin Trans. 1993;1:2309. [Google Scholar]; (b) Kato J, Funa N, Watanabe H, Ohnishi Y, Horinouchi S. Proc. Natl. Acad. Sci. USA. 2007;104:2378. doi: 10.1073/pnas.0607472104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ohnishi Y, Kameyama S, Onaka H, Horinouchi S. Mol. Microbiol. 1999;34:102. doi: 10.1046/j.1365-2958.1999.01579.x. [DOI] [PubMed] [Google Scholar]; (d) Miyake K, Horinouchi S, Yoshida M, Chiba N, Mori K, Nogawa N, Morikawa N, Beppu T. J. Bacteriol. 1989;171:4298. doi: 10.1128/jb.171.8.4298-4302.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Campbell MM, Fox JL, Sainsbury M, Liu Y. Tetrahedron. 1989;45:4551. [Google Scholar]
- (6).Li WM, Kan KK, Carlier PR, Pang YP, Han YF. Current Alzheimer Research. 2007;4:386. doi: 10.2174/156720507781788918. [DOI] [PubMed] [Google Scholar]
- (7).Garcia-Carrasco M, Escárcega RO, Fuentes-Alexandro S, Riebeling C, Cervera R. Autoimmunity Reviews. 2007;6:373. doi: 10.1016/j.autrev.2007.01.001. [DOI] [PubMed] [Google Scholar]
- (8).Kaur J, Zhang M-Q. Curr. Med. Chem. 2000;3:273. doi: 10.2174/0929867003375254. [DOI] [PubMed] [Google Scholar]
- (9).(a) Holm C, Osterlund T, Laurell H, Conteras JA. Annu. Rev. Nutr. 2000;20:365. doi: 10.1146/annurev.nutr.20.1.365. [DOI] [PubMed] [Google Scholar]; (b) Yeaman SJ. Biochem J. 2004;379:11. doi: 10.1042/BJ20031811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Bandyopadhyay S, Dutta S, Spilling CD, Dupureur CM, Rath NP. J. Org. Chem. 2008;73:8386. doi: 10.1021/jo801453v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For examples see: Gadir SA, Smith Y, Taha AA, Thaller V. J. Chem. Res (S) 1986:222.. Yoda H, Mizutani M, Takabe K. Synlett. 1998:855.. Sengoku T, Suzuki T, Kakimoto T, Takahashi M, Yoda H. Tetrahedron. 2009;65:2415..
- (12).For examples see: Banfi L, Basso A, Guanti G, Zannetti MT. Tetrahedron: Asymmetry. 1997;8:4079.. Parsons PJ, Lacrouts P, Buss AD. Chem. Commun. 1995:437.. Crawforth JM, Fawcett J, J. Rawlings B. J. Chem. Soc., Perkin Trans. 1998:1721.. Posner GH, Wietzberg M, Jew S-S. Synth. Commun. 1987;17:611.. Takabe K, Mase N, Matsumura H, Hasegawa T, Iida Y, Kuribayashi H, Adachi K, Yoda H, Ao M. Bioorg. Med. Chem. Lett. 2002;12:2295. doi: 10.1016/s0960-894x(02)00458-4.. Mori K, Chiba N. Liebigs Ann. Chem. 1989:957.. Takabe K, Tanaka M, Sugimoto M, Yamada T, Yoda H. Tetrahedron: Asymmetry. 1992;3:1385.
- (13).For examples see: Jacks TE, Nibbe H, Wiemer DF. J. Org. Chem. 1993;58:4584.. Singh AK, Rao MN, Simpson JH, Li W-S, Thornton JE, Kuehner DE, Kacsur DJ. Org. Process Res. Dev. 2002;6:618.. Krawczyk H, Wasek K, Kedzia J, Wojciechowski J, Wolf WM. Org. Biomol. Chem. 2008;6:308. doi: 10.1039/b712145h.
- (14).The reaction of phosphates with NaI is generally performed in acetone, but other solvent including MeCN also work well. See Zervas L, Dilaris I. J. Am. Chem. Soc. 1955;77:5354.
- (15).(a) Smith M, Drummond GI, Khorana HG. J. Am. Chem. Soc. 1961;83:698. [Google Scholar]; (b) Smith M, Moffatt JG, Khorana HG. J. Am. Chem. Soc. 1958;80:6204. [Google Scholar]; (c) Khorana HG, Tener GM, Wright RS, Moffatt JG. J. Am. Chem. Soc. 1957;79:430. [Google Scholar]
- (16).The details of the structure determination have been deposited at the Cambridge Crystallographic Data Center as CCDC 812559.
- (17).(a) Rai AN, Basu A. Tetrahedron Lett. 2003;44:2267. [Google Scholar]; (b) Nakajima N, Horita K, Abe R, Yonemitsu O. Tetrahedron Lett. 1988;29:4139. [Google Scholar]
- (18).Dutta S, Malla RK, Bandyopadhyay S, Spilling CD, Dupureur CM. Bioorg. Med. Chem. 2010;18:2265. doi: 10.1016/j.bmc.2010.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).(a) White JD, Kawasaki M. J. Org. Chem. 1992;57:5292. [Google Scholar]; (b) Lichtenenthaler FW. Chem. Rev. 1961;61:607. [Google Scholar]
- (20).Ellman GJ, Courtney KD, Jr., Featherstone RM. Biochem Pharm. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.