Abstract
Genomic perturbations that challenge normal signaling at the pluripotent stage may trigger unforeseen ontogenic aberrancies. Anticipatory systems biology identification of transcriptome landscapes that underlie latent phenotypes would offer molecular diagnosis before the onset of symptoms. The purpose of this study was to assess the impact of calreticulin-deficient embryonic stem cell transcriptomes on molecular functions and physiological systems. Bioinformatic surveillance of calreticulin-null stem cells, a monogenic insult model, diagnosed a disruption in transcriptome dynamics, which re-prioritized essential cellular functions. Calreticulin-calibrated signaling axes were uncovered, and network-wide cartography of undifferentiated stem cell transcripts suggested cardiac manifestations. Calreticulin-deficient stem cell-derived cardiac cells verified disorganized sarcomerogenesis, mitochondrial paucity, and cytoarchitectural aberrations to validate calreticulin-dependent network forecasts. Furthermore, magnetic resonance imaging and histopathology detected a ventricular septal defect, revealing organogenic manifestation of calreticulin deletion. Thus, bioinformatic deciphering of a primordial calreticulin-deficient transcriptome decoded at the pluripotent stem cell stage a reconfigured multifunctional molecular registry to anticipate predifferentiation susceptibility toward abnormal cardiophenotype.
Keywords: Cardiogenesis, Cardiopoiesis, Network biology, Transcriptome, Pluripotent stem cells, Predictive medicine
Introduction
Embryonic gene expression profiles comprise pluripotent, primordial stem cell transcriptomes that provide the foundation for differentiation into diverse cell types essential for proper organogenesis [1–3]. Critical to fate commitment, regulated genomic access and precise transcriptomic control ensure lineage-specific progression and integration of multiple incipient cell phenotypes within an organism, with significant congenital consequences upon programmatic deviation [4, 5]. Genome-wide profiling has been used to categorize cellular development and specification, providing initial insights into transcriptional patterns evoked during normal differentiation [6–8]. As a method of dissecting developmental cues, such systems biology profiling can track and distinguish molecular programs recruited for specified functions, such as stem cell-derived cardiopoiesis [9, 10]. Indeed, charted transcriptome relationships permit surveillance of comprehensive signaling that underlies developmental metamorphosis to yield a molecular map for phenotype prediction in cases of genetic perturbation [11–13]. Yet, the value of system-based dissection of the pluripotent state to anticipate phenotype abnormality upon differentiation has not been assessed.
Monogenic insults result in dysregulated transcriptional control that trigger a spectrum of phenotypic changes, impacting embryonic viability. A case in point is calreticulin, a 46 kDa chaperone protein that facilitates N-glycosylated protein folding and participates in eukaryotic calcium maintenance [14, 15]. Targeted calreticulin gene downregulation results in accumulation of misfolded proteins [16], decreased calcium-dependent cell adhesion [17] and dysfunction of calcium homeostasis [18]. In fact, mice that lack calreticulin die in utero [19], and while lethal outcome has been related to abnormal calcium handling [20], the effects of calreticulin deficiency on global gene expression dynamics regulating embryogenesis remain untested.
Here, an embryonic stem cell-based model was used for transcriptional analysis to diagnose aberrant predifferentiation cues. Wild-type (WT) and calreticulin null embryonic stem cell (crt−/−) transcriptomes, deconstructed in silico, revealed differential programming that impacted specific molecular functions and physiological systems. Analysis of an extended calreticulin-dependent framework identified subnetwork functions, beyond the known calcium handling and protein chaperoning properties, which implicated regulatory control of cellular metabolism and structure before embryonic differentiation. Accordingly, mitochondrial and cytoarchitectural aberrations were observed in calreticulin-deficient stem cell-derived progeny as predicted by bioinformatic surveillance of embryonic cytotypes. Absence of calreticulin recalibrated the genomic framework to implicate altered developmental progression of cardiac morphogenesis, culminating in a congenital defect as confirmed in the midgestation embryo. Resolution of the primordial calreticulin-deficient transcriptome defined a reconfigured molecular registry to anticipate abnormal cardiac phenotype outcome before differentiation.
Materials and Methods
Stem Cell Culture and Field-Emission Scanning Electron Microscopy
WT (D3) and crt−/− murine embryonic stem cells were cultured in 7.5% fetal bovine serum in Glasgow’s modified Eagle’s medium, then fixed in situ with 1% glutaraldehyde and 4% formaldehyde in 0.1 mM phosphate-buffered saline (PBS) (pH 7.2). For field-emission scanning electron microscopy, specimens were rinsed in 0.1 mM phosphate buffer (pH 7.2) supplemented with 1% osmium, dehydrated with ethanol and dried in a critical point dryer (Ted Pella, Redding, CA, www.tedpella.com). On coating with platinum, samples were examined on a Hitachi S-4700 field emission scanning electron microscope (Hitachi, Japan, http://www.hitachi.com) using an Ion Tech indirect argon ion-beam sputtering system (South Bay Technology, Inc., San Francisco, CA, http://www.southbaytech.com) operating at accelerating voltages of 9.5 kV and 4.2 mA [21].
Microarrays
Total RNA isolation, from WT and calreticulin-deficient embryonic stem cells was performed using the Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA) to investigate transcriptome dynamics in a calreticulin-deficient background before differentiation. Double-stranded complementary cDNA and labeled complementary cRNA were obtained from isolated total RNA, with the latter hybridized against the Mouse 430 2.0 GeneChip (Affymetrix, Santa Clara, CA, http://www.affymetrix.com). Arrays were scanned using an argon-ion laser, and visualized using MAS 5.0 Affymetrix software to assess quality of hybridization. Data were deposited to the Gene Expression Omnibus (GEO) repository under accession number GSE13805.
Expression Analysis and Gene Clustering
Gene expression data from GSE13805 were analyzed using Genespring GX 7.3 (Agilent Technologies, Palo Alto, CA, http://www.agilent.com) [10, 22] and all probesets were quality filtered using the volcano plot function, defining gene cutoffs using 1.5-fold change with p < .05. Based on the established flag values of present (P), marginal (M), or absent (A) assigned to probeset measurements, genes flagged as absent (A) in all samples for WT and calreticulin-deficient conditions were removed. Next, samples were filtered according to background noise levels to remove genes expressing signals below threshold. Expression heatmaps with hierarchical dendrograms were used to establish molecular fingerprints for all samples, and were generated using the Pearson coefficient statistic (r) applied to determine correlation between gene pairs in each condition as follows:
| (1) |
Equation 1 indicates the summation notation for Pearson coefficient used to establish molecular fingerprint of crt−/− stem cells. In Equation 1, (A) and (B) are respective sample means for genes Ai and Bi for sample (i) out of the total number of samples (n), with standard deviation terms for Ai and Bi used as denominator. Condition clustering was performed to determine sample similarity using Euclidean distance as a measure of sample “nearness” [23], and plotted as gene heatmaps. To interrogate embryonic Hdac1−/− and Hnf4a−/− transcriptomes, respective GEO records with accession identifiers GDS2294 and GDS1916, were downloaded from publicly available GEO datasets into Genespring for independent comparative analysis.
Analysis of Functional Categories and Network Mapping
To expand analysis of the calreticulin network, quality-filtered transcripts from a calreticulin-deficient background were cross-referenced with Kyoto Encyclopedia of Genes and Genomes (KEGG) designated lists for calcium handling and chaperone activity to identify genes related to innate molecular properties of calreticulin. Using an established network analysis program, that is, Ingenuity Pathways Analysis (www.ingenuity.com), curated molecular interactions of genes within calcium handling and chaperone activity were generated that incorporated fold change values for both down-and upregulated genes. Furthermore, global functional categories associated with quality-filtered genes were identified in the ingenuity pathways knowledge base and ranked with a right-tailed Fisher’s exact test. Curated gene relationships, characterized by ingenuity pathways analysis, provided a template for predictive network generation using Cytoscape 2.4.1 (www.cytoscape.org) [10].
Taqman Assays
RNA (1 µg) was prepared using a high-capacity cDNA archive kit (Applied Biosystems, Foster City, CA, http://www.appliedbio-systems.com) and assayed using Taqman gene expression kits for Calr, Cib1, Cdh2, St13, and H2ke2 as randomly selected markers of calcium binding and chaperone function. Samples were loaded in duplicate onto an optical 96-well plate for polymerase chain reactions performed using an ABI 7900HT fast real-time system with cycling parameters set for a 15 seconds, 95°C duplex denaturing step followed by primer annealing/extending for 1 minute at 60°C per cycle for 40 cycles. Relative fold change determination performed using the 2−ΔΔC T method [24], with WT embryonic stem cells as baseline, normalized to Gapdh. Taqman IDs for assay kits were as follows: Calr (Mm00482936_m1), Cdh2 (Mm00483213_m1), Cib1 (Mm00501944_m1), H2ke2 (Mm00468798_m1), and St13 (Mm00505171_m1).
Cardiomyocyte Derivation of Embryonic Stem Cells
Differentiation was carried out using the hanging drop method, where ~400 WT or crt−/− stem cells were placed for 2 days on the lid of a nonadherent bacterial dish to allow embryoid body formation [25]. Embryoid bodies were then incubated in suspension for 3 days and plated on gelatin-coated dishes for 7 days. Stem cell-derived cardiomyocytes were obtained after dissociation of embryoid bodies and subsequent centrifugation through a discontinuous two-layer Percoll gradient [26].
Transmission Electron Microscopy and Immunofluorescence
Fixed stem cell-derived cardiomyocytes were processed in phosphate-buffered 1% OsO4, stained en bloc with 2% uranyl acetate, dehydrated in ethanol and propylene oxide, and embedded in low-viscosity epoxy resin for transmitted electron microscopy. Thin (90 nm) sections were cut with an ultramicrotome (Reichert Ultracut E), placed on 200-µm mesh copper grids, and stained with lead citrate. Micrographs were taken on a JEOL 1200 EXII electron microscope (JEOL, Peabody, MA, http://www.jeol.com). Separately, isolated stem cell-derived cardiomyocytes were fixed in 3% paraformaldehyde, permeabilized with 0.5% Triton X-100, and immunostained with ventricular myosin light chain two (MLC2v) antibodies. Images were acquired on a Leica microscope with an objective-mounted piezo-electric controller (Physik Instrumente, Auburn, MA, http://www.physikinstrumente.com) driven by the Metamorph software (Molecular Devices, Sunnyvale, CA, http://www.moleculardevices.com), and recorded with a 1300YHS CCD camera (Micromax).
Calcium Transients and Line Tracing
Stem cell-derived cardiomyocytes were loaded with 5 µ M of the calcium indicator Fluo-3 and transferred to a Zeiss confocal microscope for analysis (Carl Zeiss MicroImaging, Inc., Thornwood, NY, http://www.zeiss.com). Microscopy parameters were set equal for visualization of WT and calreticulin-deficient stem cell-derived cardiomyocytes and fluorescence was recorded at 37°C [25]. Ca2+ transient intensity along a single line within contractile cells was tracked and plotted as a function of time. Contractility in Fluo-3 loaded cardiomyocytes was quantified by edge detection. Analysis of calcium transients, cardiomyocyte contraction, and beat frequency were carried out using MetaMorph software.
Magnetic Resonance Imaging and Pathohistology of Embryos
WT and crt−/− embryos at 14.5 days post-coitus (dpc) were harvested in ice-cold PBS and were fixed in 4% paraformaldehyde at 4°C for ~1 week, and photographed using a digital camera. For magnetic resonance imaging, an embryo was placed in a thin-walled 10-mm outer diameter glass tube and embedded in 1% agarose gel (Cambrex, East Rutherford, NJ, http://www.cambrex.com) containing 2 mM GdTPA (Magnevist) (Bayer Schering Pharma AG, Bergkamen, Germany, http://www.bayer.com). The specimen was scanned in a 300 MHz NMR spectrometer (vertical, 7 T wide bore magnet) equipped with microimaging accessories (Bruker Bio-Spin, Billerica, MA, http://www.bruker-biospin.com) using a 10-mm volume coil. The sample temperature was maintained at 20°C by thermoregulated air flow. Gradient-echo 3D images were acquired with TR 50 milliseconds, TE 10 milliseconds, FA 30°, NA 16, FOV 13×9×9 mm3, and matrix size 512 × 256 × 256 pixels. Data were processed using Paravision v3 (Bruker Bio-Spin) yielding images with axial resolution of 25.4 µm/pixel and transverse resolution of 35 µm/pixel. Total experimental time was 15 hours/image. Paraformaldehyde fixed crt−/− embryos were dehydrated in a series of ethanol washes and placed in 100% isopropyl, followed by a stage of vacuum extraction of residual alcohol before paraffin infiltration in a negative-pressurized closed system. All steps were done using a Milestone RHS-1 microwave histoprocessing system (Milestone, Shelton, CT, http://www.milestonesci.-com). Tissue was embedded in paraffin blocks, cut with rotary microtome into 5-µm thick sections, and stained with hematoxylin and eosin.
Results
Embryonic Stem Cell Transcriptome Remodeled by Calreticulin Deficiency
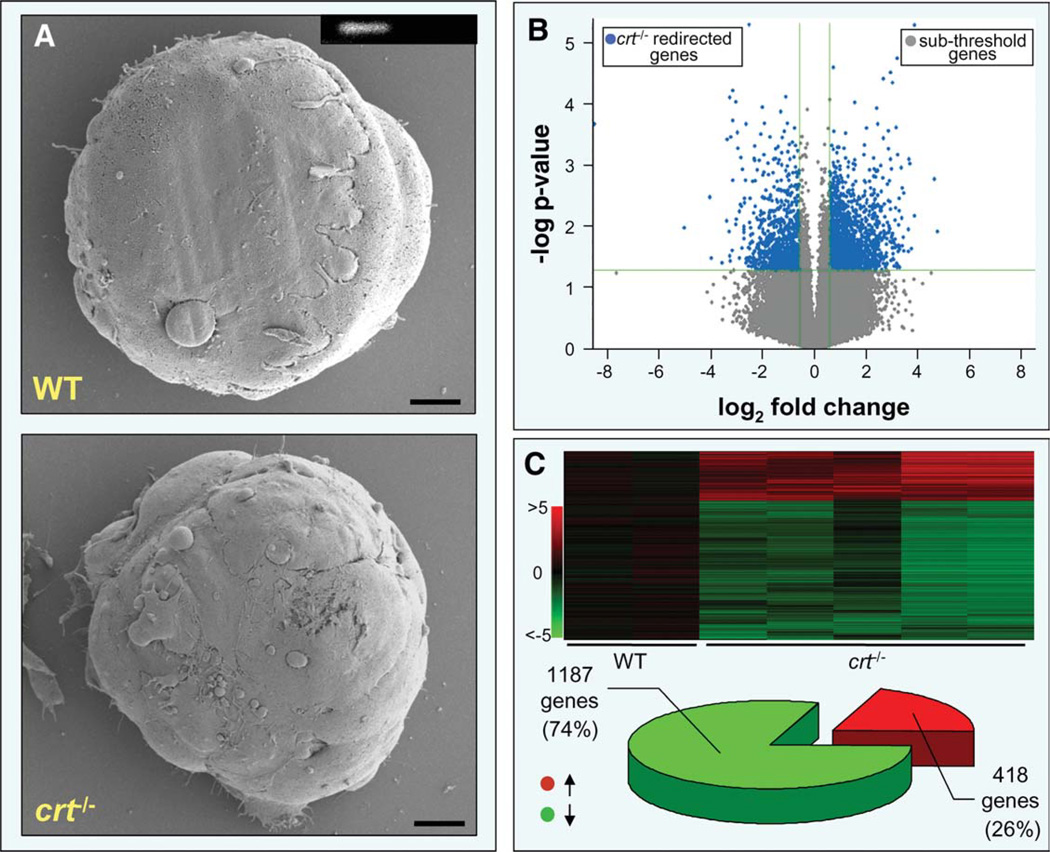
WT and crt−/− embryonic stem cells, visualized by scanning electron microscopy, displayed no apparent differences in morphologies (Fig. 1A). However, interrogation and comparison of expression profiles demonstrated significant transcriptomic disparity in crt−/− embryonic stem cells compared with WT counterparts, with significant gene expression changes ≥1.5-fold observed for 2,904 genes out of a total of 36,000 transcripts (~8%; Fig. 1B). Quality control restriction refined the pool of genes impacted by calreticulin deletion to 1,605 Pearson-correlated transcripts (Fig. 1C, upper). Significantly, 1,187 probes (74%) of the crt−/− transcriptome were downregulated, whereas 418 (26%) exhibited increased expression (Fig. 1C, lower). Thus, calreticulin deletion reshaped gene expression profiles at the embryonic stem cell stage of development.
Figure 1.
Embryonic stem cells lacking calreticulin harbor significant transcriptome shifts. (A): Field-emission scanning electron microscopy, of WT and crt−/− embryonic stem cells, shows no significant morphological differences. Scale bar, 10 µm. Inset: Reverse transcription-polymerase chain reaction confirms absence of crt in knockout stem cells (left, WT; right, crt−/−). (B): Volcano plot analysis of total RNA changes between WT and crt−/− embryonic stem cells revealed 2,904 significant down- and upregulated genes (blue), distinct from genes falling below significance threshold or minimum 1.5-fold change cutoff (gray). (C): Sample reproducibility allowed construction of an expression heatmap of 1,605 quality restricted genes (upper panel). Composition of the shifted transcriptome was 74% downregulated (green) and 26% upregulated (red) (lower panel). Abbreviation: WT, wild type.
Calreticulin Deficiency Reprograms Molecular Ontologies
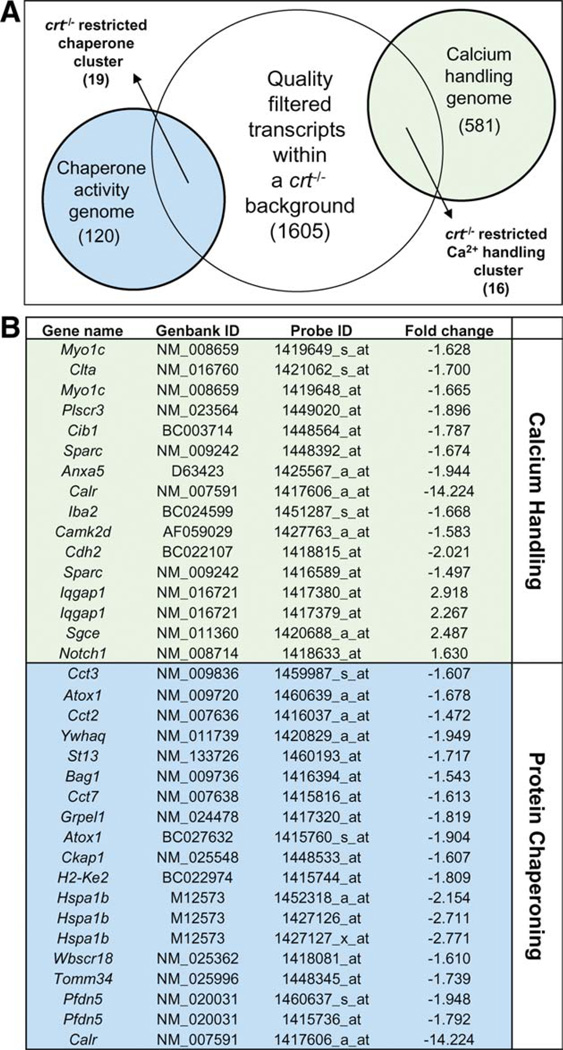
Calreticulin harbors endogenous cation binding properties critical for calcium homeostasis and cisternal calcium regulation, and possesses a separate capacity to guide conformational adoption of glycosylated proteins [15, 27]. To identify ontologies impacted by calreticulin deletion, molecular processes in the crt−/− remodeled transcriptome were cross-referenced with 581 calcium handling and 120 protein chaperoning KEGG designated pathways. A total of 16 calcium handling and 19 protein chaperoning probesets were discovered (Fig. 2A). Changes in 16 of 581 genes associated with calcium handling presented upregulated and downregulated expression profiles, whereas 19 of 120 probesets associated with chaperone activity demonstrated unilateral decreases in expression (Fig. 2B). Such statistical delimitation is a standard strategy used to restrict false-positive results during gene ontology enrichment analyses and provide high-confidence lists [28–30].
Figure 2.
Compromised functions related to innate properties of calreticulin. (A): Cross-reference of quality-filtered genes within the calreticulin knockout background against Kyoto Encyclopedia of Genes and Genomes pathway lists for calcium handling and chaperone activity, identified 16 and 19 transcripts, respectively, significantly affected. (B): Identities of affected transcripts with Affymetrix Probe and Genbank IDs. Associated fold changes are listed on the right.
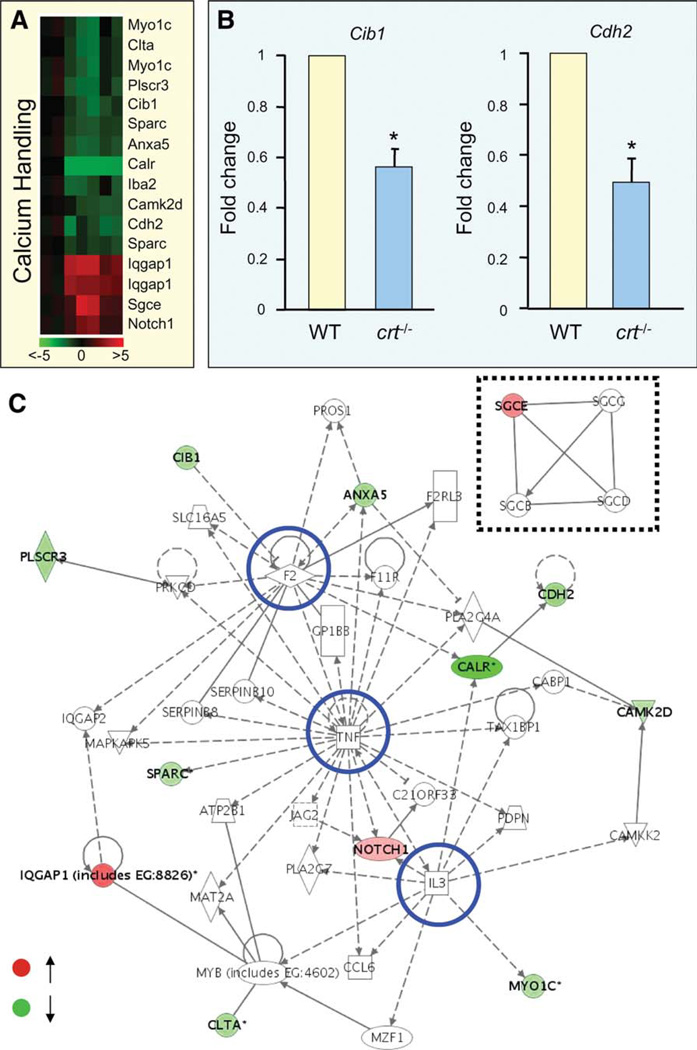
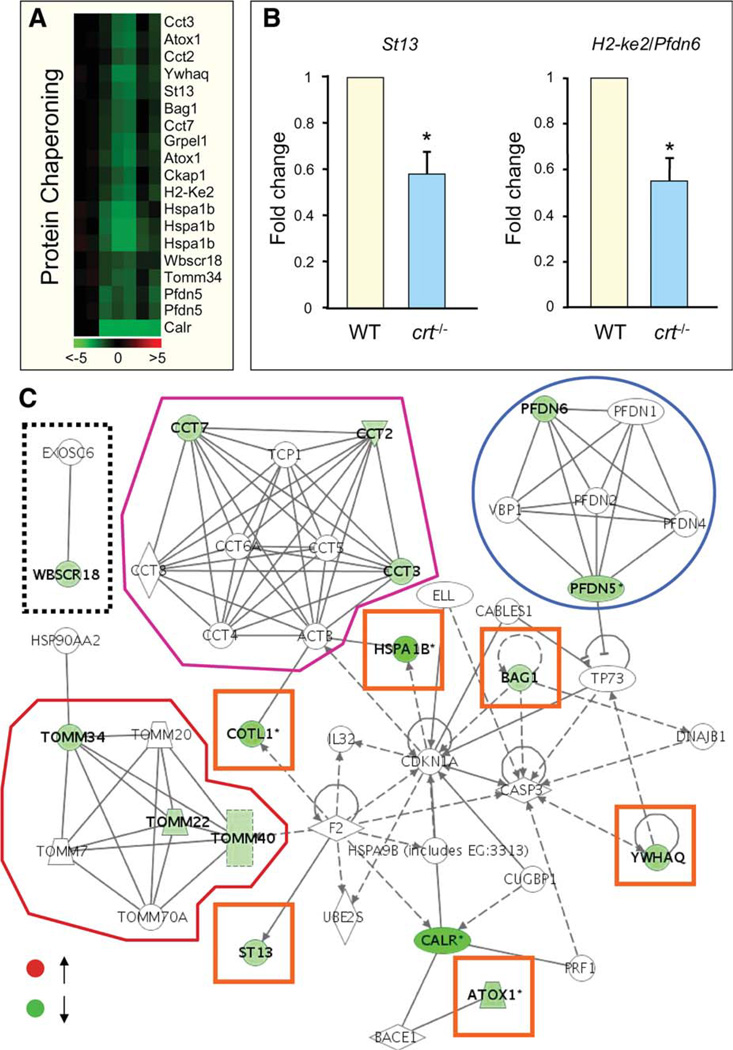
Expression profiles identified by microarray were confirmed through molecular validation for representative markers (Fig. 3A, 3B). Calcium handling related genes in crt−/− embryonic stem cells formed a web of molecular interactions that revealed a coherent framework exposing highly connected genes (Fig. 3C). These hubs include the cardiogenic cytokine, tumor necrosis factor (Tnf) [31], α-thrombin (F2), and interleukin-3 (Il3) (Fig. 3C, blue circles), as well as a discrete module formed by membrane-associated, dystrophin-interacting sarcoglycans [32] (Fig. 3C, dashed box). Genes involved in protein chaperoning were also hierarchically clustered (Fig. 4A), and profiles were confirmed through amplification of selected genes (Fig. 4B). Protein chaperoning genes integrated to form a network that included elements of the stabilizing prefoldin (Pfdn) complex (Fig. 4C, blue circle), implicated in transport of unfolded proteins to cytosolic chaperonin [33, 34]; components of the hetero-oligomeric chaperonin, TRiC/CCT (Fig. 4C, purple outline), that facilitates folding of WD-40 repeat containing proteins [35]; and Tomm genes that code for subunits of an outer membrane complex responsible for localization of mitochondrial preproteins [36, 37] (Fig. 4C, red outline). The Pfdn, TRiC/CCT, and Tomm modules were found interconnected by a fourth module embedded within the overall chaperoning-centered framework that contained genes for the heat shock protein, Hspa1b [38], the actin-binding protein coactosin-like 1, Cotl1 [39], the Hsp70 interacting tumor suppressor, St13 [40], the metallochaperone, Atox1 [41], the phosphorylation regulatory 14-3-3 theta/tau protein, Ywhaq [42], and the antiapoptotic Bcl2-associated athanogene 1, Bag1 [43] (Fig. 4C, orange boxes). Separately, the Wbscr18 node possessed a curated interaction with Exosc6, a component of the RNA degrading exosome complex [44] (Fig. 4C, dashed box). Involvement of calreticulin in global and fundamental intracellular functions against a pluripotent background is thus demonstrated by crt−/−-shifted molecular profiles within expanded networks, uncovering calreticulin-dependent gene ontologies associated with developmental program execution.
Figure 3.
Loss of calreticulin impacts calcium handling processes. (A): Down- and upregulated calcium handling genes within a calreticulin-deficient background integrate into a heatmap and display pairwise correlation of gene expression changes. Color scale indicates range of fold change. (B): Calmyrin (Cib1) and cadherin 2 (Cdh2) as random examples of microarray-identified fold changes confirmed by reverse tran-scription-polymerase chain reaction. (C): Discrete sarcoglycan module (dashed box) and main network with highly connected hubs (blue circles) integrates downregulated (green) and upregulated (red) genes that together visualizes organization of transcripts affected by calreticulin knockout associated with calcium handling. Abbreviations: WT, wild type; TNF, tumor necrosis factor.
Figure 4.
Affected protein chaperoning genes organize into a calreticulin inclusive network. (A): Calreticulin-impacted chaperoning genes were hierarchically clustered according to Pearson correlation. (B): Taqman validation of randomly selected chaperoning molecules, suppressor of tumorigenicity 13 (St13) and prefoldin six (Pfdn6). (C): Genes were downregulated (green) and organized into a network of associated modules. Clockwise from left: Discrete curated interaction of Exosc6 and Wbscr18 (boxed in dashed line); TRiC/ CCT module with Cct2 Cct3, and Cct7 downregulated (outlined in purple); prefoldin subnetwork showing downregulation in Pfdn5 and Pfdn6 (circled in blue); central network that integrated nonmodular genes (orange boxes); and the Tomm complex with affected genes highlighted in green (outlined in red). Abbreviation: WT, wild type.
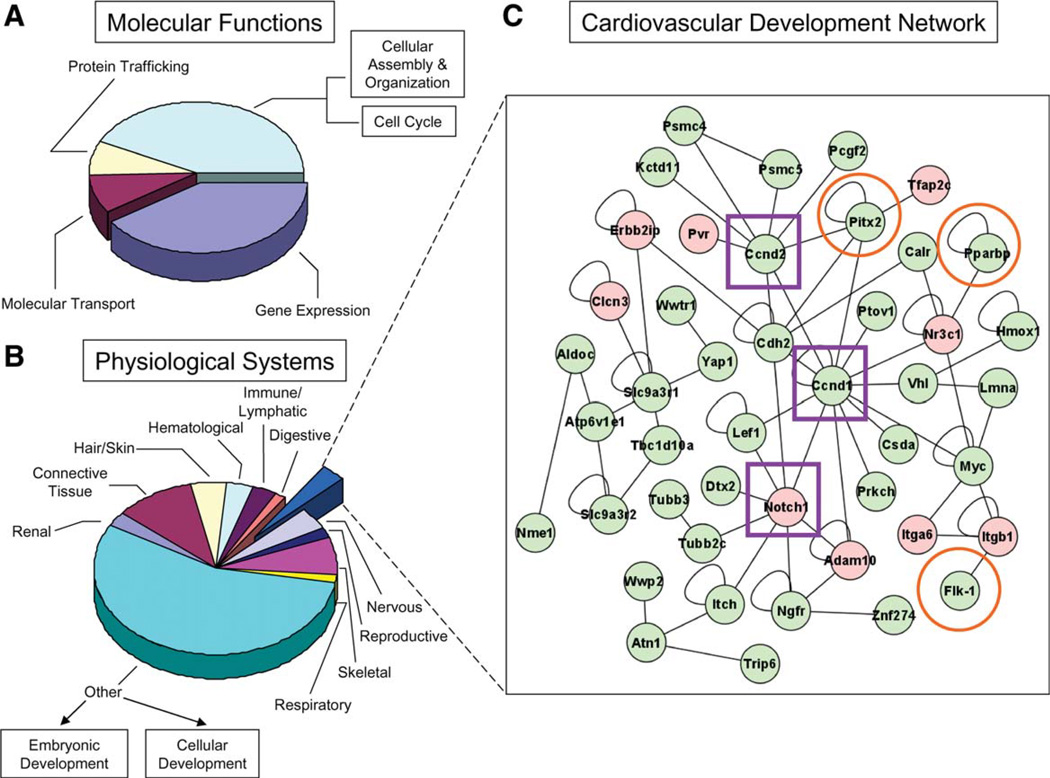
Recalibrated Calreticulin Null Networks Predict Ontological Impact
Ingenuity pathway-based comparison between crt−/− and WT transcriptomes revealed that calreticulin deletion stimulated system-wide ontological reprioritization (Supporting Information Table 1). Of 66 prioritized gene functions, calreticulin deficiency excluded protein folding as a network priority, and demoted ontological imperatives of gene expression, molecular transport, and protein trafficking (Fig. 5A). Parsing of the calreticulin-null transcriptome showed that several developmental networks were affected, including cardiovascular, nervous, reproductive, skeletal, respiratory, renal, connective tissue, hair/skin, hematological, immune/lymphatic, and digestive systems (Fig. 5B). As cardiogenesis is the first organogenically engaged program during early embryogenesis, and calreticulin is specifically expressed in the developing heart [19, 45, 46], a focused analysis on genes associated with cardiovascular function was performed. A curated framework incorporated nine upregulated and 36 downregulated transcripts (Fig. 5C). Within this crt-dependent subtranscriptome, cell cyclins Ccnd1, Ccnd2 and the developmental regulator Notch1 (Fig. 5C, boxed in purple), genes associated with cardiac malformations [47–49], were network hubs affected by calreticulin expression. The predictive aptitude of these prioritized cardiac network hubs was validated with conserved expression dynamics noted in an independent model of cardiac dysgenesis. Specifically, Hdac1 (histone deacetylase one) deficiency [50] revealed, upon transcriptome interrogation, Notch1, Ccnd1, and Ccnd2 expression trends that matched those found with calreticulin deficiency (Supporting Information Fig. 1). However, interrogation of the Hdac1−/− dataset to compare expression profiles of genes identified within the calreticulin-deficient cardiac subnetwork showed that of nine genes identified as upregulated in the crt−/− transcriptome, five were present in an Hdac1-deficient background. Of these, Notch1 and Adam10 were upregulated similar to their profile in crt deficiency, whereas Clcn3, Pvr, and Itgb1 were downregulated. Also, of 36 downregulated transcripts in the crt−/− “cardiovascular development” network, 19 of these were present in the Hdac1−/− transcriptome, 12 of which had similar expression profiles to the crt−/− dataset, including Psmc4, Psmc5, Pitx2, Hmox1, Csda, Myc, Trip6, Lef1, Nme1, Atp6v1e1, Yap1, and Cdh2. The remaining genes, that is, Pcgf2, Ptov1, Prkch, Flk1, Tubb2c, Tubb3, and Slc9a3r1, were, however, upregulated within an Hdac1 null background. Although conserved hub alteration identifies consistent organ level abnormalities, differential collective dynamics within the cardiovascular network highlights differences in overall phenotype depending on the initial monogenic lesion. Organ specificity of hub-based prediction was verified with analysis of a hepatocyte nuclear factor alpha (Hnf4a) knockout model [51] resulting in noncardiac developmental abnormality. Indeed, dissimilar hub profile changes were noted, with upregulation of Ccnd1 and no changes in Ccnd2 and Notch1 (data not shown). Thus, transcriptome deconvolution of independent crt−/− and Hdac1−/− datasets crossvalidated hub identities and concomitant network expression profiles that underlies the observed ventricular septal defect. Furthermore, bioinformatic resolution provided by the current algorithm demonstrated specificity, supported by dissection of the noncardiac Hnf4a transcriptome. Collectively, these data suggest that crt ablation reorganizes ontological priorities through privileged relationship of calreticulin with vital cardiac regulators predicting compromised heart development.
Figure 5.
Molecular attenuation of calreticulin impacts a transcriptome network with repercussions on cardiovascular development. (A): Onto-logical analysis of genes affected by calreticulin deficiency reveals transcripts that impact global processes of gene expression, protein trafficking, and molecular transport, with numerous accessory functions. (B): Re-analysis of the calreticulin-deficient transcriptome according to physiological development prioritized 11 distinct systems. (C): Genes associated with cardiac differentiation organized into a network of curated relationships within a calreticulin-deficient background. Composed of 45 network nodes, nine displayed increases in expression (red), while the remaining genes were downregulated (green), after calreticulin deletion. Within this network, Ccnd1 Ccnd2, and Notch1 are identified as network hubs (purple boxes), defined as nodes that possess the greatest number of connections to neighboring genes. Orange circles highlight genes with characterized effects on cardiac formation.
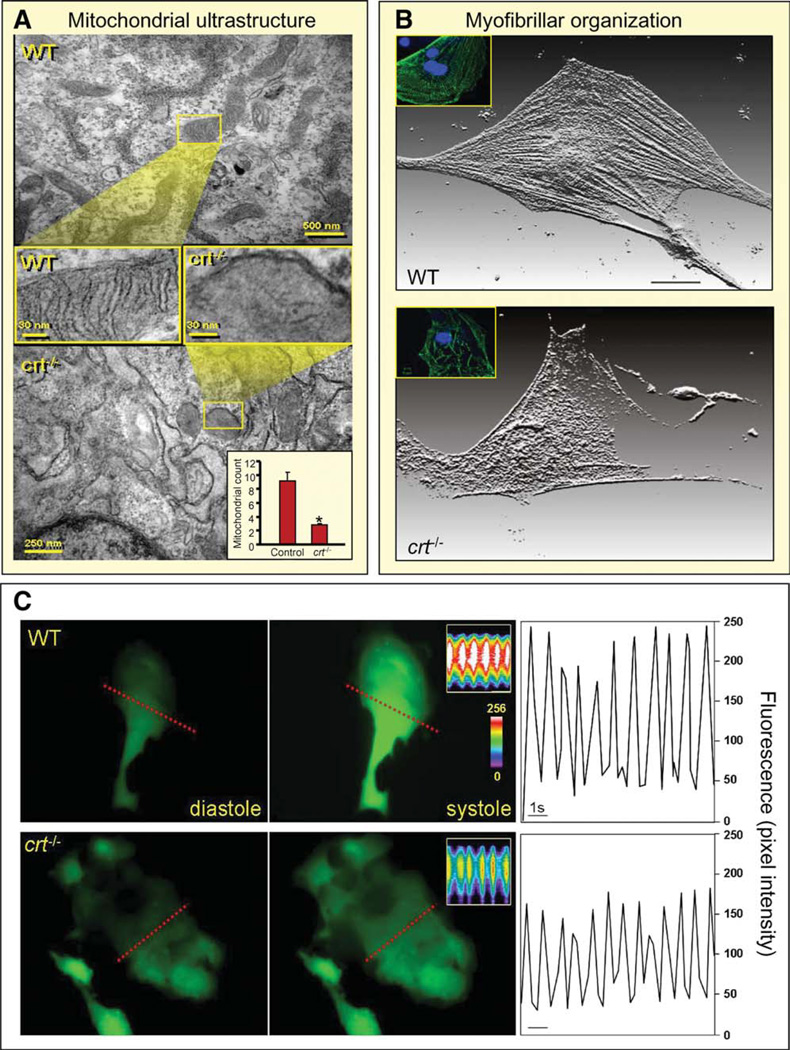
Anticipated Mitochondrial and Sarcomeric Derangement in Cardiac crt−/− Progeny
Altered Tomm gene expression and perturbed Pfdn and TRiC/ CCT network submodules, extrapolated from the protein chaperoning network (Fig. 4C), forecast mitochondrial disturbance and myofibrillogenic derangement, respectively. Indeed, ultra-structural analysis of calreticulin-deficient stem cell-derived cardiomyocytes showed diminution in mitochondrial density and architecture with compromised cristae development (Fig. 6A). Light microscopy and immunofluorescent analysis directly demonstrated impaired sarcomeric organization with decreased content of sarcomeric proteins in crt−/− derived cardiomyocytes compared with WT counterparts (Fig. 6B and Supporting Information Fig. 2). Measurement of contractile areas revealed attenuated Ca2+ transients in calreticulin knockout-derived cardiomyocytes compared with WT counterparts (Fig. 6C), whereas functional assessment demonstrated diminished cell shortening and beating frequency (Supporting Information Fig. 3). Thus, calreticulin omission inflicted transcriptome shifts in specific subnetwork modules that precipitated cellular aberrancies in derived cardiac cell progeny.
Figure 6.
Embryonic transcriptome shift precipitated by calreticulin deletion translates into phenotype derangement. (A): Comparison of mitochondria, visualized by electron microscopy, in WT (upper) and calreticulin-deficient embryonic stem cells (lower). Cross-sectional magnification revealed absence of cristae in the latter. Inset, lower right: Enumeration of mitochondrial number in WT versus crt−/−. Mitochondrial count indicated on y-axis. *, p < .05, n = 5. (B): Individual stem cell-derived cardiomyocytes stained for α-actinin, displayed here as a three-dimensional reconstruction of fluorescence. Proper sarcomere formation in WT stem cell-derived cardiomyocytes is illustrated (upper), and disarrayed myofibrillogenesis or no sarcomere generation is present in cardiomyocytes from crt−/− stem cells. Inset: Stem cell-derived cardiomyocytes stained for MLC2V (green) and DAPI-counterstained nuclei (blue). (C): Calcium transient measurements. Left and middle panels: Ca2+ levels in diastole and systole in WT and crt−/− stem cell-derived cardiomyocytes loaded with the calcium indicator, Fluo-3. Inset: Line scan (indicated by dashed line) records calcium transients in contractile cells. Color scale indicates high (white) and low (blue/purple) calcium concentrations. Right: Analysis of calcium transients as a function of time demonstrate attenuated peak intensity in crt−/− knockouts (bottom) compared with WT counterparts (top). Bar = 1 second. Abbreviation: WT, wild type.
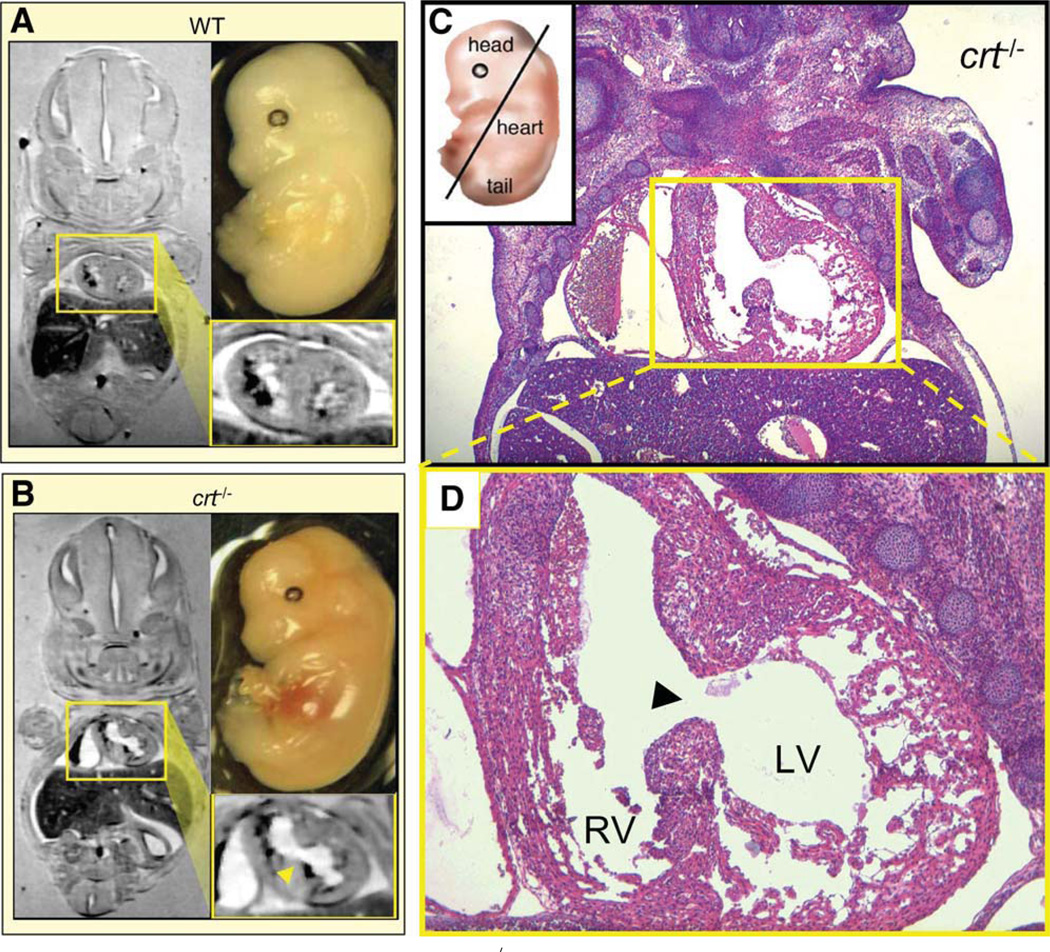
Predicted Impact of crt−/− Genome on Cardiac Morphogenesis
WT and crt−/− embryos at E14.5 of gestation were analyzed by nuclear magnetic resonance imaging and histological evaluation. Gross assessment of crt−/− embryos did not exhibit overt extracoelomic irregularities compared with WT (Fig. 7A, 7B, upper right), with coronal nuclear magnetic resonance planes exhibiting dimensional similarity of embryos (Fig. 7A, 7B, left). Magnification of cardiac sections revealed a prominent ventricular septal defect (Fig. 7A, 7B, lower right). Histological sections of crt−/− hearts confirmed the ventricular septal defect, and exposed a thin left ventricular wall with deep recesses and predominant fenestrated tissue (Fig. 7C, 7D). The right ventricle, in direct communication with the left side of the heart, demonstrated multilayer thickness without gross abnormalities throughout the myocardium. Cardiac defects, bioinformatically forecasted in calreticulin-deficient embryonic stem cells, were thus directly confirmed at the embryo level validating a network-driven algorithm of phenotype prediction.
Figure 7.
Magnetic resonance reveals ventricular septal defect in crt−/− embryo. Comparative in situ magnetic resonance imaging of coronal planes from 14.5 dpc-old WT and crt−/− embryos demonstrates normal (A) versus defective (B) cardiac structure, respectively, as a predicted manifestation of cardiac phenotype. Magnified cardiac digital cross-section (yellow boxes) shows prominent ventricular septal defect with interventricular communication in crt−/− (arrowhead) in contrast to distinct left and right ventricular lumens in WT hearts characteristic for this stage of cardiogenesis. (C): Pathoanatomical verification of crt−/− dysmorphic cardiac structure (right). Coronal hematoxylin-eosin stained thin section of embryo visualizes a direct communication (ventricular septal defect) between LV and RV through a patent ventricular septum in the crt-null mutant. (D): Magnification of cardiac section highlights incomplete septal development, a common manifestation of congenital heart disease. Histology confirms known defect of a thin left ventricular myocardium characterized by deep intertrabecular recesses with the predominance of large fenestrations. Right ventricle demonstrates normal muscularization without pathological thinning or gross abnormalities. Abbreviations: LV, left ventricle; RV, right ventricle; WT, wild type.
Discussion
As differentiation manifests from pluripotent transcriptomes [26, 52], stem cells offer a unique platform for early identification of ensuing phenotype impairment. To expose signal pathways within intracellular networks that precipitate phenotypic deviation, bioinformatic deconvolution was used here to resolve gene expression profiles associated with a discrete monogenic insult caused by calreticulin deletion. Calreticulin deficiency imposed pleiotropic deficits and altered ontological classifications that forecasted organ malformation within the selected category of “cardiac development,” exemplifying a paradigm to anticipate a pathological phenotype.
Procurement of early embryonic transcripts captures genomic alteration that is independent of differentiation, used here to identify potential predilection toward abnormalities within a physiological system or organ. Although no apparent morphologic aberrancy could be observed in undifferentiated crt−/− stem cells, underlying genotypic differences were evident upon high throughput analysis. Genetic deletion of calreticulin affected cellular networks within canonical functions of calcium homeostasis and glycosylated protein folding. Beyond these classical roles, the unbiased bioinformatic approach here uncovered novel molecular associations involved in subcellular cardiovascular development. Indeed, recent studies have demonstrated the crucial regulatory role of calreticulin during cardiomyocyte development [53]. The current work presents a systems biology deconstruction of the influence of calreticulin deficiency on essential cardiomyogenic processes before differentiation.
Inspection of calreticulin-dependent calcium handling networks revealed integration of up- and downregulated genes into a single subnetwork linked to Tnf, a cytokine identified as a significant contributor to cardiogenesis [26]. In addition, examination of chaperone-related functions identified indirect relationships of calreticulin with Tomm, Pfdn, and TRiC/CCT modules that impacted myofibrillogenesis and mitochondrial formation. Discomposure of Tomm complexes predicts subcellular, mitochondrial effects [54, 55], confirmed by the presence of poorly developed mitochondria in crt−/− stem cell-derived cardiomyocytes. Prefoldin and TRiC/CCT are multisubunit complexes that control actin dynamics [56, 57], with disruptions precluding proper actin filament generation and maturation, suggesting that derangement in myofibrillogenesis can be extrapolated from perturbation of prefoldin and TRiC/CCT network submodules. This was confirmed here as myofibrillar disarray was observed in calreticulin deficient, stem cell-derived cardiomyocytes [58]. Previous ultrastructural studies have demonstrated a significant role for Cdh2 and Vcl in myofibrillogenesis associated with calreticulin deficiency [59]. Bioinformatic mining of the present transcriptome revealed downregulation of these two genes in a calreticulin null background, in agreement with these previous observations. Together, these data suggest an expanded, calreticulin-organized network responsible for proper cardiac myofibril formation. Moreover, we observed diminished calcium transients consistent with canonical calreticulin functions [27]. Therefore, disrupted sarcomerogenesis, mitochondrial abnormalities, as well as dysregulated calcium handling were all present in stem cell-derived cardiomyocytes, validating predictions based on analysis of cellular networks affected by calreticulin deficiency.
As the heart is the first organ to develop and exclusively express high levels of calreticulin early in embryogenesis, developmental timing prioritized predictive focus on cardiogenesis among other systems [60, 61]. Inspection of genes that comprised cardiovascular development identified a framework of interactions that included three network hubs, Ccnd1, Ccnd2, and Notch1. Similar expression dynamics of these prioritized hubs in the transcriptome of an independent model of cardiogenic abnormality, Hdac1−/− [50], validated predictive capacity. Furthermore, organ specificity was demonstrated with analysis of a noncardiac Hnf4a−/− model [51] exhibiting alternative profiles for Ccnd1, Ccnd2, and Notch1. Thus, expression patterns of prioritized hubs within the cardiovascular development network provides, at an early pluripotent stage, the potential to forecast ensuing organ abnormality upon differentiation.
It has been previously shown that Ccnd1 and Ccnd2 are critical for cardiac differentiation and are upregulated as part of normal cardiac development [47, 49]. Moreover, heart formation is impaired when cyclins Ccnd1 and Ccnd2 are deleted [49]. Here, cyclins Ccnd1 and Ccnd2, identified as elements in the calreticulin-deficient cardiovascular development network, were downregulated. This previously unrecognized association of procardiac cyclins as hubs in the crt−/− network expanded the role of calreticulin-dependent gene regulation, and suggested that absence of calreticulin precludes normal cardiac development.
Also within the resolved transcriptome, Notch1 emerged as a targeted hub in a crt−/− background where calreticulin deficiency abnormally upregulated Notch1 expression. Downregulation of Notch1 is a requisite for proper cardiogenesis [48, 62], and this developmental role is supported by observations that Notch1 overexpression leads to impaired myocardial development [63]. Moreover, dysregulated Notch activity impacts cardiac differentiation [64, 65], and critical mutations in Notch, as well as its ligands, lead to clinically relevant pathologies, such as Alagille syndrome, characterized by ventricular septal defects and right ventricular hypertrophy [66]. Also, atrial and ventricular septal defects were recently demonstrated in mice with conditionally overexpressed Notch1, providing further evidence of Notch1 effects on cardiogenesis [67]. Thus, maintenance of balanced Notch activity is critical for proper development and function, and linkage of calreticulin-dependent pathways to Notch signaling here uncovered a new regulatory axis for cardiac determination. Other affected nodes included the transcription factor Pitx2, the receptor Flk-1/ VEGFR-2, and the regulatory binding protein Pparbp/Med1, all downregulated in the extant calreticulin-deficient cardiovascular development network. These genes contribute to proper myocardial developmental architecture [68, 69], predicting that their dysregulation would induce cardiac malformations.
Collectively, cardiac defects suggested by impaired cardiovascular development in calreticulin-deficient backgrounds, is anticipated by molecular alterations related to cardiac specific expression in early stages of embryogenesis, which may compromise heart development and impact embryo survival. Investigation of crt−/− cardiac anatomy revealed ventricular thinning and septal defect, consistent with calreticulin-deficiency cardiopathologies predicted from unbiased resolution of a primordial transcriptome. The current systems biology approach demonstrates capacity to navigate stochasticity of a pluripotent background to collectively resolve cardiogenic axes. Within the broader context of a regenerative paradigm, networked signaling cascades can provide an integrated molecular atlas to monitor and anticipate consistency of phenotype recapitulation upon developmental execution [70]. Bioinformatic construction and dynamic curation of mapped transcriptomes refines interrogation of cellular prototypes that harbor insufficient regenerative capacity, to efficiently identify critical targets for molecular intervention and/or salvage. Furthermore, network prognosis of a deficient embryonic transcriptome with corrupted pathways effectively captures phenotypes within a hierarchy of organizational complexity, from single molecule to whole organ disease manifestation.
Conclusion
In summary, the monogenic deficiency model presented here demonstrates how bioinformatic surveillance permits incisive transcriptomic resolution to anticipate pathophysiological deficits that corrupt normal development. Thus, network biology applied at the stem cell level demonstrates potential for enhancement and expansion of diagnostic protocols, through increased resolution of forecasted phenotypes from aberrant primordial genomes.
Supplementary Material
Acknowledgments
We thank Lois A. Rowe for excellent technical assistance. This work was supported by grants from the National Institutes of Health (HL85208, HL83439, and HL07111), American Heart Association, Marriott Heart Disease Research Program, Marriott Foundation, Ted Nash Long Life Foundation, Ralph Wilson Medical Research Foundation, Asper Foundation, Mayo Graduate School, Mayo Clinic Clinician-Investigator Program, Mayo Clinic FUTR Career Development Award, and the Canadian Institute of Health Research (MOP-53050).
Footnotes
R.S.F.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; AC: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; N.J.N.: provision of study material, collection and assembly of data, data analysis and interpretation, final approval of manuscript; T.J.N.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; A.B.: conception and design, provision of study material, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; P.K.M.: collection and assembly of data, final approval of manuscript; S.M.: collection and assembly of data, final approval of manuscript; M.M.: provision of study material, manuscript writing, final approval of manuscript; A.T.: conception and design, financial support, administrative support, manuscript writing, final approval of manuscript; C.P.T.: conception and design, provision of study material, data analysis and interpretation, financial support, administrative support, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Suzuki A, Raya Á, Kawakami Y, et al. Maintenance of embryonic stem cell pluripotency by Nanog-mediated reversal of mesoderm specification. Nat Clin Pract Cardiovasc Med. 2006;3:S114–S122. doi: 10.1038/ncpcardio0442. [DOI] [PubMed] [Google Scholar]
- 2.Boyer LA, Lee TI, Cole MF, et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell. 2005;122:947–956. doi: 10.1016/j.cell.2005.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loh Y-H, Wu Q, Chew J-L, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–440. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 4.Faustino RS, Terzic A. Interactome of a cardiopoietic precursor. J Cardiovasc Trans Res. 2008;1:120–126. doi: 10.1007/s12265-008-9019-z. [DOI] [PubMed] [Google Scholar]
- 5.Komili S, Silver PA. Coupling and coordination in gene expression processes: A systems biology view. Nat Rev Genet. 2008;9:38–48. doi: 10.1038/nrg2223. [DOI] [PubMed] [Google Scholar]
- 6.Olson EN. Gene regulatory networks in the evolution and development of the heart. Science. 2006;313:1922–1927. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boiani M, Schöler HR. Regulatory networks in embryo-derived pluripotent stem cells. Nat Rev Mol Cell Biol. 2005;6:872–884. doi: 10.1038/nrm1744. [DOI] [PubMed] [Google Scholar]
- 8.Soen Y, Mori A, Palmer TD, et al. Exploring the regulation of human neural precursor cell differentiation using arrays of signaling microen-vironments. Mol Syst Biol. 2006;2:37. doi: 10.1038/msb4100076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiriac A, Nelson TJ, Faustino RS, et al. Cardiogenic induction of pluripotent stem cells streamlined through a conserved SDF-1/VEGF/ BMP2 integrated network. PLoS One. 2010;5:e9943. doi: 10.1371/journal.pone.0009943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faustino RS, Behfar A, Perez-Terzic C, et al. Genomic chart guiding embryonic stem cell cardiopoiesis. Genome Biol. 2008;9:R6. doi: 10.1186/gb-2008-9-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bell J. Predicting disease using genomics. Nature. 2004;429:453–456. doi: 10.1038/nature02624. [DOI] [PubMed] [Google Scholar]
- 12.Hood L, Heath JR, Phelps ME, et al. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306:640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- 13.Lee I, Lehner B, Crombie C, et al. A single gene network accurately predicts phenotypic effects of gene perturbation in Caenorhabditis elegans. Nat Genet. 2008;40:181–188. doi: 10.1038/ng.2007.70. [DOI] [PubMed] [Google Scholar]
- 14.Qiu Y, Michalak M. Transcriptional control of the calreticulin gene in health and disease. Int J Biochem Cell Biol. 2009;41:531–538. doi: 10.1016/j.biocel.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 15.Michalak M, Corbett EF, Mesaeli N, et al. Calreticulin: One protein, one gene, many functions. Biochem J. 1999;344(Pt 2):281–292. [PMC free article] [PubMed] [Google Scholar]
- 16.Molinari M, Eriksson KK, Calanca V, et al. Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control. Mol Cell. 2004;13:125–135. doi: 10.1016/s1097-2765(03)00494-5. [DOI] [PubMed] [Google Scholar]
- 17.Szabo E, Papp S, Opas M. Differential calreticulin expression affects focal contacts via the calmodulin/CaMK II pathway. J Cell Physiol. 2007;213:269–277. doi: 10.1002/jcp.21122. [DOI] [PubMed] [Google Scholar]
- 18.Michalak M, Robert Parker JM, Opas M. Ca2+ signaling and calcium binding chaperones of the endoplasmic reticulum. Cell Calcium. 2002;32:269–278. doi: 10.1016/s0143416002001884. [DOI] [PubMed] [Google Scholar]
- 19.Mesaeli N, Nakamura K, Zvaritch E, et al. Calreticulin is essential for cardiac development. J Cell Biol. 1999;144:857–868. doi: 10.1083/jcb.144.5.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch JM, Chilibeck K, Qiu Y, et al. Assembling pieces of the cardiac puzzle; calreticulin and calcium-dependent pathways in cardiac development, health, and disease. Trends Cardiovasc Med. 2006;16:65–69. doi: 10.1016/j.tcm.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Perez-Terzic C, Faustino RS, Boorsma BJ, et al. Stem cells transform into a cardiac phenotype with remodeling of the nuclear transport machinery. Nat Clin Pract Cardiovasc Med. 2007;4:S68–S76. doi: 10.1038/ncpcardio0763. [DOI] [PubMed] [Google Scholar]
- 22.Nelson TJ, Faustino RS, Chiriac A, et al. Cxcr4+/Flk-1+ Biomarkers select a cardiopoietic lineage from embryonic stem cells. Stem Cells. 2008;26:1464–1473. doi: 10.1634/stemcells.2007-0808. [DOI] [PubMed] [Google Scholar]
- 23.Faustino RS, Chiriac A, Terzic A. Bioinformatic primer for clinical and translational science. Clin Trans Sci. 2008;1:174–180. doi: 10.1111/j.1752-8062.2008.00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Terzic C, Behfar A, Méry A, et al. Structural adaptation of the nuclear pore complex in stem cell-derived cardiomyocytes. Circ Res. 2003;92:444–452. doi: 10.1161/01.RES.0000059415.25070.54. [DOI] [PubMed] [Google Scholar]
- 26.Behfar A, Perez-Terzic C, Faustino RS, et al. Cardiopoietic programming of embryonic stem cells for tumor-free heart repair. J Exp Med. 2007;204:405–420. doi: 10.1084/jem.20061916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gelebart P, Opas M, Michalak M. Calreticulin, a Ca2+-binding chaperone of the endoplasmic reticulum. Int J Biochem Cell Biol. 2005;37:260–266. doi: 10.1016/j.biocel.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 28.Cassar-Malek I, Passelaigue F, Bernard C, et al. Target genes of myostatin loss-of-function in muscles of late bovine fetuses. BMC Genomics. 2007;8:63. doi: 10.1186/1471-2164-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colak D, Kaya N, Al-Zahrani J, et al. Left ventricular global transcriptional profiling in human end-stage dilated cardiomyopathy. Genomics. 2009;94:20–31. doi: 10.1016/j.ygeno.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doherty JM, Geske MJ, Stappenbeck TS, et al. Diverse adult stem cells share specific higher-order patterns of gene expression. Stem Cells. 2008;26:2124–2130. doi: 10.1634/stemcells.2008-0380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behfar A, Faustino RS, Arrell DK, et al. Guided stem cell cardiopoiesis: Discovery and translation. J Mol Cell Cardiol. 2008;45:523–529. doi: 10.1016/j.yjmcc.2008.09.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ozawa E, Mizuno Y, Hagiwara Y, et al. Molecular and cell biology of the sarcoglycan complex. Muscle Nerve. 2005;32:563–576. doi: 10.1002/mus.20349. [DOI] [PubMed] [Google Scholar]
- 33.Stirling PC, Bakhoum SF, Feigl AB, et al. Convergent evolution of clamp-like binding sites in diverse chaperones. Nat Struct Mol Biol. 2006;13:865–870. doi: 10.1038/nsmb1153. [DOI] [PubMed] [Google Scholar]
- 34.Hartl FU, Hayer-Hartl M. Molecular chaperones in the cytosol: From nascent chain to folded protein. Science. 2002;295:1852–1858. doi: 10.1126/science.1068408. [DOI] [PubMed] [Google Scholar]
- 35.Spiess C, Miller EJ, McClellan AJ, et al. Identification of the TRiC/ CCT substrate binding sites uncovers the function of subunit diversity in eukaryotic chaperonins. Mol Cell. 2006;24:25–37. doi: 10.1016/j.molcel.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rapaport D. How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane? J Cell Biol. 2005;171:419–423. doi: 10.1083/jcb.200507147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Endo T, Yamamoto H, Esaki M. Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J Cell Sci. 2003;116:3259–3267. doi: 10.1242/jcs.00667. [DOI] [PubMed] [Google Scholar]
- 38.Seo HR, Chung D-Y, Lee Y-J, et al. Heat shock protein 25 or inducible heat shock protein 70 activates heat shock factor 1. J Biol Chem. 2006;281:17220–17227. doi: 10.1074/jbc.M600062200. [DOI] [PubMed] [Google Scholar]
- 39.Doucet J, Provost P, Samuelsson B, et al. Molecular cloning and functional characterization of mouse coactosin-like protein. Biochem Biophys Res Commun. 2002;290:783–789. doi: 10.1006/bbrc.2001.6236. [DOI] [PubMed] [Google Scholar]
- 40.Fan GH, Yang W, Sai J, et al. Hsc/Hsp70 interacting protein (Hip) associates with CXCR2 and regulates the receptor signaling and trafficking. J Biol Chem. 2002;277:6590–6597. doi: 10.1074/jbc.M110588200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hamza I, Faisst A, Prohaska J, et al. The metallochaperone Atox1 plays a critical role in perinatal copper homeostasis. Proc Natl Acad Sci USA. 2001;98:6848–6852. doi: 10.1073/pnas.111058498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X, Lee WH, Sobott F, et al. Structural basis for protein-protein interactions in the 14–3-3 protein family. Proc Natl Acad Sci USA. 2006;103:17237–17242. doi: 10.1073/pnas.0605779103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Götz R, Wiese S, Takayama S, et al. Bag1 is essential for differentiation and survival of hematopoietic and neuronal cells. Nat Neurosci. 2005;8:1169–1178. doi: 10.1038/nn1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C-Y, Gherzi R, Ong S-E, et al. AU binding proteins recruit the exosome to degrade ARE-containing mRNAs. Cell. 2001;107:451–464. doi: 10.1016/s0092-8674(01)00578-5. [DOI] [PubMed] [Google Scholar]
- 45.Guo L, Nakamura K, Lynch J, et al. Cardiac-specific expression of calcineurin reverses embryonic lethality in calreticulin-deficient mouse. J Biol Chem. 2002;277:50776–50779. doi: 10.1074/jbc.M209900200. [DOI] [PubMed] [Google Scholar]
- 46.Lynch J, Guo L, Gelebart P, et al. Calreticulin signals upstream of calcineurin and MEF2C in a critical Ca2+-dependent signaling cascade. J Cell Biol. 2005;170:37–47. doi: 10.1083/jcb.200412156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busk PK, Bartkova J, Strøm CC, et al. Involvement of cyclin D activity in left ventricle hypertrophy in vivo and in vitro. Cardiovasc Res. 2002;56:64–75. doi: 10.1016/s0008-6363(02)00510-2. [DOI] [PubMed] [Google Scholar]
- 48.High FA, Epstein JA. The multifaceted role of Notch in cardiac development and disease. Nat Rev Genet. 2008;9:49–61. doi: 10.1038/nrg2279. [DOI] [PubMed] [Google Scholar]
- 49.Kozar K, Ciemerych MA, Rebel VI, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 50.Zupkovitz G, Tischler J, Posch M, et al. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol Cell Biol. 2006;26:7913–7928. doi: 10.1128/MCB.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Battle MA, Konopka G, Parviz F, et al. Hepatocyte nuclear factor 4alpha orchestrates expression of cell adhesion proteins during the epithelial transformation of the developing liver. Proc Natl Acad Sci USA. 2006;103:8419–8424. doi: 10.1073/pnas.0600246103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang J, Rao S, Chu J, et al. A protein interaction network for pluripotency of embryonic stem cells. Nature. 2006;444:364–368. doi: 10.1038/nature05284. [DOI] [PubMed] [Google Scholar]
- 53.Papp S, Dziak E, Opas M. Embryonic stem cell-derived cardiomyogenesis: A novel role for calreticulin as a regulator. Stem Cells. 2009;27:1507–1515. doi: 10.1002/stem.85. [DOI] [PubMed] [Google Scholar]
- 54.Mukhopadhyay A, Avramova LV, Weiner H. Tom34 unlike Tom20 does not interact with the leader sequences of mitochondrial precursor proteins. Arch Biochem Biophys. 2002;400:97–104. doi: 10.1006/abbi.2002.2777. [DOI] [PubMed] [Google Scholar]
- 55.Neupert W, Brunner M. The protein import motor of mitochondria. Nat Rev Mol Cell Biol. 2002;3:555–565. doi: 10.1038/nrm878. [DOI] [PubMed] [Google Scholar]
- 56.Gao Y, Thomas JO, Chow RL, et al. A cytoplasmic chaperonin that catalyzes β-actin folding. Cell. 1992;69:1043–1050. doi: 10.1016/0092-8674(92)90622-j. [DOI] [PubMed] [Google Scholar]
- 57.Simons CT, Staes A, Rommelaere H, et al. Selective contribution of eukaryotic prefoldin subunits to actin and tubulin binding. J Biol Chem. 2004;279:4196–4203. doi: 10.1074/jbc.M306053200. [DOI] [PubMed] [Google Scholar]
- 58.Li J, Puceat M, Perez-Terzic C, et al. Calreticulin reveals a critical Ca(2+) checkpoint in cardiac myofibrillogenesis. J Cell Biol. 2002;158:103–113. doi: 10.1083/jcb.200204092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lozyk MD, Papp S, Zhang X, et al. Ultrastructural analysis of development of myocardium in calreticulin-deficient mice. BMC Dev Biol. 2006;6:54. doi: 10.1186/1471-213X-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cordes KR, Srivastava D. MicroRNA regulation of cardiovascular development. Circ Res. 2009;104:724–732. doi: 10.1161/CIRCRESAHA.108.192872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Srivastava D. Making or breaking the heart: From lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 62.Nemir M, Croquelois A, Pedrazzini T, et al. Induction of cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ Res. 2006;98:1471–1478. doi: 10.1161/01.RES.0000226497.52052.2a. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe Y, Kokubo H, Miyagawa-Tomita S, et al. Activation of Notch1 signaling in cardiogenic mesoderm induces abnormal heart morphogenesis in mouse. Development. 2006;133:1625–1634. doi: 10.1242/dev.02344. [DOI] [PubMed] [Google Scholar]
- 64.Nemir M, Pedrazzini T. Functional role of Notch signaling in the developing and postnatal heart. J Mol Cell Cardiol. 2008;45:495–504. doi: 10.1016/j.yjmcc.2008.02.273. [DOI] [PubMed] [Google Scholar]
- 65.Niessen K, Karsan A. Notch signaling in cardiac development. Circ Res. 2008;102:1169–1181. doi: 10.1161/CIRCRESAHA.108.174318. [DOI] [PubMed] [Google Scholar]
- 66.Niessen K, Karsan A. Notch signaling in the developing cardiovascular system. Am J Physiol Cell Physiol. 2007;293:C1–C11. doi: 10.1152/ajpcell.00415.2006. [DOI] [PubMed] [Google Scholar]
- 67.Kratsios P, Catela C, Salimova E, et al. Distinct roles for cell-autonomous notch signaling in cardiomyocytes of the embryonic and adult heart. Circ Res. 2010;106:559–572. doi: 10.1161/CIRCRESAHA.109.203034. [DOI] [PubMed] [Google Scholar]
- 68.Lu MF, Pressman C, Dyer R, et al. Function of Rieger syndrome gene in left-right asymmetry and craniofacial development. Nature. 1999;401:276–278. doi: 10.1038/45797. [DOI] [PubMed] [Google Scholar]
- 69.Sylvius N, Tesson F. Lamin A/C and cardiac diseases. Curr Opin Cardiol. 2006;21:159–165. doi: 10.1097/01.hco.0000221575.33501.58. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y-C, Chen B-S. Integrated cellular network of transcription regulations and protein-protein interactions. BMC Syst Biol. 2010;4:20. doi: 10.1186/1752-0509-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.