Abstract
In August 2008, a team from the National Environmental Agency conducted an entomological investigation of a chikungunya cluster in Singapore, with the primary aim of identifying the vector responsible for the outbreak and to assess the vector control operation. A total of 173 adult mosquitoes were caught using both the sweep-net method and the BG Sentinel Traps in and around the affected workers' quarters. Of these, 120 (69.4%) were Aedes albopictus and the rest were Culex quinquefasciatus. More than 2700 Ae. albopictus larvae were also collected from 33 breeding habitats detected. No Aedes aegypti was found. During the preintervention period, 6 (8.4%) out of 71 adult female Ae. albopictus were found positive for the chikungunya virus (CHIKV). Vector control measures resulted in a 90% reduction of adult Ae. albopictus caught by BG Sentinel Traps. Postintervention surveillance revealed the presence of CHIKV-positive mosquitoes. These findings led to continued intensive vector control operation in the affected area that further reduced vector population and interrupted the transmission of the disease. The E1 gene sequence of the CHIKV was identical to those of CHIKV isolated from human chikungunya cases working in the affected area, and contained the A226V mutation. The incrimination of Ae. albopictus as a major vector involved in the transmission of A226V CHIKV had led to the revision of chikungunya control strategy in Singapore. This study suggests the benefit of a vector control program that includes the evaluation of control measures in conjunction to virological surveillance in vector population.
Key Words: Aedes albopictus, Chikungunya, Pirimiphos-methyl, Singapore, Vector control
Introduction
Chikungunya fever is a mosquito-borne disease caused by an Alphavirus belonging to the Family Togaviridae. In Africa, though the virus had been transmitted mainly by the sylvatic mosquitoes, Aedes africanus, Aedes furcifer, Aedes taylori, Aedes leutocephalus, and Aedes cordellieri, involvement of Aedes aegypti and Aedes albopictus in recent years have been reported (Diallo et al. 1999, Pastorino et al. 2004, Peyrefitte et al. 2007, Sang et al. 2008). In contrast, outbreaks of chikungunya virus (CHIKV) in Asia have mainly been associated with urban Ae. aegypti and more recently Ae. albopictus (Powers and Logue 2007, Ng et al. 2009b). CHIKV was first isolated in 1953 in the former Tanganyika (present day Tanzania) (Robinson 1955). Between 1960 and 2000, transmissions were evident in numerous countries in Western, Central, and Southern Africa. In the same period, sporadic localized outbreaks have also been reported in South Asia (India, Sri Lanka, and Pakistan) and South East Asia (Malaysia, Philippines, Indonesia, Cambodia, Vietnam, Myanmar, and Thailand) (Halstead 2007, Powers and Logue 2007, Ng et al. 2009a).
Chikungunya had largely been a neglected disease until an unprecedented outbreak occurred in the Indian Ocean Islands in early 2005, when several hundred thousands of cases were reported from Comoros, Reunion, Seychelles, Mauritius, and Mayotte (Chastel 2005, Josseran et al. 2006, Renault et al. 2007, Beesoon et al. 2008, Kariuki Njenga et al. 2008, Sang et al. 2008, Sissoko et al. 2010). Since then, the spread of the virus has caused fresh outbreaks in Asia. India saw an epidemic wave that swept throughout the country, with >1 million cases reported (Yergolkar et al. 2006, Kumar et al. 2008). Chikungunya epidemics were also reported in Sri Lanka, Maldives, Malaysia, and Thailand (Noridah et al. 2007, Sam et al. 2009, Hapuarachchi et al. 2010). In 2007, >200 cases of chikungunya were reported in Italy, indicating that the disease was not restricted to developing tropical countries (Rezza et al. 2007).
The first documented emergence of chikungunya in Singapore in January 2008 was limited to a cluster of 13 reported cases, which was successfully contained by combining aggressive vector control operations with active case detection and isolation of patients (Ministry of Health 2008, Leo et al. 2009, Ng et al. 2009b). The virus belonged to the East, Central, and South African (ECSA) genotype and adult vector surveillance conducted in the cluster area only yielded Ae. aegypti. After 3 months of quiescence, local outbreaks of chikungunya resurfaced in May 2008 and the circulating virus was found to be the A226V variant of the ECSA genotype.
In August 2008, a team comprising field and laboratory personnel from the National Environmental Agency conducted an entomological investigation and control of a chikungunya cluster in an industrial area of Singapore. This study suggests the importance of integrating field and laboratory tools in the control, and the value of evaluation of control operations in the management of a chikungunya cluster.
Methods
Study site and chikungunya cases
Twenty-six cases of chikungunya were reportedly linked to a concrete slabs factory with 70 workers and scores of people visiting the factory each day (Fig. 1). The premises of the factory, which included a workers' dormitory, was located in Kranji (1°25′30′′N, 103°45′43′′E), an industrial estate in the rural part of northwest Singapore. The vegetation (grass, banana, and papaya plants) within the 20 ha premises was sparse and mostly found along the perimeter of the premise.
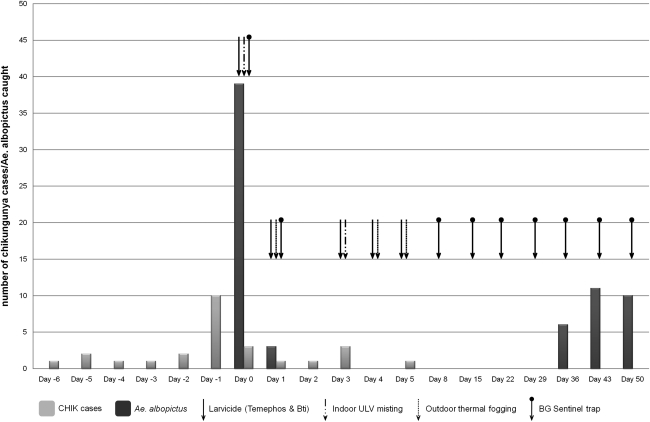
FIG. 1.
Number of chikungunya cases, number of Aedes albopictus caught with BG Sentinel Traps, and vector control activities at the study site (July–September 2008). In response to a notification of a cluster on day 0, adult mosquito surveillance using sweep net and search and destroy of breeding habitats were concurrently conducted in the afternoon. By 18:00 h of day 0, BG Sentinel Traps were set up and indoor ULV misting was carried out. On day 1, mass outdoor thermal fogging was conducted after removal of the traps. Finding of continued presence of infected mosquitoes in the morning of day 2 triggered more vector control activities on days 3, 4, and 5. Meanwhile, search and destroy of breeding sites continued daily for a month (not shown).
Mosquito collections
Adult mosquito surveillance
Adult mosquito surveillance was first carried out to determine the vector involved in the chikungunya cluster, and subsequently to evaluate the impact of vector control intervention. Due to the urgency in managing the cluster, the preintervention surveillance was carried out within 16 h (14:00 h of day 0 to 06:00 h of day 1) using two methods—sweep-net method for collecting resting mosquitoes and BG Sentinel mosquito trap (BGS Trap) (Biogents GMbH) with BG-Lure™ for host-seeking mosquitoes. The sweep-net method was performed by 12 officers from 14:00 to 17:00 h (day 0, preintervention), before the start of chemical fogging and misting activities. Nets were moved swiftly over tables, beds, clothing, vegetation, and so on, and were periodically checked for any mosquitoes captured. In areas where the net could not access, the surrounding area was tapped/disturbed to cause resting mosquitoes to escape to the open. Escaping mosquitoes were then caught with the nets. All mosquitoes caught were transferred to screw-cap plastic vials and labeled accordingly. Sweep-net catches were done inside and around the workers' quarters, in an adjacent open shed that houses machineries and in nearby vegetation opposite the workers' quarters. For the collection of host-seeking mosquitoes, four BGS Traps were set outdoor, around the vicinity of the workers' quarters and in the open shed, according to the manufacturer's recommendation, previously described (Maciel-de-Freitas et al. 2006). During the preintervention phase (day 0), the four BGS Traps were set at 17:00 h and retrieved at 06:00 h, the following day (day 1). Catch bags were checked for the presence of mosquitoes, and all mosquitoes trapped were transferred by a mechanical aspirating device to screw capped vials. Identification and analysis were performed by the Environmental Health Institute (EHI), a mosquito reference laboratory in Singapore. To determine the effectiveness of the vector control activities, the same number of BGS Traps was placed at the exact location and the area was monitored weekly for 7 weeks (Fig. 1). During this period, the traps were set from 16:00 to 10:00 h the following day.
Adult mosquito processing and identification
Mosquitoes were freeze-killed, at −20°C, sorted according to sex and species, and identified using the taxonomic key of Rueda (2004). All Aedes sp. mosquitoes were tested for CHIKV by reverse transcription polymerase chain reaction (RT-PCR) on the day that they were caught. Individual mosquitoes or pools of male mosquitoes were homogenized in 200 μL of MEM with Earle's Salts (PAA Laboratories) supplemented with 1× fungizone. The homogenate was clarified by centrifugation at 14,000 rpm for 10 min, before 140 μL of the supernatant was used for the viral RNA extraction.
CHIKV detection by RT-PCR
The presence of CHIKV in mosquito samples was determined by RT-PCR detection of CHIKV nonstructural protein 1 gene (Ng et al. 2009b). Briefly, CHIKV RNA was extracted from mosquito homogenate by QIAamp viral RNA minikit (Qiagen), and the amplification was performed in a LightCycler 2.0 system by using LightCycler RNA Master SYBR Green Kit I (Roche Diagnostics, GMbH) according to the manufacturer's recommendations.
CHIKV isolation
CHIKV isolation was performed on all samples that were positive by RT-PCR assay. Fifty microliters of each mosquito supernatant was inoculated into a 25-cm3 flask with 90% confluent monolayer Vero cells. The cells were maintained on M199 medium; supplemented with 2% heat-inactivated fetal bovine serum, 1% HEPES buffer, 1% penicillin, 1% streptomycin, 1% L-glutamine, 1% nonessential amino acids, and 1% sodium pyruvate; and incubated at 37°C in a 5% CO2 incubator. The supernatant was harvested when 75% of the cells showed cytopathic effects, and stored at −80°C. CHIKV in the cell supernatant was confirmed by the RT-PCR assay mentioned above.
Sequencing of the CHIKV E1 gene
CHIKV E1 cDNA was synthesized directly from the RNA extracted from each or pooled Ae. albopictus isolates using the Superscript III First-Strand synthesis system (Invitrogen Corp.) and the PCR products were purified using QIAquick PCR purification kit (Qiagen) following the manufacturer's protocol. Sequencing of CHIKV E1 genes was performed as previously described (Ng et al. 2009b).
Sequence and phylogenetic analyses of the CHIKV E1 gene
Consensus sequences were obtained by assembling a contiguous sequence from raw sequencing data using Seqman software (Lasergene, DNASTAR) and aligned using Clustal W (MegAlign software; Lasergene, DNASTAR). The sequences of CHIKV E1 gene from mosquito samples were compared with those from the local human cases from the premises, and sequences obtained from the GenBank database. The phylogenetic tree was constructed according to the neighbor-joining method (Saito and Nei 1987) using software MEGA version 3. The model used for all analyses was Kimura-2-parameter, including transition and transversion. Sites containing missing data and alignment gaps were removed before the analysis begin. Reliability of the internal nodes of the tree was assessed by the bootstrap method based on 1000 replicates.
Vector control operations
On notification of a cluster of 22 chikungunya cases on 1 August 2010 (day 0), a total of 70 officers from National Environmental Agency were deployed on day 0 (13:00–17:00 h) to conduct search and destroy of mosquito-breeding habitats in the premises of the factory and other surrounding factories. All detected mosquito larvae and/or pupae were collected in screw-cap plastic vials, labeled according to the type of habitat, and sent to EHI for identification. Larviciding using Abate 1% SG (Temephos) was dispensed in all breeding sites found, at 1 g/10 L of water. Bti (Vectobac WG) misting using water-dispersable granules formulation was carried out in areas (500 g/ha) where potential breeding sites can be observed but inaccessible. Overgrown vegetation was also trimmed or removed. On the same day, after the initial adult mosquito surveillance was conducted as described above, indoor ultra low volume (ULV) misting of workers' quarters was carried out using Actellic 50EC (Pirimiphos-methyl) at an application rate of 200 g active ingredient/ha. In addition, extensive outdoor thermal fogging was carried out the next morning at 06:00 h (Fig. 1), with the same insecticide dispensed by 48 portable and one vehicle mounted with thermal fogging machines at an application rate of 100 g a.i./ha. Approximately 180 ha covering the factory premise and eight other industrial premises were targeted.
In response to results from postintervention evaluation, chemical larviciding and adulticiding were repeated on days 3, 4, and 5 (Fig. 1). An average of 24 personnel were also deployed daily continue the search and destroy operation for a month.
Results
Adult mosquito surveillance
During the study, a total of 173 adult mosquitoes, comprising 120 Ae. albopictus and 53 Culex sp. (Table 1), were collected around the workers' quarters, adjacent shed, and the nearby vegetation and drains. Before the commencement of vector control activities (day 0), a total of 140 mosquitoes were caught using both the sweep-net method and the BGS Trap. Of these, 64.3% were Ae. albopictus and 35.7% were Culex sp. (Table 1).
Table 1.
The Species, Number of Mosquitoes Collected by Sweep-Net Method and BG Sentinel Mosquito Trap, and the Number of Aedes albopictus Found Positive for the Chikungunya Virus
| |
|
Mosquitoes collected |
|
|||
|---|---|---|---|---|---|---|
| |
|
Aedes albopictus |
|
|
||
| Week of collection | Method of collection | Female (%) | Males (%) | Total | Culex sp. | Number of CHIKV-positive Ae. albopictus |
| Day 0a | Sweep-net | 35 (68.6)b | 16 (31.4)c | 51 | 30 | 5 |
| BGS Trap | 36 (92.3) | 3 (7.7) | 39 | 20 | 1 | |
| Day 1d | BGS Trap | 3 (100) | 0 | 3 | 3 | 1 |
| Day 8 | BGS Trap | 0 | 0 | 0 | 0 | 0 |
| Day 15 | BGS Trap | 0 | 0 | 0 | 0 | 0 |
| Day 22 | BGS Trap | 0 | 0 | 0 | 0 | 0 |
| Day 29 | BGS Trap | 0 | 0 | 0 | 0 | 0 |
| Day 36 | BGS Trap | 5 (83.3) | 1 (16.7) | 6 | 0 | 0 |
| Day 43 | BGS Trap | 8 (72.7) | 3 (27.3) | 11 | 0 | 0 |
| Day 50 | BGS Trap | 10 (100) | 0 | 10 | 0 | 0 |
| Total | 97 | 23 | 120 | 53 | 7 | |
Preintervention.
Fourteen (40%) and 21 (60%) were caught indoor and outdoor, respectively.
Four (25%) and 12 (75%) were caught indoor and outdoor, respectively.
Postintervention (initial thermal fogging activity).
BGS, BG Sentinel mosquito Trap; CHIKV, chikungunya virus.
Out of the 90 Ae. albopictus caught before intervention, 51 were caught using the sweep-net method and the rest (n = 39) were trapped using BGS trap (Table 1 and Fig. 1). Among those caught with sweep net methods, 35 (68.6%) were female Ae. albopictus; of these, 21 (60%) were caught outdoor (Table 1). Ten (62.5%) of the male Ae. albopictus caught using the sweep-net methods were from outdoor locations. Among the 39 caught with BGS trap, 36 (92.3%) were females. Majority (n = 26) of the females were caught near the vicinity of the workers' living quarters, whereas the rest was caught in the open shed. No Ae. aegypti was caught throughout the study.
CHIKV detection by RT-PCR and virus isolation
All female Ae. albopictus were individually tested for the presence of CHIKV using the real-time RT-PCR assay. For male Ae. albopictus, nine were individually assayed, whereas the rest were pooled with two or four mosquitoes per pool. None of the male Ae. albopictus was found to be positive for the virus. During the initial preintervention surveillance, six (9.1%) female Ae. albopictus were found positive with the CHIKV (Table 1). Three of the positive Ae. albopictus were analyzed using whole mosquitoes; we thus could not confirm whether these mosquitoes would have disseminated CHIKV in their body (Table 2). However, the other three positive mosquitoes were found to have disseminated viral infection, as shown by the presence of CHIKV RNA in the head and thorax region (Table 2). Four of the six CHIKV-positive mosquitoes were caught outdoor, whereas two were caught in the living quarters of the workers, including a case's quarter.
Table 2.
The Crossing Point Value: Methods, Place, and Timing of Collection of Chikungunya Virus-Infected Aedes albopictus and Parts of the Mosquitoes Used in the Detection and Isolation of Chikungunya Virus
| Mosquito | CPavalue | Method of collection | Place caught | Pre-/postintervention | Parts of mosquitoes used in the detection of CHIKV |
|---|---|---|---|---|---|
| M-Kr15 | 19 | Sweep-net | Outdoor, nearby vegetation | Preintervention | Whole mosquitoes |
| M-Kr2 | 21 | Sweep-net | Outdoor, living quarters | Preintervention | Whole mosquitoes |
| M-Kr42 | 25 | Sweep-net | Outdoor, nearby vegetation | Preintervention | Whole mosquitoes |
| M-Kr58 | 30.78 | Sweep-net | Outdoor, living quarters | Preintervention | Head/thorax only |
| M-Kr71 | 21.01 | Sweep-net | Indoor, living quarters | Preintervention | Head/thorax only |
| M-Kr79 | 19.55 | BGS Trap | Indoor, living quarters | Preintervention | Head/thorax only |
| M-Kr84 | 30.5 | BGS Trap | Outdoor, living quarters | Postintervention | Head/thorax only |
Crossing point (CP) (equivalent to Ct value) derived from the Roche Lightcycler. CP represents the cycle number that generates a threshold amount of product, and correlates inversely to the amount of virus in the sample.
Among the CHIKV-disseminated mosquitoes, the viral load was found to be in the range of 50 pfu to 5 × 104 pfu per mosquitoes, determined by PCR using an external standard curve generated by plotting 10-fold serially diluted virus from a concentration of 108 pfu/mL. CHIKV from all RT-PCR-positive Ae. albopictus were successfully isolated. Together with the larvae surveillance (described below), the results from the adult surveillance strongly suggest that Ae. albopictus was the vector responsible for the CHIKV transmission.
Sequence and phylogenetic analyses of the CHIKV E1 gene
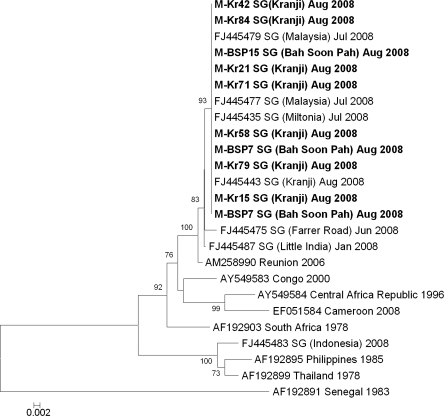
Sequencing of the E1 gene was performed on all seven RT-PCR-positive mosquitoes. Phylogenetic inferences (Fig. 2) showed that all CHIKV isolated from Ae. albopictus clustered with those sequences obtained from human cases from the most recent outbreak (Ng et al. 2009b), including those isolated from human cases working in the concrete slabs-manufacturing factory. They belonged to the ECSA genotype, and had a valine at residue 226 in the E1 gene.
FIG. 2.
Phylogenetic tree based on the E1 envelope glycoprotein genes of chikungunya produced by the neighbor-joining method. Sequences highlighted in bold are obtained from Ae. albopictus and those underlined are from human cases detected in Singapore. Whenever available, all sequences are labeled with GenBank accession numbers and country of origin and isolated by year/month. In addition, Singapore isolates are labeled with the reported area or country (imported human cases). Figures on the branches are bootstrap percentages based on 1000 replicates and only those above 70% are shown. SG, Singapore.
Search and destroy operations cum larvae surveillance
During the first 4 days of operation (days 0–4, excluding day 2), a total of about 2275 Ae. albopictus larvae was collected from 34 breeding habitats detected in the factory premise. The most common breeding habitats were discarded receptacles, canvas/plastic sheets, and domestic containers. A summary of the Ae. albopictus larval habitats and average number of immature per habitat found in the study site is listed in Table 3. No Ae. aegypti was found.
Table 3.
Summary of Aedes albopictus Larval Habitats and Average Number of Immatures per Habitat Detected in the Premises of the Concrete Slab Manufacturing Factory During the 3 Days of Search and Destroy Operation
| Larval habitat | Number of habitats found with Ae. albopictus larvae | Average number of immatures per habitat |
|---|---|---|
| Discarded receptacles | 8 | 43 |
| Canvas/plastic sheets | 6 | 70 |
| Domestic containers | 8 | 93 |
| “U”-channel metal racks | 3 | 83 |
| Discarded boots | 2 | 30 |
| Tires | 2 | 35 |
| Machinery parts | 1 | 30 |
| Concrete slab | 1 | 50 |
| Safety barrier | 1 | 200 |
| Ground puddle | 1 | 50 |
| Coconut husk | 1 | 50 |
Assessment of vector control operations
To assess the impact of the vector control measures on Ae. albopictus population, the same number of BGS traps were set up at the same locations. Postintervention surveillance using the BG Trap (days 1–2) showed a reduction of 92.3% in the number of female Ae. albopictus caught (Table 1 and Fig. 1), demonstrating a significant reduction in the Ae. albopictus population. However, RT-PCR revealed that one of the three Ae. albopictus caught was infected and had disseminated CHIKV (Tables 2 and 3). Despite a reduction in mosquito population, there was still evidence of infected mosquitoes. The findings led to a follow-up indoor ULV misting and outdoor thermal fogging on days 3, 4, and 5 to further reduce the risk of transmission. Subsequently, adult mosquito surveillance using the BGS Trap did not yield any adult mosquitoes for 4 weeks (Table 2 and Fig. 1). However, on week 5 (day 36), six adult Ae. albopictus were caught and the number of Ae. albopictus caught increased in the following weeks (Table 2). The adult mosquito surveillance was discontinued after week 7 (day 50). All Ae. albopictus collected from day 36 onward were found to be negative for the CHIKV. In addition, no further cases were notified after day 5 (Fig. 1).
Discussion
The interruption of transmission at the study site showed that the comprehensive combination vector control strategies were effective in reducing the risk of chikungunya. Chemical adulticiding was to effect an immediate sharp reduction of Ae. albopictus population; of particular importance was to remove infective vectors. Thorough source reduction and larviciding were to minimize emergence of new mosquitoes that could cause a next wave of transmission through acquiring the virus from viraemic patients in the area. Clearing of unkempt vegetations and general housekeeping aimed to minimize further breeding of vectors. A similar integrated approach has previously been reported to be successful for malaria control in forested areas in Singapore (Lee et al. 2010). Although it was not feasible to quantify the contribution of each component to the reduction in Aedes population, it is unlikely that the weekly use of four BGS traps had any trap-out effect. The total number of 70 Ae. albopictus adults caught by the traps is small compared to the >2200 larvae found and destroyed (Table 3). It is also supported by other studies on Ae. albopictus in Singapore (C.H. Tan, unpublished data) and a previous report on Ae. aegypti (Williams et al. 2007), where the population of the Aedes mosquitoes were not reduced by weekly and long-term use of the trap.
Although five more cases were detected postintervention from day 2 to 5 (Fig. 1), it is unlikely that the cases were infected postintervention. By day 2, they were likely already in the incubation period, which usually lasts 3–7 days. No further cases were reported from the study site.
Entomological investigation conducted at the concrete slab factory yielded only Ae. albopictus and Culex sp. mosquitoes. No Ae. aegypti larvae or adults were found. The finding of Ae. albopictus females disseminated with CHIKV that had the E1 gene sequence identical to those isolated from the patients from the study area confirmed the vector role of Ae. albopictus. It led to the revision of chikungunya control strategy in Singapore (Ng et al. 2009b). As Ae. albopictus are generally exophagic and exophilic mosquitoes in contrast to Ae. aegypti, we included measures such as outdoor thermal fogging and expansion of source reduction effort to include Ae. albopictus habitats, in controlling this and subsequent clusters.
The viruses isolated in this cluster were of the A226V variant of the ECSA genotype. Globally, the A266V mutation has been acquired by CHIKV in at least three independent events (de Lamballerie et al. 2008, Hapuarachchi et al. 2010), in Reunion, India, Cameroon, and possibly Sri Lanka. It has been shown that the single A226V mutation in the CHIKV has increased the susceptibility of Ae. albopictus to the CHIKV. This effective partnership between Ae. albopictus and the A226V mutant CHIKV conferred a higher vector competence to Ae. albopictus when compared to Ae. aegypti (Tsetsarkin et al. 2007, Vazeille et al. 2007). Studies have also shown the short extrinsic incubation period of the mutant CHIKV, which can be detected virus in the saliva of Ae. albopictus and Ae. aegypti 2 days after oral infection (Dubrulle et al. 2009). EHI has also observed that 33.3% of Ae. aegypti and 60% of Ae. albopictus had disseminated A226V CHIKV in the salivary glands on day 3 postinfectious blood meal (C.H. Tan, unpublished data). Together, these suggested a high epidemic potential of the Ae. albopictus–CHIKV–A226V partnership.
The high epidemic potential of CHIKV in Ae. albopictus was also reflected in the high infection rate (9.5%) of the female Ae. albopictus caught in this study. In another cluster (Bah Soon Pah area), 13% of female Ae. albopictus caught were found to have disseminated infection (C.H. Tan, unpublished data).
Like Ae. aegypti, Ae. albopictus is highly anthropophagic mosquitoes. Ae. albopictus has been shown to prefer human as their source of blood meal (Ponlawat and Harrington 2005). The relatively high proportion of Ae. albopictus caught in and around living quarters in our study further demonstrated such feeding behavior. Their multiple feeds within a single gonotrophic cycle (Ponlawat and Harrington 2005) coupled with a short extrinsic incubation period for CHIKV makes it a highly efficient vector to transmit the virus. In the Reunion island, 35% of 770,000 residents were infected within 15 months. Similarly, in India, Mayotte, Lamu Island, and Comoros, attack rate ranges from 37.5% to 75% (Ng et al. 2009a).
In Singapore, large clusters of chikungunya attributable to the A226V strain were seen in less urbanized areas of Singapore where Ae. albopictus was the predominant species (Ng et al. 2009b). Although sporadic cases of chikungunya fever caused by the mutant strain have also been reported in urban parts of Singapore where Ae. aegypti is the predominant mosquitoes, local transmission in these areas was limited. This could be due to Ae. aegypti being a less competent vector and/or the aggressive dengue control program, which mainly targets the urban and suburban parts of Singapore where dengue fever is transmitted by Ae. aegypti.
The success in interrupting transmission in Kranji demonstrated the benefit of a program that includes good coordination between field and laboratory personnel. The data gathered using field and laboratory tools had assisted in situation assessment on the ground, and in operational decision-making in controlling the spread of chikungunya in Singapore. First, through the field and laboratory investigation, it became clear that we had to target Ae. albopictus that were resting both indoors and outdoors. Second, the postintervention evaluation that found infected mosquitoes revealed the need for more intervention, without which, we would probably have seen more cases due to the continued presence of infected mosquitoes in the area.
Acknowledgment
We thank the staff from the Environmental Health Department who facilitated the field investigation, colleagues at EHI, especially Dr. Chanditha Hapuarachchige and Dr. Indra Vythilingam, for their critical reading of this article. We also thank the Ministry of Finance for the Reinvestment Fund made available for the study.
Authors' Contributions
C.H.T. led the field investigation and the molecular aspect of the laboratory investigation, and prepared the article. P.S.W. and M.Z.I.L. participated in the field and laboratory investigations. S.C.P. and S.G.L.P. were involved in the field investigation and mosquito identification. S.Y.S.T., T.K.C.L., A.B.P., S.Y.K., and D.L. participated in the field investigation. NM coordinated the vector control operations. L.C.N. conceptualized and oversaw the study, and participated in the field investigation and the writing of article.
Disclosure Statement
No competing financial interests exists.
References
- Beesoon S. Funkhouser E. Kotea N. Spielman A. Robich RM. Chikungunya fever, Mauritius, 2006. Emerg Infect Dis. 2008;14:337–338. doi: 10.3201/eid1402.071024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastel C. [Chikungunya virus: its recent spread to the southern Indian Ocean and Reunion Island (2005–2006)] Bull Acad Natl Med. 2005;189:1827–1835. [PubMed] [Google Scholar]
- de Lamballerie X. Leroy E. Charrel RN. Ttsetsarkin K, et al. Chikungunya virus adapts to tiger mosquito via evolutionary convergence: a sign of things to come? Virol J. 2008;5:33. doi: 10.1186/1743-422X-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diallo M. Thonnon J. Traore-Lamizana M. Fontenille D. Vectors of Chikungunya virus in Senegal: current data and transmission cycles. Am J Trop Med Hyg. 1999;60:281–286. doi: 10.4269/ajtmh.1999.60.281. [DOI] [PubMed] [Google Scholar]
- Dubrulle M. Mousson L. Moutailler S. Vazeille M. Failloux AB. Chikungunya virus and Aedes mosquitoes: saliva is infectious as soon as two days after oral infection. PLoS ONE. 2009;4:e5895. doi: 10.1371/journal.pone.0005895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB. Dengue and dengue hemorrhagic fever. In: Kliegman RM, editor; Behrman RF, editor; Jenson HB, editor. Nelson Textbook of Pediatrics, 18th edition, chapter 266. Philadelphia, WB: Saunders; 2007. online edition. [Google Scholar]
- Hapuarachchi HC. Bandara KB. Sumanadasa SD. Hapugoda MD, et al. Re-emergence of Chikungunya virus in South-east Asia: virological evidence from Sri Lanka and Singapore. J Gen Virol. 2010;91:1067–1076. doi: 10.1099/vir.0.015743-0. [DOI] [PubMed] [Google Scholar]
- Josseran L. Paquet C. Zehgnoun A. Caillere N, et al. Chikungunya disease outbreak, Reunion Island. Emerg Infect Dis. 2006;12:1994–1995. doi: 10.3201/eid1212.060710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki Njenga M. Nderitu L. Ledermann JP. Ndirangu A, et al. Tracking epidemic Chikungunya virus into the Indian Ocean from East Africa. J Gen Virol. 2008;89:2754–2760. doi: 10.1099/vir.0.2008/005413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K. Chhabra M. Katyal R. Patnaik PK, et al. Investigation of an outbreak of chikungunya in Malegaon Municipal areas of Nasik district, Maharashtra (India) and its control. J Vector Borne Dis. 2008;45:157–163. [PubMed] [Google Scholar]
- Lee V. Ow S. Heah H. Tan MY, et al. Elimination of mosquito-borne disease risk through integrated combination strategies in a Tropical Military Training Island. Am J Trop Med Hyg. 2010;82:1024–1029. doi: 10.4269/ajtmh.2010.09-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo YS. Chow AL. Tan LK. Lye DC, et al. Chikungunya outbreak, Singapore, 2008. Emerg Infect Dis. 2009;15:836–837. doi: 10.3201/eid1505.081390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel-de-Freitas R. Eiras AE. Lourenco-de-Oliveira R. Field evaluation of effectiveness of the BG-Sentinel, a new trap for capturing adult Aedes aegypti (Diptera: Culicidae) Mem Inst Oswaldo Cruz. 2006;101:321–325. doi: 10.1590/s0074-02762006000300017. [DOI] [PubMed] [Google Scholar]
- Ministry of Health Singapore. Singapore's first chikungunya outbreak—surveillance and response. www.moh.gov.sg/mohcorp/publicationsnewsbulletins.aspx?id=19542 Epidemiol News Bull. 2008;35:25–28. [Google Scholar]
- Ng LC. Lam S. Teo D. Epidemiology of dengue and chikungunya viruses and their potential impact on the blood supply. ISBT Sci Ser. 2009a;4:357–367. [Google Scholar]
- Ng LC. Tan LK. Tan CH. Tan SSY, et al. Entomologic and virologic investigation of Chikungunya, Singapore. Emerg Infect Dis. 2009b;15:1243–1249. doi: 10.3201/eid1508.081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noridah O. Paranthaman V. Nayar SK. Masliza M, et al. Outbreak of chikungunya due to virus of Central/East African genotype in Malaysia. Med J Malays. 2007;62:323–328. [PubMed] [Google Scholar]
- Pastorino B. Muyembe-Tamfum JJ. Bessaud M. Tock F, et al. Epidemic resurgence of Chikungunya virus in democratic Republic of the Congo: identification of a new central African strain. J Med Virol. 2004;74:277–282. doi: 10.1002/jmv.20168. [DOI] [PubMed] [Google Scholar]
- Peyrefitte CN. Rousset D. Pastorino BA. Pouillot R, et al. Chikungunya virus, Cameroon, 2006. Emerg Infect Dis. 2007;13:768–771. doi: 10.3201/eid1305.061500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponlawat A. Harrington LC. Blood feeding patterns of Aedes aegypti and Aedes albopictus in Thailand. J Med Entomol. 2005;42:844–849. doi: 10.1093/jmedent/42.5.844. [DOI] [PubMed] [Google Scholar]
- Powers AM. Logue CH. Changing patterns of chikungunya virus: re-emergence of a zoonotic arbovirus. J Gen Virol. 2007;88:2363–2377. doi: 10.1099/vir.0.82858-0. [DOI] [PubMed] [Google Scholar]
- Renault P. Solet JL. Sissoko D. Balleydier E, et al. A major epidemic of chikungunya virus infection on Reunion Island, France, 2005–2006. Am J Trop Med Hyg. 2007;77:727–731. [PubMed] [Google Scholar]
- Rezza G. Nicoletti L. Angelini R. Romi R, et al. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- Robinson MC. An epidemic of virus disease in Southern Province, Tanganyika Territory, in 1952–53. I. Clinical features. Trans R Soc Trop Med Hyg. 1955;49:28–32. doi: 10.1016/0035-9203(55)90080-8. [DOI] [PubMed] [Google Scholar]
- Rueda LM. Pictorial keys for the identification of mosquitoes (Diptera: Culicidae) associated with dengue virus transmission. Zootaxa. 2004;589:1–60. [Google Scholar]
- Saito N. Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Sam IC. Chan YF. Chan SY. Loong SK, et al. Chikungunya virus of Asian and Central/East African genotypes in Malaysia. J Clin Virol. 2009;46:180–183. doi: 10.1016/j.jcv.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Sang RC. Ahmed O. Faye O. Kelly CL, et al. Entomologic investigations of a chikungunya virus epidemic in the Union of the Comoros, 2005. Am J Trop Med Hyg. 2008;78:77–82. [PubMed] [Google Scholar]
- Sissoko D. Ezzedine K. Moendandze A. Giry C, et al. Field evaluation of clinical features during chikungunya outbreak in Mayotte, 2005–2006. Trop Med Int Health. 2010;15:600–607. doi: 10.1111/j.1365-3156.2010.02485.x. [DOI] [PubMed] [Google Scholar]
- Tsetsarkin KA. Vanlandingham DL. McGee CE. Higgs S. A single mutation in chikungunya virus affects vector specificity and epidemic potential. PLoS Pathog. 2007;3:e201. doi: 10.1371/journal.ppat.0030201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazeille M. Moutailler S. Coudrier D. Rousseaux C, et al. Two Chikungunya isolates from the outbreak of La Reunion (Indian Ocean) exhibit different patterns of infection in the mosquito, Aedes albopictus. PLoS ONE. 2007;2:e1168. doi: 10.1371/journal.pone.0001168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CR. Long SA. Webb CE. Bitzhenner M, et al. Aedes aegypti population sampling using BG-Sentinel traps in north Queensland Australia: statistical considerations for trap deployment and sampling strategy. J Med Entomol. 2007;44:345–350. doi: 10.1603/0022-2585(2007)44[345:aapsub]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Yergolkar PN. Tandale BV. Arankalle VA. Sathe PS, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–1583. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]