Abstract
Purpose of the Report
This study tested the feasibility of C11-acetate (acetate) positron emission tomography (PET) imaging to assess response to therapy in men with bone metastatic prostate cancer and compared results for disease detection and response evaluation with F-18 fluorodeoxyglucose (FDG) PET.
Materials and Methods
Men with ≥3 prostate cancer bone metastases identified by Tc-99m methylene diphosphonate (MDP) bone scintigraphy and/or computed tomography were enrolled in a prospective study of serial acetate and FDG PET imaging. Patients were imaged before and 6 to 12 weeks after initial androgen deprivation therapy for new metastatic prostate cancer or first-line chemotherapy with docetaxel for castration-resistant prostate cancer. Qualitative assessment and changes in the tumor:normal uptake ratio were used to assess response by both acetate and FDG PET. In addition, the detection of bone metastases pretherapy was compared for acetate and FDG PET.
Results
A total of 8 patients with documented bone metastases were imaged, of which 6 were imaged both pre- and post-therapy. Acetate PET detected bone metastases in all 8 patients, whereas FDG PET detected lesions in 6 of the 7 imaged patients. Acetate PET generally detected more metastases with a higher tumor:normal uptake ratio. Qualitative and quantitative assessments of post-treatment response correlated with composite clinical designations of response, stable disease, or progression in 6 of 6 and 5 of 6 by acetate and 4 of 5 and 3 of 5 by FDG PET, respectively.
Conclusions
In this pilot study, results indicate that acetate PET holds promise for response assessment of prostate cancer bone metastases and is complementary to FDG PET in bone metastasis detection.
Keywords: positron emission tomography (PET), prostate cancer, bone metastases, C11-acetate (acetate), F-18 fluorodeoxyglucose (FDG), response
Prostate cancer is the most commonly diagnosed malignancy in men in the United States of America and is second in cancer-related mortality only to lung cancer.1 Earlier diagnosis, attributable to increased screening, has led to the discovery of many potentially “curable” lesions, yet approximately 30% to 40% of patients will ultimately relapse after definitive local surgery or radiation. Eventually, these patients may develop metastatic prostate cancer lesions that require treatment with androgen deprivation therapy (ADT)2 and eventually chemotherapy for more advanced castration-resistant disease.3,4
Identifying prostate cancer metastases, especially determining response to therapy, is a major challenge for clinicians. As a result, the recent Prostate Cancer Working Group 2 recommendations5 for clinical trials recommend evaluating time to event endpoints rather than response endpoints. This is because clinically meaningful prostate cancer treatment responses have been difficult to define, and no uniformly accepted modality predicts clinical outcome. This is partially because the majority of men with prostate cancer harbor bone metastases, and only a minority of patients have measurable soft tissue lesions that shrink in a small fraction of patients,3,4 making the Response Evaluation Criteria in Solid Tumors criteria challenging for use in metastatic prostate cancer. Planar bone scintigraphy is hindered with issues such as the “flare response,” slow resolution, and a lack of quantitative ability. Prostate-specific antigen (PSA) decline does not provide information on the anatomy or the heterogeneity of disease response, and is not a universally accepted surrogate for long-term outcomes. Establishing an effective biomarker for men with metastatic prostate cancer would provide useful prognostic information, individualize systemic treatment, and aid in clinical trial development of novel therapeutics.
The use of molecular imaging as a means of monitoring response to prostate cancer therapy is appealing. Both magnetic resonance spectroscopy and diffusion magnetic resonance imaging offer functional and anatomic information, but additional work is necessary to demonstrate ability as a response measure.6,7 Positron emission tomography (PET) imaging is another modality with excellent potential to measure response, relying on differential tumor uptake and metabolism of substances such as glucose, acetate, or choline. Recent studies report decline in F-18 fluorodeoxyglucose (FDG) standard uptake value (SUV) with response to therapy in prostate cancer bone metastases,8,9 which correlate with decrease in PSA. Another study has shown that a >33% increase in FDG PET SUVmax or the appearance of a new lesion by FDG PET indicates progression for castration-resistant metastatic prostate cancer patients receiving antimicrotubule chemotherapy.10 Work from our institution in bone-dominant breast cancer has shown FDG PET changes to correlate with therapeutic response, tumor marker levels, and time to progression.11 However, FDG PET may have limited sensitivity for imaging prostate cancer, especially for those patients with earlier stage disease.12
Early studies with C11-acetate (acetate) PET imaging have generally demonstrated superiority in detection of prostate cancer over FDG PET.13–15 In one report, Fricke et al15 reported a higher rate of overall lesion detection with acetate (83%) than FDG (75%); however, median SUV was higher for acetate in local recurrences (2.9 vs. 1.0) and lymph node metastases (3.8 vs. 1.1), whereas median SUV was higher for FDG in distant metastases (3.2 vs. 2.3). The focus of the previously reported studies has been to detect the location(s) of cancer during an otherwise biochemically recurrent state.14–17 To our knowledge, no studies have focused on evaluation of bone metastases and response to systemic therapy with clinical endpoints. Therefore, the purpose of this pilot study was to evaluate acetate PET as a method to assess the response of prostate bone metastases to therapy. A secondary goal was to compare acetate uptake with FDG uptake in patients with prostate cancer bone metastases. Our hypothesis was that qualitative and quantitative changes in acetate uptake would occur with response and/or progression to systemic therapy. We compared acetate PET results with FDG PET, which has previously demonstrated utility for response assessment for some patients.10
MATERIALS AND METHODS
Patients
Men, 18 years or older, with prostate cancer and ≥3 bone metastases identified by Tc-99m bone scintigraphy and/or computed tomography (CT), who were preparing to receive systemic treatment with either ADT for new prostate cancer bone metastases or first-line chemotherapy with docetaxel for castration-resistant prostate cancer, were recruited from the Seattle Cancer Care Alliance and the University of Washington Medical Center. Patients were excluded from the study if a bisphosphonate, radiation to bone, or radiopharmaceutical treatment was given within 4 weeks, lifespan was limited to 12 weeks or less, patient was unable to lay still, patient was too heavy (>300 lbs) for the imaging table, or the patient had a condition that would impair ability to receive imaging and treatment or to understand and authorize informed consent.
We acquired acetate and FDG PET images before and 6 to 12 weeks after initiation of systemic therapy. All patients underwent chest, abdomen, and pelvic CT and Tc-99m MDP bone scintigraphy at similar intervals for comparison. After the first patient was enrolled, we amended the protocol to exclude patients who were treated with granulocyte-colony stimulating factor (GCSF) or granulocyte-macrophage colony stimulating factor within 4 weeks of the first PET scan or during the interval between the 2 PET scans. The rationale for this exclusion is presented in the results section.
Radiosynthesis
Acetate was made by reacting C11-CO2 with methylmagnesium bromide (Sigma Aldrich, St. Louis, MO) in a Grignard’s reaction as previously reported.18 The radiochemical purity was >98%. Acetate (0.3 mCi/kg of radiotracer up to a maximum of 30 mCi, containing <400 µg) was administered by intravenous injection of a 10-mL solution of isotonic saline. FDG was synthesized by the method of Hamacher et al.19 The radiochemical purity of the FDG was consistently >99%. Acetate and FDG met all of the quality control limits specified in their United States Pharmacopeia monographs.
Imaging Protocols
The PET studies were performed on a GE Advance PET tomograph (GEMS, Waukesha, WI) providing 35 image planes over a 15-cm axial field-of-view (FOV) with a 4.25-mm spacing.20 Although a 25-minute transmission scan with a Ge-68 rotating sector source was underway, intravenous lines were introduced for isotope injection and blood sampling. Acetate emission images were acquired in 1 FOV over the index lesion with a dynamic sequence: 16 × 5 seconds, 7 × 10 seconds, 5 × 30 seconds, 5 × 1 minutes, 5 × 3 minutes, and 1 × 5 minutes time frames for a total of 30 minutes. The index lesion was chosen as a site of prominent and easily identified disease, on the basis of the review of conventional imaging (CT, bone scan) performed before PET. The dynamic imaging data served primarily to provide quantitative uptake data for evaluation of change with therapy. After the dynamic emission scan and body transmission imaging, additional emission images were acquired for approximately 5 minutes per bed position from mid-thigh to head (3–5 FOV). Emission PET images were corrected for scattered and random coincidences, and reconstructed by filtered back-projection using a Hanning filter, which resulted in reconstructed spatial resolution of approximately 10 mm.
Immediately after the acetate study, and while the patient was in the same position, FDG (7–10 mCi) was injected intravenously into one arm, followed by both dynamic emission imaging over the same dynamic FOV as acetate and a body sweep (3–5 FOV). FDG body emission images were reconstructed with and without segmentation, applied to the transmission and transaxial slices, and reconstructed by conventional reconstruction, with corrections for scatter and random events.
Imaging Analysis
The primary goal of the study was to determine if qualitative and quantitative changes in acetate uptake occur in response to systemic therapy, and secondarily to compare acetate and FDG uptake pretherapy and changes in uptake in response to therapy. For the qualitative analysis, 2 independently blinded nuclear medicine specialists reviewed each patient’s CT and bone scans to determine sites of bone metastases. Furthermore, they reviewed both FDG and acetate PET torso survey images to determine if any metastases were detectable, and if so, whether the PET imaging modalities detect the known sites of metastases seen on CT and bone scan over the extent of the body covered by the PET studies (neck to pelvis). They also qualitatively described a change in response to treatment as response, stable disease, or progression based on qualitative changes in the intensity and/or extent of abnormal uptake seen on the PET studies. All lesions with tracer uptake were taken into account for the qualitative analysis, including any discrepancies between the index lesion used for quantitative analysis (see below) and other sites of disease. Any discordance in interpretation was resolved by consensus.
Quantitative analysis focused on the single dynamic imaging field where high-count data were available for both acetate and FDG. For the single FOV images of acetate and FDG, quantitative analysis was performed by constructing regions of interest over the index lesion(s) on the summed SUV scans acquired from 5 to 25 minutes for acetate21 and 40 to 60 minutes for FDG. Because the analysis considered images acquired at least 40 minutes postinjection of FDG and at least 100 minutes postinjection of acetate, there was minimal, if any, inclusion of counts from acetate injection in the FDG images that were analyzed. Mean and Max SUV from these regions were recorded using PMOD image analysis software (PMOD Technologies; Zurich, CH, Switzerland). For the early analysis in this study, we chose the lesion with the highest pretherapy uptake in the dynamic imaging field as the index lesion for quantitative comparisons with treatment. Because we observed considerable variability in acetate SUV in both tumor and normal bone, the tumor:normal tissue ratio served as the primary means for evaluating uptake and changes in response to therapy for both tracers. In each case, a normal comparable bone, documented to be free of disease by standard imaging and having no lesions by either FDG or acetate PET, served as the basis for the tumor:normal ratio for the index tumor site.
Clinical Response Determination
Each patient served as his own control, and specific qualitative and quantitative changes in both acetate and FDG PET uptake were compared with clinical response to treatment. To serve as a “gold standard” response, 2 medical oncologists, blinded to the PET results, reviewed each case before treatment and again 6 to 12 weeks after initiation of therapy. Clinical symptoms, bone scans, CT scans, and PSA levels were extracted for medical oncologist review, and the patients were designated as either demonstrating a response to therapy, stable disease, or progressive disease. Both, improvement in cancer pain or ≥50% PSA decline from the pretreatment baseline level were included in the designation of clinical response.22 Worsening of cancer pain, ≥2 new abnormal malignant bone scan lesions, or ≥25% increase in PSA from baseline were included in designation of clinical progression.5 Stable disease was designated if patients did not meet the criteria for clinical response or progression. If there was any discordance in clinical classification, a consensus was reached by the 2 blinded readers by discussion. The clinical classification was further compared directly with both qualitative and quantitative changes in PET uptake measures, as determined by methods described above.
Statistics
Statistical comparisons before and after therapy were made using Student t test (paired) for continuous variables and Spearman ρ for categorical variables, using the statistical software package JMP (SAS; Cary, NC).
RESULTS
A total of 8 patients were enrolled in this pilot study. Patient characteristics are provided in Table 1. All patients underwent pretreatment acetate PET scans, while 7 underwent pretreatment FDG PET imaging (1 patient refused the baseline FDG PET after developing pain while lying on the scanning table). Two patients underwent pretreatment scans only, one patient withdrew from the study early to pursue naturopathic therapies and another patient was unable to undergo the second set of imaging studies due to equipment malfunction.
TABLE 1.
Patient Characteristics
| PSA | |||||
|---|---|---|---|---|---|
| Patient | Gleason Score | Primary Therapy |
Disease Status On Study |
Baseline | Post-Rx |
| 1 | 5 + 4 = 9 | RP | CRPC | 6.3 | 1.2 |
| 2 | 4 + 5 = 9 | ADT | HS | 451 | N/A* |
| 3 | 4 + 3 = 7 | ADT | CRPC | 234 | 263 |
| 4 | Cytology positive, no Gleason given | ADT | HS | 432 | 0.2 |
| 5 | Moderately differentiated, no Gleason given | ADT | CRPC | 60 | 75 |
| 6 | 5 + 4 = 9 | ADT | CRPC | 2010 | 1690 |
| 7 | 3 + 4 = 7 | RP | HS | 26 | 0.7 |
| 8 | 3 + 4 = 7 | Brachytherapy | HS | 98 | N/A* |
N/A: not applicable because patient did not undergo a second post-Rx PET.
PSA indicates prostate-specific antigen; RP, radical prostatectomy; ADT, androgen deprivation therapy; CRPC, castration resistant prostate cancer; HS, hormone sensitive.
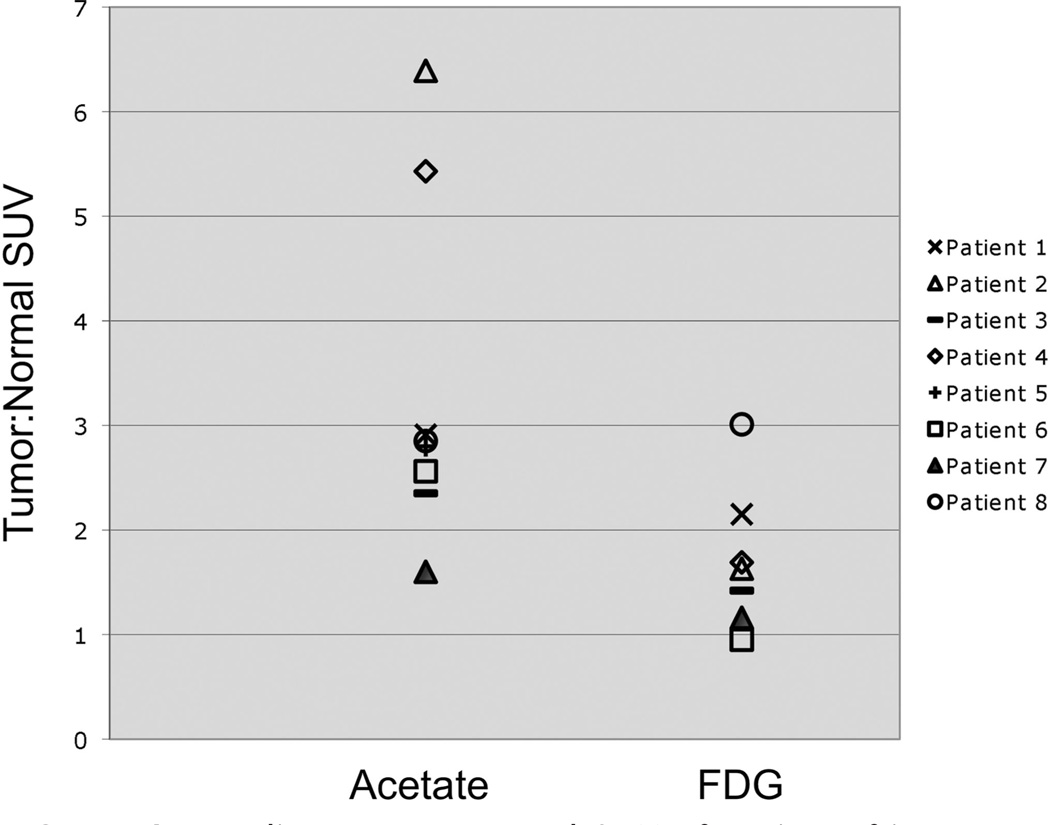
Of the patients who underwent baseline scans, all 8 patients had metastatic bone lesions detected by acetate PET and 6 of 7 detected by FDG PET. Five of 8 of the acetate PET images and 2 of 7 of the FDG PET images detected all metastatic bone lesions visualized by conventional imaging over the imaging field covered by PET. In 6 patients, acetate detected more lesions than FDG PET, and occasionally, lesions were detected that were not found on bone scan. In one patient, FDG detected more lesions than acetate PET, and in another patient, the 2 radiotracers were equivalent. Uptake was overall heterogeneous, where acetate would illuminate some and FDG other lesions; however, overall demonstration of metastases was more reliable by acetate as compared with FDG PET. Additionally, 2 patients had lymph node metastases, and although none were detected by FDG PET, acetate PET detected all as well as some additional sites that were not visualized on CT imaging. By quantitative analysis, there was higher tumor:normal background SUV ratio by acetate when compared with FDG PET (Fig. 1).
FIGURE 1.
Baseline tumor:normal SUV of region of interest. For each PET scan, the region of interest was defined and the ratio of the acetate SUV for that region to the SUV for a normal region of bone was used to determine the tumor: normal SUV. This figure illustrates that acetate PET imaging of the metastatic bone lesions generally demonstrated a higher tumor:normal SUV.
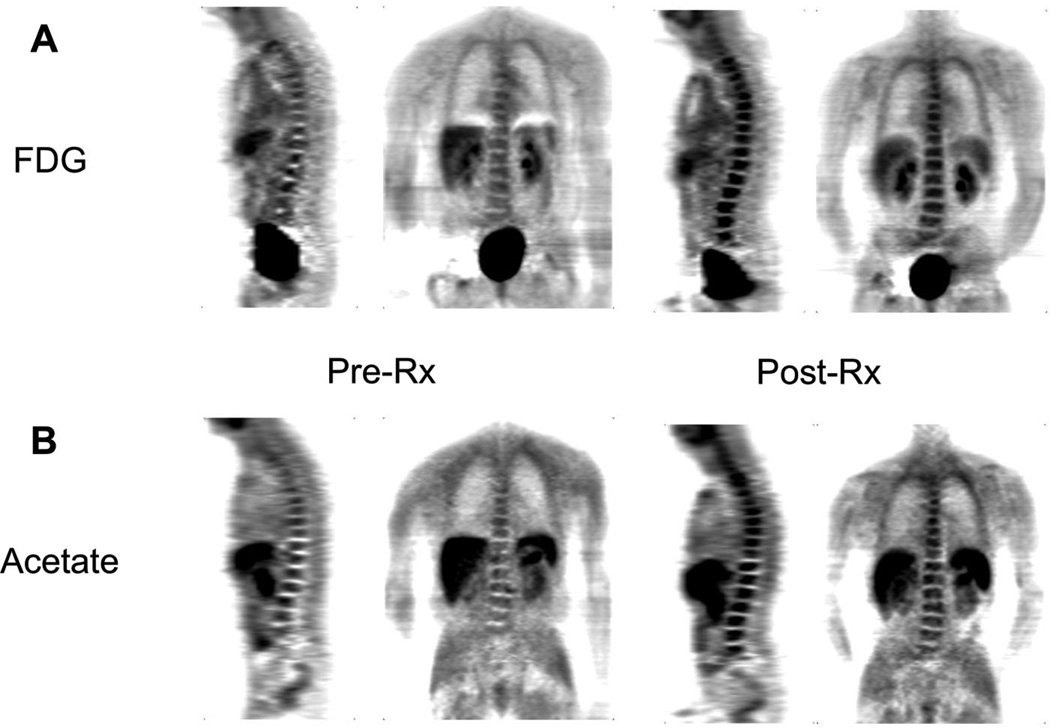
Six patients underwent both pretreatment and post-treatment imaging studies and were evaluable for treatment response. Two patients had hormone treatment naive prostate cancer and received first-line ADT with goserelin combined with bicalutamide. Four patients had received prior ADT and were initiating every 3-week docetaxel chemotherapy, with prednisone for castration-resistant (serum testosterone, <50 ng/dL) disease. Of the 4 patients receiving docetaxel, the first patient developed asymptomatic neutropenia as a result of his first treatment with docetaxel and received prophylactic GCSF after his second treatment of docetaxel. His second PET imaging studies showed diffuse marrow signal on both FDG and acetate PET (Fig. 2), preventing the classification of bone metastasis treatment response by PET. This is well described in the FDG PET literature,23 but to our knowledge has not been described in the acetate literature. However, he had an assessable enlarged right hilar lymph node, visualized by CT and acetate PET but not by FDG PET; evaluation of this lymph node was not hampered by GCSF administration. Clinically, he had a response to docetaxel, with decrease in pain and PSA. In the remaining 5 patients, both patients receiving first-line ADT had dramatic response to therapy by composite clinical parameters assessed by our panel of medical oncologists, specifically improvement in pain and significant PSA decline. Of the remaining 3 patients receiving docetaxel chemotherapy, 2 progressed (1 patient by PSA and the other by restaging CT and bone scan) and one had response by PSA decline with stable restaging scans.
FIGURE 2.
Diffuse marrow signal in response to GCSF. In the first study patient receiving docetaxel chemotherapy, GCSF was used for prophylaxis from neutropenia. Not surprisingly, the second FDG PET scan (A, right: post-treatment [post-Rx]) to determine response to therapy revealed a diffuse intense marrow signal in the bone compared with the first (A, left: pretreatment [pre-Rx]). The second acetate PET scan showed a similar result (B, left: pre-Rx and B, right: post-Rx). As a result, the protocol was amended to prohibit imaging of patients who would receive growth factors that might lead to such a result, confounding assessment of treatment response.
Changes observed by qualitative analyses in response to therapy correlated with changes in composite clinical parameters in 6 of 6 by acetate PET and 4 of 5 by FDG PET (Table 2). Examples of both response to therapy and progression of disease are provided in Figures 3 and 4, respectively.
TABLE 2.
Qualitative Therapeutic Response Assessment
| Patient | Disease Status | Treatment Received | Clinical Response | Acetate | FDG |
|---|---|---|---|---|---|
| 1 | CRPC | Docetaxel + OGX011* | Response by PSA | Response | Response |
| 3 | CRPC | Docetaxel + Prednisone | Progression by PSA | Progression | Progression |
| 4 | HS | Goserelin + Bicalutamide | Response by PSA | Response | Response |
| 5 | CRPC | Docetaxel + Prednisone | Progression by bone scan and PSA | Progression | N/A—no baseline scan |
| 6 | CRPC | Docetaxel + Prednisone | Response by PSA after 12 wk | Response | Response |
| 7 | HS | Goserelin + Bicalutamide | Response by PSA | Response | No uptake‡ |
OGX011: experimental agent being tested in clinical trials as an antisense to clusterin.
This patient was deemed to lack FDG uptake at baseline and on subsequent PET imaging after therapy.
CRPC indicates castration resistant prostate cancer; HS, hormone sensitive.
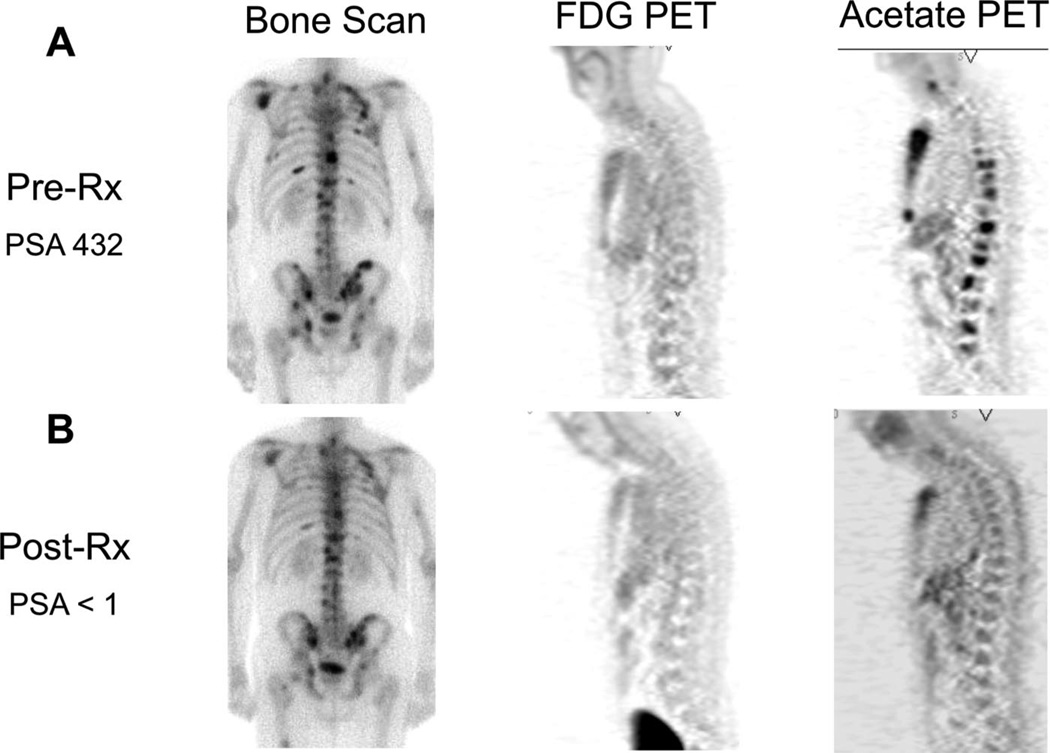
FIGURE 3.
Response to androgen deprivation therapy. This patient presented with painful bone metastases with a PSA of 432 ng/mL. After 12 weeks of combined androgen deprivation therapy (goserelin + bicalutamide), his pain resolved and his PSA declined to <1 ng/mL. His pre-Rx (A, left) and post-Rx (B, left) bone scans do not show significant change in response to therapy. The bone metastases were not detected in the baseline FDG scan (A, center), and therefore, no assessment can be made in terms of response to therapy (B, center). The acetate PET imaging studies (A, B, right) show almost no uptake after 12 weeks of therapy.
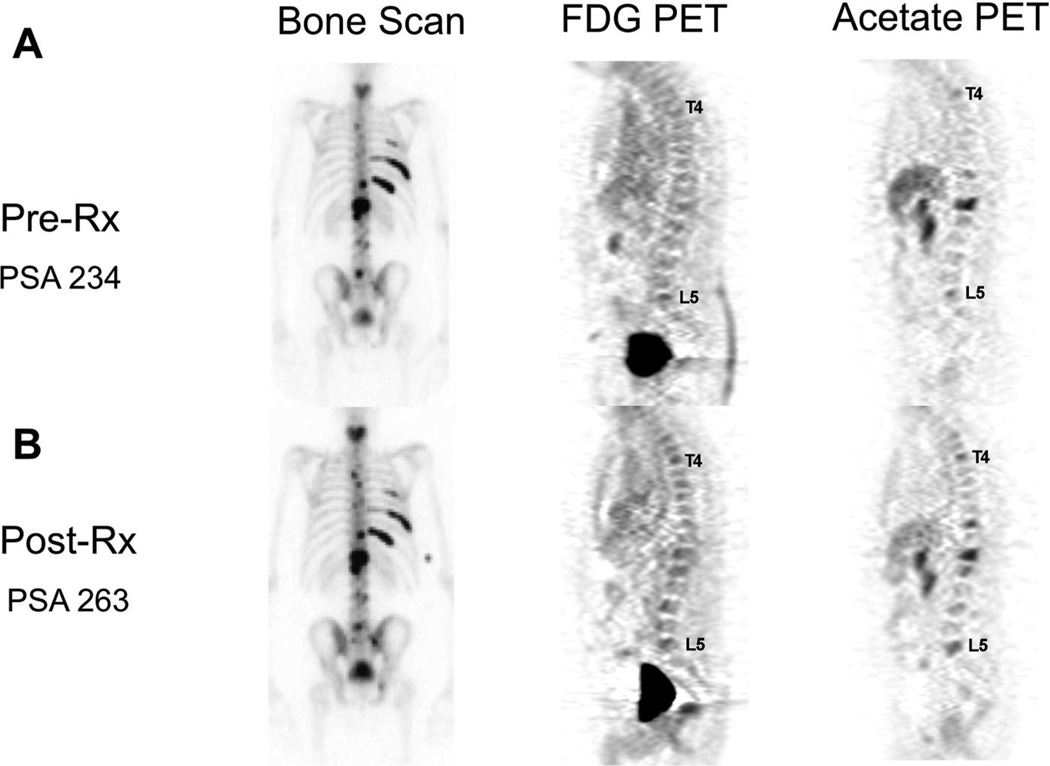
FIGURE 4.
Progression on docetaxel. This patient with castration-resistant prostate cancer received 3 cycles of docetaxel chemotherapy with prednisone, with subjective improvement in bone pain. He had no obvious change in response to therapy on bone scan (A, B, left), yet very subtle worsening on both FDG (A, B, center) and acetate PET (A, B, right) imaging studies. Specifically, the lesions at L5 may be worse and there may be a new lesion at T4 by PET (see labels). This matched a subtle increase in his PSA from 234 to 263 ng/mL.
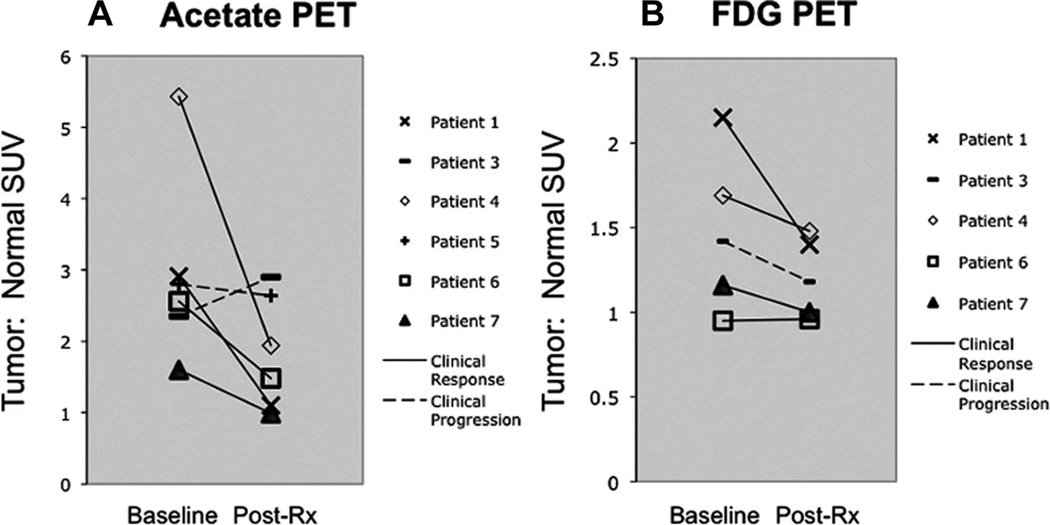
Tumor:normal ratio was most useful in assessing quantitative response (Fig. 5A, B), given the greater variability in normal background for acetate, possibly due to the conversion of acetate to 11C-CO2. The quantitative analysis, using tumor:normal ratio, revealed the lesion with the highest pretherapy uptake in the dynamic imaging field to be the same site in all cases by both acetate and FDG PET. There were no instances in which the index lesion behaved substantially differently in treatment response to the other lesions evaluated on the qualitative analysis. Changes in PET in response to therapy correlated with changes in composite clinical parameters in 5 of 6 by acetate PET and 3 of 5 by FDG PET. Additionally, 2 patients with lymph node metastases detected only by acetate PET underwent changes in response to therapy that corresponded with the composite clinical parameters.
FIGURE 5.
Quantitative change in PET tumor:normal SUV in response to therapy. A, Acetate PET: Six patients were assessable for response to therapy by undergoing both baseline and post-treatment response acetate PET scans. Four patients were deemed to have a clinical response to therapy and that matched in all 4 patients with declines in tumor:normal SUV by acetate PET. Two patients had clinical progression. Of those 2 patients, 1 had increase in tumor:normal SUV and another had little change. B, FDG PET: Five patients were assessable for response to therapy by undergoing both baseline and post-treatment response FDG PET scans. Four patients were deemed to have a clinical response to therapy and that matched with declines in tumor:normal SUV by FDG PET in 3 of 4 patients. Another had essentially no change in tumor:normal SUV. One patient had clinical progression and this did not match with the change in tumor:normal SUV that showed decrease in response to therapy rather than increase.
DISCUSSION
In this prospective pilot trial, acetate PET detected more bone metastases and had higher tumor:normal background SUV for prostate cancer bone metastases when compared with FDG PET in most patients. This assessment was true for the majority of both hormone-sensitive and castration-resistant patients. Response to therapy was assessable by both qualitative and quantitative acetate PET, corresponding with early response and progression to therapy in most patients, as measured by composite criteria incorporating bone scans, CT scans, PSA changes, and clinical symptoms. Interestingly, we observed diffuse marrow uptake of acetate PET in one patient who received GCSF while undergoing docetaxel chemotherapy, similar to diffuse marrow FDG uptake in response to GCSF in other types of cancer patients.23 We observed, as others have,10 that serial FDG PET also measured response, although low pretherapy uptake hampered evaluation in some patients.
The biologic mechanisms for the accumulation of acetate are not yet fully understood, but there is a reasonable rationale supporting its use in prostate cancer. Acetate uptake in tumor cells is regulated by fatty acid synthase (FASN) and distributed into biosynthetic pathways for phospholipid membrane synthesis and maintenance.24,25 FASN expression occurs early in prostate cancer development,26 yet androgen-independent bone metastases display the highest FASN expression.27 Therefore, overexpression of FASN in prostate cancer may increase the accumulation of acetate as a marker of tumor activity and lipid metabolism.26,28,29
Normal tissue uptake varied between patients and for the same patient in serial studies. We therefore chose to use the tumor:normal ratio to quantify changes in acetate uptake with therapy. The reason for this variability is unknown, but may relate to acetate clearance and/or metabolism. Planned future analysis of dynamic data using kinetic modeling may shed light on this finding and decrease variability of measures of acetate uptake.
Although our data suggest that acetate has potential as a response measure for men with prostate cancer bone metastases, we recognize that there may be limitations to its use in clinical trials and clinical practice. In particular, acetate has a very short 20-minute half-life and not all institutions can perform these studies. Additionally, biologic heterogeneity exists within individual patient bone metastases,30 and combination with other imaging agents or modalities may improve detection and response assessments. Other agents that are also thought to image lipid metabolism, such as C11-choline31–33 and F-18 Fluorocholine,34 may have similar value for imaging prostate cancer.
Our study population consists patients who are earlier in the natural history of prostate cancer progression than other recent publications evaluating FDG PET for measuring prostate cancer bone metastasis response.10 This small study sample offers a mixed but representative population of patients with hormone-sensitive and castration-resistant prostate cancer, who were receiving first-line treatment for their respective disease states. Potential differences in results compared with other studies10,15 may be related to heterogeneity of patient populations or to our limited sample size. Future studies would benefit from a more comprehensive analysis of the entire burden of metastatic disease9 than was possible in this pilot study.
CONCLUSIONS
Our early data suggest that the acetate PET may offer an assessment of treatment response and is complementary to FDG PET for detection of bone metastatic prostate cancer. Further studies in this setting are warranted.
Acknowledgments
Supported by the following NIH grants: Pacific Northwest Prostate Cancer SPORE P50-CA97186, Cancer Center Support Grant P30-CA015704, and Nuclear Medicine P01-CA42045.
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evelhoch J, Garwood M, Vigneron D, et al. Expanding the use of magnetic resonance in the assessment of tumor response to therapy: workshop report. Cancer Res. 2005;65:7041–7044. doi: 10.1158/0008-5472.CAN-05-0674. [DOI] [PubMed] [Google Scholar]
- 7.Kurhanewicz J, Swanson MG, Nelson SJ, et al. Combined magnetic resonance imaging and spectroscopic imaging approach to molecular imaging of prostate cancer. J Magn Reson Imaging. 2002;16:451–463. doi: 10.1002/jmri.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oyama N, Akino H, Suzuki Y, et al. FDG PET for evaluating the change of glucose metabolism in prostate cancer after androgen ablation. Nucl Med Commun. 2001;22:963–969. doi: 10.1097/00006231-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Morris MJ, Akhurst T, Osman I, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–918. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 10.Morris MJ, Akhurst T, Larson SM, et al. Fluorodeoxyglucose positron emission tomography as an outcome measure for castrate metastatic prostate cancer treated with antimicrotubule chemotherapy. Clin Cancer Res. 2005;11:3210–3216. doi: 10.1158/1078-0432.CCR-04-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Specht JM, Tam SL, Kurland BF, et al. Serial 2-F18-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) to monitor treatment of bone-dominant metastatic breast cancer predicts time to progression (TTP) Breast Cancer Res Treat. 2007;105:87–94. doi: 10.1007/s10549-006-9435-1. [DOI] [PubMed] [Google Scholar]
- 12.Schoder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34:274–292. doi: 10.1053/j.semnuclmed.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Oyama N, Miller TR, Dehdashti F, et al. C11-acetate PET imaging of prostate cancer: detection of recurrent disease at PSA relapse. J Nucl Med. 2003;44:549–555. [PubMed] [Google Scholar]
- 14.Oyama N, Akino H, Kanamaru H, et al. C11-acetate PET imaging of prostate cancer. J Nucl Med. 2002;43:181–186. [PubMed] [Google Scholar]
- 15.Fricke E, Machtens S, Hofmann M, et al. Positron emission tomography with C11-acetate and F18-FDG in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2003;30:607–611. doi: 10.1007/s00259-002-1104-y. [DOI] [PubMed] [Google Scholar]
- 16.Kotzerke J, Volkmer BG, Neumaier B, et al. Carbon-11 acetate positron emission tomography can detect local recurrence of prostate cancer. Eur J Nucl Med Mol Imaging. 2002;29:1380–1384. doi: 10.1007/s00259-002-0882-6. [DOI] [PubMed] [Google Scholar]
- 17.Wachter S, Tomek S, Kurtaran A, et al. C11-acetate positron emission tomography imaging and image fusion with computed tomography and magnetic resonance imaging in patients with recurrent prostate cancer. J Clin Oncol. 2006;24:2513–2519. doi: 10.1200/JCO.2005.03.5279. [DOI] [PubMed] [Google Scholar]
- 18.Pike VW, Eakins MN, Allan RM, et al. Preparation of C11-acetate—an agent for the study of myocardial metabolism by positron emission tomography. Int J Appl Radiat Isot. 1982;33:505–512. doi: 10.1016/0020-708x(82)90003-5. [DOI] [PubMed] [Google Scholar]
- 19.Hamacher K, Coenen HH, Stocklin G. Efficient stereospecific synthesis of no-carrier-added 2-F18-fluoro-2-deoxy-D-glucose using aminopolyether supported nucleophilic substitution. J Nucl Med. 1986;27:235–238. [PubMed] [Google Scholar]
- 20.Lewellen TK, Kohlmyer SG, Miyaoka RS, et al. Investigation of the performance of the general electric ADVANCE positron emission tomograph in 3D mode. IEEE Trans Nucl Sci. 1996;43:2199–2206. [Google Scholar]
- 21.Muzi M, Swanson K, Spence A, et al. Initial assessment of an acetate model for membrane biosynthesis in glioma patients. J Nucl Med. 2003;44:216P. [Google Scholar]
- 22.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 23.Jacene HA, Ishimori T, Engles JM, et al. Effects of pegfilgrastim on normal biodistribution of F18-FDG: preclinical and clinical studies. J Nucl Med. 2006;47:950–956. [PubMed] [Google Scholar]
- 24.Yoshimoto M, Waki A, Yonekura Y, et al. Characterization of acetate metabolism in tumor cells in relation to cell proliferation: acetate metabolism in tumor cells. Nucl Med Biol. 2001;28:117–122. doi: 10.1016/s0969-8051(00)00195-5. [DOI] [PubMed] [Google Scholar]
- 25.Swinnen JV, Van Veldhoven PP, Timmermans L, et al. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898–903. doi: 10.1016/s0006-291x(03)00265-1. [DOI] [PubMed] [Google Scholar]
- 26.Swinnen JV, Roskams T, Joniau S, et al. Overexpression of fatty acid synthase is an early and common event in the development of prostate cancer. Int J Cancer. 2002;98:19–22. doi: 10.1002/ijc.10127. [DOI] [PubMed] [Google Scholar]
- 27.Rossi S, Graner E, Febbo P, et al. Fatty acid synthase expression defines distinct molecular signatures in prostate cancer. Mol Cancer Res. 2003;1:707–715. [PubMed] [Google Scholar]
- 28.Swinnen JV, Vanderhoydonc F, Elgamal AA, et al. Selective activation of the fatty acid synthesis pathway in human prostate cancer. Int J Cancer. 2000;88:176–179. doi: 10.1002/1097-0215(20001015)88:2<176::aid-ijc5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 29.Pizer ES, Pflug BR, Bova GS, et al. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–110. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 30.Roudier MP, True LD, Higano CS, et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum Pathol. 2003;34:646–653. doi: 10.1016/s0046-8177(03)00190-4. [DOI] [PubMed] [Google Scholar]
- 31.Kotzerke J, Volkmer BG, Glatting G, et al. Intraindividual comparison of C11-acetate and C11-choline PET for detection of metastases of prostate cancer. Nuklearmedizin. 2003;42:25–30. [PubMed] [Google Scholar]
- 32.Luboldt W, Kufer R, Blumstein N, et al. Prostate carcinoma: diffusion-weighted imaging as potential alternative to conventional MR and C11-choline PET/CT for detection of bone metastases. Radiology. 2008;249:1017–1025. doi: 10.1148/radiol.2492080038. [DOI] [PubMed] [Google Scholar]
- 33.Tuncel M, Souvatzoglou M, Herrmann K, et al. C11-Choline positron emission tomography/computed tomography for staging and restaging of patients with advanced prostate cancer. Nucl Med Biol. 2008;35:689–695. doi: 10.1016/j.nucmedbio.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Beheshti M, Vali R, Waldenberger P, et al. Detection of bone metastases in patients with prostate cancer by F18-fluorocholine and F18-fluoride PET-CT: a comparative study. Eur J Nucl Med Mol Imaging. 2008;35:1766–1774. doi: 10.1007/s00259-008-0788-z. [DOI] [PubMed] [Google Scholar]