Abstract
A deeper understanding of how the relationships between impulsivity, reward systems and executive function deficits may be similar or different in attention-deficit/hyperactivity disorder (ADHD) and pediatric bipolar disorder (PBD) is fundamental for better defining phenotypy in these two developmental illnesses, and moving towards improved treatment and intervention. We focus our article on recent neurocognitive and neuroimaging data examining the behavioral and neural aspects of poor behavior regulation, response inhibition and reward systems in ADHD and PBD. In light of recent research evidence, we propose that the common behavioral manifestations of impulsivity in ADHD and PBD may indeed originate from different neural mechanisms mediated by altered reward systems. In order to define and differentiate these mechanisms, unlike previous approaches, our theoretical model examines the interface of the dorsal frontostriatal circuit, involved in behavior regulation, and the ventral frontostriatal circuit, which is involved in reward-related and affect processes. Preliminary evidence suggests that the neural systems involved in impulsivity, reward systems and executive function engage differently in the two illnesses. In PBD, `emotional impulsivity' is predominantly `bottom-up' and emotionally/motivationally driven, and stems from ventral frontostriatal circuitry dysfunction. By contrast, in ADHD `cognitive impulsivity' is predominantly `top-down' and more `cognitively driven', and stems from dorsal frontostriatal dysfunction. We discuss this evidence in view of clinically relevant questions and implications for illness-based intervention. We conclude that the reward-related mechanisms underlying the interactions between executive function, behavior regulation and impulsivity in PBD and ADHD may be differentially compromised, and in accordance differently shape the clinical symptoms of impulsivity and goal-directed behavior.
Keywords: ADHD, adolescents, affect, behavior regulation, children, executive function, functional MRI, impulsivity, inhibition, neurocognitive, pediatric bipolar disorder, reward
At present it is extremely difficult to delineate the phenotypy of impulsivity in pediatric bipolar disorder (PBD) and attention-deficit/hyperactivity disorder (ADHD). A potentially debatable concept is whether impulsivity is a common dimensional dysfunction across the two disorders, with a unified neurobiological mechanism, or if it is driven by two distinct neural mechanisms with merely a common behavioral manifestation. A clear understanding of the mechanistic route to psychopathology will lead to accurate and neurobiologically informed interventions, including pharmacotherapy or psychosocial therapy. Therefore, we critically evaluate the existing literature and present our current conceptual and methodological framework to study and potentially differentiate the neurobiological bases of impulsivity in ADHD and PBD. We will examine impulsivity in terms of poor inhibition function, which results from altered interaction between regulation and motivational/reward processes. This article will conclude with the presentation of a new model to pave the path for future directions in translational neuroscience and symptom-based characterization [1] towards a better understanding of PBD and ADHD psychopathology that will inform precise illness-specific intervention.
ADHD & PBD phenotypy & neural mechanisms involved
Profiles of pathophysiology in ADHD & PBD: similarities & differences
Attention-deficit/hyperactivity disorder and PBD are two developmental syndromes with a high degree of comorbidity, ranging from 60 to 90% [2–4]. Even in the absence of comorbidity, they share the common symptoms of inattention, impulsivity, excessive talk and hyperactivity [4], which are often associated with cognitive dysfunction and affect dysregulation [2,4–6]. This apparent overlap in clinical phenotypes may lead to misdiagnosis or delay in diagnosis and treatment with adverse consequences for the individual and families.
Attention-deficit/hyperactivity disorder is one of the most common pediatric mental illnesses, with an estimated prevalence of 5–8% in children and often persisting into adulthood [7] with impaired inhibition, attention and executive functions, as well as altered sensitivity to reward contingencies [8–13], all of which may contribute to impulsivity, risky behavior, poor social, academic and occupational skills, increased rates of substance abuse, and traffic accidents [5,11,14,15]. We will focus here on the ADHD combined subtype with hyperactivity, impulsivity and inattention (DSM-IV-TR) [16].
Bipolar spectrum disorder has an estimated prevalence of 1–5% [17]. However, in this article, we will focus on studies that examined the narrow phenotype of PBD, Type I and II (DSM-IV-TR) [16] presenting with mania and hypomania, elation, grandiosity, irritability, racing thoughts, decreased need for sleep, and hypersexuality [4,18]. Note that there is still an ongoing debate on how euphoria or irritability may contribute to mania in youth [18,19]. Either way, in these children, severe and persistent affect dysregulation, impulsivity and altered response to reward with low frustration tolerance [20,21] often leads to considerable impairment in social, cognitive and school functioning [22–24] maladaptive reward-seeking behaviors and high rates of substance abuse [4,25–27].
Common phenotypy & neural operations underlying executive function deficits in ADHD & PBD
Executive functions, which rely heavily on lateral and medial prefrontal cortex (PFC) function [28], include behavior control, inhibition processes, attention, working memory, planning, decision making, problem solving and emotional self-regulation. Protracted myelination and maturation of PFC regions through adolescence [29] have been associated with an improvement in executive functions [30–32], and better emotional self-regulation with development has been ascribed to improved executive functions and attentional control of emotion processing [33,34]. Deficits in the realm of executive function affect cognitive control operations that support complex cognitive behaviors and regulate impulses or reward processing.
There is recent evidence of cognitive deficits in PBD in the domains of executive function, sustained attention, verbal learning and working memory [6,23,24] that are independent of illness status [23] and may worsen rather than diminish with development. This broad range of executive function deficits has been demonstrated to directly impact reward-related processes, motivation and goal-oriented behavior in bipolar disorder (BD) in youths [20,35–37].
Children with ADHD exhibit deficits in executive functions, attention, vigilance, working memory, planning and response inhibition [14,38], with more severe neurocognitive impairment than children with PBD with or without comorbid ADHD diagnosis [39]. Moreover, it has recently been acknowledged that in addition to the core attention deficits, poor emotional self-regulation is common in ADHD [40,41], including problems with facial affect recognition [42], with resisting the impact of emotions on cognitive processes during emotional challenge [8,43,44] and underlying hyperactivity of limbic brain regions involved in emotion processing [45]. Such emotional deficits need to be better understood in their relation to executive function deficits and mood dysregulation as observed in PBD.
In summary, executive function deficits are common across ADHD and PBD – although they may be more severe across different domains in ADHD, and while future research needs to ascertain whether the underlying mechanisms may be different, these deficits impact cognitive control of behavior in a similar manner in the two illnesses.
Inhibitory functions: common external phenotype & differential neural operations of inhibition in ADHD & PBD
Inhibitory function, that is the ability to inhibit or delay an action, or to inhibit conflicting cognitive and affective processes, is a major component of executive function and a prerequisite for self-regulation of behavior, affect and arousal [8,46,47]. This function relies on frontostriatal circuits including the dorsolateral PFC (DLPFC), ventrolateral PFC (VLPFC), anterior cingulate cortex (ACC) and dorsal striatum [30,48,49]. Developmental studies indicate that maturation of PFC and dorsal striatum through late adolescence leads to improvement in inhibitory functions [31,32,50,51].
Deficits in inhibition and self-regulation of affect and arousal are core components of impulsivity [8,47]. Impulsivity has multiple facets to its behavioral manifestation, such as being too quick to act, the inability to stop or postpone action, exhibiting emotional outbursts short of considering the consequences, poor ability to plan, bypassing multiple steps towards a goal, or inability to delay a reward and wait for a better outcome. Behavioral studies on response inhibition have confirmed that problems in inhibition are a core deficit in children with ADHD (see [52–55] for reviews). Given the long history of characterizing ADHD (see [40] for a historical perspective), there have been several influential neuroscience-based models that attempt to explain impulsivity in this illness. Abnormal patterns of cortical maturation have been found in ADHD, suggesting an overall developmental delay in brain maturation, including delayed maturation of prefrontal regions [56]. Building from Barkley's (1997) theory of ADHD as a dysfunction in `inhibitory control' [8], Sonuga-Barke [11,57], Nigg and Casey [58] and Castellanos et al. [59] ascribe deficits in executive function to frontostriatal or `cool' pathways and deficits in emotion regulation to fronto–limbic or `hot' pathways (see also Rubia et al. for a similar theory [60]). Moreover, given the specific role of dopamine in the frontal cortex and striatal functioning related to attention, cognition, inhibition and reward processes [61], dopaminergic system dysfunction has played a particular role in ADHD theories.
Dopamine has been found to affect brain function early on in development. Dopamine levels in dorsolateral PFC have been documented to affect performance in working memory and inhibition tasks in humans from infancy through to early childhood [62]. Moreover, nigrostriatal and mesolimbic projections in dopamine circuits are important for reinforcement learning mechanisms [12]. Sagvolden et al. proposed that impulsive emotions and low frustration tolerance in ADHD are caused by hypodopaminergic activation in the mesolimbic pathway and striatum, which control affect and motivation [63]. Important clues in this regard come from recently developed animal models of ADHD that may aid in understanding the biochemical and neurological substrates of this pathophysiology in striatum and prefrontal regions [64]. For example, Paine et al. examined whether dopamine signaling within the medial PFC modulates attention processes in rats [65]. They performed intramedial PFC micro-infusion of D1 receptor agonists and antagonists of cAMP-dependent protein kinase, a target of D1 receptor stimulation, to examine effects on attention performance in a five-choice serial reaction time task similar to the continuous performance task in humans. The results indicate that these manipulations in rats led to both reduced attentional performance and hyperactivity, which are two distinctive features of ADHD in humans – suggesting that ADHD may involve protein kinase dysregulation within medial PFC resulting in inattention and hyperactivity.
Among imaging studies, functional MRI (fMRI) findings from response inhibition tasks point to frontostriatal dysfunction as responsible for poor inhibition in children with ADHD [48] and, more recently, in children with PBD, with or without ADHD comorbidity [66–68]. Response inhibition has been mainly studied using the Stop Signal Task or `go/no-go' tasks. The Stop Signal Task is a well-known paradigm, based on animal [69] and human [46,70] models of response inhibition, that examines the ability to inhibit a prepotent and already on-the-way motor response when a stop cue appears at varying delays from the presentation of a go cue. This task has been widely used with healthy adults and children [71,72], as well as in the clinical population [66,70]. Performance on this task improves steadily from childhood to adulthood [72,73] and has been found to strongly correlate with levels of impulsivity in ADHD [52,72]. Studies of adolescents with ADHD that used either the Stop Signal Task [48,74], or go/no-go tasks, where subjects respond or not based on the presence or absence of target stimuli [30,75–77], found decreased activation in response inhibition circuits. Specifically, Durston et al. employed an event-related (ER) fMRI go/nogo task with parametric manipulation of the number of go trials preceding a no-go trial to manipulate levels of cognitive interference [75]. As expected, healthy controls (HC) showed increased susceptibility to interference with increasing numbers of go trials preceding nogo trials, but children with ADHD had response inhibition difficulties even with a single go trial preceding a nogo trial. Moreover, this performance pattern in ADHD was accompanied by reduced frontostriatal activation, in favor of a more diffuse network of posterior brain regions, suggesting that poor inhibition in ADHD is associated with executive function deficits. Similar results were obtained in a later ER-fMRI study by Durston et al., where male children and adolescents with ADHD showed decreased correlation between performance on successful nogo trials and activation in VLPFC, relative to HC [76]. Rather, their performance correlated with parietal activation, possibly suggesting compensatory activation in posterior brain regions to counterbalance poor activation in prefrontal regulatory centers. Moreover, using the Stop Signal Task, Rubia et al. compared a small sample of adolescent boys with ADHD with HC on stop trials (both correct and incorrect ones), and found reduced activation in the VLPFC, ventromedial PFC (VMPFC) and caudate in the ADHD group relative to HC [48]. Similarly, Plitzka et al. found that during unsuccessful stop trials in a Stop Signal Task, in spite of no behavioral group differences, children with ADHD (combined type) exhibited reduced activation in dorsal ACC and VLPFC, and increased activation of DLPFC, relative to HC [74]. These fMRI findings are in line with findings of reduced volumes in prefrontal and caudate regions, especially in the right hemisphere, in children with ADHD during the developmental years [78–80].
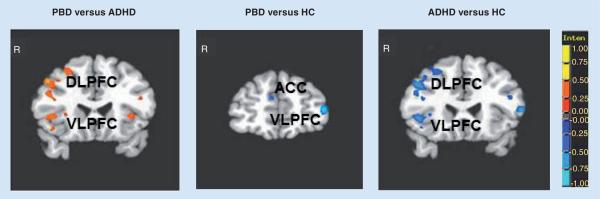
In PBD studies, several block design and ER fMRI studies have linked inhibition deficits to VLPFC dysfunction [66,67,81], which seems to be independent of mood state [81,82]. Important differences in the neural substrate of response inhibition were found in a first block design fMRI study comparing children with PBD and ADHD. Passarotti et al. compared adolescents with PBD without ADHD comorbidity and adolescents with ADHD without PBD comorbidity on a response inhibition task [67]. Comparable response inhibition deficits and ADHD symptom scores were found in both patient groups. However, at the neurological level, the ADHD group, relative to both HC and PBD, failed to engage the VLPFC, which is implicated in inhibition and interference control [14,83], and the DLPFC and dorsal striatum, which are responsible for sustained behavior control selection [84], action suppression and working memory processes [49,85]. The greater level of VLPFC and DLPFC dysfunction in ADHD relative to PBD (Figure 1) is in agreement with a diffusion tensor imaging (DTI) study that illustrated extensive white matter abnormality in prefrontal fiber tracts involved in cognitive function in ADHD relative to PBD [86]. These findings support dual deficits within the dorsal and ventral regulation and inhibition circuits in ADHD relative to PBD.
Figure 1. Between-group differences in significant clusters of brain activation for the Stop vs Go condition.
(A) PBD versus ADHD; (B) PBD versus HC; (C) ADHD versus HC. Red indicates greater activation in the first group compared with the second group. Blue indicates greater activation in the second group compared with the first group.
ACC: Anterior cingulate cortex; ADHD: Attention-deficit/hyperactivity disorder group; DLPFC: Dorsolateral prefrontal cortex; HC: Healthy controls; PBD: Pediatric bipolar disorder group; VLPFC: Ventrolateral prefrontal cortex.
Consistent findings emerged in two other block design studies that directly compared PBD and ADHD using paradigms that examined inhibition function as the ability to inhibit challenging emotional information during cognitive processing, such as during a color matching task with emotional words [87] and a working memory task with emotional faces [88]. These findings collectively imply shared functionality of the brain regions such as VLPFC, DLPFC and dorsal ACC in motor response inhibition and inhibition of conflicting processes [87]. However, when it comes to the differential dysfunction across the disorders, despite deficits in response control in both disorders, deficits in ADHD are associated with more extensive dorsal and ventral PFC dysfunction, involving both dorsal frontostriatal dysfunction and ventral VLPFC dysfunction (i.e., Brodmann area [BA] 45,47) [15,30,48]. By contrast, the neural underpinnings of response inhibition deficits in PBD, relative to HC, are associated with a more localized neural dysfunction, which, based on our studies [67,81,87,88], seems to lie in a more superior region at the junction of VLPFC/DLPFC, at the interface of cognitive and affective systems [89] in BA 46, 47 and 10, and in ventral ACC (vACC), a region involved in automatic inhibition of amygdala activation [81,82,90]. In spite of these interesting initial results, it is important to further determine what drives these fMRI group differences in response inhibition dysfunction.
In summary, the phenotype of impulsivity manifests similarly in ADHD and PBD, but it is differentially driven by cognitive and emotional brain regions, respectively, across these two disorders. Precision to act or not to act and the associated inhibition processes: performance monitoring, sustained vigilance, and response selection towards cognitive and affective regulation, are all critical for overcoming impulsivity in the clinical population. We propose that reward-related processes are closely associated with such cognitive and motor behavior regulatory functions.
Different phenotypy & neural operations of reward sensitivity in ADHD & PBD
Until recently, behavior regulation deficits were mainly examined as attention or executive function problems [8,70,91]. However, recently reward-related processes are re-emerging as a concept of motivating forces shaping the regulation of behavior. Reward sensitivity is linked to the motivational drive underlying goal-oriented behavior because in order to provide appropriate response to the environment, information about motivation and reward needs to be integrated with an action plan to attain goals [92].
Human and animal studies [93,94] have revealed a complex and extended neural circuitry for reward processes that includes brain areas involved in processing of reward and punishment contingencies [95], as well as circuits involved in decision-making, motivation and emotion processing [96]. There are several parallel loops within the frontostriatal system that initiate from and project to DLPFC, supplementary motor area, ACC, VLPFC, lateral orbitofrontal cortex and striatum [92,95,96].
Frontostriatal circuits involved in self-regulation keep maturing until late adolescence [31,32] where neural development is accompanied by reorganization of cognitive, affective and social functions [31,97–99]. Given that studies on reward systems in ADHD and PBD are in their infancy, we will start by discussing developmental studies on reward systems that can provide a framework to help understand the role of frontostriatal and limbic response to reward contingencies in normally and abnormally developing children [100,101].
An important study by Ernst et al. compared reward systems in adolescents and adults during an ER fMRI study using the `wheel of fortune' task: a computerized two-choice task involving probabilistic monetary outcomes [100]. The fMRI results indicated that the amygdala and nucleus accumbens (NAcc) showed greater activation when winning than losing, or with greater than smaller incentives, in both adolescents and adults. Nevertheless, the signal differences between positive and negative outcomes and between smaller or greater incentives involved more the NAcc in adolescents and the amygdala in adults. Moreover, using a gambling task with reward or losses May et al. found greater sensitivity to reward delivery in adolescents than in adults in the ventral striatum and orbitofrontal cortex [102]. An important distinction is also the one between anticipation of rewards, that is the `motivational' aspect, and response to delivered rewards, that is the `consummatory' aspect. Using a monetary incentive task, Bjork et al. found that while both adolescents and adults exhibited similar consummatory responses, associated with increased activation in ventral striatum and VMPFC, during reward anticipation, adolescents relative to adults exhibited reduced recruitment of the ventral striatum and amygdala [103]. While preliminary, these findings suggest that adolescents may engage in risky behaviors that may lead to negative and dangerous outcomes because they need greater reward stimulation in order to reach levels of motivation to action comparable to those in adults.
Prefrontal regions are also highly involved in reward-directed behavior. To examine whether risk-taking bias during decision-making correlates with development of PFC, a study by Eshel et al. used a monetary decision-making task requiring participants to choose among options with different degrees of risk [104]. The results indicated that adolescents engaged the PFC less than adults when making risky monetary decisions. There was also a correlation between less efficient recruitment of prefrontal regulatory regions (VMPFC, VLPFC and ACC) and preference for risky options in adolescents. Similarly, using a monetary game where participants decided when to press a button to collect their winnings, before risking to lose everything, Bjork et al. found that medial ACC regions in BA 24 become more sensitive to risk options with age [103]. These same PFC and ACC regions are highly implicated in both ADHD and PBD. In summary, these developmental studies suggest that the biological bases for impulsive behavior in adolescents may result from a combination of hyperactive ventral striatum for `approach' behavior, and hypoactive amygdala supporting `avoidance' mechanisms, as well as reduced regulation of PFC that is still maturing and not yet primed to inhibit excessive subcortical activity [98,100,101]. While the dual pathway theory by Sonuga-Barke posits that deficits in inhibition and in motivation are separate and may lead to two different subtypes of ADHD, we propose that inhibition and motivation deficits are interlaced and lead to behavior regulation deficits in ADHD [11,57]. Initial research evidence suggests that ADHD may involve abnormalities not only in executive but also in motivational pathways. Recent behavioral studies point at altered reinforcement sensitivity in ADHD, although results are sometimes inconsistent, and we still know very little about the cognitive or neurobiological mechanisms underlying these deficits (see [105,106] for a comprehensive review). Behavioral research suggests that similar to healthy children, children with ADHD benefit from positive reinforcement and there is some evidence that reinforcement effects on cognitive skills are larger in ADHD children than in HC [105]. However, children with ADHD prefer immediate over delayed reward even if the immediate reward is smaller [107,108]. Reward-seeking may lead to risky behavior such as gambling in ADHD. A gambling behavior study by Faregh and Derevensky found that adolescents with ADHD were significantly more likely than non-ADHD adolescents to engage in gambling and later develop gambling problems [109].
In terms of the neurobiological mechanisms that may underlie these deficits, Sheres et al. found that 12–17-year-old adolescents with ADHD, relative to HC, demonstrated similar behavioral responses but reduced ventral striatal activation for reward vs non-reward trials [13]. Importantly, reduced striatal activation for reward anticipation correlated with hyperactivity and impulsivity, but not with general cognitive measures of inattention. Similarly, reduced activation in VMPFC and ventral striatum during a response inhibition task with monetary contingencies was found in adults with ADHD relative to HC [110]. Moreover, both experimental evidence and clinical observations suggest that children with ADHD respond well to immediate rewards, but are less responsive to rewards that are delivered intermittently or with a temporal delay [9], possibly because of weak anticipatory dopamine signal to cues predicting reinforcement [12]. These results in ADHD likely suggest more prominent impairment in motivational response to reward rather than skewed appraisal of the actual reward contingencies once they are delivered, which may affect or be associated with compromised executive function and impulsivity.
With regard to PBD, recent studies suggest impaired `top-down' prefrontal regulation of over-reactive limbic activity as key in its pathology [67,68,81,87,88,111,112]. Studies on reward-related processes in BD have shown increased frustration and emotional reactivity, especially to negative contingencies and feedback, with poor ability to adapt to changing contingencies during reversal learning tasks, both in children with PBD [20,35] and adults with BD [37]. Gorrindo et al. compared performance in children with PBD and HC on a probabilistic reversal learning task [35]. Their results showed worse performance in PBD relative to HC while learning the reward object in a repetitively presented pair of stimuli, and then re-identifying the reward object after it had been switched to the other stimulus in the pair. Similar results were obtained in a reversal learning study by Pizzagalli et al. where adults with BD exhibited, relative to HC, reduced and delayed reward-related learning, which correlated with self-reported mood symptoms [37].
Children with PBD also show reduced ability to deal with frustration in other cognitive flexibility tasks specifically designed to engender frustration. For example, in an event-related potential (ERP) study by Rich et al., participants underwent an affective Posner attention task, with three conditions: a condition with feedback but no contingencies, a condition with contingencies, and a third condition with rigged feedback in order to cause frustration [20]. ERP data showed that the PBD group relative to HC had impaired ability to adapt to contingencies, in that it exhibited reduced amplitude in parietal P3 (an ERP component originating in the parietal cortex and reflecting attention processes) with the rigged task, suggesting that these children deploy attention to their internal frustration rather than to the task. Using the same affective Posner task, a magnetoencephalography study by Rich et al. showed altered magnetoencephalography patterns of activity in right ACC and bilateral parietal lobe, related to frustration-inducing negative feedback [36]. Similar results were obtained by Dickstein et al. using a fMRI probabilistic reversal learning task, that showed greater frontoparietal activation in PBD patients relative to HC, especially in response to punished reversal errors [113]. Finally, a fMRI study with adult bipolar patients with mania by Abler et al. found NAcc dysfunction in patients during a monetary incentive paradigm, where after an expectation interval participants either underwent reward trials or reward omission trials [114]. Different from HC, patients showed a lower differential signal in the NAcc for the receipt as compared with the omission of an expected reward. It is still to be ascertained whether the same abnormalities in NAcc would be observed with euthymic patients and in BD children and adolescents.
In summary, dysfunctional development of reward systems may lead to excessive reward-seeking behavior such as pathological gambling [109] or substance abuse [115] and vulnerability to mental illness, impulsivity and deficient behavior regulation [97,101,116,117], and therefore remain central to our interpretation and application of our findings. Both ADHD and PBD exhibit altered reward-related processes. However, preliminary evidence suggests that while in ADHD there are deficits in executive functions that may be associated with reward anticipation (i.e., motivational aspects), in PBD, altered evaluation and appraisal of reward contingencies (i.e., consummatory aspect), with increased frustration and emotionality, especially with negative contingencies, may create interference with executive function. Therefore, the behavioral manifestations vary between the two disorders and the neural operations also differ across ADHD and PBD.
An integrated neurobiological model of response inhibition, reward & executive function systems to differentiate PBD & ADHD
The interaction between dorsal and ventral frontostriatal circuits in PBD & ADHD
Unlike previous approaches, our theoretical model aims to differentiate PBD and ADHD phenotypes for impulsivity by examining the interface of the interlinked processes of response inhibition and reward as fundamental to understand the complexity across these two illnesses.
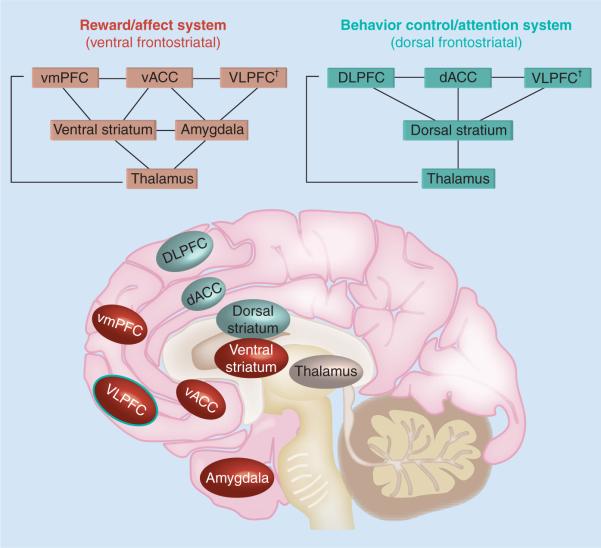
Building from our previous work [23,65,67,68,81,87,88] and expanding from current neuroscience models of ADHD [8,11,40,57,58,60,80], we conceptualize behavior regulation as a function that relies on the integration of interfacing systems involved in cognition, reward processes and inhibition [92,96]. As shown in Figure 2, we propose a neurobiological model of the reward and behavior control systems, presenting two distinct but parallel and interfacing systems that regulate behavior. A fronto–dorsal–striatal circuit regulates behavior in the context of `stimulus–reward' and `action–reward' association and includes the DLPFC, the dorsal ACC (dACC) and the VLPFC, which have reciprocal connections with the dorsal striatum (i.e., caudate), via thalamic mediation [96]. The DLPFC and dACC are involved in behavior control, working memory and decision-making [85], while the VLPFC is involved both in affect regulation [111,117,118] and inhibition [83,87,112]. Finally, the dorsal striatum is involved in learning, error signal and reinforcement of actions potentially leading to reward [119], where caudate signal associating an action with either a positive or negative outcome could be used to either reinforce or weaken the action [120, 121].
Figure 2. Schematic representation of reward and behavior control systems.
In the brain image, regions that are part of the reward/affect system are depicted in dark circles, while regions that are part of the behavioral control/attention system are depicted in light circles.
†The VLPFC is included in both systems because of its integrative role for behavioral inhibition and emotional regulation.
dACC: Dorsal anterior cingulate cortex; DLPFC: Dorso-lateral prefrontal cortex; vACC: Ventral anterior cingulate cortex; VLPFC: Ventro-lateral prefrontal cortex; VmPFC: Ventro-medial prefrontal cortex.
A fronto–ventral striatal circuitry is involved in reward processes and includes VLPFC, VMPFC (including BA 10,11 and 32), the ventral ACC (vACC), ventral striatum and amygdala. The vACC aids VLPFC in limbic regulation [122]. The VMPFC is important for evaluation and prediction of reward contingencies [122–124], and provides the main cortical input to the ventral striatum (i.e., nucleus accumbens), which is also involved in reward evaluation and prediction [121,125,126].
Importantly, while both the ventral and dorsal striatum receive input from PFC and project back to PFC through the medial thalamus, only the ventral striatum, involved in motivational aspects of goal-oriented behavior [127,128], receives abundant projections from the amygdala. The amygdala is a key region for emotional processing, which is not limited to aversive stimuli [129]. Moreover, single-cell recording studies conducted with nonhuman primates suggest amygdala contributions to reward processing in terms of affective evaluation of both positive and negative stimuli [130,131]. In addition, fMRI studies with adult humans [132] and patients with bilateral amygdala lesion [124] suggest that the amygdala aids the VMPFC in encoding the value of reward representations and in reward expectancy. An important point is that while NAcc motivates approaching behavior towards the most advantageous course of action, the amygdala relates the affective valence of stimuli to NAcc as well as to brainstem and arousal systems, warning to avoid negative stimuli [101,133]. In view of these considerations, we included the amygdala in the ventral system circuits.
Amygdala dysfunction is present predominantly in PBD [111,112,118] and to a degree in ADHD [45] and may affect interactions between the dorsal and ventral frontostriatal systems. For example, our group found preliminary evidence of reduced functional integration of amygdala, VLPFC and VMPFC regions within affect regulation and working memory networks in PBD relative to HC during a working memory task with emotional faces [134]. Moreover, a study by Foland et al. found reduced functional connectivity between VLPFC and amygdala in BD patients relative to HC during emotion processing tasks [135]. These patterns of dysfunctional connectivity may contribute to the persistent fronto–limbic dysfunction seen in this disorder. To highlight the limbic influence of affective arousal on the ventral circuitry, we have called the ventral frontostriatal system the `reward/affect system'.
Cognitive versus emotional impulsivity in ADHD & PBD
Based on our current understanding of the brain mechanisms underlying inhibition processes, it is becoming evident that prefrontal regions are involved both in motor inhibition and in inhibition of emotions, such as anger, fear and sadness (see also [47] as well as in reward-directed behavior [11,92,101,104]. Yet, we propose that different mechanisms of interaction between inhibition and reward/affect circuits characterize `cognitive impulsivity' in ADHD and `emotional impulsivity' in PBD. With regard impulsivity, we think it is important to make a distinction that has utmost clinical relevance, between `cognitive' versus `emotional' drives to impulsivity, based on the mechanistic root the behavioral impulsivity. Barkley recently proposed emotional dysregulation as a `core feature' of ADHD in both children and adults, where executive failure to inhibit normally intense emotions of anger, impatience and frustration can lead to emotional over-reactivity, for which he coined the term `emotional impulsivity' [10,40]. The issues of impulsivity, self-regulation and the relationship between ADHD and irritable mood are currently being considered and may lead to significant changes in the DSM-V [136–138]. In agreement with Barkley's view, and based on our own studies comparing ADHD children with no severe mood dysregulation and children with PBD, we propose that the emotional disinhibition seen in the typical child with ADHD is in fact resulting from generalized inhibition problems across cognition and affect, and is qualitatively different from the abnormal and extremely intense emotional reactions that severely challenge prefrontal regulation systems in PBD. Moreover, given the new directions towards disorder classification based on neurobiological mechanisms underlying symptom dimension [1], we propose the term `cognitive impulsivity' for ADHD, in order to highlight the type of impulsivity that is primarily driven by a generalized `top-down' inhibition problem, based on poor executive and attention functions, which extends both to cognitive and emotional processes. This emotional dysregulation in a child with the typical ADHD profile is a potential but minor affect dysregulation in proportion to the `emotional impulsivity' seen in severe affect disorders such as PBD, where persistent and exaggerated subcortical limbic activity that undermines PFC regulation in a `bottom-up' fashion may lead to explosive emotionality, rage, violent outbursts, self-harm, suicide attempts and risky behavior.
Based on our model (Figure 2), our overarching hypothesis is that `emotional impulsivity' in PBD is more robustly modulated by dysfunction of the reward/affect system, that is the ventral frontostriatal circuitry. We hypothesize that emotional impulsivity results from subcortical overactivity of the amygdala and ventral striatum, associated with reduced emotion regulatory function of the VLPFC, VMPFC and vACC. Moreover, because of the research evidence on worsening of affect dysregulation in PBD with negative valence stimuli [81,87,88] or negative contingencies such as punishment or loss of expected reward [20,36], we predict that over-reactivity in limbic and ventral striatal regions will be worse in the presence of perceived or experienced negative emotions, such as anger, frustration and fear, that may be elicited by negative contingencies. By contrast, based on previous literature [8,11,13,40,57–60,110] we hypothesize that in ADHD, impulsivity would be predominantly modulated by poor attentional, inhibitory and regulatory function in the dorsal frontostriatal system, with reduced capacity of DLPFC and dACC to attend to and evaluate reward-related contingencies and to regulate amygdala and ventral striatum activity elicited by these contingencies.
A key aspect in our model for differentiating between ADHD and PBD relates to the interface of inhibition and reward. Based on previous studies with cognitive and affective paradigms [23,67,81,87,88,112,118], we propose that overall, children with ADHD have greater dorsal frontostriatal inhibition dysfunction relative to children with PBD, but that when we tap on the interface of reward and inhibition circuits by introducing performance-based reward or punishment contingencies, children with PBD would show worse dysfunction in the ventral reward/affect system and the dorsal behavioral control system (Figure 2) relative to ADHD. In other words, positive and negative contingencies should affect the ability to inhibit pre-potent responses over subsequent trials in a response inhibition task more severely in PBD than in ADHD, because of the more severe affect dysfunction in PBD that impacts more on the interface between reward and regulation systems in PBD relative to ADHD. Therefore, we would expect that in a task where the child fails to inhibit a response and receives negative feedback, we should see increased limbic and ventral striatum activity in children with PBD relative to children with ADHD, accompanied by reduced activity in the regulatory dorsal frontostriatal system. Ventral striatum activity has been found to respond to both occurrence or anticipation of positive and negative contingencies because of its involvement in motivation and goal-directed behavior. For example, a fMRI study by Levita et al. showed increased NAcc activation at the onset of both rewarding and aversive stimuli [128], which is likely due its role in modulating goal-directed behavior [133]. Moreover, Carter et al. found increases and decreases in NAcc in anticipation of both gain and losses during a monetary incentive delay task, which may reflect anticipatory activation related to the motivational relevance of upcoming events [127].
Preliminary studies using cognitive and affective paradigms provide initial support to our model. Pilot data using a stop signal task with positive and negative monetary feedback indicate in fact that after errors on stop trials, the PBD group relative to the ADHD group exhibits reduced ability to adjust inhibitory response based on the immediate feedback, with increased ventral striatal activity and reduced ventral and dorsal PFC regulation of emotional over-reactivity in response to negative contingencies [PASSAROTTI AM, PAVULURI MN. UNPUBLISHED DATA]. Moreover, in line with our hypotheses that negative emotional challenge hinders regulatory functions in PBD more than in ADHD, it was found that while in a response inhibition task with no feedback, those with PBD showed increased VLPFC and DLPFC activation compared with those with ADHD [67], during an emotional task, namely a `n-back task' with angry, happy and neutral faces, it was only with negative-valence stimuli such as angry faces that PBD exhibited a reduction in DLPFC activation relative to ADHD [88].
Finally, while in our discussion we are mainly referring to the typical ADHD Combined Type profile, with no chronic irritability, there is a third important group that is positioned symptomatically in between PBD and ADHD and that deserves further attention. Specifically, children with severe mood dysregulation (SMD), as defined by Leibenluft [137,138], present not only high ADHD comorbidity rates, but also severe irritability and hyperarousal, which are chronic and non-episodic. Moreover, different from children with PBD, children with SMD do not exhibit discrete hypomanic or manic episodes [138]. It will be very important to better differentiate, in research and clinical practice, between the phenotypes for ADHD, PBD or SMD in order to inform more specific intervention and treatments [138].
A few studies have already confirmed neural differences between ADHD, PBD and SMD with regard to limbic activity related to face emotion processing [45] and attention to emotional stimuli [139]. The amygdala is often hyperactive in PBD relative to HC, contributing to persistent mood dysregulation [20,81,112]. While our studies with emotional challenge did not find differences in amygdala activation in PBD relative to ADHD [87,88], Brotman et al. report hyperactivity in left amygdala activation in children with ADHD relative to either PBD, SMD or HC, while giving emotional ratings of subjective fear to neutral faces [45]. This novel finding suggests potentially different neural correlates of face emotion processing in ADHD relative to PBD or SMD that warrant future investigations. Moreover, a study by Rau et al. suggests that youth with SMD and with narrow BD phenotype did not differ in their ability to select between differently valued rewards and punishments during a decision-making task, but we still do not know whether the two groups may differ at the neural level in this regard [140]. It will be important that future studies further differentiate between different profiles of mood dysregulation also in terms of impulsive behavior and reward-related processes within a neurobiological framework.
Neurobiologically-informed treatments of cognitive & emotional regulation deficits in ADHD & PBD: clinical applications
While the proposed neurobiological model, with its predictions and the preliminary results, is just an initial step towards better understanding impulsivity mechanisms in ADHD and PBD, it nevertheless provides a crucial framework for future studies that will foster development of neurobiologically informed intervention (Figure 3 & Table 1). In line with the proposed concept of either emotionally-driven or cognitively-driven impulsivity, one aspect that is gaining confirmation experimentally is that in PBD, impairment in reward-related learning [20,35,36] may be due to abnormal reactions to punishment or negative contingencies, with emotional over-reactivity (i.e., frustration and irritability), leading to diminished ability to attend to task-related processes and inhibit interfering affective or cognitive processes [20,36]. On the contrary, in ADHD, reward-related learning is within normal range as long as positive and negative contingencies are administered consistently and without delays [8,9,105,106], possibly because of altered reward anticipation and delay aversion [11] accompanied by poor working memory, performance monitoring and inhibitory function for goal maintenance [59,67,71]. This initial evidence suggests that different types of feedback and reward contingencies need to be integrated with intervention at school and home for PBD and ADHD (Table 1). In agreement with these findings, our model and preliminary findings suggest that negative contingencies affect children with PBD more than those with ADHD and that ADHD may benefit more than PBD from feedback for improving response inhibition. These are crucial predictions to be tested in the quest to differentiate PBD and ADHD pathology and inform new intervention strategies.
Figure 3. Model of dimensions informing disorders: pediatric bipolar disorder and attention-deficit/hyperactivity disorder.
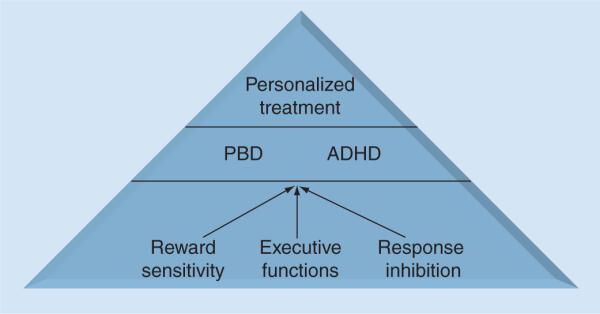
ADHD: Attention-deficit/hyperactivity disorder; PBD: Pediatric bipolar disorder.
Table 1.
Model of neurobiologically informed treatment.
| Dimension | Behavior | Neural operations | Treatment |
|---|---|---|---|
| Response inhibition | Impulsivity | PBD: mainly driven by amygdala and striatum in interaction with ventral frontostriatal system (VLPFC, VMPFC, vACC) | PBD: mood stabilizers as first line to aid ventral frontostriatal–limbic system, followed by stimulants to aid dorsal frontostriatal system |
| ADHD: mainly driven by cognitive dorsal frontostriatal system (DLPFC, VLPFC, dACC, dorsal striatum) | ADHD: stimulants as first line to aid dorsal frontostriatal system | ||
| Reward | Frustration Altered sensitivity | PBD: mainly driven by amygdala and striatum in interaction with ventral frontostriatal system (VLPFC, VMPFC, vACC) | PBD: child and family-focused CBT (self-psychology + CBT). No negative contingencies |
| ADHD: mainly driven by ventral striatum in interaction with dorsal frontostriatal system (DLPFC, VLPFC, dACC, dorsal striatum) | ADHD: contingency-based training and management | ||
| Executive functions | Cognitive regulation (working memory, attention, decision-making, planning) | PBD and ADHD: mainly driven by dorsal frontostriatal system (DLPFC, VLPFC, dACC, dorsal striatum) | PBD and ADHD: computer-based cognitive enhancement training with feedback, focused on cognitive functions (i.e., working memory, attention, inhibition, problem-solving) |
ADHD: Attention-deficit/hyperactivity disorder; CBT: Cognitive–behavioral therapy; dACC: Dorsal anterior cingulate cortex; DLPFC: Dorsolateral prefrontal cortex; PBD: Pediatric bipolar disorder; vACC: Ventral anterior cingulate cortex; VLPFC: Ventrolateral prefrontal cortex; VMPFC: Ventromedial prefrontal cortex.
In ADHD, several studies support the efficacy of behavioral parent training and classroom management based on social learning aspects to cope with impulsivity, inattention and poor behavioral regulation (see [141,142] for reviews), with the use of reinforcement (e.g., praise, positive attention and concrete rewards) to reinforce appropriate behavior and immediate punishment (e.g., ignoring the child or time out) to extinguish inappropriate behavior (Table 1) [8]. Studies with ADHD on cognitive–behavioral therapy (CBT) with parental training revealed some improvement in parental ratings of hyperactivity and self-esteem but no improvements on parent or teacher ratings of inattention and impulsivity [143] Behavioral classroom intervention studies relying on structured environment and direct contingency management together with medication suggest improved behavioral control in ADHD [144].
What is quintessentially different in CBT-based treatments for PBD is that the negative contingency-based behavior therapy will be unhelpful in PBD, as explained by our neurobiological model (Table 1) where PBD children over-react to negative contingencies and cannot regulate their behavior, based on contingencies. Therefore, Pavuluri et al. designed the child and family-focused CBT that is based on cognitive therapy limited to positive self-statements and self-psychology concepts, which are integrated to foster cognitive self-statements and collaborative problem solving between parent and child, rather than implementing negative consequences to the child maladaptive reactions [145]. This integrated or modified child and family focused-CBT model, which helps facilitate long-term management of symptoms together with medication [146], may address the therapeutic needs complicated by affect/motivation dysregulation, impulsivity, explosive rage, anger, depression, mania, sleep problems, poor executive functions, negative thinking and risky behavior in PBD [145–147].
It is also noteworthy that there has recently been a development of cognitive enhancement programs, usually in the form of structured computerized games with feedback, such as Cogmed (Cognitive Medical Systems AB, Stockolm, Sweden) [148,149] to aid with cognitive deficits related to poor executive function (e.g., in attention and working memory) (Table 1). Previous studies that trained healthy children and children with ADHD using Cogmed showed improvements in working memory function [148] and changes in related brain networks [150], although it is a current challenge to prove that improvements in functions closely related to those engaged in the training program can generalize to response inhibition and executive functions, as well as academic skills. Our laboratory is currently examining the mechanisms that may improve working memory in computerized training programs such as the Cogmed program, towards developing modified Cogmed protocols with additional response inhibition training tasks that are more tailored to the type of executive problems seen in PBD and ADHD.
In terms of pharmacological treatment in ADHD, stimulants modulate dopaminergic systems and improve response inhibitory functions in children with ADHD (Table 1) [151]. Sheridan et al. used a working memory task in adolescents with ADHD who were either on their usual dose of stimulants or off stimulants, and found increased function in basal ganglia accompanied by decreased effort-related DLPFC activation, suggesting that stimulants may improve saliency of basal ganglia signal to PFC, thereby reducing cognitive effort [152]. There is also initial evidence that by affecting dopaminergic reward systems in the striatum [153], stimulants reduce reward-seeking behavior and substance abuse [154].
Moreover, a few studies on methylphenidate (MPH) treatment in children with ADHD have shown improved function in caudate and prefrontal regions during attentional tasks [155,156], and there is some recent evidence that MPH may normalize functioning of both attentional and motivation networks, probably because of strong dopamine involvement in both interacting circuits. In fact, a fMRI study with adolescents with ADHD by Rubia et al., which used a rewarded continuous performance task that measured vigilance and the effects of reward, found that acute dose of MPH upregulated functional connectivity in the attentional fronto–striato–parieto–cerebellar circuit, and downregulated hypersensitive motivational orbitofrontal networks [157]. However, the clinical significance of these results may need further confirmation, since clinical applications of MPH are usually titrated.
Other recently approved treatment options are available for pediatric population with ADHD who do not respond well to stimulants (see [158] for a comprehensive review). In general, though, it seems that effects of medications and reward contingencies used in psychosocial therapy for ADHD are additive rather than synergistic with regard to improving inhibitory control. In a recent ERP study, Groom et al. found improvements in electro physiological markers of response inhibition during a go/nogo task in children with ADHD as an effect of both MPH treatment and reward contingencies [159]. Even if to a lesser degree than medication, motivational incentives also increased electrophysiological correlates of attention (P3 component) and response conflict monitoring (N2 component) in ADHD children, suggesting that they have the ability to improve based on feedback and contingencies. It is noteworthy that the effects of motivational incentives were not differentially greater in patients than in HC, suggesting normal response to immediate and consistent rewards, but given the reduced inhibition performance in ADHD at baseline, this suggests that children with ADHD may need qualitatively or quantitatively different incentives relative to healthy peers. Future studies may need to better examine various aspects of reward such as reward magnitude, reward schedule and reinforcement history in ADHD intervention. In summary, medication makes the child more able to attend and in control, which facilitates learning and task completion, enabling a more concerted and time-sensitive reaction to the prevailing motivation or reinforcement contingencies [8].
In line with the neurobiologically informed treatment model that we describe in Table 1, while in ADHD stimulants are mainstream pharmacotherapy that work by aiding the dorsal frontostriatal system, in PBD, mood stabilizers alone or followed by stimulants are widely used to treat mood dysregulation and improve function in fronto–limbic regions. Stimulants by themselves will only worsen mood stability in PBD (see [160] for a review). Recent pharmacological fMRI studies in PBD showed improvement in inhibitory function and mood stability with lamotrigine, divalproex and risperidone [68,161–164]. For example, lamotrigine treatment in hypomanic pediatric patients led to increased activation in ventromedial PFC during a motor response inhibition task [68] and an affective color matching task that probed the interface of cognition and emotion control [161]. In another study using an affective working memory paradigm in PBD, Passarotti et al. showed that antipsychotic medication followed by lamotrigine monotherapy resulted in improvement of manic and depressive symptoms and normalization of activity in higher cortical emotional (i.e., VLPFC) and cognitive (i.e., DLPFC) regions patients relative to HC [112]. These initial studies suggest that lamotrigine may have a dual action on affective and cognitive systems and, therefore, may be useful in PBD where the interface of cognition and affect is severely compromized. There is also initial evidence that lamotrigine treatment may improve working memory performance [163]. Another study by Pavuluri et al. was a double-blind randomized controlled trial to examine how 6-week treatment with an antipsychotic (i.e., risperidone) and an anti-epileptic agent (i.e., divalproex) would affect brain function during a working memory task under emotional challenge in adolescents with bipolar mania [162]. The results suggested drug-specific mechanisms of action, in that while with treatment both patient groups showed reduced activation in manic symptoms, the divalproex group showed greater increase over treatment in VLPFC and middle temporal gyrus, whereas the group treated with risperidone, showed a greater increase in ventral striatum, rich in D2 receptors. At present there are no studies examining whether pharmacotherapy directly affects reward systems in PBD. Future research will need to ascertain whether an improvement in mood regulation may also lead to improved regulation in motivational mechanisms, with benefits to cognitive and executive functions.
Conclusion
Understanding the brain function and behavior that underlie the overlapping clinical feature of impulsivity and poor inhibition and how that shapes the response to reward and loss, and vice versa, will reveal the bio-signatures of ADHD and PBD. We propose a testable neurobiological model of impulsivity positing that in PBD a predominantly `emotionally-driven' or `bottom-up' impulsivity is associated with a reward/affect system or ventral frontostriatal circuitry. By contrast, in ADHD a more `cognitively-driven' or `top-down' impulsivity predominates, stemming from dysfunction in the behavior regulation system or dorsal frontostriatal circuit. It will be important that future studies integrate fMRI, neurocognitive and clinical measures to test new theories on impulsivity and better differentiate the neurobiological mechanisms of PBD and ADHD. This in turn will inform disorder-specific interventions such as cognitive remediation or reward-based psychosocial interventions.
Expert commentary
Approximately 50% of adults with mental disorders have illness onset by the age of 14 years [165]. Therefore, it is imperative to understand the developmental aspects of pediatric mental illnesses, as well as how the psychopathology develops into adult life, and provide early diagnosis and interventions during the formative years. The past two decades have witnessed considerable progress in the diagnosis and treatment of ADHD and PBD, thanks also to developmental cognitive neuroscience studies and the use of brain imaging techniques to uncover brain dysfunction in these mental illnesses [166], which led to the discovery of abnormal patterns of cortical maturation in ADHD [56] and of dysfunction in prefrontal control of limbic over-reactivity in PBD [66,112,117,118]. Still, there is a persistent debate, in the media and the scientific community alike, on the risk of misdiagnosis in the pediatric clinical population, which is particularly true with regard to ADHD and PBD, because of high comorbidity and overlapping psychiatric and cognitive symptoms that confuse diagnosis and delay treatment.
While the notion of ADHD as a pediatric and adult illness has been present for over 100 years [8], it is only very recently that PBD has been acknowledged as a distinct pediatric illness, and that clinical evidence has emerged of continuity of child bipolar I disorder into adulthood, for frequency of manic episodes and substance use disorder [4]. For PBD the focus was originally on persistent affect dysregulation [6,23], but recently it became increasingly clear that PBD also presents with cognitive problems and problems at the interface of affect and cognition [6,20,36,81,85,87], therefore raising the issue of behavior regulation deficits. Similarly, for ADHD, in addition to the core symptoms of inattention and hyperactivity [8,5, 14,11,57], it is now proposed that regulatory deficits in ADHD affect both cognition and emotion processes [10,40,41]. Therefore, new theories trying to explain the neurobiological bases of PBD and ADHD need to take into account and be guided by the distinctive clinical manifestations present in each illness, as well as by comorbidity issues. Both in ADHD and PBD, deficits in PFC executive function prevent the child from normally acquiring cognitive and affective regulation skills. While it seems that in ADHD PFC regulatory systems exhibit genetic developmental delay [56], it is still an open question whether in PBD PFC regulatory systems are genetically dysfunctional or whether they are weakened during the course of brain development by the interaction with a persistently overactive and dysfunctional limbic system [98].
Furthermore, in view of explaining the high comorbidity rates between mental illnesses, there has been a recent push towards creating biologically validated approaches to the diagnosis of mental illness, using research domain criteria, or basic dimensions of functioning, such as cognition (e.g., inhibition, attention or working memory), affect (e.g., fear, aggression or reward) and social processes (e.g., theory of mind, face processing or attachment). These basic dimensions can be studied across disorders and across multiple levels of ana lysis, from behavior to neural circuits and to genes [1]. This new classification system does not necessarily exclude discrete disorder classification per se, rather it proposes that different illnesses may be better diagnosed in terms of biologically identifiable combinations of symptoms (e.g., impulsivity and affect dysregulation) or varying degrees of symptom severity (Figure 3). This perspective promotes a dual neuroscience–intervention approach, where rigorous neuroscience research can help clinical classification, for example of underlying behavioral manifestation of poor impulse control, the bias for immediate gratification, and reduced frustration tolerance. This knowledge in turn will help develop intervention to foster development of executive functions to better regulate both affect and cognitive processes in PBD and ADHD.
Five-year view
In the past decade, much research has been undertaken to better understand the pathophysiology of ADHD and PBD separately. In the next 5 years an important direction will be that of comparing and differentiating several clinical groups with overlapping clinical features that lead to a diagnosis of comorbidity (e.g., ADHD, PBD, SMD, conduct disorder and autism spectrum disorder) in order to better characterize and differentiate these illnesses based on functional domains that inform clinical diagnosis (Figure 3 & Table 1) [1,45,60,66]. For example, along the mood dysregulation spectrum, in addition to PBD and ADHD, SMD [137,138] presents not only high ADHD comorbidity rates, but also severe and chronic irritability and hyperarousal. A few studies have already validated this clinical classification by showing neural differences between ADHD, PBD and SMD with regard to emotion processing and frustration [45,167], and it will be clinically important to further differentiate between these different profiles of mood dysregulation also in terms of impulsive behavior and inhibition.
A stronger interdisciplinary multimodal imaging approach, for example including resting state, DTI, fMRI, and independent component analysis (ICA) [168,169] will also tackle the pathophysiology of these disorders in a more comprehensive way by examining and comparing structural and functional dysfunction and by identifying different biomarkers (i.e., biologically based diagnostic indicators of the disease). One of these studies with adult population, combining fMRI and multivariate analyses methods such as ICA, differentiated between adult BD and schizophrenia in temporal regions and `default' network, a network involved with the stream of consciousness and self-reflection during idle time, which disengages during information processing [170]. Studies in children with ADHD have also found possible dysfunction of brain regions that support the `default network' [171,172] comprising VMPFC, posterior cingulate gyrus and inferior parietal regions. In line with these findings, a DTI study showed more extensive white matter abnormality in prefrontal fiber tracts involved in cognitive processes in ADHD relative to PBD [86]. A few preliminary studies have also found altered functional connectivity in cognitive and affective networks in PBD. Rich et al. found that compared with HC, children with PBD had significantly reduced connectivity between the left amygdala and right temporal association cortical regions implicated in processing facial expressions and social stimuli [173]. Passarotti et al. found that during an affective working memory task relative to HC, adolescents with PBD showed altered engagement in amygdala, VMPFC and VLPFC both within a network for affect regulation and a network for working memory function [134].
In the future, combining DTI study data with neurocognitive data on behavior regulation and functional connectivity data on network interactions will provide converging evidence on the specific circuitries and mechanisms of altered function related to response inhibition [174] that may differentiate PBD and ADHD. Future studies will need to address the issue of how the dysfunction in default network may affect behavior regulation. It will also be important to correlate clinical measures with brain function and neurocognitive measures to obtain a comprehensive characterization of symptom–brain–behavior dysfunction in PBD and ADHD that will better inform illness-specific intervention.
Another goal that is central to the future of studies in child psychiatry and psychology is the quest to understand how genes and environment interact in mental illness [80,166]. Both in ADHD [175–177] and PBD [178], there is robust evidence for heritability; however, molecular genetic studies have provided only few conclusive results because of the trait complexity of pediatric mental illnesses (see [179] for a review). In the meantime, an ancillary approach to evaluate early vulnerability markers for disease risk is that of identifying intermediate phenotypes, in patients and in unaffected family members, which are highly heritable biological and behavioral traits prevalent in the illness [178]. The endophenotype approach has helped to genetically define more homogeneous ADHD subtypes [38] and has revealed that unaffected siblings and relatives of patients with ADHD exhibit neuropsychological impairment in attention, response inhibition and executive functions [179,180]. Durston et al. found that similar to children and adolescents with ADHD, their unaffected siblings also showed decreased activation in VLPFC, a region that is important for inhibition, during no-go trials that required response inhibition [76].
Preliminary evidence also indicates that first-degree relatives of BD patients may exhibit deficits in verbal memory [181,182], executive functions [182,183], face emotion processing [45] and gray matter volume abnormality in parahippocampal and hippocampal gyri [184], suggesting intermediate phenotypes for this illness in family members. In summary, while only in its beginnings, the endophenotype approach is very promising and further points at the urgent public health need for early intervention and prevention even in unaffected relatives during the formative child and adolescent years of brain development.
Finally, we need to multiply current efforts towards developing new interventions to improve behavior regulation deficits that may lead to risky and maladaptive behavior in ADHD [5,11,14,185] and PBD [17,22,25,186]. To this goal, it will be important to keep developing theoretical models such as the one we propose here that can be easily tested using neurocognitive and neuroimaging methodologies while feeding information to new intervention methodologies (Table 1). In particular, given the proposed relationship between impulsivity and reward systems, it will be important to better differentiate various aspects of reward-related processes, such as reward expectation, response to reward delivery, reward-related learning, and preference for immediate versus delayed response, which may be differently affected in different pathologies [11,97]. A few published studies in ADHD suggest a bias for immediate reward and steeper temporary discounting, which has been ascribed to aversion for delay [11]. Related to this finding, children with ADHD seem to show normal reward-related learning when positive and negative contingencies are administered consistently and without delays [8], possibly because of delay aversion and poor working memory and cognitive maintenance systems [11,59]. On the contrary, findings in PBD studies suggest that negative contingencies severely affect regulatory functions, and that impairment in reward-related learning may be due to abnormal emotional reactions and frustration to punishment or negative contingencies [20,35,36]. This initial evidence is relevant to approaches in CBT and warrants future research because it possibly suggests that different types of feedback and reward contingencies from parents, teachers and counselors may be needed for PBD and ADHD to reduce impulsive behavior during social interactions and learning at school and home.
Key issues
Poor behavior control, risky behavior, emotional dysfunction and impulsivity are signature deficits in both pediatric bipolar disorder (PBD) and attention-deficit/hyperactivity disorder (ADHD): two developmental syndromes with high comorbidity rates and severe deficits in academic, social and interpersonal domains.
Similar neurocognitive deficits in PBD and ADHD are reported, although ADHD children may exhibit more severe neurocognitive impairment. We still do not know whether similar or different mechanisms underlie these deficits in the two illnesses. Finally, mood instability and poor emotional self-regulation are also reported in ADHD.
The National Institute of Mental Health has recently fostered efforts towards new ways of classifying psychopathology based on dimensions of observable behavior and neurobiological measures. The Research Domain Criteria project defines basic dimensions of functioning (e.g., affect, inhibition and working memory) that can be studied across disorders and across multiple levels of analysis, to further validate disorder classification based on neurobiological measures.
Knowledge on the neurobiological mechanisms underlying impulsivity and risky behavior in PBD and ADHD is currently scarce. Both ADHD and PBD exhibit behavioral deficits in response inhibition that are associated with frontostriatal dysfunction. In spite of similar attention and inhibition performance deficits, there is now initial brain imaging evidence that inhibitory function in prefrontal cortex may be more impaired in ADHD than in PBD, suggesting possibly different neural mechanisms for inhibition deficits in the two illnesses.
While up until now behavior regulation deficits were examined mainly as attention or executive function problems, more recently a re-emerging and renewed concept is that of reward-related processes as motivating forces for behavior regulation. Only recently these aspects have been considered in development or illnesses such as ADHD and PBD. Reward circuits interface with cognitive circuits and play a key role in affect and behavior regulation.
To better disentangle the neurological underpinnings of impulsivity and poor inhibition we propose a neurobiological circuit involved in behavior regulation, including the dorsal frontostriatal system, involved in behavior control, and the ventral frontostriatal circuits, involved in reward and affect. We suggest that in PBD a predominantly `emotionally driven' or `bottom-up' impulsivity is associated with ventral frontostriatal circuitry. By contrast, in ADHD a more `cognitively driven' or `top-down' impulsivity predominates, stemming from dorsal frontostriatal dysfunction.
Future studies should integrate multimodal brain imaging, neurocognitive and clinical measures to test new theories on impulsivity and to better differentiate the neurobiological and behavioral mechanisms of impulsivity in PBD and ADHD.
Initial evidence suggests that ADHD and PBD may respond optimally to different types of feedback and reward contingencies, and therefore different interventions across disorders may work at school and home. A deeper understanding of these issues will inform disorder-specific interventions in the fields of pharmacotherapy, cognitive remediation or reward-based psychosocial therapy.
Acknowledgments
Financial & competing interests disclosure Alessandra M Passarotti's work, unrelated to this manuscript, is supported by the NARSAD Young Investigator Award. Mani N Pavuluri's work, unrelated to this manuscript, is supported by NARSAD Independent Investigator Award, NIMH, NICHD, DANA foundation and American Foundation for Suicide Prevention. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: •• of considerable interest
- 1.Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol. Psychiatry. 2009;66(11):988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Galanter CA, Leibenluft E. Frontiers between attention deficit hyperactivity disorder and bipolar disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2008;17:325–346. doi: 10.1016/j.chc.2007.11.001. [DOI] [PubMed] [Google Scholar]; •• Influential paper that examines and compares the two developmental disorders based on their symptoms, and addresses the issue of irritability and severe mood dysregulation.
- 3.Singh MK, Chang KD, Mazaika P, et al. Neural correlates of response inhibition in pediatric bipolar disorder. J. Child Adolesc. Psychopharmacol. 2010;20(1):15–24. doi: 10.1089/cap.2009.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geller B, Warner K, Williams M, Zimerman B. Prepubertal and young adolescent bipolarity versus ADHD: assessment and validity using the WASH-U-KSADS, CBCL, and TRF. J. Affect. Disord. 1998;51(2):93–100. doi: 10.1016/s0165-0327(98)00176-1. [DOI] [PubMed] [Google Scholar]
- 5.Biederman J, Mick E, Faraone SV, Spencer T, Wilens TE, Wozniak J. Pediatric mania: a developmental subtype of bipolar disorder? Biol. Psychiatry. 2000;48:458–466. doi: 10.1016/s0006-3223(00)00911-2. [DOI] [PubMed] [Google Scholar]
- 6.Dickstein DP, Garvey M, Pradella AG, et al. Neurologic examination abnormalities in children with bipolar disorder or attention deficit/hyperactivity disorder. Biol. Psychiatry. 2005;58:517–524. doi: 10.1016/j.biopsych.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–248. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- 8.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]; •• Influential paper on attention and executive deficits in attention-deficit/ hyperactivity disorder (ADHD).
- 9.Barkley RA. Psychosocial treatments for attention-deficit/hyperactivity disorder in children. J. Clin. Psychiatry. 2002;63(Suppl. 12):36–43. [PubMed] [Google Scholar]
- 10.Barkley RA, Fischer M. The unique contribution of emotional impulsiveness to impairment in major life activities in hyperactive children as adults. J. Am. Acad. Child Adolesc. Psychiatry. 2010;49(5):503–513. doi: 10.1097/00004583-201005000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Sonuga-Barke EJS. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neurosci. Biobehav. Rev. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]; •• Influential dual pathway model of ADHD.
- 12.Tripp G, Wickens JR. Research review: dopamine transfer deficit: a neurobiological theory of altered reinforcement mechanisms in ADHD. J. Child Psychol. Psychiatry. 2008;49(7):691–704. doi: 10.1111/j.1469-7610.2007.01851.x. [DOI] [PubMed] [Google Scholar]
- 13.Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyper-responsiveness during reward anticipation in attention deficit/hyperactivity disorder. Biol. Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- 14.Rubia K, Taylor E, Smith H, Oksannen H, Overmeyer S, Newman S. Neuropsychological analyses of impulsiveness in childhood hyperactivity. Br. J. Psychiatry. 2001;179:138–143. doi: 10.1192/bjp.179.2.138. [DOI] [PubMed] [Google Scholar]
- 15.Bush G, Frazier JA, Rauch SL, et al. Anterior cingulate cortex dysfunction in attention-deficit/hyperactivity disorder revealed by fMRI and the counting stroop. Biol. Psychiatry. 1999;45:1542–1552. doi: 10.1016/s0006-3223(99)00083-9. [DOI] [PubMed] [Google Scholar]
- 16.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders IV-TR. American Psychiatric Association; Washington DC, USA: 2000. [Google Scholar]
- 17.Pavuluri MN, Birmaher B, Naylor M. Pediatric bipolar disorder: ten year review. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44(9):846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- 18.Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS. Defining clinical phenotypes of juvenile mania. Am. J. Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- 19.Geller B, Zimerman B, Williams M, et al. DSM-IV mania symptoms in a prepubertal and early adolescent bipolar disorder phenotype compared to attention-deficit hyperactive and normal controls. J. Child Adolesc. Psychopharmacol. 2002;12(1):11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- 20.Rich BA, Schmajuk M, Perez-Edgar KE, Pine DS, Fox NA, Leibenluft E. The impact of reward, punishment, and frustration on attention in pediatric bipolar disorder. Biol. Psychiatry. 2005;58:532–539. doi: 10.1016/j.biopsych.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein DP, Nelson EE, McClure EB, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(3):341–355. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- 22.Biederman J, Faraone S, Mick E, et al. Attention-deficit hyperactivity disorder and juvenile mania: an overlooked comorbidity? J. Am. Acad. Child Adolesc. Psychiatry. 1996;35(8):997–1008. doi: 10.1097/00004583-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Pavuluri MN, Schenkel LS, Aryal S, et al. Neurocognitive function in unmedicated manic and medicated euthymic pediatric bipolar patients. Am. J. Psychiatry. 2006;163:286–293. doi: 10.1176/appi.ajp.163.2.286. [DOI] [PubMed] [Google Scholar]
- 24.Pavuluri MN, West A, Hill K, Jindal K, Sweeney JA. Neurocognitive function in pediatric bipolar disorder: three-year follow-up shows cognitive development lagging behind healthy youth. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48(3):299–307. doi: 10.1097/CHI.0b013e318196b907. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First study on a 3-year follow-up on cognitive deficits in pediatric bipolar disorder (PBD). This study showed persistence of deficits over time, and even with optimal treatment outcome in PBD.
- 25.Lewinsohn PM, Shankman SA, Gau JM, Klein DN. The prevalence and co-morbidity of subthreshold psychiatric conditions. Psychol. Med. 2004;34(4):613–622. doi: 10.1017/S0033291703001466. [DOI] [PubMed] [Google Scholar]
- 26.DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am. J. Psychiatry. 2007;164(4):582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 27.Birmaher B. Longitudinal course of pediatric bipolar disorder. Am. J. Psychiatry. 2007;164(4):537–539. doi: 10.1176/ajp.2007.164.4.537. [DOI] [PubMed] [Google Scholar]
- 28.Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- 29.Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nat. Neurosci. 1999;2(10):861–863. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 30.Casey BJ, Castellanos FX, Giedd JN, et al. Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1997;36:374–383. doi: 10.1097/00004583-199703000-00016. [DOI] [PubMed] [Google Scholar]
- 31.Luna B, Thulborn KR, Munoz DP, et al. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- 32.Luna B, Garver KE, Urban TN, Lazar N, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- 33.Posner MI, Rothbart MK. Developing mechanisms of self-regulation. Dev. Psychopathol. 2000;12:427–441. doi: 10.1017/s0954579400003096. [DOI] [PubMed] [Google Scholar]
- 34.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J. Child Psychol. Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 35.Gorrindo T, Blair RJR, Budhani S, Dickstein DP, Pine DS, Leibenluft E. Deficits on a probabilistic response-reversal task in patients with pediatric bipolar disorder. Am. J. Psychiatry. 2005;162:1975–1977. doi: 10.1176/appi.ajp.162.10.1975. [DOI] [PubMed] [Google Scholar]
- 36.Rich BA, Holroyd T, Carver FW, et al. A preliminary study of the neural mechanisms of frustration in pediatric bipolar disorder using magnetoencephalography. Depress. Anxiety. 2010;27(3):276–286. doi: 10.1002/da.20649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pizzagalli DA, Goetz E, Ostacher M, Iosifescu DV, Perlis RH. Euthymic patients with bipolar disorder show decreased reward learning in a probabilistic reward task. Biol. Psychiatry. 2008;64(2):162–168. doi: 10.1016/j.biopsych.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Doyle AE, Willcutt EG, Seidman LJ, et al. Attention-deficit/hyperactivity disorder endophenotypes. Biol. Psychiatry. 2005;57:1324–1335. doi: 10.1016/j.biopsych.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 39.Rucklidge JJ. Impact of ADHD on the neurocognitive functioning of adolescents with bipolar disorder. Biol. Psychiatry. 2006;60:921–928. doi: 10.1016/j.biopsych.2006.03.067. [DOI] [PubMed] [Google Scholar]
- 40.Barkley RA. Deficient emotional self-regulation: a core component of attention deficit/hyperactivity disorder. J. ADHD Rela. Disord. 2009;1(2):5–37. [Google Scholar]
- 41.Skirrow C, McLoughlin G, Kuntsi J, Asherson P. Behavioral, neurocognitive and treatment overlap between attention-deficit/hyperactivity disorder and mood instability. Expert Rev. Neurother. 2009;9(4):489–503. doi: 10.1586/ern.09.2. [DOI] [PubMed] [Google Scholar]
- 42.Casey BJ. Emotional competence in children with externalizing and internalizing disorders. In: Lewis M, Sullivan M, editors. Emotional Development in Atypical Children. Erlbaum; NJ, USA: 1996. pp. 161–183. [Google Scholar]
- 43.Braaten EB, Rosen LA. Self-regulation of affect in attention-deficit hyperactivity disorder (ADHD) and non-ADHD boys: differences in empathic responding. J. Consult. Clin. Psychol. 2000;68:313–321. doi: 10.1037/0022-006X.68.2.313. [DOI] [PubMed] [Google Scholar]
- 44.Friedman SR, Rapport LJ, Lumley M, et al. Aspects of social and emotional competence in adult attention-deficit/hyperactivity disorder. Neuropsychology. 2003;17(1):50–58. [PubMed] [Google Scholar]
- 45.Brotman MA, Rich BA, Guyer AE, et al. Amygdala activation during emotion processing of neutral faces in children with severe mood dysregulation versus ADHD or bipolar disorder. Am. J. Psychiatry. 2010;167(1):61–69. doi: 10.1176/appi.ajp.2009.09010043. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First functional MRI (fMRI) study comparing ADHD, PBD and severe mood dysregulation during emotion processing.
- 46.Logan GD, Cowan WB, Davis KA. On the ability of inhibit simple and choice reaction time responses: a model and a method. J. Exp. Psychol. Hum. Percept. Perform. 1984;10:276–291. doi: 10.1037//0096-1523.10.2.276. [DOI] [PubMed] [Google Scholar]
- 47.Hoeksma JB, Osterlaan J, Schipper E. Emotion regulation and the dynamics of feelings: a conceptual and methodological framework. Child Dev. 2004;75(2):354–360. doi: 10.1111/j.1467-8624.2004.00677.x. [DOI] [PubMed] [Google Scholar]
- 48.Rubia K, Overmeyer S, Taylor E, et al. Hypofrontality in attention deficit hyperactivity disorder during higher-order motor control: a study with functional MRI. Am. J. Psychiatry. 1999;156:891–896. doi: 10.1176/ajp.156.6.891. [DOI] [PubMed] [Google Scholar]
- 49.Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- 50.Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol. Psychol. 2000;54(1–3):241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- 51.Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;2:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- 52.Oosterlaan J, Logan GD, Sergeant JA. Response inhibition in AD/HD, CD, comorbid AD/HD + CD, anxious, and control children: a meta-analysis of studies with the stop task. J. Child Psychol. Psychiatry. 1998;39(3):411–425. [PubMed] [Google Scholar]
- 53.Nigg JT. Is ADHD a disinhibitory disorder? Psychol. Bull. 2001;127:571–598. doi: 10.1037/0033-2909.127.5.571. [DOI] [PubMed] [Google Scholar]
- 54.Adams ZW, Derefinko KJ, Milich R, Fillmore MT. Inhibitory functioning across ADHD subtypes: recent findings, clinical implications, and future directions. Dev. Disabil. Res. Rev. 2008;14(4):268–275. doi: 10.1002/ddrr.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fillmore MT, Milich R, Lorch EP. Inhibitory deficits in children with attention-deficit/hyperactivity disorder: intentional versus automatic mechanisms of attention. Dev. Psychopathol. 2009;21(2):539–554. doi: 10.1017/S0954579409000297. [DOI] [PubMed] [Google Scholar]
- 56.Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc. Natl Acad. Sci. USA. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonuga-Barke EJS, Sergeant JA, Nigg J, Willcutt S. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child Adolesc. Psychiatry Clin. N. Am. 2008;17:367–384. doi: 10.1016/j.chc.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Nigg JT, Casey BJ. An integrative theory of attention-deficit/hyperactivity disorder based on the cognitive and affective neurosciences. Dev. Psychopathol. 2005;17(3):785–806. doi: 10.1017/S0954579405050376. [DOI] [PubMed] [Google Scholar]
- 59.Castellanos FX, Sonuga-Barke EJ, Milham MP, Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 2006;10:117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 60.Rubia K. `Cool' inferior frontostriatal dysfunction in attention-deficit/hyperactivity disorder versus `hot' ventromedial orbitofrontal-limbic dysfunction in conduct disorder: a review. Biol. Psychiatry. 2010 doi: 10.1016/j.biopsych.2010.09.023. DOI: 10.1016/j.biopsych.2010.09.023 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 61.Solanto MV. Dopamine dysfunction in AD/HD: integrating clinical and basic neuroscience research. Behav. Brain Res. 2002;130:65–71. doi: 10.1016/s0166-4328(01)00431-4. [DOI] [PubMed] [Google Scholar]
- 62.Diamond A, Prevor MB, Callender G, Druin DP. Prefrontal cortex cognitive deficits in children treated early and continuously for PKU. Monogr. Soc. Res. Child Dev. 1997;62(4):i–v. 1–208. [PubMed] [Google Scholar]
- 63.Sagvolden T, Johansen EB, Aase H, Russell VA. A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav. Brain Sci. 2005;28:397–419. doi: 10.1017/S0140525X05000075. [DOI] [PubMed] [Google Scholar]
- 64.Russell VA, Sagvolden T, Johansen EB. Animal models of attention-deficit hyperactivity disorder. Behav. Brain Funct. 2005;1:9. doi: 10.1186/1744-9081-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paine TA, Neve RL, Carlezon A. Attention deficits and hyperactivity following inhibition of cAMP-dependent protein kinase within the medial prefrontal cortex of rats. Neuropsychopharmacology. 2009;34:2143–2155. doi: 10.1038/npp.2009.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leibenluft E, Rich BA, Vinton DT, et al. Neural circuitry engaged during unsuccessful motor inhibition in pediatric bipolar disorder. Am. J. Psychiatry. 2007;164:52–60. doi: 10.1176/ajp.2007.164.1.A52. [DOI] [PubMed] [Google Scholar]; •• First fMRI study on response inhibition deficits in PBD, which found reduced activation in striatal and ventrolateral prefrontal cortex during failed inhibition trials in PBD relative to healthy controls.
- 67.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of response inhibition deficits in pediatric bipolar disorder and attention deficit hyperactivity disorder. Psychiatry Res. 2010;181(1):36–43. doi: 10.1016/j.pscychresns.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• First fMRI study comparing response inhibition in ADHD and PBD. The results indicate more extensive prefrontal deficits in ADHD relative to PBD in spite of similar attention problems.
- 68.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. Enhanced prefrontal function with pharmacotherapy on a response inhibition task in adolescent bipolar disorder. J. Clin. Psychiatry. 2010;71:1–9. doi: 10.4088/JCP.09m05504yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eagle DM, Robbins TW. Inhibitory control in rats performing a stop-signal reaction-time task: effects of lesions of the medial striatum and D-amphetamine. Behav. Neurosci. 2003;117(6):1302–1317. doi: 10.1037/0735-7044.117.6.1302. [DOI] [PubMed] [Google Scholar]
- 70.Aron AR, Poldrack RA. The cognitive neuroscience of response inhibition: relevance for genetic research in attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2005;57:1285–1292. doi: 10.1016/j.biopsych.2004.10.026. [DOI] [PubMed] [Google Scholar]; •• Excellent review on cognitive neuroscience theories of response inhibition and their relevance for clinical research.
- 71.Verbruggen F, Logan GD. Response inhibition in the stop-signal paradigm. Trends Cogn. Sci. 2008;12(11):418–424. doi: 10.1016/j.tics.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tillman CM, Thorell LB, Brocki KC, Bohlin G. Motor response inhibition and execution in the stop signal task: development and relation to ADHD behaviors. Child Neuropsychology. 2008;14(1):42–59. doi: 10.1080/09297040701249020. [DOI] [PubMed] [Google Scholar]
- 73.Johnstone SJ, Dimoska A, Smith JL, et al. The development of stop-signal and Go/Nogo response inhibition in children aged 7–12 years: performance and event-related potential indices. Int. J.Psychophysiol. 2007;63(1):25–38. doi: 10.1016/j.ijpsycho.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 74.Plitzka SR, Glahn DC, Semrud-Clikeman M, et al. Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naïve or in long-term treatment. Am. J. Psychiatry. 2006;163(6):1052–1060. doi: 10.1176/ajp.2006.163.6.1052. [DOI] [PubMed] [Google Scholar]
- 75.Durston S, Tottenham NT, Thomas KM, et al. Differential patterns of striatal activation in young children with and without ADHD. Biol. Psychiatry. 2003;53(10):871–878. doi: 10.1016/s0006-3223(02)01904-2. [DOI] [PubMed] [Google Scholar]
- 76.Durston S, Mulder M, Casey BJ, Ziermans T, van Engeland H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention deficit hyperactivity disorder. Biol. Psychiatry. 2006;60:1062–1070. doi: 10.1016/j.biopsych.2005.12.020. [DOI] [PubMed] [Google Scholar]; •• fMRI study linking deficits in ventrolateral prefrontal cortex to genetic vulnerability for ADHD.
- 77.Tamm L, Menon V, Ringel J, Reiss AL. Event-related fMRI evidence of frontotemporal involvement in aberrant response inhibition and task switching in attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43:1430–1440. doi: 10.1097/01.chi.0000140452.51205.8d. [DOI] [PubMed] [Google Scholar]
- 78.Castellanos FX, Giedd JN, Marsh WL, et al. Quantitative brain magnetic resonance imaging in attention-deficit hyperactivity disorder. Arch. Gen. Psychiatry. 1996;53(7):607–616. doi: 10.1001/archpsyc.1996.01830070053009. [DOI] [PubMed] [Google Scholar]
- 79.Filipek PA, Semrud-Clikeman M, Steingard RJ, Renshaw PF, Kennedy DN, Biederman J. Volumetric MRI analysis comparing subjects having attention-deficit hyperactivity disorder with normal controls. Neurology. 1997;48(3):589–601. doi: 10.1212/wnl.48.3.589. [DOI] [PubMed] [Google Scholar]
- 80.Castellanos FX, Tannock R. Neuroscience of attention-deficit hyperactivity disorder: the search for endophenotypes. Nat. Neurosci. 2002;3:617–628. doi: 10.1038/nrn896. [DOI] [PubMed] [Google Scholar]
- 81.Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. An fMRI study of the interface between affective and cognitive neural circuitry in pediatric bipolar disorder. Psychiatry Res. 2008;162(3):244–255. doi: 10.1016/j.pscychresns.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blumberg HP, Leung HC, Skudlarski P, et al. A functional magnetic resonance imaging study of bipolar disorder: state-and trait-related dysfunction in ventral prefrontal cortices. Arch. Gen.Psychiatry. 2003;60:601–609. doi: 10.1001/archpsyc.60.6.601. [DOI] [PubMed] [Google Scholar]
- 83.Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122:981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- 84.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–338. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 85.D'Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp. Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- 86.Pavuluri MN, Yang S, Kamineni K, et al. DTI Study of white matter fiber tracts: in pediatric bipolar disorder and attention deficit hyperactivity disorder. Biol. Psychiatry. 2009;65(7):586–593. doi: 10.1016/j.biopsych.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Passarotti AM, Sweeney JA, Pavuluri MN. Differential engagement of cognitive and affective neural systems in pediatric bipolar disorder and attention deficit hyperactivity disorder. J. Int. Neuropsychol. Soc. 2010;16(1):106–117. doi: 10.1017/S1355617709991019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Passarotti AM, Sweeney JA, Pavuluri MN. Emotion processing influences working memory circuits in pediatric bipolar disorder and attention deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2010;9(10):1064–1080. doi: 10.1016/j.jaac.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Petrides M, Pandya DN. Comparative cytoarchitectonic analysis of the human and the macaque ventrolateral prefrontal cortex and corticocortical connection patterns in the monkey. Eur. J. Neurosci. 2002;16:291–310. doi: 10.1046/j.1460-9568.2001.02090.x. [DOI] [PubMed] [Google Scholar]
- 90.Frangou S, Haldane M, Roddy D, Kumari V. Evidence for deficit in tasks of ventral, but not dorsal, prefrontal executive function as an endophenotypic marker for bipolar disorder. Biol. Psychiatry. 2005;58(10):838–839. doi: 10.1016/j.biopsych.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 91.Bush G. Attention-deficit hyperactivity disorder and attention networks. Neuropsychopharmacology. 2010;35:278–300. doi: 10.1038/npp.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacol. Rev. 2009:1–23. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr. Opin. Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- 94.Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–294. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- 95.McClure SM, Laibson DI, Lowenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306:503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 96.Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27:8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fareri D, Martin L, Delgado M. Reward-related processing in the human brain: Developmental considerations. Dev. Psychopathol. 2008;20:1191–1211. doi: 10.1017/S0954579408000576. [DOI] [PubMed] [Google Scholar]
- 98.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc. Cogn. Affect. Neurosci. 2009;4:387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Geier C, Luna B. The maturation of incentive processing and cognitive control. Pharmacol. Biochem. Behav. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- 101.Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.May JC, Delgado MR, Dahl RE, et al. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol. Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 103.Bjork JM, Smith AR, Danube CL, Hommer DW. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. J. Neurosci. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: Development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luman M, Oosterlaan J, Sergeant JA. The impact of reinforcement contingencies on AD/HD: a review and theoretical appraisal. Clin. Psychol. Rev. 2005;25(2):183–213. doi: 10.1016/j.cpr.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 106.Luman M, Tripp G, Scheres A. Identifying the neurobiology of altered reinforcement sensitivity in ADHD: a review and research agenda. Neurosci. Biobehav. Rev. 2010;34:744–754. doi: 10.1016/j.neubiorev.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 107.Sonuga-Barke EJS, Taylor E, Sembi E, Smith J. Hyperactivity and delay aversion. I. The effect of delay on choice. J. Child Psychol. Psychiatry. 1992;33:387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- 108.Drechsler R, Rizzo P, Steinhausen HC. Decision-making on an explicit risk-taking task in preadolescents with attention-deficit/hyperactivity disorder. J. Neural Transm. 2008;115:201–209. doi: 10.1007/s00702-007-0814-5. [DOI] [PubMed] [Google Scholar]
- 109.Faregh N, Derevensky J. Gambling behavior among adolescents with attention deficit/hyperactivity disorder. J. Gambl. Stud. 2010 doi: 10.1007/s10899-010-9211-3. DOI: 10.1007/s10899-010-9211-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 110.Dibbets P, Evers L, Hurks P, Marchetta N, Jolles J. Differences in feedback- and inhibition- related neural activity in adult ADHD. Brain Cogn. 2009;70:73–83. doi: 10.1016/j.bandc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Pavuluri MN, O'Connor MM, Harral EM, Sweeney JA. Affective neural circuitry during facial emotion processing in pediatric bipolar disorder. Biol. Psychiatry. 2007;62:158–167. doi: 10.1016/j.biopsych.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 112.Passarotti AM, Sweeney JA, Pavuluri MN. Fronto-limbic dysfunction in mania pre-treatment and persistent amygdala over-activity post-treatment in pediatric bipolar disorder. Psychopharmacology (Berl.) 2011 doi: 10.1007/s00213-011-2243-2. DOI: 10.1007/s00213-011-2243-2. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12(7):707–719. doi: 10.1111/j.1399-5618.2010.00863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abler B, Greenhouse I, Ongur D, Walter H, Heckers S. Abnormal reward system activation in mania. Neuropsychopharmacology. 2008;33(9):2217–2227. doi: 10.1038/sj.npp.1301620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strakowski SM, DelBello MP, Fleck DE, Arndt S. The impact of substance abuse on the course of bipolar disorder. Biol. Psychiatry. 2000;48(6):477–485. doi: 10.1016/s0006-3223(00)00900-8. [DOI] [PubMed] [Google Scholar]
- 116.Marsh R, Zhu H, Schultz RT, et al. A developmental fMRI study of self-regulatory control. Hum. Brain Mapp. 2009;27(11):848–863. doi: 10.1002/hbm.20225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pavuluri MN, Passarotti AM. Neural bases of emotional processing in pediatric bipolar disorder. Expert Rev. Neurother. 2008;8(9):1381–1387. doi: 10.1586/14737175.8.9.1381. [DOI] [PubMed] [Google Scholar]
- 118.Pavuluri MN, Passarotti AM, Harral EM, Sweeney JA. An fMRI study of the neural correlates of incidental versus directed emotion processing in pediatric bipolar disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:308–319. doi: 10.1097/CHI.0b013e3181948fc7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 120.Barto AG. Adaptive critics and the basal ganglia. In: Houk JC, Davis JL, Beiser DG, editors. Models of Information Processing in the Basal Ganglia. MIT Press; MA, USA: 1995. pp. 215–232. [Google Scholar]
- 121.O'Doherty J, Dayan P, Schultz J, Deichmann R, Friston K, Dolan RJ. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- 122.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 123.Knutson B, Fong GW, Bennett SM, Adams CM, Homme D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18:263–272. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- 124.Hampton AN, Adolphs R, Tyszka MJ, O'Doherty JP. Contributions of the amygdala to reward expectancy and choice signals in human prefrontal cortex. Neuron. 2007;55(4):545–555. doi: 10.1016/j.neuron.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 125.Knutson B, Fong GW, Adams CS, Hommer D. Dissociation of reward anticipation versus outcome with event-related fMRI. Neuroreport. 2001;12:3683–3687. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- 126.Hare TA, O'Doherty J, Camerer CF, Schultz W, Rangel A. Dissociating the role of the orbitofrontal cortex and the striatum in the computation of goal values and prediction errors. J. Neurosci. 2008;28(22):5623–5630. doi: 10.1523/JNEUROSCI.1309-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carter R, MacInnes J, Huettel SA, Adcock R. Activation in the VTA and nucleus accumbens increases in anticipation of both gains and losses. Front. Behav. Neurosci. 2009;3:21. doi: 10.3389/neuro.08.021.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44(3):1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Adolphs R. Neural systems for recognizing emotion. Curr. Opin. Neurobiol. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- 130.Paton JJ, Belova MA, Morrison SE, Salzman CD. The primate amygdala represents the positive and negative value of visual stimuli during learning. Nature. 2006;439(7078):865–870. doi: 10.1038/nature04490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Salzman CD, Paton JJ, Belova MA, Morrison SE. Flexible neural representations of value in the primate brain. Ann. NY Acad. Sci. 2007;1121:336–354. doi: 10.1196/annals.1401.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gottfried JA, O'Doherty J, Dolan RJ. Encoding predictive reward value in human amygdala and orbitofrontal cortex. Science. 2003;301(5636):1104–1107. doi: 10.1126/science.1087919. [DOI] [PubMed] [Google Scholar]
- 133.Cardinal RN, Parkinson JA, Lachenal G, et al. Effects of selective excitotoxic lesions of the nucleus accumbens core, anterior cingulate cortex, and central nucleus of the amygdala on autoshaping performance in rats. Behav. Neurosci. 2002;116:553–567. doi: 10.1037//0735-7044.116.4.553. [DOI] [PubMed] [Google Scholar]
- 134.Passarotti AM, Ellis J, Wegbreit E, Varghese B, Stevens MC, Pavuluri MN. Working memory networks are influenced by facial emotions in pediatric bipolar disorder. The Pediatric Bipolar Conference; Boston, MA, USA. 24–25 March (2011). [Google Scholar]
- 135.Foland LC, Altshuler LL, Bookheimer SY, Eisenberger N, Townsend J, Thompson PM. Evidence for deficitent modulation of amygdala response by prefrontal cortex in bipolar mania. Psychiatry Res. 2008;162:27–37. doi: 10.1016/j.pscychresns.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coghill D, Seth S. Do the diagnostic criteria for ADHD need to change? Comments on the preliminary proposals of the DSM-5 ADHD and disruptive behavior disorders committee. Eur. J. Adolesc. Psychiatry. 2011;20:75–81. doi: 10.1007/s00787-010-0142-4. [DOI] [PubMed] [Google Scholar]
- 137.Leibenluft E, Blair RJ, Charney DS, Pine DS. Irritability in pediatric mania and other childhood psychopathology. Ann. NY Acad. Sci. 2003b;1008:201–218. doi: 10.1196/annals.1301.022. [DOI] [PubMed] [Google Scholar]
- 138.Leibenluft E. Severe mood dysregulation, irritability, and the diagnostic boundaries of bipolar disorder in youths. Am. J. Psychiatry. 2011;168(2):129–142. doi: 10.1176/appi.ajp.2010.10050766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rich BA, Brotman MA, Dickstein DP, Mitchell DG, Blair RJ, Leibenluft E. Deficits in attention to emotional stimuli distinguish youth with severe mood dysregulation from youth with bipolar disorder. J. Abnorm. Child Psychol. 2010;38(5):695–706. doi: 10.1007/s10802-010-9395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Rau G, Blair KS, Berghorst L, et al. Processing of differentially valued rewards and punishments in youths with bipolar disorder or severe mood dysregulation. J. Child Adolesc. Psychopharmacol. 2008;18(2):185–196. doi: 10.1089/cap.2007.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Daly BP, Creed T, Xanthopoulos M, Brown RT. Psychosocial treatments for children with attention deficit/hyperactivity disorder. Neuropsychol. Rev. 2007;17(1):73–89. doi: 10.1007/s11065-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 142.Pelham WE, Jr, Fabiano GA. Evidence-based psychosocial treatments for attention-deficit/hyperactivity disorder. J. Clin. Child Adolesc. Psychol. 2008;37(1):184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- 143.Fehlings DL, Roberts W, Humphries T, Dawe G. Attention deficit hyperactivity disorder: does cognitive behavioral therapy improve home behavior? J. Dev. Behav. Pediatr. 1991;12(4):223–228. [PubMed] [Google Scholar]
- 144.Chronis AM, Fabiano GA, Gnagy EM, Wymbs B, Burrows-Mac-Lean L, Pelham WE. Comprehensive, sustained behavioral and pharmacological treatment for ADHD: a case study. Cogn. Behav. Practice. 2001;8:346. [Google Scholar]
- 145.Pavuluri MN, Graczyk PA, Henry DB, Carbray JA, Heidenreich J, Miklowitz DJ. Child- and family-focused cognitive-behavioral therapy for pediatric bipolar disorder: development and preliminary results. J. Am. Acad. Child Adolesc. Psychiatry. 2004;43(5):528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 146.West AE, Henry DB, Pavuluri MN. Maintenance model of integrated psychosocial treatment in pediatric bipolar disorder: a pilot feasibility study. J. Am. Acad. Child Adolesc. Psychiatry. 2007;46(2):205–212. doi: 10.1097/01.chi.0000246068.85577.d7. [DOI] [PubMed] [Google Scholar]
- 147.West AE, Pavuluri MN. Psychosocial treatments for childhood and adolescent bipolar disorder. Child Adolesc. Psychiatr. Clin. N. Am. 2009;18(2):471–482. doi: 10.1016/j.chc.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 148.Klingberg T, Fernell E, Olesen PJ, et al. Computerized traininig of working memory in children with ADHD – a randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry. 2005;44:177–186. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 149.Holmes J, Gathercole SE, Dunning GL. Adaptive training leads t osustained enhancement of poor working memory in children. Dev. Sci. 2009;12(4):F9–15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 150.Thorell LB, Linqvist S, Bergman nutley S, Bolin G, Klingberg T. Training and transfer effects of executive function in preschool children. Dev. Sci. 2009;12(1):106–113. doi: 10.1111/j.1467-7687.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- 151.Tannock R. Attention deficit hyperactivity disorder: advances in cognitive, neurobiological, and genetic research. J. Child Psychol. Psychiatry. 1998;39:65–99. 1998. [PubMed] [Google Scholar]
- 152.Sheridan MA, Hinshaw S, D'Esposito M. Stimulant medication and prefrontal functional connectivity during working memory in ADHD: a preliminary report. J. Atten. Disord. 2010;14(1):69–78. doi: 10.1177/1087054709347444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J. Neurosci. 2001;21(2):RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wilens TE. Attention-deficit/hyperactivity disorder and the substance use disorders: the nature of the relationship, subtypes at risk, and treatment issues. Psychiatr. Clin. North Am. 2004;27(2):283–301. doi: 10.1016/S0193-953X(03)00113-8. [DOI] [PubMed] [Google Scholar]
- 155.Vaidya CJ, Austin G, Kirkorian G, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance imaging study. Proc. Natl Acad. Sci. USA. 1998;95:14494–14499. doi: 10.1073/pnas.95.24.14494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shafritz KM, Marchione KE, Gore JC, Shaywitz SE, Shaywitz BA. The effects of methylphenidate on neural systems of attention in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2004;161:1990–1997. doi: 10.1176/appi.ajp.161.11.1990. [DOI] [PubMed] [Google Scholar]
- 157.Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naïve children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57(7–8):640–652. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 158.Wigal SB, Chae S, Patel A. Steinberg-Epstein R. Advances in the treatment of attention-deficit/hyperactivity disorder: a guide for pediatric neurologists. Semin. Pediatr. Neurol. 2010;17(4):230–236. doi: 10.1016/j.spen.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 159.Groom MJ, Scerif G, Liddle PF, et al. Effects of motivation and medication on electrophysiological markers of response inhibition in children with attention deficit/hyperactivity disorder. Biol. Psychiatry. 2010;67:624–631. doi: 10.1016/j.biopsych.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chen J, Fang Y, Kemp DE, Calabrese JR, Gao K. Switching to hypomania and mania: differential neurochemical, neuropsychological, and pharmacologic triggers and their mechanisms. Curr. Psychiatry Rep. 2010;12(6):512–521. doi: 10.1007/s11920-010-0157-z. [DOI] [PubMed] [Google Scholar]
- 161.Pavuluri MN, Passarotti AM, Parnes SA, Fitzgerald JM, Sweeney JA. A pharmacological fMRI study probing the interface of cognitive and emotional brain systems in pediatric bipolar disorder. J. Child Adolesc. Psychopharmacol. 2010;20(5):395–406. doi: 10.1089/cap.2009.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Pavuluri MN, Passarotti AM, Fitzgerald JM, Sweeney JA. Risperidone and divalproex differentially engange the fronto–striato–temporal circuitry in pediatric mania: a pharmacological fMRI study. J. Am. Acad. Child Adolesc. Psychiatry. 2011 doi: 10.1016/j.jaac.2011.10.019. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pavuluri MN, Passarotti AM, Mohammed T, Carbray J, Sweeney JA. Enhanced working and verbal memory after lamotrigine treatment in pediatric bipolar disorder. Bipolar Disord. 2010;12(2):213–220. doi: 10.1111/j.1399-5618.2010.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Pavuluri MN, Passarotti AM, Lu LH, Carbray JA, Sweeney JA. Double-blind randomized trial of risperidone versus divalproex in pediatric bipolar disorder: fMRI outcomes. Psychiatry Res. doi: 10.1016/j.pscychresns.2011.01.005. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kessler RC, Berglund P, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national Comorbidity Survey replication. Arch. Gen. Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 166.Insel TR. Foreword. Developmental cognitive neuroscience. Dev. Cogn. Neurosci. 2011;1:1–2. doi: 10.1016/j.dcn.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Rich BA, Schmajuk M, Perez-Edgar KE, Fox NA, Pine DS, Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am. J. Psychiatry. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- 168.Calhoun V, Wu L, Kiehl K, Eichele T, Pearlson G. Aberrant processing of deviant stimuli in schizophrenia revealed by fusion of fMRI and EEG data. Acta Neuropsychiatr. 2010;22(3):127–138. doi: 10.1111/j.1601-5215.2010.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Stevens MC. The developmental cognitive neuroscience of functional connectivity. Brain Cogn. 2009;70:1–12. doi: 10.1016/j.bandc.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 170.Calhoun VD, Maciejewski PK, Pearlson GD, Kiehl KA. Temporal lobe and `default' hemodynamic brain modes discriminate between schizophrenia and bipolar disorder. Hum. Brain Mapp. 2008;29(11):1265–1275. doi: 10.1002/hbm.20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhu CZ, Zang YF, Cao QJ, et al. Fisher discriminative analysis of resting-state brain function for attention-deficit/hyperactivity disorder. Neuroimage. 2008;40:110–120. doi: 10.1016/j.neuroimage.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 172.Castellanos FX, Margulies DS, Kelly C, et al. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63:332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rich BA, Fromm SJ, Berghorst LH, et al. Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. J Child Psychol. Psychiatry. 2008;49(1):88–96. doi: 10.1111/j.1469-7610.2007.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav. Brain Res. 2007;181(1):12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Faraone SV, Perlis RH, Doyle AE, et al. Molecular genetics of attention-deficit/ hyperactivity disorder. Biol. Psychiatry. 2005;57(11):1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 176.Stein MA, Waldman ID, Sarampote CS, et al. Dopamine transporter genotype and methylphenidate dose–response in children with ADHD. Neuropsychopharmacology. 2005;30:1374–1382. doi: 10.1038/sj.npp.1300718. [DOI] [PubMed] [Google Scholar]
- 177.Stollstoff MA, Foss-Feig J, Cook EH, Stein MA, Gaillard WD. Neural response to working memory load varies by dopamine transporter genotype in children. Neuroimage. 2010;53:970–977. doi: 10.1016/j.neuroimage.2009.12.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.MacQueen GM, Hajek T, Alda M. The phenotypes of bipolar disorder: relevance for genetic investigations. Mol. Psychiatry. 2005;10(9):811–826. doi: 10.1038/sj.mp.4001701. [DOI] [PubMed] [Google Scholar]
- 179.Gau SS, Shang CY. Executive functions as endophenotypes in ADHD: evidence from the Cambridge Neuropsychological Test Battery (CANTAB) J. Child Psychol. Psychiatry. 2010;51(7):838–849. doi: 10.1111/j.1469-7610.2010.02215.x. [DOI] [PubMed] [Google Scholar]
- 180.Fan J, Fossella J, Sommer T, Wu Y, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proc. Natl Acad. Sci. USA. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gourovitch ML, Torrey EF, Gold JM, Randolph C, Weinberger DR, Goldberg TE. Neuropsychological performance of monozygotic twins discordant for bipolar disorder. Biol. Psychiatry. 1999;45:639–646. doi: 10.1016/s0006-3223(98)00148-6. [DOI] [PubMed] [Google Scholar]
- 182.Kulkarni SK. Re-engineering pharmacology teaching and research: can we adopt translational approach? Indian J. Pharmacol. 2010;42(5):259–260. doi: 10.4103/0253-7613.69941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zalla T, Joyce C, Szoke A, et al. Executive dysfunctions as potential markers of familial vulnerability to bipolar disorder and schizophrenia. Psychiatry Res. 2004;121(3):207–271. doi: 10.1016/s0165-1781(03)00252-x. [DOI] [PubMed] [Google Scholar]
- 184.Ladouceur CD, Almeida JR, Birmaher B, et al. Subcortical gray matter volume abnormalities in healthy bipolar offspring: potential neuroanatomical risk marker for bipolar disorder? J. Am. Acad. Child Adolesc. Psychiatry. 2008;47(5):532–539. doi: 10.1097/CHI.0b013e318167656e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Donnelly M, Haby MM, Carter R, Andrews G, Vos T. Cost-effectiveness of dexamphetamine and metylphenidate for the treatment of childhood attention deficit hyperactivity disorder. Aust. NZ J. Psychatry. 2004;38:592–601. doi: 10.1080/j.1440-1614.2004.01422.x. [DOI] [PubMed] [Google Scholar]
- 186.Wozniak J, Biederman J, Mundy E, Mennin D, Faraone SV. A pilot family study of childhood-onset mania. J. Am. Acad. Child Adolesc. Psychiatry. 2005;34(12):1577–1583. doi: 10.1097/00004583-199512000-00007. [DOI] [PubMed] [Google Scholar]