Abstract
Q fever, a zoonotic disease, is caused by a gram-negative intracellular bacterium, Coxiella burnetii. Although normally transmitted during exposure to infectious aerosols, C. burnetii is also found in arthropod vectors. In the environment, ticks are thought to play a crucial role in bacterial maintenance and transmission by infecting various mammalian species. However, the nature of the pathogen–tick relationship is not well defined. To determine C. burnetii's interactions with a cultured tick cell line, we introduced purified C. burnetii NMII into Ixodes scapularis–derived IDE8 cells and assayed for bacterial presence, replication, gene expression, and subsequent infectivity for mammalian cells. Tick cells were harvested at 24 h, 72 h, 7 days, and 11 days postinfection (PI). C. burnetii uptake and subsequent replication was demonstrated by indirect immunofluorescence assay, electron microscopy, and real-time polymerase chain reaction (PCR). When a genome equivalent multiplicity of infection of 30 was used, 30%–40% of exposed cells were seen to have small, rounded, vacuoles at 72 h PI, whereas at 7 and 11 days PI, 60%–70% of cells contained enlarged vacuoles harboring large numbers of bacteria. Quantitative PCR analysis of total genomic DNA confirmed that C. burnetii genome numbers increased significantly from 24 h to 11 days PI. Expression of C. burnetii type four secretion system homologs at 7 days PI was demonstrated by reverse transcriptase PCR. Finally, indirect immunofluorescence assay demonstrated that C. burnetii propagated within IDE8 cells were infectious for mammalian cells. These studies demonstrate the utility of cultured tick cell lines as a model to investigate C. burnetii's molecular interactions with its arthropod vectors.
Key Words: Coxiella, IDE8 cells, Intracellular replication
Introduction
Q fever is a zoonotic disease found throughout the world, with the exception of New Zealand (Kaplan and Bertagna 1955, Hilbink et al. 1993). The disease is caused by the Gram-negative intracellular bacterial pathogen Coxiella burnetii. Human infection occurs mainly through inhalation of contaminated particulates shed from infected goats, sheep, and cattle (Babudieri 1959, Woldehiwet 2004). Transmission to animals and humans is facilitated by the ability of C. burnetii to survive for extended periods in a spore-like state on objects contaminated with infected tick feces, in water, and in soil (McCaul and Williams 1981). Additionally, wild and domestic mammals, birds, and ticks act as reservoirs for the bacterium (Babudieri 1959, Maurin and Raoult 1999). C. burnetii infections are usually not clinically apparent in animals; however, acute and chronic infection can lead to abortion in sheep and goats, and low birth weights and infertility in cattle (Aitken 1989). Since ticks are a reservoir, it is thought that they act as vectors in the transmission of C. burnetii among animals (Eklund et al. 1947, Babudieri 1959, Beaman and Hung 1989) as well as maintaining the pathogen in the environment. Early investigations indicate that C. burnetii may replicate in the middle gut or stomach of ticks and subsequently be excreted in the feces (Kordova and Rehacek 1959). Moreover, studies indicate that transovarial and transstadial transmission of C. burnetii may occur in Hyalomma asiaticum, Hyalomma lusitanicum, and Dermacentor marginatus (Daiter 1977, Walker and Fishbein 1991, Toledo et al. 2009). Although there is evidence that C. burnetii is able to replicate in crude primary tick cell cultures (Rehacek and Brezina 1964), recently established continuous tick cell lines have not been employed to study the host cell–pathogen interactions of C. burnetii and these vectors.
Blood feeding Ixodid ticks (subphylum Chelicerata; class Arachnida; subclass Acari; family Ixodidae) are known to transmit a variety of bacterial, rickettsial, viral, and protozoan diseases (Estrada-Pena and Jongejan 1999). Ixodid ticks have recently been shown to harbor Coxiella spp. and Coxiella-like pathogens in the wild (Vilcins et al. 2009). Due to the efficiency of Ixodes spp. ticks as vectors of pathogens and their worldwide distribution, we have chosen an Ixodes scapularis–derived cell line (IDE8) to investigate as an in vitro model for studying the tick-pathogen cellular interactions of C. burnetii. This cell line has been used to successfully propagate multiple tick-borne pathogens, including Anaplasma phagocytophilum, A. marginale, Ehrlichia canis, E. ruminantium, Borrelia spp., and Rickettsia spp. (Bell-Sakyi et al. 2007). In the current study, we sought to determine C. burnetii's infectivity, growth rate, gene expression, as well as its ability to reinfect mammalian cells after growth in cultured tick cells. Using the indirect fluorescent antibody (IFA) microscopy assay, electron microscopy (EM), quantitative polymerase chain reaction (qPCR), and reverse transcriptase-PCR (RT-PCR), we determined the ability of C. burnetii to invade and replicate within the IDE8 tick cell line, expression levels of genes of the type four secretion system (T4SS) within tick cells, and the ability of tick cell-derived C. burnetii to invade mammalian cells.
Materials and Methods
Bacterial cultivation and purification
Coxiella burnetii Nine Mile Phase II Clone 4 (NMII) was propagated in African green monkey kidney (Vero) cells in RPMI 1640 medium and 5% fetal bovine serum (FBS) at 37°C in an atmosphere of 5% CO2, and the small cell variant (SCV) form of the organism was isolated as previously described (Coleman et al. 2004). The SCVs were resuspended in SPG buffer (0.7 M sucrose, 3.7 mM KH2PO4, 6.0 mM K2HPO4, 0.15 M KCl, and 5.0 mM glutamic acid, pH 7.4) and stored at −80°C. C. burnetii genome equivalents were calculated using qPCR (Brennan and Samuel 2003).
Tissue culture cells
Uninfected Vero cells were propagated as described in the medium containing 20 μg/mL gentamicin. The medium was exchanged with fresh RPMI 1640 and 5% FBS without antibiotics 2 h before bacterial infection. The tick cell line IDE8 (ATCC CRL 11973), derived from embryos of I. scapularis and maintained in continuous passage for several years, was maintained in a modified Liebovitz's L15 medium at 34°C following the procedures of Munderloh et al. (1994). Cultures were washed with the antibiotic-free medium before C. burnetii infections.
Infection of IDE8 tick cells
The optimal C. burnetii multiplicity of infection (MOI, based on genome equivalents) for IDE8 cells was empirically determine (data not shown). Thereafter, 25 cm2 flasks containing 1 × 107 IDE8 cells were infected with C. burnetii NMII at a genome equivalent MOI of 30 in 2 mL of L15 medium at 34°C for 4 h. The flask volume was then brought up to a total of 5 mL with L15 medium. Infected cells were incubated at 34°C with culture flask caps closed. The medium was replaced every 24–48 h as needed.
IDE8 cell sample harvest
The 25 cm2 flasks containing 1 × 107 IDE8 cells were divided into five sections. One section of the flask was harvested by scraping just before infection (uninfected), and 24, 72, 168 (7 days), and 264 h (11 days) postinfection (PI). The medium was removed before each sampling and replaced immediately afterward, and flasks returned to incubation. Parallel aliquots of infected cells from each time point were (1) seeded in 24-well plastic tissue culture plates for 3–4 h at 34°C to allow for re-attachment, and then fixed with a 4% paraformaldehyde, Tween 20 (0.05%), and phosphate-buffered saline; (2) fixed to glass slides using a cytospin centrifuge followed by fixation for 10 min using ice-cold methanol; and (3) centrifuged and the total genomic DNA isolated using the Genomic Isolation Kit (Promega). A minimum of three biological samples were isolated for each condition and time point.
Indirect immunofluorescence antibody assay
The 24-well plate was seeded and cytospin samples were analyzed by IFA microscopy using rabbit polyclonal antibody against whole-killed C. burnetii NMII followed by an Alexa-fluor 488–tagged goat anti-rabbit IgG (Molecular Probes). Fluorescent images were captured at 400× magnification using a Nikon eclipse TE-2000 S inverted microscope equipped with a Nikon DS-Fi1 digital camera.
Vero cells seeded on 24-well tissue culture plates were inoculated with C. burnetii isolated from 7-day PI IDE8 cell lysates. Lysates were created by scraping C. burnetii–infected IDE8 cells into phosphate-buffered saline, and freeze–thawing the cells twice at −80°C followed by repeatedly passing the thawed cells through a 26.5-gauge needle. C. burnetii were separated from cell debris by differential centrifugation, and resuspended in RPMI 1640 medium and 5% FBS, which was used to inoculate the Vero cells within 24-well culture plates. Cultures were grown at 37°C in an atmosphere of 5% CO2, for 72 h, and then fixed to the culture plates using methanol. IFA microscopy analysis was performed directly in the culture plates as described above.
qPCR analysis
Ten-fold serial dilutions of purified C. burnetii genomic DNA (106, 105, 104, and 103 genomes/sample well) were used to generate a standard quantitative curve in each experiment. C. burnetii genome equivalents in infected IDE8 cell samples were estimated using qPCR and the SYBR Green Master Mix kit (Applied Biosystems) in an Applied Biosystems 7500 real-time cycler, with forward [f] and reverse [r] primers CB594—5′-CGCTTCATGAATTAGCAGCA-3′[f] and CB595—5′-TGCAGTCAAACGGTTCTTCA-3′[r]. These primers target the C. burnetii icmW gene (GenBank accession no. AF318146). Briefly, the reaction mixture contained 0.3 μM of each primer, and 10 ng of sample template DNA in a total volume of 15 μL. The resulting fluorescent plots were analyzed, and estimated numbers of C. burnetii genomes in the experimental samples were determined based on the standard curve. An increase in genome equivalents was observed relative to infected IDE8 cells collected 24 h PI. A minimum of three biological and three technical samples were used in the analysis of each time point.
RNA isolation and quality control
One half of a 25 cm2 flask containing infected IDE8 cells was scraped at 7 days PI and cells were pelleted by centrifugation. Total RNA was then harvested using Tri Reagent (Ambion) following the manufacturer's recommendations. All RNA samples were DNase treated to remove contaminating DNA with RQ1 DNase (Promega) and confirmed DNA-free by PCR before RNA analysis assays.
RT-PCR analysis
RT-PCR analysis was carried out using the Access Quick RT-PCR Kit (Promega) and total RNA isolated from C. burnetii–infected IDE8 cells following the manufacturer's directions. Primers CB40, 5′-ATGCCAGATCTGTCGC-3′[f] and CB41, 5′-TAAACCACCTTCCTCAAGAG-3′[r] (icmW), CB70, 5′-ATGATTCTTTTGGAGTCTTCC-3′[f] and CB71, 5′-TTGTTTGGACCCCTTAAAGGTG-3′[r] (icmV), and CB703, 5′-ATTGGGGCCAGTATCATTCC-3′[f] and CB696, 5′-ATGGAGTGTGCGGATTTGAT-3′[r] (dotH) were used in RT-PCR analysis.
Electron microscope analysis
One half of a 25 cm2 flask containing infected IDE8 cells was scraped at 7 days PI and cells were pelleted by centrifugation. The C. burnetii–infected IDE8 cells were fixed with 2.5% paraformaldehyde (v/v)/2.5% glutaraldehyde (v/v) for transmission EM analysis as previously described (Morgan et al. 2010). The Imaging Facility in the Department of Molecular Microbiology Center for Infectious Disease Research, Washington University, St. Louis, MO, performed the subsequent sample processing and transmission EM analyses following published techniques (Belland et al. 2003).
Results
C. burnetii infection of IDE8 tick cells
To determine whether C. burnetii infects IDE8 cells, we used an approximate genome equivalent MOI of 30. The infected cells were cytospun to a microscope slide followed by methanol fixation and IFA. These analyses indicate that C. burnetii containing vacuoles are present by 72 h PI and large, spacious, immunostained C. burnetii vacuoles were prominent by 7 days PI (data not shown). Although this indicated that C. burnetii were infecting IDE8 cells and replicating within them, the cytospin centrifugation method causes distortion and/or disruption of infected cells, resulting in dispersion of many of the bacteria.
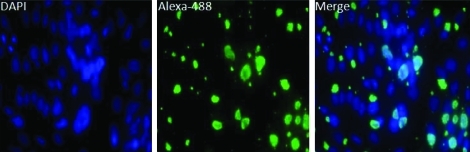
To observe infected IDE8 cells that are physiologically intact, an alternative method was employed where the tick cells were re-seeded to 24-well tissue culture plates and allowed to adhere before fixation and IFA analysis. Figure 1A shows that after 72 h PI ∼30%–40% of infected cells had small, rounded vacuoles, and at 168 h (7 days) PI, swollen, enlarged vacuoles containing large numbers of bacteria were present. By 264 h (11 days) PI, the infected IDE8 cells had large, fragile vacuoles such that intact infected cells could not be transferred from larger flasks to 24-well culture plates for microscopy analysis without rupturing the cells.
FIG. 1.
IFA of Coxiella burnetii NMII infection of IDE8 cells. (A) Left panel, DAPI-stained cells. Middle panel, Alexa-488 labeling of C. burnetii. Right panel, merge of left and middle panels. Time of fixation PI is indicated at the left of each corresponding row. (B) Electron micrograph of C. burnetii–infected IDE8 cells fixed at 7 days PI. Arrows indicate C. burnetii large cell variants. Arrowheads indicate C. burnetii small cell variants. Scale bar is 1.0 μm. PI, postinfection.
To determine whether C. burnetii were growing within membrane-bound parasitophorous vacuoles, EM was performed on infected IDE8 cells fixed at 7 days PI (Fig. 1B). EM micrographs indicate that C. burnetii is replicating in a membrane-bound compartment (Fig. 1B, left panel) and that both replicative large cell variants and environmentally stable SCV forms of the bacteria appear to be present within the vacuole (Fig. 1B arrows and arrowheads, respectively) at 7 days PI. Combined, these experiments demonstrate that C. burnetii can be internalized, survive, and grow within IDE8 tick cells in vitro. The appearance of spacious vacuoles at the beginning of what might be thought of as the exponential growth phase (72 h PI) is similar to that seen in C. burnetii infection of cultured mammalian cells (Coleman et al. 2004).
C. burnetii genome numbers increase after an extended lag phase
In an effort to quantitate the growth characteristics of C. burnetii NMII in IDE8 tick cells, we estimated the number of C. burnetii genomes during the course of infection using qPCR. Using primers designed to the C. burnetii icmW homolog, and 24 h PI as a base line, C. burnetii genome equivalents were observed to decrease slightly between 24 and 72 h PI, although the decrease was not statistically significant (p < 0.05). This was followed by a 3.10 and 17.83-fold increase at 7 and 11 days PI (Fig. 2), respectively. After a lag, C. burnetii double every 10 h in mammalian cell models (Zamboni et al. 2001, Coleman et al. 2004). Using our data to calculate the replication rate of C. burnetii in the IDE8 cells, a doubling time of nearly 40 h can be derived over the entire time period. However, if the calculation is made following the ∼72 h lag phase, C. burnetii genomes double every 10.87 h (Fig. 2) in the IDE8 cells. This rate is very similar to the 10.2 h (qPCR assay) rate found during the exponential phase of C. burnetii growth in mammalian cells following a 48 h lag phase (Coleman et al. 2004).
FIG. 2.
C. burnetii genome levels during infection of IDE8 cells. Fold changes in genome numbers relative to 24 h PI. An equal amount of total genomic DNA from each sample was analyzed by quantitative qPCR. The time (in hours and days) PI when DNA was harvested is indicated below the X-axis. Results represent the mean of three biological samples with no fewer than three technical replicates of each sample. Standard error bars represent the combined standard error of the mean per time point. PCR, polymerase chain reaction.
Expression of C. burnetii T4SS during infection of IDE8 cells
Secretion systems have been shown to be crucial for the delivery of effector proteins in a number of bacterial pathogens. In particular, the type-three secretion system is required for the virulence of bacteria, including Escherichia coli, Shigella, and Salmonella spp. (Hueck 1998). C. burnetii possesses T4SS homologs, a system that has been shown to be required for virulence in its closely related neighbor, Legionella pneumophila (Marra et al. 1992, Berger and Isberg 1993, Ensminger and Isberg 2009). C. burnetii T4SS homologs are expressed at the RNA and protein level during infection of mammalian cells in culture (Zamboni et al. 2003, Zusman et al. 2003, Coleman et al. 2004, Morgan et al. 2010). To determine whether this virulence determinant is expressed by C. burnetii during infection of IDE8 cells, RT-PCR was used to analyze total RNA isolated from infected cells 7 days PI (Fig. 3). Amplification products following RT-PCR clearly demonstrate that icmW, icmV, and dotH are expressed by the bacterium during infection of the IDE8 cell line.
FIG. 3.
RT-PCR detection of C. burnetii T4BSS transcripts icmW, icmV, and dotH during infection of IDE8 cells. Total RNA template was isolated at 7 days PI from C. burnetii–infected IDE8 cells. L, 100 bp DNA ladder. RT, reverse transcriptase; +RT, with reverse transcriptase; –RT, without reverse transcriptase; C, genomic DNA-positive PCR control.
IDE8-derived C. burnetii infectivity for mammalian cells
To determine whether the C. burnetii surviving within IDE8 cells were infectious for mammalian cells, IFA microscopy analysis was performed on Vero cells that were inoculated with lysates harvested from C. burnetii–infected IDE8 cells 7 days PI. Figure 4 shows an IFA of Vero cells infected with IDE8-derived C. burnetii. In this qualitative analysis, it is evident that the C. burnetii growing within IDE8 cells are infectious for mammalian cells. Large parasitophorous vacuoles containing multiple bacteria are indicated by the large, green fluorescing vacuoles present within the Vero cells (Fig. 4). This finding indicates that the C. burnetii growing within tick cells are readily infectious for mammalian cells, increasing the likelihood that tick-borne exposure to the pathogen could lead to disease.
FIG. 4.
IFA of IDE8-derived C. burnetii NMII infecting Vero cells. Left panel, DAPI (4′,6 diamidino-2-phenylindole)-stained cells. Middle panel, Alexa-488 labeling of C. burnetii. Right panel, merge of left and middle panels. Infected cells were fixed at 72 h PI.
Discussion
Since the earliest studies of C. burnetii, it has been known that this pathogen has an association with arthropod vectors (Davis and Cox 1938, Davis 1939). However, an understanding of whether C. burnetii is passively carried in ticks or it is amplified by replication within the tick is not clear. Additionally, it has been demonstrated that C. burnetii can grow in a myriad of mammalian cell lines (Brezina et al. 1969, Burton et al. 1978, Baca et al. 1981, Voth et al. 2007), yet its ability to invade, replicate, and produce infectious progeny in tick cell lines had not been reported.
Our findings indicate that C. burnetii readily infects cultured IDE8 cells. qPCR of the icmW gene indicates that C. burnetii grown in IDE8 cells undergoes a prolonged lag phase before replication begins relative to growth in mammalian cells (Coleman et al. 2004). When replication does begin, C. burnetii's doubling time in IDE8 cells appears to approach the ∼10 h observed in mammalian cells. These findings suggest that a period of adjustment may be required for successful growth. It may be that the organism has to adjust to the lower temperature (34°C) of IDE8 cell culture. It could also be hypothesized that the bacterium has to adjust to a substantially different host cell environment relative to in vitro growth in a mammalian cell line. Both factors may influence C. burnetii's replication during early infection of these tick cells. Interestingly, while C. burnetii genome numbers remained relatively constant between 24 and 72 h of infection (Fig. 2), vacuoles with appreciable numbers of C. burnetii are evident in 72 h PI IFA analyses (Fig. 1). Although difficult to appreciate in the low light fluorescent images (Fig. 1), a large number of free, or single, bacteria were observed 24 after C. burnetii infection of IDE8 cells, suggesting that many of the bacteria used to initiate an infection did not cause a productive infection within a given cell. We speculate that this is the result of overestimation of the MOI due to the inability of the qPCR assay to discriminate between DNA from viable and nonviable bacteria, or that the IDE8 cells were capable of ingesting and killing a portion of the bacteria. In either case, the bacteria that invaded and survived within the IDE8 cells had formed visible vacuoles by 72 h PI (Fig. 1).
The ability of facultative and obligate intracellular pathogens to subvert host cell processes is crucial to their survival. Secretion systems are one of the primary means by which pathogens interact with the host. Our evidence that C. burnetii is expressing RNA for the production of a T4SS during infection of IDE8 cells (Fig. 3) leads us to hypothesize that the pathogen is interacting with the tick cells and are likely manipulating the host cell response, as in mammalian cells (Voth and Heinzen 2009). In mammalian cells, expression of T4SS homologs has been shown at the RNA level throughout infection and that the T4SS machinery is localized to the poles of the bacterial cell (Zamboni et al. 2003, Zusman et al. 2003, Coleman et al. 2004, Morgan et al. 2010). Moreover, it is likely that the C. burnetii T4SS is crucial to the pathogens ability to subvert cellular pathways for its own benefit.
Ultimately, C. burnetii's ability to infect a mammalian host after replication within a tick vector makes ticks a viable and crucial environmental reservoir of this pathogen. Our demonstration that C. burnetii isolated from IDE8 cells could readily infect cultured Vero cells (Fig. 4) would indicate that the C. burnetii propagated within a tick would be capable of causing disease in humans and other mammals. In addition, the production of environmentally stable SCV forms of the pathogen would enable a tick vector to shed infectious particles with the ability to cause disease long after they enter the environment.
The results of these experiments show that C. burnetii capable of infecting mammalian cells are produced IDE8 tick cells after an extended lag phase. Further, we demonstrate that C. burnetii expresses homologs of a suggested virulence determinant during infection of the IDE8 cultured cells. These findings demonstrate that cultured tick cells represent a viable in vitro model to study the pathogens cellular interactions with tick cells in comparison to those found in mammalian cells while expanding our understanding of C. burnetii growth within the tick vector.
Acknowledgments
We wish to thank Dr. Wandy Beatty, Washington University School of Medicine, for technical expertise and advice on the EM analysis. We also thank Dr. Susan Little and Mr. Brandon Luedtke for critical reading and discussion of this article. This research was supported by National Institutes of Health grant A1072710 (E.I.S.).
Disclosure Statement
No competing financial interests exist.
References
- Aitken ID. Clinical aspects and prevention of Q fever in animals. Eur J Epidemiol. 1989;5:420–424. doi: 10.1007/BF00140132. [DOI] [PubMed] [Google Scholar]
- Babudieri B. Q fever: a zoonosis. Adv Vet Sci. 1959;5:82–182. [Google Scholar]
- Baca OG. Akporiaye ET, et al. Fate of phase I and phase II Coxiella burnetii in several macrophage-like tumor cell lines. Infect Immun. 1981;33:258–266. doi: 10.1128/iai.33.1.258-266.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman MH. Hung J. Pericarditis associated with tick-borne Q fever. Aust NZ J Med. 1989;19:254–256. doi: 10.1111/j.1445-5994.1989.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belland RJ. Zhong G, et al. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc Natl Acad Sci USA. 2003;100:8478–8483. doi: 10.1073/pnas.1331135100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-Sakyi L. Zweygarth E, et al. Tick cell lines: tools for tick and tick-borne disease research. Trends Parasitol. 2007;23:450–457. doi: 10.1016/j.pt.2007.07.009. [DOI] [PubMed] [Google Scholar]
- Berger KH. Isberg RR. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Brennan RE. Samuel JE. Evaluation of Coxiella burnetii antibiotic susceptibilities by real-time PCR assay. J Clin Microbiol. 2003;41:1869–1874. doi: 10.1128/JCM.41.5.1869-1874.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezina R. Kazar J, et al. Difference in the multiplication of phase I and II Coxiella burnettii in cell cultures. Acta Virol. 1969;13:455. [PubMed] [Google Scholar]
- Burton PR. Stueckemann J, et al. Some ultrastructural effects of persistent infections by the Rickettsia Coxiella burnetii in mouse L cells and green monkey kidney (Vero) cells. Infect Immun. 1978;21:556–566. doi: 10.1128/iai.21.2.556-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SA. Fischer ER, et al. Temporal analysis of Coxiella burnetii morphological differentiation. J Bacteriol. 2004;186:7344–7352. doi: 10.1128/JB.186.21.7344-7352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daiter AB. Transovarial and transspermal transmission of Coxiella burnetii by the tick Hyalomma asiaticum and its role in the ecology of Q-rickettsiosis. Parazitologiia. 1977;11:403–411. [PubMed] [Google Scholar]
- Davis GE. Ricksttsia diaporica: recovery of three strains from Dermacentor andersoni collected in southeastern Wyoming: their identity with Montana strain. Public Health Rep. 1939;54:2219–2225. [Google Scholar]
- Davis GE. Cox HR. A filter-passing infectious agent isolated from ticks. I. Isolation from Dermacentor andersoni, reactions in animals, and filtration experiments. Public Health Rep. 1938;53:2259–2261. [Google Scholar]
- Eklund CM. Parker RR. Lackman DB. A case of Q fever probably contracted by exposure to ticks in nature. Public Health Rep. 1947;62:1413–1416. [PubMed] [Google Scholar]
- Ensminger AW. Isberg RR. Legionella pneumophila Dot/Icm translocated substrates: a sum of parts. Curr Opin Microbiol. 2009;12:67–73. doi: 10.1016/j.mib.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada-Pena A. Jongejan F. Ticks feeding on humans: a review of records on human-biting Ixodoidea with special reference to pathogen transmission. Exp Appl Acarol. 1999;23:685–715. doi: 10.1023/a:1006241108739. [DOI] [PubMed] [Google Scholar]
- Hilbink F. Penrose M. Kovacova E. Kazar J. Q fever is absent from New Zealand. Int J Epidemiol. 1993;22:945–949. doi: 10.1093/ije/22.5.945. [DOI] [PubMed] [Google Scholar]
- Hueck CJ. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan MM. Bertagna P. The geographical distribution of Q fever. Bull World Health Organ. 1955;13:829–860. [PMC free article] [PubMed] [Google Scholar]
- Kordova N. Rehacek J. Experimental infection of ticks in vivo and their organs in vitro with filterable particles of Coxiella burnetii. Acta Virol. 1959;3:201–209. [PubMed] [Google Scholar]
- Marra A. Blander SJ, et al. Identification of a Legionella pneumophila locus required for intracellular multiplication in human macrophages. Proc Natl Acad Sci USA. 1992;89:9607–9611. doi: 10.1073/pnas.89.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurin M. Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul TF. Williams JC. Developmental cycle of Coxiella burnetii: structure and morphogenesis of vegetative and sporogenic differentiations. J Bacteriol. 1981;147:1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JK. Luedtke BE, et al. Polar localization of the Coxiella burnetii type IVB secretion system. FEMS Microbiol Lett. 2010;305:177–183. doi: 10.1111/j.1574-6968.2010.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munderloh UG. Liu Y, et al. Establishment, maintenance and description of cell lines from the tick Ixodes scapularis. J Parasitol. 1994;80:533–543. [PubMed] [Google Scholar]
- Rehacek J. Brezina R. Propagation of Coxiella burneti in tick tissue cultures. Acta Virol. 1964;8:380. [PubMed] [Google Scholar]
- Toledo A. Jado I, et al. Detection of Coxiella burnetii in ticks collected from Central Spain. Vector Borne Zoonot Dis. 2009;9:465–468. doi: 10.1089/vbz.2008.0070. [DOI] [PubMed] [Google Scholar]
- Vilcins IM. Old JM, et al. Molecular detection of Rickettsia, Coxiella and Rickettsiella DNA in three native Australian tick species. Exp Appl Acarol. 2009;49:229–242. doi: 10.1007/s10493-009-9260-4. [DOI] [PubMed] [Google Scholar]
- Voth DE. Heinzen RA. Coxiella type IV secretion and cellular microbiology. Curr Opin Microbiol. 2009;12:74–80. doi: 10.1016/j.mib.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voth DE. Howe D, et al. Coxiella burnetii inhibits apoptosis in human THP-1 cells and monkey primary alveolar macrophages. Infect Immun. 2007;75:4263–4271. doi: 10.1128/IAI.00594-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DH. Fishbein DB. Epidemiology of rickettsial diseases. Eur J Epidemiol. 1991;7:237–245. doi: 10.1007/BF00145672. [DOI] [PubMed] [Google Scholar]
- Woldehiwet Z. Q fever (coxiellosis): epidemiology and pathogenesis. Res Vet Sci. 2004;77:93–100. doi: 10.1016/j.rvsc.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Zamboni DS. McGrath S, et al. Coxiella burnetii express type IV secretion system proteins that function similarly to components of the Legionella pneumophila Dot/Icm system. Mol Microbiol. 2003;49:965–976. doi: 10.1046/j.1365-2958.2003.03626.x. [DOI] [PubMed] [Google Scholar]
- Zamboni DS. Mortara RA, et al. Infection of Vero cells with Coxiella burnetii phase II: relative intracellular bacterial load and distribution estimated by confocal laser scanning microscopy and morphometry. J Microbiol Methods. 2001;43:223–232. doi: 10.1016/s0167-7012(00)00223-2. [DOI] [PubMed] [Google Scholar]
- Zusman T. Yerushalmi G, et al. Functional similarities between the Icm/dot pathogenesis systems of Coxiella burnetii and Legionella pneumophila. Infect Immun. 2003;71:3714–3723. doi: 10.1128/IAI.71.7.3714-3723.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]