Abstract
Vitamin D is an important regulator of immune function. T cells express the vitamin D receptor (VDR) and have been shown to be direct and indirect vitamin D targets. Why should T cells be responsive to vitamin D? The data suggest that expression of the VDR is required for the development of two cells types, NKT cells and CD8αα T cells, that inhibit autoimmunity. In addition, effector T cell cytokine production is regulated by vitamin D. I propose that NKT and CD8αα T cells express the VDR as part of the selection process to protect against the generation of autoimmunity, particularly in the gut.
Keywords: Vitamin D receptor, T cells, Agonist selection, autoimmunity
Before 1980 no one imagined that vitamin D had a role as a regulator of immune function. The description of vitamin D receptors (VDR) in peripheral blood monoculear cells led to some early interest in the role of vitamin D as an immune system regulator.1,2 The first experiments added active vitamin D (1,25(OH)2D3) to peripheral blood mononuclear cells and showed the T cell proliferation, IL-2, and IFN-γ production were inhibited in vitro. 3–5 All cells of the immune system that have been tested express the VDR and activation of T cells, macrophage and other cells induces additional expression of the VDR.6 It was recognized as early as 1984 that the thymus expressed large amounts of the VDR and calf thymus was used as a source of the VDR for competitive binding assays for 1,25(OH)2D3.7 The role of vitamin D in thymocytes was however not investigated until more recently.
Vitamin D status and immune mediated diseases
The incidence of immune mediated diseases like multiple sclerosis (MS) and inflammatory bowel disease (IBD) have increased in developed countries over the last 50 years. To explain the increased incidence of immune mediated diseases as well as the geographical restriction of these diseases to the developed world the hygiene hypothesis has been put forward. The hygiene hypothesis states that reduced exposure to microbial components results in immune-dysregulation and T cell responses that drive immune mediated disease.8 There is evidence that support the hygiene hypothesis including studies that show that infections with several worms ameliorates autoimmunity in both mice and humans.9 However, it would be naïve to think that the environmental effect on autoimmunity could be explained completely by the hygiene hypothesis.
Vitamin D has also been linked to the development of autoimmunity.10 In particular, MS patients in the highest quintile for vitamin D intakes were shown to have a 40% reduction in the risk of developing MS.11–13 Autoimmunity occurs in areas that experience fluctuations in vitamin D status due to seasonal changes in sunlight exposure and when it’s been measured circulating levels of vitamin D are lower in patients with IBD and MS than in healthy controls.12–15 We propose that in addition to the hygiene hypothesis vitamin D status is an environmental factor important in the development of autoimmunity.
Decreased outdoor activity and increased pollution and diets that lack adequate vitamin D have combined to create large fluctuations in vitamin D status in developed countries and especially in populations that experience winter. Specker et. al have shown that in the first year of life children undergo significant alterations in the level of circulating vitamin D.16 The vitamin D hypothesis proposes that vitamin D regulates the development and function of the immune system and that changes in vitamin D status especially prenatal as well as childhood alterations affect the development of the resultant immune response and the development of autoimmunity.
The effects of 1,25(OH)2D3 on immunity and Th cell responses
1,25(OH)2D3 has been shown by several different investigators in several different laboratories to suppress autoimmunity driven by Th1 and Th17 cells. The evidence suggests that 1,25(OH)2D3 functions to directly and indirectly regulate Th1/Th17 and Treg function. 1,25(OH)2D3 has been shown to reduce the production and/or expression of the Th1 associated cytokines IL2, TNF-α, and IFNγ in T cells 17–20. These cytokines are characteristic of Th1 cell responses, and associated with the progression of several autoimmune diseases. More recently Th17 associated cytokines have also been shown to be inhibited by 1,25(OH)2D3.20 The effects of 1,25(OH)2D3 on Th17 cells have been shown both in vitro and in vivo in experimental uveitus.20 The production of the Th2 associated cytokine IL4 has been shown to be upregulated by 1,25(OH)2D3 treatment in vivo 21. 1,25(OH)2D3 addition to purified CD4 + T cells inhibited Th1 cell development and cytokine production and resulted in Th2 cell expansion and increased IL4 production 22. The induction of IL-4 secreting cells would be an indirect method of inhibiting Th1 cells and 1,25(OH)2D3 treatment is less effective at treating experimental autoimmune encephalomyelitis (EAE) in IL-4 KO mice.23 1,25(OH)2D3 inhibits experimental autoimmunity in part by directly inhibiting Th1 and Th17 function. Because CD4+ T cells control experimental autoimmunity, the data suggest that vitamin D regulates the differentiation and activity of CD4+ T cells both directly and indirectly to suppress autoimmune disease pathology.24
In addition to Th1 and Th2 cells CD4+ T cells can also develop into regulatory/suppressive T cells.25 The regulatory CD4+ T cells main function appears to be the maintenance of self-tolerance. Expression of the regulatory T cell receptor CD25 heterodimeric partner (IL-2 receptor β) was increased by 1,25(OH)2D3 treatment of CD4+ T cells.19 Inhibition of IBD symptoms by 1,25(OH)2D3 was ineffective in the IL-2 KO mouse and IL-2 KO mice do not make CD4+ CD25+ regulatory T cells.26,27 Furthermore; in vivo, 1,25(OH)2D3 treatment of experimental autoimmune diabetes induced a population of CD4+ CD25+ regulatory T cells that correlated with the protection of the mice from diabetes.28 Barrat et al.29 have shown that a combination of 1,25(OH)2D3 and dexamethasone induced IL-10-producing regulatory T cells in human and mouse CD4+ T cells. In addition, the regulatory T cell associated cytokine TGF-β1 was increased by 1,25(OH)2D3 treatment.21 Some of the identified targets of 1,25(OH)2D3 in CD4+ T cells have been shown to include genes which indicate an increase in either the number or function of the regulatory T cell compartment. At this point the effects of 1,25(OH)2D3 on T cell function have been well established. What is less clear is what the physiological role of VDR expression is in T cells.
The VDR and T cells
One approach to determine what the physiological role of the VDR is in T cells is to study the VDR KO mouse. The VDR is a nuclear receptor that in the presence of 1,25(OH)2D3 binds to vitamin D response elements and regulates transcription of targeted genes. Mice lacking the vitamin D receptor (VDR KO) showed impaired bone formation,30 but revealed no gross abnormalities in the numbers and types of cells present.31 The numbers and percentages of CD4, CD8, CD4/CD8 double positive (DP) or CD4/CD8 double negative (DN) T cells are normal in the VDR KO thymus. There is no effect of VDR deficiency on the number of naïve, or memory CD4+ cells including T regs. FoxP3+ T cell numbers were similar from WT and VDR KO mice.32 Functionally, the VDRKO T regs were able to suppress proliferation in vitro and IBD in vivo as well as their WT counterparts.32 When analyzed ex vivo Th cells from VDR KO mice responded more readily in a mixed lymphocyte reaction and produced slightly more IFN-γ.33 The relatively modest changes in CD4+ T cell responses failed to explain the increased autoimmunity (especially IBD) that develop in VDR KO mice using several different models.33–35
The expression of the VDR is required for NKT cell development and function.36 VDR KO mice have invariant (i)NKT cells that are blocked at a less mature state and lack the full effector functions of WT iNKT cells.36 The VDR is required for normal expression of CD1d in the thymus, and the DP VDR KO thymocytes are poor selectors of iNKT cells in part because of decreased expression of CD1d.36 The remaining iNKT cells from VDR KO mice are intrinsically defective and lack T-bet expression.36 The data show that development of iNKT cells requires the expression of the VDR both intrinsically and extrinsically.
The VDR also controls mucosal T cell responses in the gut. VDR KO mice contain similar numbers of intraepithelial lymphocytes (IEL) and the IEL have normal number of conventional CD4+, CD8+ and FoxP3+ T cells.32 The VDR KO gut has normal numbers of TCRγδ CD8αα but is missing half of all the TCRαβ CD8αα expressing cells.32 In particular CD4/CD8αα TCRαβ are missing in the VDR KO gut.32 VDR expression is required for these gut specific T cells to develop and reach the gastrointestinal tract. The CD8αα T cells that are left in the gut fail to produce IL-10 to WT levels.32 The development of TCRαβ CD8αα T cells requires the VDR. Expression of the VDR is critical for two different populations of T cells; iNKT cells and TCRαβ CD8αα T cells.
Agonist selected iNKT cells and CD8αα T cells
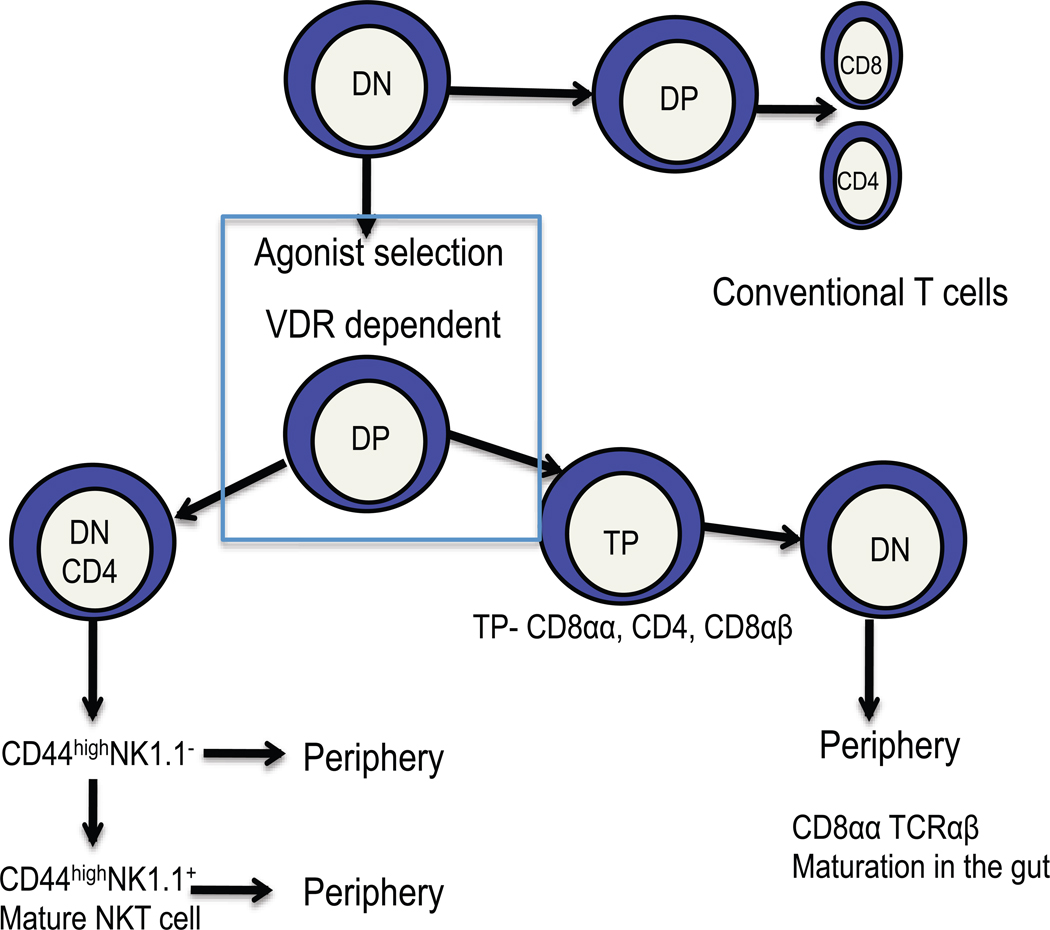
iNKT cells and TCRαβ CD8αα have several similarities to one another.37–39 An understanding of the similarities and differences between these two vitamin D targets should give insight into what the role of the VDR is in these cells. iNKT cells and TCRαβ CD8αα T cells require the thymus for development, are autoreactive and undergo agonist selection in the thymus (Fig.1).37–39 Both cell types develop in the absence of classical MHC class I or class II molecules but require the expression of β2m.37–39 For iNKT cells the non-classical MHC molecule required for selection is known to be CD1d but for the CD8αα T cells it has yet to be identified. Both NKT and CD8αα T cells derive from DP thymocytes that undergo agonist signaling and selection for autoreactivity during development (Fig. 1). The iNKT cell precursors go on to downregulate CD8, and in some cases both CD8 and CD4, undergo rapid expansion, followed by upregulation of CD44 and eventually NK1.1 (Fig. 1).37 Fully mature NK1.1 expressing and less mature NK1.1- iNKT cells can leave the thymus and make their way into the periphery (Fig.1).37 The fate of the CD8αα precursor is a bit different. The DP thymocytes upregulate CD8αα and become triple positive (TP) then downregulating CD4, CD8 and CD8αα to become double negative (DN, Fig. 1).39 These immature DN TCRαβ precursors then migrate to the gut and develop into mature CD8αα TCRαβ cells in the gut.39 The NKT cells and CD8αα T cells have a memory phenotype in the periphery and both cell types rapidly produce cytokines upon stimulation.37,38 There are several common features of NKT cell and CD8αα T cell development. VDR KO mice have selective defects in both NKT cells and CD8αα T cells. Conventional T cells develop normally in VDR KO mice and their function is only moderately affected by deletion of the VDR. It therefore seems likely that the VDR is critical for parts of the developmental pathway that are shared between NKT cells and CD8αα T cells (Fig. 1). The possibilities include a requirement for the VDR in agonist selection in the thymus or a common precursor in the thymus. Following rearrangement of the TCR and agonist selection the common precursor would then commit to either the TP or DN/CD4+ NKT cells (Fig. 1). There have been other agonist selected T cells identified in the thymus and they include the FoxP3+ CD4+ T cells and Th17 cells.40,41 Th17 and FoxP3+ T cells require class II MHC for development so the mechanisms for agonist selection between the NKT/CD8αα T cells are likely different.40,41 It has already been shown that at least for the FoxP3+ T cells, expression of the VDR is not required in the developmental pathway.32 Future work will be required to determine if there is a common NKT/CD8αα T cell precursor that requires expression of the VDR and/or a requirement for the VDR in agonist selection by non-classical MHC type I molecules in the thymus.
Figure 1.
The role of vitamin D and the VDR in development of iNKT cells and TCRαβ CD8αα T cells. VDR KO mice have normal numbers of conventional CD4 and CD8 positive T cells. Conversely, VDR KO mice develop fewer less mature iNKT cells and TCRαβ CD8αα T cells. iNKT cells and TCRαβ CD8αα T cells diverge from conventional T cells at the DP stage when they undergo agonist selection under the influence of non-classical MHC class I molecules. Following selection the iNKT cells and TCRαβ CD8αα T cells diverge. It is likely that there is a common and vitamin D regulated step that occurs at this point in the development of these cell types.
Predictions as to the role of the VDR as an immune system regulator
What are the implications of a requirement for the VDR and vitamin D in the development of NKT/CD8αα T cells? Diseases or protective immune responses that rely on these cell types will be most affected. NKT cells have been shown to be protective in animal models of experimental autoimmunity and low numbers of NKT cells are associated with increased susceptibility to autoimmunity.42,43 Vitamin D has been shown to regulate all Th1/Th17 mediated autoimmune diseases that have been studied.10 Vitamin D or VDR deficiency results in more severe type-1 diabetes, EAE, and IBD.10 The role for CD8αα T cells has been shown to be mainly in the gut.44 CD8αα T cells are important in the gut to dampen the immune response to the large number of gut antigens present there. VDR KO mice develop a fulminating form of IBD. The severe effects of vitamin D and/or VDR deficiency on autoimmunity of the gut support the gut as a tissue that is extremely sensitive to changes in vitamin D. Perhaps the dual role of both NKT cells and CD8αα T cells as providing protection against inflammation in the gut explains the increased IBD susceptibility of the VDR KO mice.
Conclusions
I propose that NKT and CD8αα T cells require vitamin D and expression of the VDR at a common point in their developmental pathway. Development of conventional T cells and other agonist selected T cells like the FoxP3+ T cells does not require vitamin D or the VDR. However, the function of many different types of T cells are regulated by vitamin D. Expression of the VDR is needed to induce development of NKT cells and CD8αα T cells and to regulate T cell effector response. Vitamin D is an inhibitor of Th1 and Th17 cytokine production and induces production of IL-10 and T reg cells. The consequence of low vitamin D status is then a higher propensity for autoimmunity and in particular for autoimmunity in the gut.
Acknowledgements
I thank Dr. Sanhong Yu and the members of the Center for Immunology and Infectious Diseases for lively discussion. This work was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases DK070781 and National Center for Complementary and Alternative Medicine and the Office of Dietary Supplements AT005378.
References
- 1.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 2.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 3.Muller K, Odum N, Bendtzen K. 1,25-dihydroxyvitamin D3 selectively reduces interleukin-2 levels and proliferation of human T cell lines in vitro. Immunol Lett. 1993;35:177–182. doi: 10.1016/0165-2478(93)90088-j. [DOI] [PubMed] [Google Scholar]
- 4.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tsoukas CD, et al. Inhibition of interleukin-1 production by 1,25-dihydroxyvitamin D3. J Clin Endocrinol Metab. 1989;69:127–133. doi: 10.1210/jcem-69-1-127. [DOI] [PubMed] [Google Scholar]
- 6.Veldman CM, Cantorna MT, DeLuca HF. Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys. 2000;374:334–338. doi: 10.1006/abbi.1999.1605. [DOI] [PubMed] [Google Scholar]
- 7.Reinhardt TA, Horst RL, Orf JW, Hollis BW. A microassay for 1,25-dihydroxyvitamin D not requiring high performance liquid chromatography: application to clinical studies. J Clin Endocrinol Metab. 1984;58:91–98. doi: 10.1210/jcem-58-1-91. [DOI] [PubMed] [Google Scholar]
- 8.Cooke A. Infection and autoimmunity. Blood Cells Mol Dis. 2009;42:105–107. doi: 10.1016/j.bcmd.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Weinstock JV, Elliott DE. Helminths and the IBD hygiene hypothesis. Inflamm Bowel Dis. 2009;15:128–133. doi: 10.1002/ibd.20633. [DOI] [PubMed] [Google Scholar]
- 10.Cantorna MT. Vitamin D and its role in immunology: multiple sclerosis, and inflammatory bowel disease. Prog Biophys Mol Biol. 2006;92:60–64. doi: 10.1016/j.pbiomolbio.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 11.Cantorna MT. Vitamin D and multiple sclerosis: an update. Nutr Rev. 2008;66:S135–S138. doi: 10.1111/j.1753-4887.2008.00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. Jama. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 13.Munger KL, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 14.Cantorna MT, Yu S, Bruce D. The paradoxical effects of vitamin D on type 1 mediated immunity. Mol Aspects Med. 2008;29:369–375. doi: 10.1016/j.mam.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappa HM, Grand RJ, Gordon CM. Report on the vitamin D status of adult and pediatric patients with inflammatory bowel disease and its significance for bone health and disease. Inflamm Bowel Dis. 2006;12:1162–1174. doi: 10.1097/01.mib.0000236929.74040.b0. [DOI] [PubMed] [Google Scholar]
- 16.Specker BL, Tsang RC. Vitamin D in infancy and childhood: factors determining vitamin D status. Adv Pediatr. 1986;33:1–22. [PubMed] [Google Scholar]
- 17.Rigby WF, Denome S, Fanger MW. Regulation of lymphokine production and human T lymphocyte activation by 1,25-dihydroxyvitamin D3. Specific inhibition at the level of messenger RNA. J Clin Invest. 1987;79:1659–1664. doi: 10.1172/JCI113004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemire JM. Immunomodulatory role of 1,25-dihydroxyvitamin D3. J Cell Biochem. 1992;49:26–31. doi: 10.1002/jcb.240490106. [DOI] [PubMed] [Google Scholar]
- 19.Mahon BD, Wittke A, Weaver V, Cantorna MT. The targets of 14 vitamin D depend on the differentiation and activation status of CD4 positive T cells. J Cell Biochem. 2003;89:922–932. doi: 10.1002/jcb.10580. [DOI] [PubMed] [Google Scholar]
- 20.Tang J, et al. Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response. J Immunol. 2009;182:4624–4632. doi: 10.4049/jimmunol.0801543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cantorna MT, Woodward WD, Hayes CE, DeLuca HF. 1,25-dihydroxyvitamin D3 is a positive regulator for the two anti-encephalitogenic cytokines TGF-beta 1 and IL-4. J Immunol. 1998;160:5314–5319. [PubMed] [Google Scholar]
- 22.Boonstra A, et al. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 23.Cantorna MT, Humpal-Winter J, DeLuca HF. In vivo upregulation of interleukin-4 is one mechanism underlying the immunoregulatory effects of 1,25-dihydroxyvitamin D(3) Arch Biochem Biophys. 2000;377:135–138. doi: 10.1006/abbi.2000.1765. [DOI] [PubMed] [Google Scholar]
- 24.Cantorna MT. Vitamin D and autoimmunity: is vitamin D status an environmental factor affecting autoimmune disease prevalence? Proc Soc Exp Biol Med. 2000;223:230–233. doi: 10.1046/j.1525-1373.2000.22333.x. [DOI] [PubMed] [Google Scholar]
- 25.Jonuleit H, Schmitt E. The regulatory T cell family: distinct subsets and their interrelations. J Immunol. 2003;171:6323–6327. doi: 10.4049/jimmunol.171.12.6323. [DOI] [PubMed] [Google Scholar]
- 26.Schimpl A, et al. IL-2 and autoimmune disease. Cytokine Growth Factor Rev. 2002;13:369. doi: 10.1016/s1359-6101(02)00022-9. [DOI] [PubMed] [Google Scholar]
- 27.Bemiss CJ, Mahon BD, Henry A, Weaver V, Cantorna MT. Interleukin-2 is one of the targets of 1,25-dihydroxyvitamin D3 in the immune system. Arch Biochem Biophys. 2002;402:249–254. doi: 10.1016/S0003-9861(02)00082-6. [DOI] [PubMed] [Google Scholar]
- 28.Gregori S, Giarratana N, Smiroldo S, Uskokovic M, Adorini L. A 1alpha,25-dihydroxyvitamin D(3) analog enhances regulatory T-cells and arrests autoimmune diabetes in NOD mice. Diabetes. 2002;51:1367–1374. doi: 10.2337/diabetes.51.5.1367. [DOI] [PubMed] [Google Scholar]
- 29.Barrat FJ, et al. In vitro generation of interleukin 10-producing regulatory CD4(+) T cells is induced by immunosuppressive drugs and inhibited by T helper type 1 (Th1)- and Th2-inducing cytokines. J Exp Med. 2002;195:603–616. doi: 10.1084/jem.20011629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kato S, et al. In vivo function of VDR in gene expression-VDR knock-out mice. J Steroid Biochem Mol Biol. 1999;69:247–251. doi: 10.1016/s0960-0760(99)00042-4. [DOI] [PubMed] [Google Scholar]
- 31.Mathieu C, et al. In vitro and in vivo analysis of the immune system of vitamin D receptor knockout mice. J Bone Miner Res. 2001;16:2057–2065. doi: 10.1359/jbmr.2001.16.11.2057. [DOI] [PubMed] [Google Scholar]
- 32.Yu S, Bruce D, Froicu M, Weaver V, Cantorna MT. Failure of T cell homing, reduced CD4/CD8alphaalpha intraepithelial lymphocytes, and inflammation in the gut of vitamin D receptor KO mice. Proc Natl Acad Sci U S A. 2008;105:20834–20839. doi: 10.1073/pnas.0808700106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Froicu M, et al. A crucial role for the vitamin D receptor in experimental inflammatory bowel diseases. Mol Endocrinol. 2003;17:2386–2392. doi: 10.1210/me.2003-0281. [DOI] [PubMed] [Google Scholar]
- 34.Cantorna MT, Munsick C, Bemiss C, Mahon BD. 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr. 2000;130:2648–2652. doi: 10.1093/jn/130.11.2648. [DOI] [PubMed] [Google Scholar]
- 35.Froicu M, Cantorna MT. Vitamin D and the vitamin D receptor are critical for control of the innate immune response to colonic injury. BMC Immunol. 2007;8:5. doi: 10.1186/1471-2172-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu S, Cantorna MT. The vitamin D receptor is required for iNKT cell development. Proc Natl Acad Sci U S A. 2008;105:5207–5212. doi: 10.1073/pnas.0711558105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 38.Cheroutre H. Starting at the beginning: new perspectives on the biology of mucosal T cells. Annu Rev Immunol. 2004;22:217–246. doi: 10.1146/annurev.immunol.22.012703.104522. [DOI] [PubMed] [Google Scholar]
- 39.Cheroutre H, Lambolez F. The thymus chapter in the life of gut-specific intra epithelial lymphocytes. Curr Opin Immunol. 2008 doi: 10.1016/j.coi.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bendelac A. Nondeletional pathways for the development of autoreactive thymocytes. Nat Immunol. 2004;5:557–558. doi: 10.1038/ni0604-557. [DOI] [PubMed] [Google Scholar]
- 41.Marks BR, et al. Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat Immunol. 2009;10:1125–1132. doi: 10.1038/ni.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsuda JL, Mallevaey T, Scott-Browne J, Gapin L. CD1d-restricted iNKT cells, the 'Swiss-Army knife' of the immune system. Curr Opin Immunol. 2008;20:358–368. doi: 10.1016/j.coi.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Singh AK, et al. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1801–1811. doi: 10.1084/jem.194.12.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Das G, et al. An important regulatory role for CD4+CD8 alpha alpha T cells in the intestinal epithelial layer in the prevention of inflammatory bowel disease. Proc Natl Acad Sci U S A. 2003;100:5324–5329. doi: 10.1073/pnas.0831037100. [DOI] [PMC free article] [PubMed] [Google Scholar]