Abstract
One mechanism to molecularly explain the strong association of maternal anti-Ro60 antibodies with cardiac disease in neonatal lupus (NL) is that these antibodies initiate injury by binding to apoptotic cardiomyocytes in the fetal heart. Previous studies have demonstrated that beta2-glycoprotein I (β2GPI) interacts with Ro60 on the surface of apoptotic Jurkat cells and prevents binding of anti-Ro60 IgG. Accordingly, the current study was initiated to test two complementary hypotheses a) competition between β2GPI and maternal anti-Ro60 antibodies for binding apoptotic induced surface translocated Ro60 occurs on human fetal cardiomyocytes and b) circulating levels of β2GPI influence injury in anti-Ro60 exposed fetuses. Initial flow cytometry experiments conducted on apoptotic human fetal cardiomyocytes demonstrated dose-dependent binding of β2GPI. In competitive inhibition experiments, β2GPI prevented opsonisation of apoptotic cardiomyocytes by maternal anti-Ro60 IgG. ELISA was used to quantify β2GPI in umbilical cord blood from 97 neonates exposed to anti-Ro60 antibodies, 53 with cardiac NL and 44 with no cardiac disease. β2GPI levels were significantly lower in neonates with cardiac NL. Plasmin-mediated cleavage of β2GPI prevented binding to Ro60 and promoted the formation of pathogenic anti-Ro60 IgG-apoptotic cardiomyocyte complexes. In aggregate these data suggest that intact β2GPI in the fetal circulation may be a novel cardioprotective factor in anti-Ro60-exposed pregnancies.
Introduction
The identification of isolated congenital heart block in utero during the mid to late second trimester is almost universally associated with maternal Abs to a component of the SSA/Ro-SSB/La ribonucleoprotein complex, even in asymptomatic women. The cardiac disease of neonatal lupus (cardiac NL), while typically characterized by fibrosis of the atrioventricular node, can extend to the working myocardium and endocardium (1). Although the accessibility of maternal Ab to a normally sequestered intracellular antigen has been difficult to reconcile, apoptosis has been proposed as a cellular event which promotes the translocation of Ro and La proteins to the cell surface and binding by cognate Abs (2, 3). This notion led to the observation that healthy cardiomyocytes are capable of engulfing apoptotic cardiomyocytes and that binding of anti-Ro/La Abs to the apoptotic cardiomyocytes inhibits this physiologic process (4). Histological studies support the in vitro findings since hearts from fetuses dying with cardiac NL reveal exaggerated apoptosis, while apoptosis is rarely detected in healthy hearts from electively terminated age matched fetuses (5).
The direct pathogenicity of maternal anti-Ro60 Abs has been questioned since cardiac NL occurs in only 2% of neonates born to mothers with the candidate Abs (1). Although Abs appear necessary, it is likely that fetal and environmental factors amplify the Ab effect to promote full expression of disease. A focus on beta2-glycoprotein I (β2GPI) as a candidate fetal factor is supported by two recent observations a) Ro60 expressed on the surface of apoptotic Jurkat cells interacts with β2GPI and b) preincubation of the apoptotic cells with β2GPI significantly blocks the binding of anti-Ro60 Abs (6, 7). β2GPI is an abundant positively charged protein comprised of five short consensus repeats with a lysine patch adjacent to a hydrophobic C-terminal loop (residues 313–316) in domain V (8). Significantly lower levels of circulating β2GPI were reported in umbilical cord plasma compared to adult plasma (9) which may be relevant to the clinical observation that the maternal heart is not affected despite continuous exposure to the identical Abs. β2GPI has been implicated in the modulation of coagulation and fibrinolysis pathways (10) and is regulated by plasmin which proteolytically cleaves β2GPI domain V (11, 12), the putative site for binding by Ro60 (6). Of further relevance to the pathogenesis of cardiac NL, the binding of anti-Ro60 IgG to apoptotic cardiomyocytes was recently shown to enhance the activity of urokinase plasminogen activator (uPA) which catalyses the conversion of plasminogen to plasmin (13). This may in turn result in an amplification cycle whereby anti-Ro60 binding results in increased plasmin generation, cleavage of β2GPI and further uncompeted binding by pathogenic Ab.
Accordingly, this study was initiated to evaluate the hypothesis that in utero levels of β2GPI influence pregnancy outcome in anti-Ro60-positive mothers. The relevance of the Ro60-β2GPI interaction to the pathogenesis of cardiac NL was approached by using the target cell, human fetal cardiomyocytes, and affinity purified anti-Ro60 IgG from a mother of an affected child. The levels of β2GPI were measured by ELISA in umbilical cord blood from affected and unaffected neonates, each exposed to maternal anti-Ro60 Abs. The Ro60-β2GPI binding site was then mapped to determine whether cleavage of β2GPI by plasmin, affects binding to Ro60.
Materials and Methods
Patients and Controls
Umbilical cord serum or plasma from neonates of anti-Ro60-positive mothers and serum from mothers were obtained, with informed consent. Pregnancies resulting in cardiac NL or no cardiac manifestations were identified from the Research Registry for Neonatal Lupus (RRNL) (14) and PR Interval and Dexamethasone Evaluation (PRIDE) (15). Each database has IRB approval for evaluation of de-identified information. Maternal serum and cord blood contained Abs reactive with Ro60 and/or Ro52 and La as measured by an NYU CLIA approved laboratory. Total IgG and affinity-purified Abs against recombinant Ro60 (16) were prepared from maternal serum (4). Anti-β2GPI Abs were measured in maternal sera by QUANTA Lite® β2GPI IgG ELISA (INOVA Diagnostics, San Diego, CA).
Induction of apoptosis in cultured human fetal cardiomyocytes and Jurkat cells
Healthy fetal hearts of gestational ages 16–24 weeks were obtained after elective termination and obtaining consent from the mothers through Dr. Brad Poulos at the Human Fetal Tissue Repository of Albert Einstein College of Medicine in accordance with the guidelines of the Institute Review Board of New York University School of Medicine. Cells were isolated and cultured as described (4). Apoptosis was induced with 1 µg/ml staurosporine for 4 h in serum free DMEM or by treating cardiomyocytes with IFN-γ for 24 h and plating on poly-HEMA in the presence of IFN-γ, TNF-α, and cycloheximide (4). Early and late apoptotic Jurkat cells were prepared as described (17). Apoptosis was confirmed by flow cytometric analysis of phosphatidylserine exposure (Annexin V binding).
Measuring β2GPI binding to apoptotic cardiomyocytes and competitive inhibition of anti-Ro60 IgG
Native purified human β2GPI (Haematologic Technologies, Essex Junction, VT) was added to apoptotic cardiomyocytes or Jurkat cells for 30 min at room temperature, washed and detected by either polyclonal rabbit anti-β2GPI antiserum or anti-β2GPI domain I mAb (a gift from Professor Steven Krilis, University of New South Wales, Australia) (18) by flow cytometry (6). A proteolytically clipped form of β2GPI was generated with human plasmin (Sigma, St. Louis, MO) (19) and binding to apoptotic cells was determined (6). In competitive inhibition experiments, increasing concentrations of β2GPI (0.5–50 µg/ml) or IgG depleted-cord blood (diluted 1/10) were co-incubated with 10 µg/ml affinity-purified anti-Ro60 IgG or healthy control IgG in the presence or absence of plasmin (0.2–3 µg/ml) and binding assessed with an anti-human IgG-FITC by flow cytometry (17). To confirm the specificity of the β2GPI mediated inhibition of anti-Ro60 IgG binding to apoptotic cardiomyocytes, recombinant human Growth Arrest Specific 6 (GAS6) or milk fat globule-EFG factor 8 (MFG-E8) were used in competitive inhibition experiments as described above. Binding of GAS6 and MFG-E8 to apoptotic cardiomyocytes was detected with an anti-penta-His mAb (Qiagen) that recognizes the C-terminal 6-His tag in both proteins.
Measuring β2GPI levels in umbilical cord blood by ELISA
β2GPI levels in umbilical cord serum or plasma were determined by a capture ELISA adapted from McNally and colleagues (20). Briefly, rabbit polyclonal anti-β2GPI antiserum diluted 1/2000 in PBS was coated onto 96-well Nunc Maxisorp™ microtitre plates. All incubations were conducted at 37°C for 1 h. Wells were blocked with 2% BSA/PBS and then washed 3 times with PBS containing 0.05% Tween 20. A standard curve was constructed with known concentrations of purified human β2GPI. In some experiments plasmin cleaved β2GPI was added to ELISA plates. Umbilical cord serum or plasma were diluted 1/1000, 1/2000, and 1/4000 in 1% BSA/PBS. After extensive washing, anti-β2GPI mAb was applied and detected with alkaline phosphatase conjugated anti-mouse IgG (Sigma).
Assessing β2GPI levels in umbilical cord blood by Immunoblot
To confirm ELISA findings, a panel of 9 umbilical cord bloods, 5 from neonates affected by cardiac NL and 4 non-cardiac NL neonates, were subjected to SDS-PAGE under non-reducing conditions and transferred to nitrocellulose membranes. Membranes were blocked with 5% non-fat skim milk powder in PBS overnight at 4°C, washed 3 times in PBS/0.1% Tween 20 and probed with either rabbit anti-β2GPI antiserum (1/2000) or mAb (0.5 µg/ml) for 1 h at room temperature. Membranes were stained with IRDye® 800CW anti-rabbit or anti-mouse IgG conjugates and analysed on the Odyssey® Infrared Imager (LI-COR® Biosciences).
Binding recombinant Ro60 to immobilized native or plasmin-clipped β2GPI
Soluble overlapping fragments of mouse Ro60 encompassing aa 82–244, 82–146, 82–192, 149–244 and 193–236 expressed as maltose-binding protein (MBP) fusion constructs were prepared from pMAL cDNAs (a gift from Kenneth Kaufman, Oklahoma Medical Research Foundation) (21). All recombinant proteins were biotinylated with Sulfo-NHS-Biotin (Pierce, Rockford, IL) according to the manufacturer’s instructions. Binding of biotinylated MBP-Ro60 aa 82–244, aa 82–146, aa 82–192, aa 149–244 or aa 193–236 to native or plasmin-cleaved β2GPI was assessed by ELISA (6).
Molecular modelling of the Ro60-β2GPI complex
A homology model of the 3-dimensional structure of human Ro60 was constructed based on the crystal structure of Xenopus laevis Ro60 using the Swiss Model Program (22). Molecular visualisation and hypothetical docking experiments of Ro60 and the crystal structure of human β2GPI was performed using Swiss Pdb Viewer (23) and manual docking of the structures based on experimental mapping data, surface charge and stereochemical complementarity.
Statistical Analysis
Dichotomous variables (sex, race, maternal Ab status, dexamethasone use, and delivery method) between cardiac NL and non-cardiac NL neonates were compared by Fisher’s exact test. Continuous variables (birth weight, gestational age, titers of anti-Ro60 Abs, and levels of β2GPI) were compared in neonates with cardiac NL and non-cardiac NL by the unpaired T-test. Levels of β2GPI in umbilical cord blood from twins were compared by paired T-test. Multivariate regression analysis was conducted including cardiac NL, dexamethasone use, and delivery method as predictor variables in the model. Two-sided p values <0.05 were considered statistically significant.
Results
β2GPI binds to apoptotic cardiomyocytes and inhibits opsonisation by maternal anti-Ro60 Abs
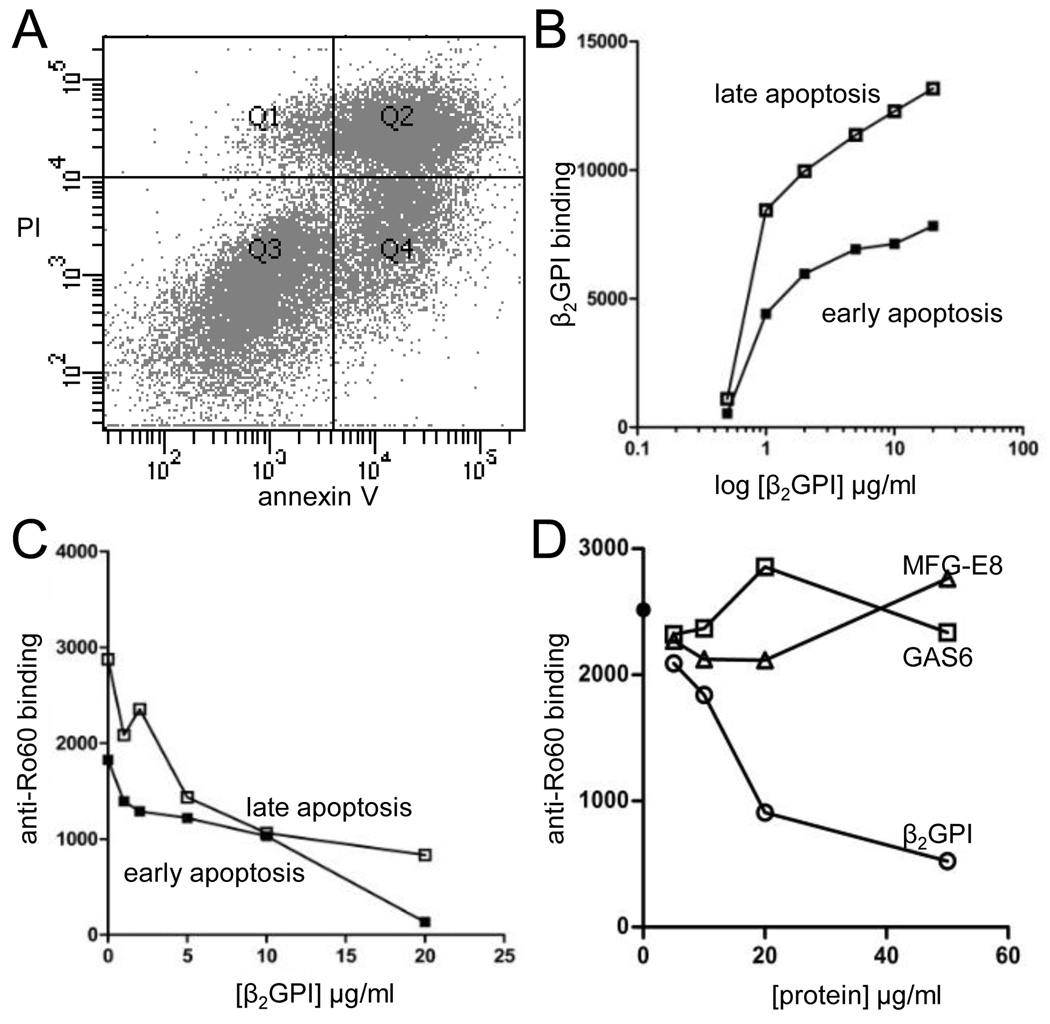
To test the hypothesis that β2GPI may be a protective factor in cardiac NL by preventing the formation of pathogenic anti-Ro60 IgG-apoptotic cardiomyocyte complexes in the fetal heart, initial experiments assessed the binding of β2GPI to apoptotic human fetal cardiomyocytes. Apoptosis was induced either by staurosporine (intrinsic pathway) or a loss of anchorage in the presence of INF-γ, TNF-α, and cycloheximide (extrinsic pathway). The latter treatment induced Annexin V- positivity consistent with apoptosis in 75% of the cell population, of which 38±5% were considered early apoptotic (PI-negative) and 37±13% late apoptotic (PI- positive) (Figure 1A). Staurosporine treatment generated approximately 66% Annexin V positive cells, of which 29±18% and 37±4% were considered early and late apoptotic respectively. Purified native human β2GPI bound to both early and late apoptotic cardiomyocytes in a saturable and dose-dependent manner, with a 2-fold increase in binding to late apoptotic cells compared to the early apoptotic population (Figure 1B). Affinity purified anti-Ro60 IgG from a mother of an infant with cardiac NL bound to both early and late apoptotic cardiomyocytes, with enhanced binding to the late apoptotic population. Increasing the concentration of β2GPI revealed a dose-dependent inhibition of anti-Ro60 IgG binding to early and late apoptotic cardiomyocytes (Figure 1C). These findings confirm that β2GPI competes with anti-Ro60 IgG for binding to the surface of apoptotic cells and extends the observation to human fetal cardiomyocytes. To address the specificity of the Ro60-β2GPI interaction on apoptotic cardiomyocytes competitive inhibition experiments were repeated using GAS6 or MFG-E8, two molecules that also bind apoptotic cells (24, 25). Neither GAS6 nor MFG-E8 altered anti-Ro60 IgG binding to apoptotic cardiomyocytes (Figure 1D). Binding of GAS6 and MFG-E8 to apoptotic cardiomyocytes was confirmed with an anti-penta-His mAb (data not shown).
FIGURE 1.
Purified native human β2GPI binds to apoptotic human fetal cardiomyocytes and inhibits binding of maternal anti-Ro60 IgG. (A) Apoptotic cardiomyocyte populations were gated based on Annexin V and propidium iodide (PI) staining. Annexin V-positive, PI-negative cells (Quadrant, Q4) were termed early apoptotic and Annexin V/PI-positive cells (Q2) were late apoptotic. (B) β2GPI bound both early and late populations in a dose-dependent and saturable manner. (C) Increasing concentrations of β2GPI inhibited the binding of anti-Ro60 IgG to both early and apoptotic cardiomyocyte populations. (D) The inhibition of anti-Ro60 IgG binding to apoptotic cardiomyocytes is specific for β2GPI as GAS6 and MFG-E8 do not alter antibody binding.
Circulating levels of β2GPI are lower in cord blood from anti-Ro60 Ab exposed neonates with cardiac NL compared to non-cardiac NL neonates
Having established in vitro proof-of-concept for the protective β2GPI hypothesis, translation to pathogenesis was sought by measurement of circulating β2GPI levels in affected and unaffected neonates exposed to maternal anti-Ro60 antibodies. Because it is not feasible to obtain fetal blood during the critical time of heart injury (18–24 weeks gestation) umbilical cord blood was used as a proxy to assess the potential influence of circulating β2GPI levels in the pathogenesis of disease. Umbilical cord blood (serum or plasma were previously reported to have no differences in β2GPI levels (20, 26)) from 97 anti-Ro60 exposed infants were studied, 53 with cardiac NL and 44 with no cardiac manifestations (Table 1). Of the 53 affected children, 49 had congenital heart block (47 3rd degree and 2 2nd degree), 3 had an isolated cardiomyopathy and 1 had sustained sinus bradycardia. Of the non-cardiac NL neonates (many of whom had affected siblings), 38 were completely healthy, 5 had cutaneous-NL and 1 had hepatic/haematological manifestations of NL. The demographic characteristics, mother’s antibody status and medication, method of delivery, and infant birth weight and gestational age are shown in Table 1. The sex, race, maternal antibody profile and titer of anti-Ro60 antibody were not significantly different between affected and unaffected neonates. As expected, mothers of children with cardiac NL were more likely to have taken dexamethasone and deliver by caesarean section (p=0.0001). Cardiac NL children were more frequently born prematurely and of lower birth weight than the non-cardiac NL controls (p=0.007).
Table I.
Clinical and demographic characteristics of anti-Ro60 exposed neonates with cardiac NL compared to those without cardiac NL
| Cardiac NL* (N=53) |
Non-Cardiac NL** (N=44) |
p Value | |
|---|---|---|---|
| Sex of Child | 0.22 | ||
| Male | 24 (45%) | 25 (57%) | |
| Female | 29 (55%) | 17 (39%) | |
| NA | 0 (0%) | 2 (4%) | |
| Race/ethnicity | 0.78 | ||
| White | 43 (81%) | 36 (82%) | |
| Black | 3 (6%) | 3 (7%) | |
| Hispanic | 1 (2%) | 4 (9%) | |
| Asian | 4 (7%) | 1 (2%) | |
| Other/NA | 2 (4%) | 0 (0%) | |
| Antibody Status | 0.68 | ||
| Anti-Ro+/La+ | 29 (55%) | 27 (61%) | |
| Anti-Ro+/La− | 24 (45%) | 17 (39%) | |
| Medication | |||
| Dexamethasone | 26 (49%) | 3 (7%) | 0.0001 |
| Delivery | |||
| C-section | 37 (70%) | 10 (23%) | 0.0001 |
| Vaginal | 3 (6%) | 16 (36%) | |
| NA | 13 (24%) | 18 (41%) | |
| Birth Weight (g) | 2682 (±647)+ | 3124 (±572)++ | 0.007 |
| Gestational age(weeks) | 37 (±2)# | 38 (±2)## | 0.018 |
3rd degree block (n=47), 2nd degree (n=2), cardiomyopathy (n=3), sinus bradycardia (n=1)
rash (n=5), liver complications (n=1)
n=36,
n=29,
n=44,
n=31
Birth weight and gestational age are presented as mean±SD. All other data are reported as N (%). NL, neonatal lupus.
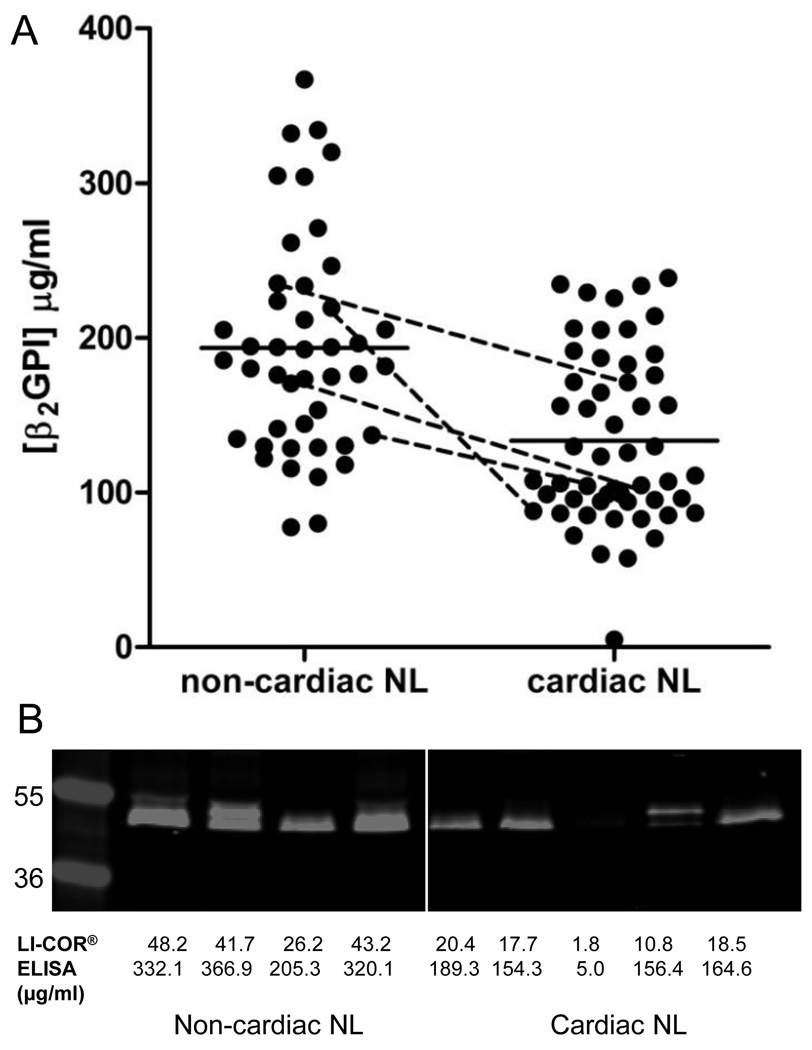
β2GPI levels as assessed by ELISA were significantly lower in the neonates with cardiac NL (133.6±7.6 µg/ml) compared to those without cardiac NL (193.5±10.6 µg/ml) (p<0.0001) (Figure 2A). Dexamethasone use and delivery status were also significantly associated with β2GPI (p=0.05, p=0.01, respectively). Gender, race, maternal antibody status, birth weight and gestational age were not significantly correlated with β2GPI. In a multivariable regression analysis which included cardiac NL status, dexamethasone use and delivery method as predictor variables in the model, β2GPI levels remained lower in cardiac NL compared to non-cardiac NL (p=0.06). However, the effects of dexamethasone use and delivery method were substantially diminished and no longer significantly associated with β2GPI levels after adjusting for cardiac NL status (p=0.53 and p=0.40, respectively). These results are likely explained by the fact that dexamethasone is generally only given to treat cardiac NL once identified, not for prophylaxis, and C-section is more common for delivery of a baby with cardiac NL than an otherwise healthy baby. Four twin pairs discordant for cardiac NL were available for study (2 monozygotic and 2 dizygotic) and proved to be highly informative. In each pair, the twin affected by cardiac NL had lower levels of β2GPI than the unaffected twin (p=0.04) (Figure 2A). In the non-cardiac NL group, there were no significant differences between β2GPI levels in the children affected by rash or those without any manifestation of NL (data not shown). Titres of anti-Ro60 antibodies were equivalent in the sera obtained at the time of delivery from mothers of children with cardiac NL (23,932±4,647, n=47) compared to non-cardiac NL (27,138±6,623 n=40) (p=0.69) and in the umbilical cord bloods from neonates with cardiac NL (11,814±2,021, n=47) vs. non-cardiac NL neonates (14,286±2,829 n=39) (p=0.47).
FIGURE 2.
β2GPI levels are lower in anti-Ro60 exposed neonates with cardiac NL compared to non-cardiac NL. (A) β2GPI concentration, measured by ELISA, in umbilical cord blood from children affected by cardiac NL (n=53) and non-cardiac NL controls (n=44). Solid horizontal lines represent mean β2GPI concentration. Dashed lines connect β2GPI levels in umbilical cord blood from twins discordant for cardiac NL. (B) Immunoblot analysis of a panel of 9 umbilical cord blood samples (4 non-cardiac NL and 5 cardiac NL) probed with anti-β2GPI antibody were quantitatively consistent with those measured by ELISA.
To substantiate the ELISA findings regarding β2GPI concentration and to investigate functional relevance, a subset of umbilical cord blood from 4 unaffected controls and 5 cardiac NL children were selected for further analysis. Immunoblot results were consistent with those of the ELISA in that the density of bands was lower in the 5 umbilical cord bloods from the neonates with cardiac NL compared to the 4 bloods from the healthy neonates (Figure 2B). Cord blood from an unaffected control (β2GPI = 367 µg/ml) and a neonate with cardiac NL (β2GPI = 154 µg/ml) were selected for competitive inhibition experiments to support the protective effects of higher circulating levels of β2GPI. Prior to the experiment, IgG was removed from each cord blood by protein A to eliminate the effect of endogenous maternal anti-Ro60 antibodies. As assessed by flow cytometery, exogenously added affinity purified anti-Ro60 IgG bound at higher levels to apoptotic cardiomyocytes in the presence of cardiac NL cord blood compared to an equal quantity of cord blood from the healthy neonate (data not shown).
It is plausible that concomitant levels of maternal anti-β2GPI antibodies may impede the protective function of β2GPI and account for the lower levels of β2GPI measured in umbilical cord blood from neonates with cardiac NL. To test this consideration, 40 maternal sera obtained at the time of birth (20 cardiac NL, 17 non-cardiac NL, and 3 discordant twins) were available for evaluation of antibodies to β2GPI. Of fourteen tested in which the associated cord blood level of β2GPI was low (<150 µg/ml), only 1 had anti-β2GPI antibodies. Overall, only two of the 40 were positive for anti-β2GPI antibodies (1 cardiac NL and 1 non-cardiac NL). These data suggest that maternal anti-β2GPI antibodies are not associated with a loss of the protective function of β2GPI or the low levels of β2GPI observed in the cord bloods.
Molecular mapping reveals β2GPI binds to Ro60 aa 82–146 via its domain V hydrophobic loop
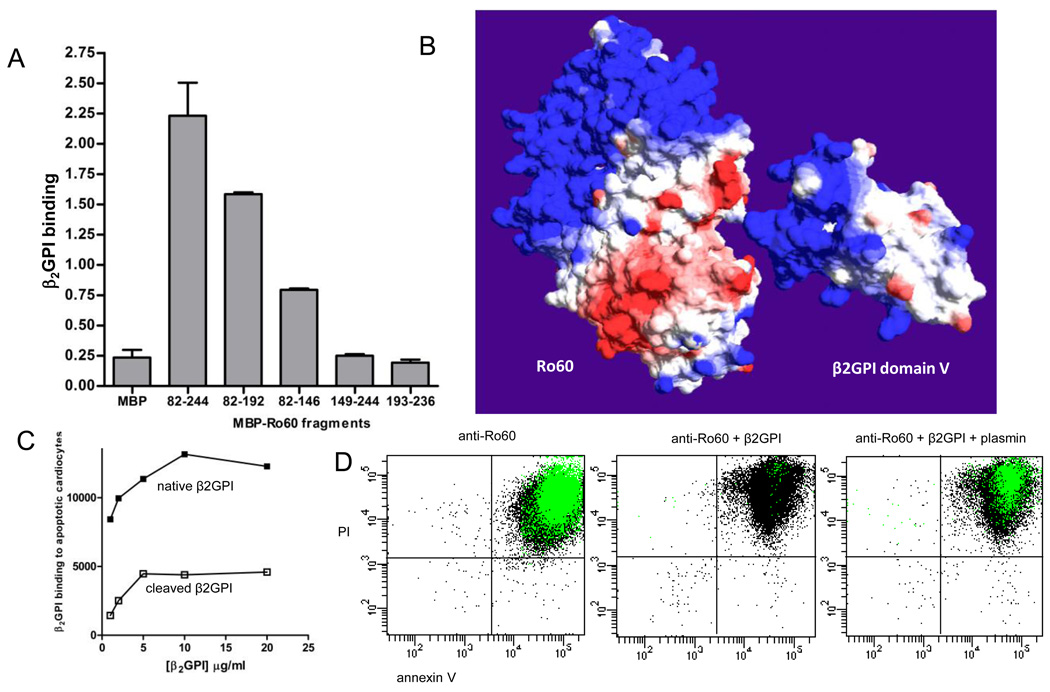
Molecular studies were conducted to assess the β2GPI-Ro60 interaction and factors that may affect this interaction and ultimately generate cardiac vulnerability. β2GPI was previously shown to bind within Ro60 amino acids (aa) 82–244, an immunodominant region of the molecule (22, 27). To more precisely map the β2GPI binding site on Ro60, soluble recombinant MBP fusion proteins expressing fragments of Ro60 within aa 82–244 were used in direct binding experiments. β2GPI bound MBP-Ro60 aa 82–192 and aa 82–146, but not MBP-Ro60 aa 149–244 or aa 193–236 or MBP control (Figure 3A). These results indicate that β2GPI binding occurs predominantly within Ro60 aa 82–146, an alpha helical region of the protein that forms a distinct cleft containing clusters of hydrophobic residues interspaced by acidic amino acids (Figure 3A). To map the β2GPI binding site on Ro60 expressed on the surface of apoptotic cells, flow cytometry competitive inhibition experiments were performed with β2GPI and the Ro60 sub-fragments. MBP-Ro60 aa 82–192 and MBP-Ro60 aa 82–146 reduced the binding of β2GPI to late apoptotic Jurkat cells by 35.3±2.7% and 18.8±0.5% respectively, whereas MBP-Ro60 aa 149–244 and aa 193–236 had a negligible effect on β2GPI binding (6.1±0.3% and 6.5±3.4% inhibition respectively). These data support Ro60 aa 82–146 as being the predominant β2GPI binding region. The 2-fold greater inhibition observed with MBP-Ro60 aa 82–192 compared to the truncated MBP-Ro60 aa 82–146 suggests that β2GPI may favor the conformation presented by the larger Ro60 fragment.
FIGURE 3.
The Ro60 binding site on β2GPI is within the plasmin cleavage site, Lys317-Thr318. (A) The β2GPI binding site on Ro60 was measured by ELISA using recombinant maltose binding protein (MBP) expressing various regions of Ro60. Ro60 fragments encompassing amino acids (aa) 82–244, aa 82–192, and aa 82–146 bound to purified native human β2GPI compared to MBP-Ro60 aa 149–244, aa 193–236, and MBP control. Values are the mean ± standard deviation (SD) of triplicate determinations (n=3). (B) Molecular surface models of Ro60 and β2GPI with the β2GPI fifth domain oriented towards a cleft in Ro60 formed by the mapped aa 82–146 region. Surface colours are calculated using a coulombic algorithm for electrostatic potential where blue is positive charge, white is neutral, and red is negatively charged. (C) The binding of β2GPI to apoptotic cardiomyocytes is abrogated by plasmin cleavage. (D) Plasmin reverses the β2GPI-mediated inhibition of anti-Ro60 binding to apoptotic cells. Representative flow cytometry dot plots depicting the binding of anti-Ro60 IgG (green) to late apoptotic cells (n=5).
In a previous study Ro60 was shown to bind β2GPI via its fifth domain (aa 244–326), (6) a structurally distinct part of the molecule defined by a lysine-rich patch and hydrophobic loop. To predict potential Ro60 binding sites within domain V of β2GPI, hypothetical docking experiments using a previously reported homology model of human Ro60 (22) and human β2GPI (8) were performed using Swiss PdbViewer. Kyte-Doolittle plots reflected a high degree of charge complementarity in the primary sequences of Ro60 aa 82–146 and β2GPI domain V. More specifically, manual docking taking into account both conformation complementarity and electrostatics indicated a potential surface interaction between the relatively acidic cleft formed by the mapped Ro60 aa 82–146 and a basic region adjacent to the β2GPI domain V hydrophobic loop (Figure 3B). Interestingly, the Ro60 binding site on β2GPI was within the plasmin cleavage site, Lys317-Thr318. This suggests that proteolytic cleavage of β2GPI by plasmin may abrogate binding to Ro60 and promote the formation of pathogenic anti-Ro60-apoptotic cardiomyocyte complexes. Precedent for this possibility is that proteolytic cleavage of β2GPI by plasmin prevents binding to phospholipids(11).
Plasmin cleavage of β2GPI prevents binding to apoptotic cardiomyocytes and promotes anti-Ro60 binding
To determine whether plasmin cleavage of β2GPI results in a loss of specificity for Ro60 on apoptotic cardiomyocytes, a plasmin-cleaved form of β2GPI was prepared (19) and binding to Ro60 assessed. Recombinant MBP-Ro60 bound to immobilized native β2GPI by ELISA (OD 1.0±0.02 compared to MBP control 0.1±0.01) but did not bind to the plasmin cleaved β2GPI (OD 0.1±0.02). Flow cytometry experiments confirmed that plasmin cleavage of β2GPI resulted in reduced binding to Ro60 on the apoptotic cardiomyocytes (Figure 3C). To determine the effect of plasmin-cleaved β2GPI on the opsonisation of apoptotic cells by anti-Ro60 antibody, maternal anti-Ro60 IgG was co-incubated with either native or cleaved β2GPI and added to late apoptotic cardiomyocytes. To assess whether plasmin reverses the protective effect of β2GPI, anti-Ro60 IgG and native β2GPI in the presence or absence of plasmin were incubated together with apoptotic cells. As expected, the opsonization of apoptotic cardiomyocytes by anti-Ro60 IgG (MFI 3,445±102) was significantly inhibited by β2GPI (MFI 436±90) (p=0.006). The addition of plasmin to this system reversed the protective effect of β2GPI and promoted anti-Ro60 IgG opsonization at levels similar to those achieved in the absence of β2GPI (MFI 3,378±66) (Figure 3D).
Plasmin cleavage of β2GPI could account for the reduced binding of umbilical cord β2GPI in the quantitative ELISA (Figure 2A). However, plasmin-cleaved β2GPI could not be directly measured by ELISA (due to unavailability of monospecific reagent). Therefore, plasmin cleaved β2GPI was compared to native purified β2GPI on ELISA. Native β2GPI gave a stronger signal on ELISA compared to cleaved β2GPI (range of reduction in binding 19–51%, p=0.0001). The reduction in binding was presumably due to the loss of an epitope which was recognised by the coating anti-β2GPI polyclonal antibody since immobilized plasmin-cleaved β2GPI showed similar reactivity with an anti-β2GPI domain I monoclonal antibody as native β2GPI (OD 1.57±0.14 vs. 1.54±0.14, n=3). This discrepancy in binding between native and cleaved β2GPI led to the speculation that there might be increased cleavage of β2GPI in fetuses with cardiac NL. To determine whether lower levels of β2GPI in umbilical cord blood from cardiac NL cases was due to a reduced concentration of β2GPI or increased cleavage of β2GPI by plasmin, immunoblots were repeated using the same panel of 9 umbilical cord bloods (used in Figure 2A) but probed with the anti-β2GPI domain I monoclonal antibody (which detects both intact and cleaved β2GPI equally). Membranes probed with this monoclonal antibody still revealed lower levels of β2GPI in the umbilical cord bloods from the 5 neonates with cardiac NL compared to the 4 non-cardiac NL neonates equivalent to the results obtained when the membranes were probed with the polyclonal antibody (Figure 2A). This finding suggests that the lower levels of β2GPI observed by ELISA and immunoblot are due to a lower concentration of circulating β2GPI protein rather than increased levels of plasmin-cleaved β2GPI in neonates with cardiac NL.
Discussion
In the present study, we show that β2GPI competes with maternal anti-Ro60 IgG for binding to the surface of human fetal cardiomyocytes rendered apoptotic. A reasonable extension of these data to the pathogenesis of cardiac NL is the prediction that fetal levels of circulating β2GPI represent a protective factor which averts tissue injury by displacing antibody binding. The displacement by β2GPI may explain, in part, the absence of anti-Ro60 IgG binding to apoptotic cells in vivo in a murine transplacental model, (28) or passive transfer xenograft model (29).
The hypothesis of β2GPI as a protective factor in cardiac NL is supported by our finding that levels of circulating β2GPI are lower in neonates with cardiac NL compared to unaffected neonates. Several factors could account for the lower levels of β2GPI observed in the neonates with cardiac NL. Previous studies of cardiac tissue from fetuses dying with cardiac NL demonstrated increased apoptotic cells in the septal region (5). Therefore, decreased levels of β2GPI might be secondary to absorption from the circulation by binding to the apoptotic cells. However, this possibility is unlikely for several reasons a) apoptosis occurs earlier in development and not necessarily at term b) the binding of β2GPI should be displaced by anti-Ro60 antibodies (if equivalent as in the case of twins) in both affected and unaffected fetuses. Alternatively, lower levels of β2GPI may be a primary factor in antibody mediated injury due to a genetic predisposition and not a secondary effect. It is known that plasma levels of β2GPI vary among individuals (ranging from undetectable to 350 µg/ml in adults) which may be under genetic control (30). For example, substitution of C with A at the β2GPI transcriptional initiation site is a causative mutation that affects gene expression at the transcriptional level and ultimately β2GPI plasma concentrations (31). Although it has been reported that African Americans have lower levels than Caucasians due to a genetic polymorphisms, the lowest levels detected were not present in the few African American patients (data not shown). The disparate levels in the discordant monozygotic twins suggest that genetics do not fully account for reduced β2GPI. Finally, maternal anti-β2GPI antibodies were not associated with a loss of the protective function of β2GPI or the low levels of β2GPI observed in the umbilical cord bloods.
Molecular studies revealed the β2GPI binding site on Ro60 to be within amino acids 82–146. In a recent report, we showed that this region of Ro60 also contains an apotope which is recognised by 45% of patients with SLE and isolated anti-Ro60 responses (22). However, β2GPI inhibited the opsonisation of apoptotic cells by IgG from patients with anti-Ro60 autoantibodies, not just those with reactivity to the aa 82–146 apotope (data not shown). This suggests that β2GPI inhibits the binding of anti-Ro60 IgG by steric hindrance, where upon binding to Ro60 aa 82–146, β2GPI undergoes a conformational change and masks other Ro60 apotopes. Interestingly, Ro60 binds to β2GPI within the plasmin cleavage site, residues 317–318, of domain V. Based on these findings, it is likely that a neonate with less circulating β2GPI would be more vulnerable to the effects of plasmin cleavage than a neonate with higher levels of β2GPI. These findings are in accord with those of Briasouli and colleagues (32) who demonstrated that binding of anti-Ro60 antibodies to apoptotic cardiomyocytes results in an increased and altered distribution of uPAR as well as enhanced activation of the urokinase-type plasminogen activator protease (uPA). In binding experiments using FACS analysis, these authors confirmed that preincubation of apoptotic cardiomyocytes with β2GPI markedly decreased the binding of IgG from a mother whose child had cardiac NL to the apoptotic cardiomyocytes. Consistent with the notion that the binding of anti-Ro60 contributes to a pathway leading to an enhanced activation of the urokinase-type plasminogen activator protease, β2GPI added prior to the cardiac NL-IgG attenuated plasminogen activation.
While the clinical association between maternal anti-Ro60 autoantibodies and cardiac NL is strong (33), the rarity and unique fetal vulnerability suggest that other variables are required for disease expression. Both the levels of circulating β2GPI and extent of plasmin cleavage may be highly permissive with regard to the balance of anti-Ro60 antibody binding. Either intrinsically lower levels based on genetic predisposition or cleavage generated by plasminogen activation would confer loss of protection in fetuses exposed to maternal anti-Ro60 antibodies. Since Ro60 is the first of the Ro/La autoantigens to be exposed on the surface of cells undergoing apoptosis (17), it is plausible that preventing the formation of pathogenic anti-Ro60-apoptotic cell complexes with β2GPI may facilitate clearance and protect the fetal heart from antibody-mediated tissue injury.
Acknowledgements
We thank Professor Steven Krilis and Dr. Bill Giannakopoulos for discussion and expertise regarding cleaved beta2-glycoprotein I.
Grant support:
This study was funded by an Australian National Health and Medical Research Council Postgraduate Training Fellowship grant 595989 (Dr. Joanne Reed), the Australian National Health and Medical Research Council grant 595907 (Dr. Tom Gordon), and NIH grant RO1 AR42455-16 and N01-AR-4-2271 (Drs. Jill P. Buyon and Robert M. Clancy).
References
- 1.Buyon JP, Friedman DM. Neonatal Lupus. In: Lahita RG, Tsokos G, Buyon JP, Koike T, editors. Systemic Lupus Erythematosus. 2nd ed. San Diego, CA: Academic Press; 2011. pp. 541–567. [Google Scholar]
- 2.Casciola-Rosen L, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miranda-Carus ME, Tseng CE, Rashbaum W, Ochs RL, Casiano CA, Di Donato F, Chan EK, Buyon JP. Accessibility of SSA/Ro and SSB/La antigens to maternal autoantibodies in apoptotic human fetal cardiac myocytes. J Immunol. 1998;161:5061–5069. [PubMed] [Google Scholar]
- 4.Clancy RM, Neufing PJ, Zheng P, O'Mahony M, Nimmerjahn F, Gordon TP, Buyon JP. Impaired clearance of apoptotic cardiomyocytes is linked to anti-SSA/Ro and -SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest. 2006;116:2413–2422. doi: 10.1172/JCI27803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clancy RM, Kapur RP, Molad Y, Askanase AD, Buyon JP. Immunohistologic evidence supports apoptosis, IgG deposition, and novel macrophage/fibroblast crosstalk in the pathologic cascade leading to congenital heart block. Arthritis Rheum. 2004;50:173–182. doi: 10.1002/art.11430. [DOI] [PubMed] [Google Scholar]
- 6.Reed JH, Giannakopoulos B, Jackson MW, Krilis SA, Gordon TP. Ro60 functions as a receptor for beta2-glycoprotein I on apoptotic cells. Arthritis Rheum. 2009;60:860–869. doi: 10.1002/art.24361. [DOI] [PubMed] [Google Scholar]
- 7.Clancy RM. When the levee doesn't break: A novel role of beta2-glycoprotein I to protect against congenital heart block. Arthritis Rheum. 2009;60:636–638. doi: 10.1002/art.24358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schwarzenbacher R, Zeth K, Diederichs K, Gries A, Kostner GM, Laggner P, Prass R. Crystal structure of human beta2-glycoprotein I: implications for phospholipid binding and the antiphospholipid syndrome. EMBO J. 1999;18:6228–6239. doi: 10.1093/emboj/18.22.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cvirn G, Gallistl S, Koestenberger M, Kutschera J, Ferstl U, Kellner J, Jurgens G, Gries A. Effects of beta2-glycoprotein-I on platelet aggregation in cord versus adult whole blood. Platelets. 2007;18:24–28. doi: 10.1080/09537100600800529. [DOI] [PubMed] [Google Scholar]
- 10.Miyakis S, Giannakopoulos B, Krilis S. Beta 2 glycoprotein I - function in health and disease. Thromb Res. 2004;114:335–346. doi: 10.1016/j.thromres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 11.Ohkura N, Hagihara Y, Yoshimura T, Goto Y, Kato H. Plasmin can reduce the function of human beta2 glycoprotein I by cleaving domain V into a nicked form. Blood. 1998;91:4173–4179. [PubMed] [Google Scholar]
- 12.Horbach DA, van Oort E, Lisman T, Meijers JC, Derksen RH, de Groot PG. Beta2-glycoprotein I is proteolytically cleaved in vivo upon activation of fibrinolysis. Thromb Haemost. 1999;81:97–95. [PubMed] [Google Scholar]
- 13.Briassouli P, Komissarova EV, Clancy RM, Buyon JP. Role of the Urokinase Plasminogen Activator Receptor in Mediating Impaired Efferocytosis of Anti-SSA/Ro-Bound Apoptotic Cardiomyocytes. Circ Res. 2010;107:374–387. doi: 10.1161/CIRCRESAHA.109.213629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buyon JP, Hiebert R, Copel J, Craft J, Friedman D, Katholi M, Lee LA, Provost TT, Reichlin M, Rider L, Rupel A, Saleeb S, Weston WL, Skovron ML. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–1666. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 15.Friedman DM, Kim MY, Copel JA, Davis C, Phoon CK, Glickstein JS, Buyon JP PRIDE Investigators. Utility of cardiac monitoring in fetuses at risk for congenital heart block: the PR Interval and Dexamethasone Evaluation (PRIDE) prospective study. Circulation. 2008;117:485–493. doi: 10.1161/CIRCULATIONAHA.107.707661. [DOI] [PubMed] [Google Scholar]
- 16.Miranda-Carus ME, Boutjdir M, Tseng C-E, DiDonato F, Chan EK, Buyon JP. Induction of antibodies reactive with SSA/Ro-SSB/La and development of congenital heart block in a murine model. J Immunol. 1998;161:5886–5892. [PubMed] [Google Scholar]
- 17.Reed JH, Neufing PJ, Jackson MW, Clancy RM, Buyon JP, Gordon TP. Different temporal expression of immunodominant Ro60/60 kDa-SSA and La/SSB apotopes. Clin Exp Immunol. 2007;148:153–160. doi: 10.1111/j.1365-2249.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng YH, Hanly JG, Reddel SW, Kouts S, Guerin J, Koike T, Ichikawa K, Sturgess A, Krilis SA. Detection of 'antiphospholipid' antibodies: a single chromogenic assay of thrombin generation sensitively detects lupus anticoagulants, anticardiolipin antibodies, plus antibodies binding beta2-glycoprotein I and prothrombin. Clin Exp Immunol. 2001;124:502–508. doi: 10.1046/j.1365-2249.2001.01555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerin J, Sheng YH, Reddel SW, Iverson GM, Chapman MG, Krilis SA. Heparin inhibits the binding of beta2-glycoprotein I to phospholipids and promotes the plasmin-mediated inactivation of this blood protein. J Biol Chem. 2002;277:2644–2649. doi: 10.1074/jbc.M110176200. [DOI] [PubMed] [Google Scholar]
- 20.McNally T, Mackie IJ, Isenberg DA, Machin SJ. Immunoelectrophoresis and ELISA techniques for assay of plasma beta 2 glycoprotein-1 and the influence of plasma lipids. Thromb Res. 1993;72:275–286. doi: 10.1016/0049-3848(93)90136-c. [DOI] [PubMed] [Google Scholar]
- 21.Gordon TP, Kinoshita G, Cavill D, Keech CL, Farris AD, Kaufman K, McCluskey J, Purcell AW. Restricted specificity of intermolecular spreading to endogenous La (SS-B) and 60 kDa Ro (SS-A) in experimental autoimmunity. Scand J Immunol. 2002;56:168–173. doi: 10.1046/j.1365-3083.2002.01115.x. [DOI] [PubMed] [Google Scholar]
- 22.Reed JH, Dudek NL, Osborne SE, Kaufman KM, Jackson MW, Purcell AW, Gordon TP. Reactivity with dichotomous determinants of Ro60 stratifies autoantibody responses in lupus and primary Sjogren's syndrome. Arthritis Rheum. 2010;62:1448–1456. doi: 10.1002/art.27370. [DOI] [PubMed] [Google Scholar]
- 23.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417:182–187. doi: 10.1038/417182a. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y, Singh S, Georgescu MM, Birqe RB. A role for Mer tyrosine kinase in alphavbeta5 integrin-mediated phagocytosis of apoptotic cells. J Cell Sci. 2005;118:539–553. doi: 10.1242/jcs.01632. [DOI] [PubMed] [Google Scholar]
- 26.Lin F, Murphy R, White B, Kelly J, Feighery C, Doyle R, Pittock S, Moroney J, Smith O, Livingstone W, Keenan C, Jackson J. Circulating levels of beta2-glycoprotein I in thrombotic disorders and in inflammation. Lupus. 2006;15:87–93. doi: 10.1191/0961203306lu2270oa. [DOI] [PubMed] [Google Scholar]
- 27.Reed JH, Jackson MW, Gordon TP. A B cell apotope of Ro60 in systemic lupus erythematosus. Arthritis Rheum. 2008;58:1125–1129. doi: 10.1002/art.23377. [DOI] [PubMed] [Google Scholar]
- 28.Tran HB, Macardle PJ, Hiscock J, Cavill D, Bradley J, Buyon JP, Gordon TP. Anti-La/SSB Antibodies transported across the placenta bind apoptotic cells in fetal organs targeted in neonatal lupus. Arthritis Rheum. 2002;46:1572–1579. doi: 10.1002/art.10316. [DOI] [PubMed] [Google Scholar]
- 29.Neufing PJ, Clancy RM, Jackson MW, Tran HB, Buyon JP, Gordon TP. Exposure and binding of selected immunodominant La/SSB epitopes on human apoptotic cells. Arthritis Rheum. 2005;52:3934–3942. doi: 10.1002/art.21486. [DOI] [PubMed] [Google Scholar]
- 30.Sodin-Semrl S, Rozman B. Beta2-glycoprotein I and its clinical significance: from gene sequence to protein levels. Autoimmun Rev. 2007;6:547–552. doi: 10.1016/j.autrev.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Mehdi H, Manzi S, Desai P, Chen Q, Nestlerode C, Bontempo F, Strom SC, Zarnegar R, Kamboh MI. A functional polymorphism at the transcriptional initiation site in beta2-glycoprotein I (apolipoprotein H) associated with reduced gene expression and lower plasma levels of beta2-glycoprotein I. Eur J Biochem. 2003;270:230–238. doi: 10.1046/j.1432-1033.2003.03379.x. [DOI] [PubMed] [Google Scholar]
- 32.Briasouli P, Komissarova EV, Alvarez D, Buyon JP, Clancy RM. Role of the urokinase plasminogen receptor in mediating impaired apoptotic cardiomyocyte clearance by anti-SSA/Ro-SSB/La antibodies in the pathogenesis of congenital heart block. Arthritis Rheum. 2009;60:S1962. [Google Scholar]
- 33.Gordon P, Khamashta MA, Rosenthal E, Simpson JM, Sharland G, Brucato A, Franceschini F, De Bosschere K, Meheus L, Meroni PL, Hughes GR, Buyon J. Anti-52 kDa Ro, Anti-60 kDa Ro, and Anti-La Antibody Profiles in Neonatal Lupus. J Rheum. 2004;31:2480–2487. [PubMed] [Google Scholar]