Abstract
Nonsteroidal anti-inflammatory drugs (NSAIDs) are a primary choice of therapy for diseases with a chronic inflammatory component. Unfortunately, long-term NSAID therapy is often accompanied by severe side effects, including cardiovascular and gastrointestinal complications. Because of this, there is critical need for identification of new and safer treatments for chronic inflammation to circumvent these side effects. Inflammatory diseases have been successfully remedied with natural herbs by many cultures. To better understand the potential of natural herbs in treating chronic inflammation and to identify their mechanism of action, we have evaluated the anti-inflammatory activities of 20 medicinal herbs commonly used in the Hispanic culture. We have established a standardized method for preparing aqueous extracts (teas) from the selected medicinal herbs and screened for inhibition of tumor necrosis factor-α-induced activation of nuclear factor κB (NF-κB), which is the central signaling pathway of the inflammatory response. A number of herbal teas were identified that exhibited significant anti-inflammatory activity. In particular, tea from the herb commonly called laurel was found to be an especially potent inhibitor of NF-κB-dependent cyclooxygenase-2 gene expression and prostaglandin E2 production in cultured murine macrophages. These findings indicate that laurel tea extract contains potent anti-inflammatory compounds that function by inhibiting the major signal transduction pathway responsible for inducing an inflammatory event. Based on these results, laurel may represent a new, safe therapeutic agent for managing chronic inflammation.
Key Words: anti-inflammatory agent, cyclooxygenase-2, nuclear factor κB, signaling, tea
Introduction
Nonsteroidal anti-inflammatory drugs (NSAIDs) remain at the forefront for treatment of rheumatoid arthritis and other types of inflammatory conditions. NSAIDs target cyclooxygenases (COXs), which are the rate-limiting enzymes involved in the conversion of arachidonic acid into inflammatory prostaglandins (PGs). In spite of their potency as anti-inflammatory agents, recent studies suggest caution in using NSAIDs for long-term therapy1–3 due to often severe side effects. For example, use of selective COX-2 inhibitors, such as rofecoxib, can lead to thrombotic cardiovascular events through inhibition of prostacyclin formation in the infarcted heart.4 Nevertheless, NSAIDs remain a primary choice of therapy for individuals afflicted with severe arthritic conditions, if the clinical benefit of anti-inflammatory therapy outweighs the risk of cardiovascular and gastrointestinal complications.
There is interest in identifying new treatments for chronic inflammation to circumvent the side effects of NSAID therapy. Herbal teas have been used for centuries in folk medicine to treat inflammatory ailments, and their use is an important holistic approach to medical care widely practiced in Latin American countries and in the southwest region of the United States. The goal of holistic medicine is to restore the balance among mind, body, and spirit in their patients to re-establish health. Of the many substances used by these holistic healers, herbal medicines are among the most common. Herbal extracts, especially teas, are widely used for a variety of ailments, including inflammatory diseases.5 In spite of their widespread acceptance and use, little information is available describing their mechanism of action. In light of the significant health risks posed by the use of NSAIDs, the time-tested use of herbal teas warrants a more in-depth scientific inquiry to prove or disprove their therapeutic potential in treatment of anti-inflammatory disorders.
To address this potential, we have evaluated the anti-inflammatory activities of herbal teas. Twenty medicinal herbs commonly used in Hispanic communities were evaluated for their capacity to inhibit activation of the nuclear factor-κB (NF-κB) signaling pathway, which is the central signaling pathway of the inflammatory response.6 NF-κB controls expression of a wide range of pro-inflammatory cytokines and enzymes.7,8 Because many purified natural products are known to inhibit the activation of NF-κB, we hypothesized that potential anti-inflammatory compounds in Hispanic medicinal herbs may also function through inhibition of this pathway. A standardized method for preparing teas from the selected medicinal herbs was developed, and the teas were screened for inhibition of tumor necrosis factor-α (TNFα)-induced activation of NF-κB using a cell-based reporter assay. A number of herbal teas were identified that exhibited significant anti-inflammatory activity. Tea from the herb commonly called laurel was found to be an especially potent inhibitor of NF-κB-dependent COX-2 gene expression and PGE2 production in cultured murine macrophages.
Materials and Methods
Source of Hispanic medicinal herbs
Commonly used Hispanic medicinal herbs were purchased from Rio Grande Herb Company, Albuquerque, NM, USA. Herbs were obtained as dried specimens. The following herbs were used: Ameranthus (common name Alegria); Anemopsis californica (Yerba del Manso); Artemisia franserioides (Altemisa); Carthamus tinctorius (Azafran); Chenopodium ambros (Epazote); Cinchona sp. (Copalquin); Ephedra viridis (Canutillo); Hibiscus sp. (Jamaica); Juglans sp. (Nogal); Laurel nobilis (laurel); Lavandula sp. (Alucema); Linum lewissii (Linasa); Marrubium vulgare (Mastronzo); Meliilotus alba (Trebol); Mentha spicata (Yerba Buena); Ocimuim basilicum (albacar); Prunus melanocarpa (Capulin); Rosa sp. (Rosa de Castillo); Rosmarinus officinalis (Romero); and Rumex hymenosepalus (Cana Agria).
Standardized preparation of herbal extracts
An often-expressed concern of holistic medicinal treatments is a lack of standardization when preparing herbal reagents for testing. In order to maintain consistency between preparations for direct quantitative comparisons in functional assays, a standard aqueous extraction protocol was designed. Extracts were prepared by heating dried, powdered herbs (0.5 g) in ultrapure water (10 mL, >15 MΩ resistance) at 85°C for 30 minutes. The aqueous fraction was then filter-sterilized and stored at 4°C in the dark until use or lyophilized for long-term storage at −80°C. This extraction procedure resulted in 9 μg of dry-weight material/μL of tea extract. Adherence to this standard protocol not only permits accurate quantitative comparisons between herbal extracts, but also affords the opportunity to compare different harvests of the same teas to account for seasonal variations. In addition, after freeze-drying and reconstituting the most active tea, consistent values were obtained in bioactivity screens, suggesting that the active ingredients in this tea are stable and that this method is a reliable method for standardizing teas.
Screening with a NF-κB/luciferase reporter cell line
An NF-κB reporter cell line, constructed using human 293T embryonic kidney cells (293T/NFκB-luc, Panomics, Inc., Redwood City, CA, USA), was used for initial screening. NF-κB activation was measured by treating cells with TNFα for a predetermined period followed by measuring luciferase activity, which is the reporter gene under control of a promoter with NF-κB binding sites. Inhibition of NF-κB activity by a medicinal tea was quantified by measuring the capacity of the tea extract to inhibit luciferase expression. For this assay, cells were grown in a humidified atmosphere at 37°C in 5% CO2/95% air with Dulbecco's Modified Eagle's Medium (high glucose containing 4 mM glutamine) supplemented with 10% fetal bovine serum, 1 mM sodium pyruvate, 100 units/mL penicillin, 100 μg/mL streptomycin, and 100 μg/mL hygromycin (Gibco/Invitrogen, Carlsbad, CA, USA). One day prior to treatment, the 293T/NFκB-luc cells were plated into 24-well cell culture plates (Costar, Cambridge, MA, USA) at approximately 70% confluency in the above medium without hygromycin. The following day fresh medium was applied to cells 1 hour prior to treatment. Medium with or without 20 ng/mL recombinant TNFα (R&D Biosciences/Clontech, Palo Alto, CA, USA) was then applied to cells followed by immediate addition with herbal tea extracts. Cells were then placed in a humidified atmosphere at 37°C in 5% CO2/95% air for 7 hours. After this incubation, wells were gently washed with phosphate-buffered saline (pH 7.4) and lysed with 1 × passive lysis buffer (Promega, Madison, WI, USA). Lysates were then analyzed with the luciferase assay system (Promega) using a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA, USA). Luciferase relative light units were normalized to protein as determined with the BCA™ protein assay kit (Pierce, Rockford, IL, USA) and standardized to percentage of control (TNFα control).
Fractionation of laurel tea extract
Lyophilized laurel tea extract (17 mg) was extracted with boiling methanol (10 mL), cooled, and centrifuged to remove insoluble material. Methanol was evaporated from supernatant, and solids were reconstituted in 1 mL of methanol:water (1:1 vol/vol) and fractionated using a Sephadex LH-20 column (1 × 15 cm; exclusion limit, 4,000–5,000 Mr) and 1:1 (vol/vol) methanol:water solvent phase. Fractions 1–5 (2 mL each) were collected, pooled, lyophilized, reconstituted in water (labeled fraction 1, λmax = 266 nm), and tested for inhibition of NF-κB activity using the Panomics NF-κB reporter cell line. Similarly, fractions 6–9 and fractions 10–26 were pooled and tested as fractions labeled 2 (λmax = 276 nm) and 3 (λmax = 266 nm with an additional peak at 350 nm), respectively. The methanol-insoluble material from the original extraction was used as fraction 4.
Quantitative real-time polymerase chain reaction (qRT-PCR) analysis of COX-2 mRNA
BV-2 macrophage cells (kindly provided by Dr. Paul M. Stemmer, Institute of Environmental Health Sciences, Wayne State University, Detroit, MI, USA) were grown in Dulbecco's Modified Eagle's Medium supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% CO2/95% air. Cells were plated in 12-well plates for assay (2 × 105 cells per well). When cells reached 80–90% confluency, they were activated with 0.2 μg/mL lipopolysaccharide (LPS) purified from Escherichia coli (catalog number L4391; Sigma-Aldrich, St. Louis, MO, USA) together with various concentrations of herbal tea for 16 hours at 37°C in 5% CO2/95% air. Total RNA was purified from cells using RNeasy (Qiagen, Valencia, CA, USA) and converted to cDNA using TaqMan® reverse transcriptase (Applied Biosystems, Branchburg, NJ, USA). COX-2 and β-actin expression levels were measured by qRT-PCR analysis of cDNA samples. Primers specific for COX-2 (GenBank accession number NM_011198) were designed to amplify a 132-basepair sequence flanking intron 7. Primer sequences for COX-2 were as follows: upstream, TGGGGTGATGAGCAACTATT; downstream, AAGGAGCTCTGGGTCAAACT. Primers specific for β-actin (GenBank accession number NM_007393) were designed to amplify 287 basepairs flanking intron 3. Primer sequences for β-actin were as follows: upstream, CCTGAACCCTAAGGCCAACC; downstream, CAGCTGTGGTGGTGAAGCTG. qRT-PCR was performed using ABsolute QPCR SYBR Green Mix (Fisher Scientific, Atlanta, GA, USA) with the following cycling parameters: one cycle, 95°C, 15 minutes; 40 cycles, 95°C, 15 seconds; 63°C, 1 minute. Because β-actin mRNA levels are unaffected by LPS treatment, they were quantified in each sample by applying identical cycling conditions and used to normalize values obtained for COX-2 expression. Changes in gene expression were determined by the comparative CT method as follows: the amount of COX-2 message in LPS-activated cells incubated with herbal tea was normalized to the internal reference (β-actin) and compared to the COX-2 message in activated cells without herbal tea treatment. Resveratrol, an established inhibitor of NF-κB activation,9–12 served as the positive control in these experiments. Values obtained for treatment with herbal teas were directly compared to those obtained with resveratrol.
Measurement of secreted PGE2 levels by enzyme-linked immunosorbent assay (ELISA)
BV-2 cells were activated with 0.2 μg/mL LPS together with various concentrations of herbal tea for 24 hours in a humidified atmosphere at 37°C in 5% CO2/95% air. After this incubation, culture medium was removed, and secreted PGE2 levels were quantified by competitive ELISA according to directions provided by the manufacturer (R&D Systems, Inc., Minneapolis, MN, USA).
Cytotoxicity assay
The WST-1 assay (Roche Molecular Biologicals, Indianapolis, IN, USA) was used to assess cytotoxicity of selected Hispanic medicinal herbs in BV-2 cells. In this assay, the WST-1 dye, a tetrazolium-based salt, is reduced by metabolically active cells, and the resulting intracellular purple formazan salt that is formed from this reaction remains soluble within cells and is directly quantified by spectrophotometric measurements. The following methodology was used to assess cell viability following treatment with herbal teas. Cells were plated into 96-well plates and allowed to attach for 24 hours. When cells reached 90–100% confluency, they were treated with fresh medium containing various amounts of herbal teas and incubated in a humidified atmosphere at 37°C in 5% CO2/95% air for 16–24 hours. WST-1 was then added directly to the cultures to a final concentration of 5% (vol/vol), and cells were incubated at 37°C for an additional 60 minutes. Absorbance was then read at 450 nm (690 nm, reference wavelength) using a Spectramax plate reader (Molecular Devices Co., Sunnyvale). Cell viability is reported as percentage relative to control cells receiving no herbal tea treatment.
Statistical analyses
All experimental measurements were carried out in triplicate. Mean values were determined from these replicate measurements, and error bars represent SD from mean values. Two or three independent assays were carried out for each experimental objective to ensure reproducibility.
Results
Inhibition of NF-κB activation by herbal teas
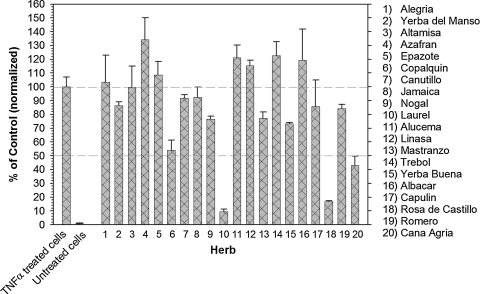
Twenty commonly used medicinal herbs were selected, and teas were prepared. Using an NF-κB/luciferase reporter cell line, the capacity of each tea to inhibit TNFα-induced activation of the pro-inflammatory transcription factor NF-κB was measured. Many of the herbal teas demonstrated NF-κB inhibitory activity, including Copalquin, laurel, Rosa de Castillo, and Cana Agria (Fig. 1). Notably, laurel and Rosa de Castillo teas were the most potent of the teas examined and were studied in greater detail.
FIG. 1.
Inhibition of TNFα-induced activation of NF-κB by Hispanic herbal teas. Herbal extracts were prepared according to a standard protocol and added to cultured NF-κB/luciferase reporter cells together with TNFα (20 ng/mL). Herbal extracts were applied at a final concentration of 50 μL of extract/1 mL of culture medium. Following a 7-hour incubation, cell lysates were prepared and assayed for luciferase activity as described in Materials and Methods.
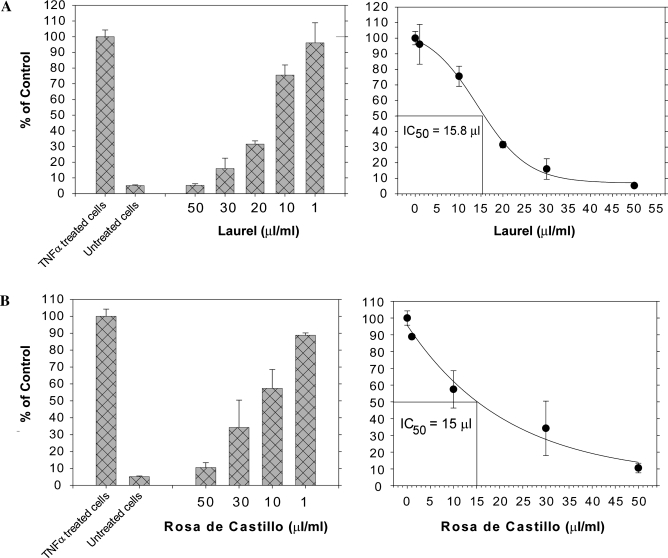
The 50% inhibitory concentration (IC50) values of Laurel and Rosa de Castillo were determined and expressed as microliters of tea added to 1 mL of standard growth medium. Laurel demonstrated an IC50 of 15.8 μL, and Rosa de Castillo showed an IC50 of 15 μL (Fig. 2). The antioxidant activities of these herbal teas were reported previously.13 There was no significant positive correlation between antioxidant activity and inhibition of activation of NF-κB, suggesting that the antioxidant activity of an herbal tea is not essential for anti-NF-κB activity. In fact, there was a significant negative correlation (r = −0.47, P = .035).
FIG. 2.
Laurel and Rosa de Castillo teas inhibit NF-κB activation in a dose-dependent manner. NF-κB/luciferase reporter cells were incubated with TNFα (20 ng/mL) together with the indicated amounts of (A) laurel or (B) Rosa de Castillo teas. Following a 7-hour incubation, cell lysates were prepared and assayed for luciferase activity as described in Materials and Methods. IC50 values were assigned as the amount of tea (in μL/mL of culture medium) that reduced luciferase activity to 50% of values obtained from cells treated with TNFα alone.
Stability of laurel and Rosa de Castillo teas
The stabilities of laurel tea and Rosa de Castillo tea were evaluated by retesting the activities of the standardized teas with the Panomics reporter assay. The standardized teas were kept at 4°C. Laurel tea was stable for months under these conditions, whereas Rosa de Castillo gradually lost activity over a similar time period (data not shown). In addition, laurel tea could be freeze-dried and reconstituted without loss of activity. Because of these observations, laurel tea was used for subsequent studies.
Fractionation of the activity of laurel tea
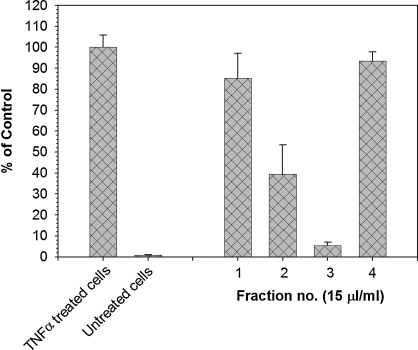
An important concept in complementary and alternative medicine is that complex mixtures, such as herbal teas, may be more active than any single component of the mixture and that there may be synergy among active components. To begin to explore this concept with laurel tea, the extract was fractionated by size exclusion chromatography. As shown in Figure 3, inhibitory activity for NF-κB activation was spread among multiple fractions, suggesting that multiple components contribute to the activity of laurel tea.
FIG. 3.
Fractionation of laurel tea demonstrates the presence of multiple active fractions. Components in laurel tea were separated into multiple fractions using size exclusion chromatography, and each fraction was tested for inhibition of NF-κB activity. Fractionated materials were applied at a final concentration of 50 μL of extract/1 mL of culture medium to NF-κB/luciferase reporter cells together with TNFα (20 ng/mL). Following a 7-hour incubation, cell lysates were prepared and assayed for luciferase activity as described in Materials and Methods. Insoluble material remaining after methanol extraction of the lyophilized laurel tea extract was also tested (fraction 4).
We also examined the partitioning of laurel's anti-inflammatory activity between aqueous and organic phases to better understand its chemical composition. A methanol extraction of the lyophilized aqueous extract isolated only a small fraction of the anti-inflammatory activity, indicating that this activity is found almost exclusively in the tea (Fig. 3). This observation was confirmed with an extract prepared from powdered laurel herb using ethyl acetate (data not shown).
Laurel tea inhibits COX-2 expression in a macrophage cell line
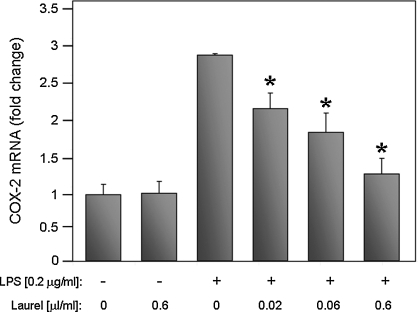
Because laurel tea proved to be a potent inhibitor of NF-κB activation and was stable following long-term storage, this tea was examined in more detail for its ability to inhibit expression of COX-2, which is an endogenous pro-inflammatory gene that is known to be regulated by NF-κB. For this analysis, the effect of laurel tea on COX-2 gene expression was measured in a murine macrophage cell line (BV-2). The NF-κB-dependent inflammatory response in BV-2 cells was stimulated using bacterial LPS. In parallel, cells were incubated for 24 hours without or with various amounts of tea or 50 μM resveratrol (positive control for NF-κB inhibition9–12). Similar to our previous findings,12 50 μM resveratrol inhibited COX-2 gene expression by 70%. Laurel tea was a potent inhibitor of COX-2 expression, with an IC50 value of 0.02 μL/mL of culture medium (Fig. 4). Interestingly, this value is far below the IC50 value measured in the cell-based reporter assay (Fig. 2; IC50 = 15.8 μL/mL), which may indicate that BV-2 cells are more sensitive to the effects of laurel tea than the reporter cell line or that laurel tea may be inhibiting COX-2 gene expression through alternative pathways in addition to inhibition of NF-κB activation.
FIG. 4.
Laurel tea reduces LPS-induced COX-2 gene expression in a murine macrophage cell line. BV-2 cells were incubated without (–) or with (+) LPS (0.2 μg/mL) together with the indicated amounts of laurel tea. After 24 hours COX-2 mRNA was quantified by qRT-PCR. *P < .05.
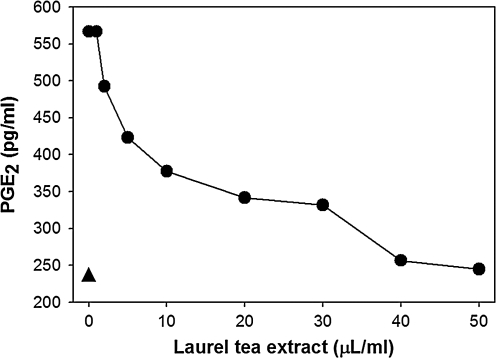
Since effects on transcriptional levels do not always reflect comparable changes in protein levels, the anti-inflammatory properties of laurel tea were also assessed by examining levels of PGE2, the downstream product of COX-2 gene activity. The inflammatory response in BV-2 cells was once again induced by LPS treatment, and, in parallel, cells were incubated with varying amounts of laurel tea. PGE2 levels in the culture medium were measured by a competitive ELISA. As shown in Figure 5, laurel tea reduced PGE2 levels in a dose-dependent manner with an IC50 value of 8 μL/mL of culture medium.
FIG. 5.
Laurel tea reduces secreted levels of PGE2. BV-2 cells were incubated with LPS (0.2 μg/mL) together with the indicated amounts of laurel tea (•). After 16 hours culture medium was removed, and PGE2 levels were quantified by a competitive ELISA. The basal level of PGE2 in the absence of LPS treatment is also given (▴).
Cytotoxicity measurements for laurel tea
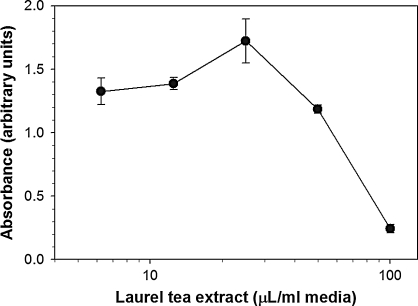
To examine the safety of laurel tea treatment at a cellular level, metabolic activity was measured in BV-2 cells following incubation with various amounts of tea for 24 hours using the tetrazolium salt-based WST-1 assay. Laurel tea showed minimal effects on BV-2 cells even when cells were exposed to amounts up to 40 μL/mL of culture medium (Fig. 6). Toxicity became measurable when cells were incubated with >50 μL/mL of culture medium, notably higher than the dose required to inhibit COX-2 gene expression and reduce secreted levels of PGE2.
FIG. 6.
Laurel tea treatment shows little cytotoxicity. BV-2 cells were incubated with the indicated amounts of tea extracts for 24 hours. The tetrazolium salt, WST-1, was added to cells followed by incubation for 1 hour. Soluble formazan was quantified by spectrophotometry (450 nm, 690 nm reference wavelength). Cells receiving no treatment generated a formazan reading of 1.55 ± 0.1 absorbance units.
Discussion
Use of herbs and other medicinal plant materials is widespread and continues to increase in popularity, especially in light of many recent examples of failed pharmaceuticals. The use of herbs is seldom monitored by practitioners of Western medicine, and as such their use can present unexpected complications due to adverse interactions with contemporary drugs or side effects, because of dosage errors or a poor understanding of their mechanism of action.14,15 Contributing to these complications is a serious lack of standardization. Standardization is necessary for comparing the mechanistic properties of herbal products and is important for monitoring potency for correct dosages.16
In the present study, a method of standardization was developed that begins with a defined procedure to prepare the tea extracts and includes a routine reporter-screening assay designed to identify inhibitors of NF-κB signal transduction. This screening assay can easily be adapted to assess batch-to-batch heterogeneity and standardize dosage regimens. From this procedure, laurel and Rosa de Castilla teas were identified as the most potent inhibitors of NF-κB activation from a survey of 20 Hispanic medicinal herbs. Laurel, but not Rosa de Castilla, was stable after repeated freeze-thawing and long-term storage. Because of this, attention was focused on laurel tea to examine its anti-inflammatory properties in greater depth. These studies revealed that laurel tea functions as a potent inhibitor of NF-κB signaling, which in turn reduces COX-2 gene expression and subsequent PGE2 production.
There is a long history supporting the use of laurel and Rosa de Castillo in herbal medicine. Laurel is the common name for L. nobilis of the Lauraceae family, but is also commonly referred to as bay laurel, bay leaf, Greek bay, sweet bay, and poet's laurel. Laurel is native to the southern Mediterranean region, where it is both wild and cultivated. Laurel is a commercial source of essential oils in Turkey, Algeria, Morocco, Portugal, Spain, Italy, Central America, the southern United States, and Mexico. The essential oils from the leaves and berries are used in perfumes, candles, and soaps.17,18 Laurel is commonly used in cooking or as an herbal treatment for a variety of ailments in the Hispanic culture. There are numerous biologically active families of compounds that have been identified from leaves and berries of laurel, including sesquiterpene lactones, alkaloids, glycosylated flavonoids, and monoterpene alcohols.19–22 The biological activities reported for isolated compounds include inhibition of gastric emptying,23 induction of apoptosis in leukemia cells,24 and trypanocidal activity.25 More complex mixtures, including essential oils, have been reported to exhibit anticonvulsant,26 antibacterial,27 and anti-inflammatory activity.28 Until the present study, there has been little information attempting to define a mechanism to explain any of these observed activities. One study showed that sesquiterpene lactones from bay leaf inhibited nitric oxide production in LPS-stimulated macrophage cells and, as a result, induced heat shock protein HSP72.29 It was suggested that HSP72 prevented activation of NF-κB, which is known to regulate expression of pro-inflammatory inhibitory nitric oxide synthase.
There are numerous biologically active compounds in extracts of rose fruit (rose hips) and petals from over 100 species of rose, including Rosa de Castillo. However, as with laurel, little information has been reported defining their mechanisms of action. R. canina extract is high in polyphenols as well as vitamin C and has been shown to inhibit the respiratory burst in neutrophils.30 R. canina extract also contains an anti-inflammatory galactolipid that inhibits neutrophil chemotaxis.31 Hips from a wide variety of rose species contains ellagic acid, an anticarcinogen and anti-inflammatory agent.32 Extracts of rose hip inhibit COX-1 and COX-2 gene expression, which may explain the efficacy of rose hip in treatment of arthritis.33 Subjects who take rose hip powder have lower levels of circulating C-reactive protein, consistent with the general view that rose species possess anti-inflammatory compounds.34 In addition, antimicrobial activities in rose hip extracts have been demonstrated.35 Monoterpenyl fatty acid esters and flavanol glycosides from rose petals contribute to the fragrance of roses but also to the antioxidant properties of rose petal tea and to the biological activities of rose essential oils.36–38
The common traditional approach would take a reductionist path to carry out complex isolation procedures to identify the component or components that are responsible for the measurable bioactivity. Although this is a logical and appealing approach for drug discovery, one must proceed cautiously so as not to lose the benefits of synergy, which often can only be found when using whole plant extracts. The use of whole or partially purified plant extracts can offer significant advantages over a single isolated component because of the inherent chemical complexity that is found in these extracts. Because of this chemical diversity, many biologically important targets can be affected simultaneously, providing the basis for synergy. This reasoning is an important consideration for use of herbal medicines and other plant-derived medicines in complementary and alternative medicine. Proponents of complementary and alternative medicine can cite many examples where a whole extract is more bioactive than any single component isolated from the extract. There are many possible explanations for this form of synergy.39–42 For example, an herbal extract may contain an inhibitor of an enzyme that degrades the active substance, thereby protecting the active substance and providing a therapeutic improvement in its biological activity. Alternatively, the herbal extract may contain a component that facilitates transport of the active substance into the target cell or may contain an inhibitor of an ATP-dependent xenobiotic transporter that normally would pump the active ingredient out of the cell. There is also the possibility that synergy results from the presence of several active components that inhibit different targets in a complex signaling pathway.
In summary, we present and validate a method for biochemical standardization that should prove useful for future studies comparing various tea extracts for bioactive properties. We also provide evidence that teas of laurel and Rosa de Castilla are inhibitors of the activation of NF-κB, which is an important transcription factor responsible for the induction of the inflammatory response. Laurel tea, selected because of its stability, was able to inhibit transcription of the NF-κB-dependent COX-2 gene and COX-2 activity, as determined by measuring PGE2.
Acknowledgments
This work was supported by grant AG027794 from the National Institutes of Health and grant-in-aid 0555647Z from the American Heart Association (to R.A.O.).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Borer JS. Simon LS. Cardiovascular and gastrointestinal effects of COX-2 inhibitors and NSAIDs: achieving a balance. Arthritis Res Ther. 2005;7(Suppl 4):S14–S22. doi: 10.1186/ar1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermann M. Ruschitzka F. Cardiovascular risk of cyclooxygenase-2 inhibitors and traditional non-steroidal anti-inflammatory drugs. Ann Med. 2007;39:18–27. doi: 10.1080/07853890601073445. [DOI] [PubMed] [Google Scholar]
- 3.Hinz B. Renner B. Brune K. Drug insight: cyclo-oxygenase-2 inhibitors—a critical appraisal. Nat Clin Pract Rheumatol. 2007;3:552–560. doi: 10.1038/ncprheum0619. quiz 551 following 589. [DOI] [PubMed] [Google Scholar]
- 4.White W. Cardiovascular risk, hypertension, and NSAIDs. Curr Pain Headache Rep. 2007;11:428–435. doi: 10.1007/s11916-007-0229-x. [DOI] [PubMed] [Google Scholar]
- 5.Meyer S. Meyer F. Traditional Herbs de Nuevo Mexico: A Complete Booklet of Traditional Uses and Beliefs of New Mexican Herbs. Rio Grande Herb Company; Albuquerque, NM: 1994. [Google Scholar]
- 6.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. Yamamoto Y. Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- 8.Nam NH. Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem. 2006;6:945–951. doi: 10.2174/138955706777934937. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S. Bueso-Ramos C. Aggarwal BB. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: role of nuclear factor-kappaB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002;62:4945–4954. [PubMed] [Google Scholar]
- 10.Bhardwaj A. Sethi G. Vadhan-Raj S, et al. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- 11.Estrov Z. Shishodia S. Faderl S, et al. Resveratrol blocks interleukin-1beta-induced activation of the nuclear transcription factor NF-kappaB, inhibits proliferation, causes S-phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood. 2003;102:987–995. doi: 10.1182/blood-2002-11-3550. [DOI] [PubMed] [Google Scholar]
- 12.Heynekamp JJ. Weber WM. Hunsaker LA, et al. Substituted trans-stilbenes, including analogues of the natural product resveratrol, inhibit the human tumor necrosis factor alpha-induced activation of transcription factor nuclear factor kappaB. J Med Chem. 2006;49:7182–7189. doi: 10.1021/jm060630x. [DOI] [PubMed] [Google Scholar]
- 13.VanderJagt TJ. Ghattas R. VanderJagt DJ. Crossey M. Glew RH. Comparison of the total antioxidant content of 30 widely used medicinal plants of New Mexico. Life Sci. 2002;70:1035–1040. doi: 10.1016/s0024-3205(01)01481-3. [DOI] [PubMed] [Google Scholar]
- 14.Bent S. Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med. 2008;23:854–859. doi: 10.1007/s11606-008-0632-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skalli S. Zaid A. Soulaymani R. Drug interactions with herbal medicines. Ther Drug Monit. 2007;29:679–686. doi: 10.1097/FTD.0b013e31815c17f6. [DOI] [PubMed] [Google Scholar]
- 16.Shan JJ. Rodgers K. Lai CT. Sutherland SK. Challenges in natural health product research: the importance of standardization. Proc West Pharmacol Soc. 2007;50:24–30. [PubMed] [Google Scholar]
- 17.Fiorini C. Fouraste I. David B. Bessiere J. Composition of the flower, leaf and stem essential oils from L. nobilis. Flavour Fragrance. 1997;12:91–93. [Google Scholar]
- 18.Putievsky E. Ravid U. Snir N. Sanderovich D. The essential oils from cultivated bay laurel. Isr J Bot. 1984;33:47–52. [Google Scholar]
- 19.De Marino S. Borbone N. Zollo F, et al. Megastigmane and phenolic components from Laurus nobilis L. leaves and their inhibitory effects on nitric oxide production. J Agric Food Chem. 2004;52:7525–7531. doi: 10.1021/jf048782t. [DOI] [PubMed] [Google Scholar]
- 20.El-Feraly F. Benigni D. Sesquiterpene lactones of Laurus nobilis leaves. J Nat Prod. 1980;43:527–531. [Google Scholar]
- 21.Novak M. A monoterpene alcohol from Laurus nobilis leaves. Phytochemistry. 1985;24:858–861. [Google Scholar]
- 22.Pech B. Bruneton J. Alkaloids of Laurus nobilis. J Nat Prod. 1982;45:560–563. [Google Scholar]
- 23.Matsuda H. Shimoda H. Ninomiya K. Yoshikawa M. Inhibitory mechanism of costunolide, a sesquiterpene lactone isolated from Laurus nobilis, on blood-ethanol elevation in rats: involvement of inhibition of gastric emptying and increase in gastric juice secretion. Alcohol Alcohol. 2002;37:121–127. doi: 10.1093/alcalc/37.2.121. [DOI] [PubMed] [Google Scholar]
- 24.Komiya T. Yamada Y. Moteki H, et al. Hot water soluble sesquiterpenes [anhydroperoxy-costunolide and 3-oxoeudesma-1,4(15),11(13)triene-12,6alpha-olide] isolated from laurel (Laurus nobilis L.) induce cell death and morphological change indicative of apoptotic chromatin condensation in leukemia cells. Oncol Rep. 2004;11:85–88. [PubMed] [Google Scholar]
- 25.Uchiyama N. Matsunaga K. Kiuchi F, et al. Trypanocidal terpenoids from Laurus nobilis L. Chem Pharm Bull (Tokyo) 2002;50:1514–1516. doi: 10.1248/cpb.50.1514. [DOI] [PubMed] [Google Scholar]
- 26.Sayyah M. Valizadeh J. Kamalinejad M. Anticonvulsant activity of the leaf essential oil of Laurus nobilis against pentylenetetrazole- and maximal electroshock-induced seizures. Phytomedicine. 2002;9:212–216. doi: 10.1078/0944-7113-00113. [DOI] [PubMed] [Google Scholar]
- 27.Dadalioglu I. Evrendilek GA. Chemical compositions and antibacterial effects of essential oils of Turkish oregano (Origanum minutiflorum), bay laurel (Laurus nobilis), Spanish lavender (Lavandula stoechas L.), and fennel (Foeniculum vulgare) on common foodborne pathogens. J Agric Food Chem. 2004;52:8255–8260. doi: 10.1021/jf049033e. [DOI] [PubMed] [Google Scholar]
- 28.Sayyah M. Saroukhani G. Peirovi A. Kamalinejad M. Analgesic and anti-inflammatory activity of the leaf essential oil of Laurus nobilis Linn. Phytother Res. 2003;17:733–736. doi: 10.1002/ptr.1197. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda H. Kagerura T. Toguchida I, et al. Inhibitory effects of sesquiterpenes from bay leaf on nitric oxide production in lipopolysaccharide-activated macrophages: structure requirement and role of heat shock protein induction. Life Sci. 2000;66:2151–2157. doi: 10.1016/s0024-3205(00)00542-7. [DOI] [PubMed] [Google Scholar]
- 30.Daels-Rakotoarison DA. Gressier B. Trotin F, et al. Effects of Rosa canina fruit extract on neutrophil respiratory burst. Phytother Res. 2002;16:157–161. doi: 10.1002/ptr.985. [DOI] [PubMed] [Google Scholar]
- 31.Larsen E. Kharazmi A. Christensen LP. Christensen SB. An antiinflammatory galactolipid from rose hip (Rosa canina) that inhibits chemotaxis of human peripheral blood neutrophils in vitro. J Nat Prod. 2003;66:994–995. doi: 10.1021/np0300636. [DOI] [PubMed] [Google Scholar]
- 32.Nowak R. Determination of ellagic acid in pseudofruits of some species of roses. Acta Pol Pharm. 2006;63:289–292. [PubMed] [Google Scholar]
- 33.Jager AK. Eldeen IM. van Staden J. COX-1 and −2 activity of rose hip. Phytother Res. 2007;21:1251–1252. doi: 10.1002/ptr.2236. [DOI] [PubMed] [Google Scholar]
- 34.Winther K. Rein E. Kharazmi A. The anti-inflammatory properties of rose-hip. Inflammopharmacology. 1999;7:63–68. doi: 10.1007/s10787-999-0026-8. [DOI] [PubMed] [Google Scholar]
- 35.Yi O. Jovel EM. Towers GH. Wahbe TR. Cho D. Antioxidant and antimicrobial activities of native Rosa sp. from British Columbia, Canada. Int J Food Sci Nutr. 2007;58:178–189. doi: 10.1080/09637480601121318. [DOI] [PubMed] [Google Scholar]
- 36.Dunphy PJ. Location and biosynthesis of monoterpenyl fatty acyl esters in rose petals. Phytochemistry. 2006;67:1110–1119. doi: 10.1016/j.phytochem.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 37.Basim E. Basim H. Antibacterial activity of Rosa damascena essential oil. Fitoterapia. 2003;74:394–396. doi: 10.1016/s0367-326x(03)00044-3. [DOI] [PubMed] [Google Scholar]
- 38.Schiber A. Mihalev K. Berardini N. Mollov P. Carle R. Flavonol glycosides from distilled petals of Rosa damascena Mill. Z Naturforsch [C] 2005;60:379–384. doi: 10.1515/znc-2005-5-602. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert B. Alves LF. Synergy in plant medicines. Curr Med Chem. 2003;10:13–20. doi: 10.2174/0929867033368583. [DOI] [PubMed] [Google Scholar]
- 40.Spelman K. Philosophy in phytopharmacology: Ockham's Razor versus synergy. J Herb Pharmacother. 2005;5:31–47. [PubMed] [Google Scholar]
- 41.Spinella M. The importance of pharmacological synergy in psychoactive herbal medicines. Altern Med Rev. 2002;7:130–137. [PubMed] [Google Scholar]
- 42.Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8:401–409. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]