Abstract
CD-NP is a novel chimeric natriuretic peptide (NP) consisting of the 22-amino-acid (AA) human C-type natriuretic peptide (CNP), a venodilating peptide with limited renal actions and minimal effects on blood pressure, and the 15-AA C-terminus of Dendroaspis NP (DNP). The rationale for the design of CD-NP was to enhance the renal actions of CNP, the ligand for natriuretic peptide receptor-B, but without inducing excessive hypotension. Here we report the first-in-human studies for CD-NP, which represent the first successful clinical testing of a chimeric NP demonstrating in normal human volunteers that CD-NP possesses cyclic guanosine monophosphate–activating, natriuretic, and aldosterone-suppressing properties without inducing excessive hypotension, laying the foundation for additional studies on this first-in-class new cardiovascular therapeutic in human heart failure, which are now underway worldwide.
Keywords: Chimeric natriuretic peptide, CD-NP, C-type natriuretic peptide, Dendroaspis natriuretic peptide
The design of novel chimeric peptides that are engineered to enhance the favorable properties of native peptides and delete or minimize unfavorable biological actions is an emerging therapeutic strategy in drug discovery.1,2 The novel chimeric natriuretic peptide, CD-NP,3 is being developed for the treatment of acute heart failure (AHF); there is a tremendous unmet need for therapy for this rapidly increasing cardiovascular syndrome, which has poor outcomes, and new drugs have repeatedly failed in clinical development.4
CD-NP represents the fusion of the 22-amino-acid (AA) human C-type natriuretic peptide (CNP) with the 15-AA C-terminus of Dendroaspis natriuretic peptide (DNP) (Figure 1).3 The rationale for its design was based on our knowledge that CNP,5 an endothelial6,7 cell–derived peptide, which mediates favorable cardiovascular hemodynamic effects via the natriuretic peptide receptor (NPR)-B and the second messenger cyclic guanosine monophosphate (cGMP), unloads the heart without inducing excessive hypotension.8 These cardiovascular properties are based on the previous demonstration that CNP primarily vasorelaxes isolated veins and not arteries.9 This is in contrast to DNP, atrial NP (ANP), and B-type NP (BNP), which activate cGMP via the NP receptor-A (NPR-A)10,11 and vasorelax both arteries and veins,12 resulting in more hypotension, which has limited their utility as cardiovascular therapeutics for AHF.2 A limitation to the use of CNP in AHF is that it has minimal renal-enhancing actions and does not suppress endogenous aldosterone synthesis.8
Figure 1.
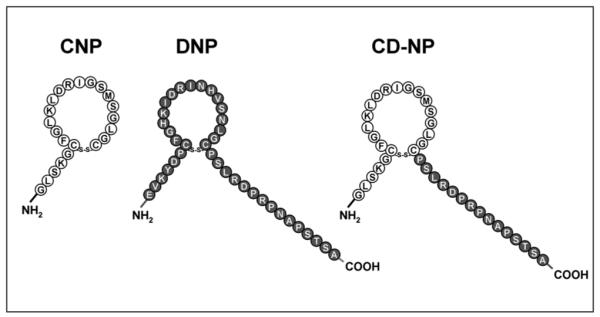
Structures and amino acid sequences of CNP, DNP, and CD-NP.
In normal anesthetized dogs, CD-NP significantly activated cGMP, the second messenger for the natriuretic peptides (NPs); exerted natriuretic and diuretic actions; enhanced glomerular filtration rate (GFR); reduced cardiac filling pressures; and suppressed the renin–angiotensin system.3,13 When CD-NP was compared with an equimolar dose of human BNP, less hypotension was observed with CD-NP in normal anesthetized dogs.3 Moreover, when compared with an equimolar dose of human CNP, CD-NP exhibited an enhanced renal and neurohumoral profile.13 In vitro evaluation of CD-NP in isolated canine glomeruli demonstrated that CD-NP resulted in an 8-fold greater cGMP response compared with an equimolar concentration of CNP.14 Moreover, the cGMP response to CD-NP in isolated glomeruli involved, at least in part, NPR-A, as evidenced by an attenuated cGMP response to CD-NP in the presence of NPR-A antagonism.14 In addition, CD-NP has been demonstrated to inhibit cardiac fibroblast proliferation,3 thus holding promise as a novel antiproliferative agent against ventricular remodeling beyond its favorable renalenhancing and cardiac-unloading actions.
Building on these promising preclinical findings, we sought to evaluate CD-NP for the first time in healthy subjects to test our hypothesis that CD-NP can be safety administered in humans and that the favorable effects of CD-NP observed in preclinical studies can be translated to humans.
METHODS
Study Design
After filing an Investigational New Drug application with the US Food and Drug Administration (no. 78,225), we proceeded to a first-in-human clinical trial in healthy volunteers (ClinicalTrials.gov identifier NCT00482937). This first-in-human clinical trial on CD-NP was conducted in accordance with the Declaration of Helsinki and its amendments, the US Food and Drug Administration Principles of Good Clinical Practice, and International Conference on Harmonization Guidelines, where applicable. Ethics approval was obtained prior to commencement of the study, which was conducted at a contract research organization in Minneapolis, Minnesota (DiVita Research Center, Hennepin County Medical Center, University of Minnesota). This clinical trial consisted of 2 phases: an open-label ascending-dose phase (phase 1) and a randomized, double-blind, placebo (PLB)–controlled phase (phase 2). In the ascending-dose phase (phase 1), cohorts of 4 subjects each were enrolled to evaluate the safety of CD-NP. The planned ascending dosage levels of CD-NP infusion were 10, 25, 50, 100, 200, and 300 ng/kg/min for administration as a continuous intravenous (IV) infusion over 4 hours (at a constant infusion rate across all dosage levels). Dose escalation was to discontinue in the event that 1 or more of the prespecified stopping rules were met, including the occurrence of clinically significant hypotension (defined as a decrease from baseline in sitting systolic blood pressure, SBP, ≥20 mm Hg and a decrease in sitting SBP to <90 mm Hg, or symptomatic hypotension, or orthostatic hypotension, or hypotension that required treatment), development of second- or third-degree atrioventricular block, intraventricular conduction defect, prolongation of the corrected QT interval to more than 450 milliseconds (where prolongation was defined as an increase from baseline ≥30 milliseconds), ventricular tachycardia greater than 5 beats; significant laboratory abnormalities; and excessive diuresis (defined as >6 L in 24 hours). In the double-blind, PLB-controlled phase (phase 2), 10 subjects were randomized in a 6:4 ratio to CD-NP versus PLB to confirm the safety and the pharmacodynamics of CD-NP at the maximum tolerated dose based on phase 1.
Screening of potential subjects was conducted within 28 days prior to dose administration. Subjects who were enrolled into the study checked into the clinic 4 days prior to dose administration and were started on a no-added-salt diet (120 mEq sodium per day, beginning 3 days prior to the study).
For determination of anti-CD-NP and CD-NP neutralizing antibodies, plasma samples were collected on day 1 (predose), day 7, and day 28.
Subjects
Main inclusion criteria were (1) healthy male, post-menopausal female, or surgically sterilized female 18 to 60 years of age; (2) a body mass index within the range of 18 to 34 kg/m2; (3) able to communicate effectively; (4) no significant disease or abnormal laboratory values; (5) a normal 12-lead electrocardiogram; (6) nonsmoker (defined as not having smoked in the past 6 months); and (7) adequately informed of the nature and risks of the study and gave written informed consent prior to receiving study medication.
Main exclusion criteria were (1) known hypersensitivity or allergy to CD-NP or its components, nesiritide, other natriuretic peptides, or related compounds; (2) women who were pregnant or breast-feeding; (3) any disease or condition (medical or surgical) which, in the opinion of the investigators, might compromise the hematologic, cardiovascular, pulmonary, renal, gastrointestinal, hepatic, or central nervous system or other conditions that might interfere with the absorption, distribution, metabolism, or excretion of study drug or would place the subject at increased risk; (4) the presence of abnormal laboratory values that were considered clinically significant; (5) positive screen for hepatitis B, hepatitis C, or human immunodeficiency virus; (6) received an investigational drug within 30 days prior to enrollment in the study; (7) received any drug therapy within 1 week, or 5 half-lives, prior to administration of the first dose of any study-related treatment (this exclusion criterion was extended to 4 weeks for any drugs that are known to induce or inhibit hepatic drug metabolism; use of nonsteroidal anti-inflammatory drugs, sulfonamides, probenecid, or other drugs known to alter renal or tubular function was prohibited for at least 5 half-lives prior to the first dose of any study-related treatment); (8) consumption of alcohol within 48 hours prior to dose administration or during any in-patient period; (9) a positive urine drug screen including ethanol, cocaine, tetrahydrocannabinol, barbiturates, amphetamines, benzodiazepines, and opiates; (10) a history (within the last 2 years) of alcohol abuse, illicit drug use, significant mental illness, physical dependence to any opioid, or any history of drug abuse or addiction; (11) a history of difficulty with donating blood; and (12) having donated blood or blood products within 45 days prior to enrollment.
Pharmacodynamic Assessments
Renal, hemodynamic, and neurohumoral responses were assessed. Main study variables included plasma cGMP and urinary cGMP excretion, urinary sodium excretion, urine flow rate, creatinine clearance (as an estimate for GFR), mean arterial pressure, and plasma angiotensin II and aldosterone levels. Treatment-emergent orthostatic hypotension was defined as a sustained decrease in SBP greater than 20 mm Hg (after standing for 3 minutes) and diastolic BP (DBP) greater than 10 mm Hg that either was not present at baseline or became worse after the start of treatment. Timed urine collections were obtained at baseline, 0-4, 4-8, 8-12, and 12-24 hours after the start of infusion.
Data Analysis
Statistical analyses were performed using Statistical Analysis Software (version 9, SAS Institute Inc, Cary, NC). Renal, hemodynamic, and neurohumoral data were analyzed for the CD-NP group and the PLB group in the randomized, double-blind phase of the clinical trial. Both within-group (comparing end of infusion vs baseline) and between-group comparisons (at baseline and at the end of infusion) were made, using parametric and nonparametric tests, where applicable.
RESULTS
Disposition of Subjects and Baseline Characteristics
A total of 22 subjects participated in this clinical trial. In phase 1, 4 subjects each received CD-NP 10, 25, and 17.5 ng/kg/min. In phase 2, 6 subjects received CD-NP 17.5 ng/kg/min (the maximum tolerated dose determined in phase 1) and 4 subjects received PLB. All 22 subjects completed the study.
The mean age was 38.7 (standard deviation [SD] 12.9) years, ranging from 19 to 60 years. Subjects across all cohorts and treatment groups were primarily males (19 subjects, 86.4%), Caucasian (20 subjects, 90.9%), and of Hispanic or Latino ethnicity (21 subjects, 95.5%).
Safety
In the dose-escalation phase (phase 1), healthy volunteers received a 4-hour infusion of CD-NP. At 10 ng/kg/min, CD-NP was well tolerated and appeared to be safe. At 25 ng/kg/min, 2 of the 4 subjects had symptomatic orthostatic hypotension following a diuretic response to CD-NP. Other adverse events observed at 25 ng/kg/min IV included flushing, dizziness, tachycardia, paresthesia, and dyspnea. The majority of these events occurred shortly after the infusion and were transient and mild in nature. Based on the above, the dose of CD-NP was lowered to 17.5 ng/kg/min IV. In the 4 subjects who received CD-NP 17.5 ng/kg/min IV, 2 non–treatment-related adverse events were observed, including upper respiratory tract infection and throat irritation. The maximum tolerated dose was set at 17.5 ng/kg/min IV.
Measurements of anti-CD-NP and CD-NP neutralizing antibodies on day 7 and day 28 fluctuated but did not raise concerns regarding immunogenicity.
Pharmacodynamics
After the maximal tolerated dose was determined to be 17.5 ng/kg/min, the double-blind study (phase 2) commenced, in which another cohort of 10 healthy subjects were randomized to CD-NP (17.5 ng/kg/min) for 4 hours or matching PLB in a 6:4 ratio. Compared with PLB, CD-NP increased plasma cGMP, urinary cGMP excretion, and urinary sodium excretion (Figures 2 and 3). Urine flow within the 4 hours of infusion increased in the CD-NP group versus baseline (mean ± standard error of the mean [SEM], 1.1 ± 0.2 to 2.3 ± 0.4 mL/min, P < .05; PLB 1.3 ± 0.2 to 1.6 ± 0.4 mL/min). A mild decrease in mean arterial pressure (MAP, Figure 2) was observed in the CD-NP group without any significant change in GFR (from 82 ± 7 to 84 ± 8 mL/min). Both MAP and GFR did not differ between the CD-NP group and the PLB group. Asymptomatic orthostatic hypotension was observed in 3 subjects in the CD-NP-treated group.
Figure 2.
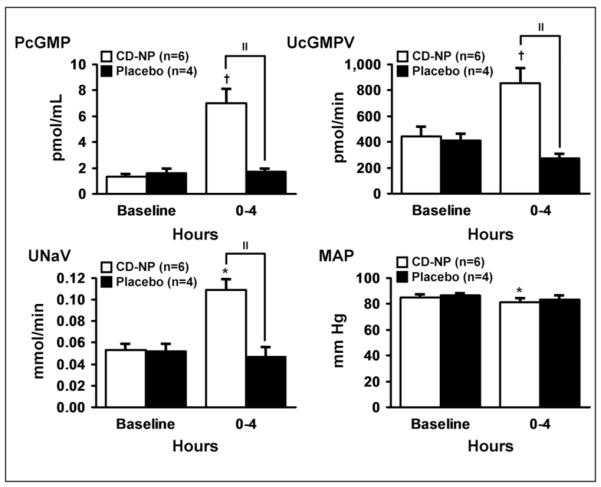
Plasma cGMP response (top left), urinary cGMP excretion (top right), natriuretic response (bottom left), and blood pressure response (bottom right). Mean ± SEM. Comparisons were made within (*P < .05, †P <.01) and between groups (“P < .01).
Figure 3.
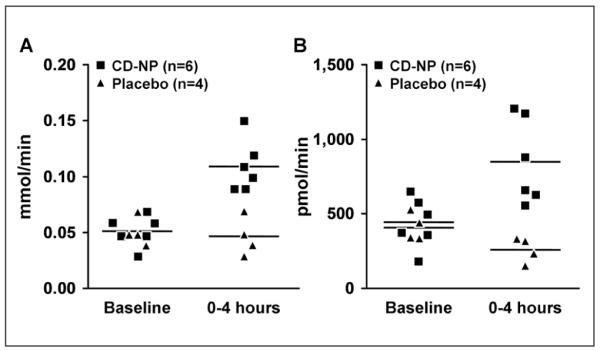
A plot of individual urinary sodium excretion (left panel, P < .05 vs baseline and P < .01 between groups) and urinary cGMP excretion (right panel, P < .01 vs baseline and P < .01 between groups) data. Mean ± SEM.
CD-NP suppressed plasma aldosterone (mean ± SD) from 21.9 ± 2.7 to 9.5 ± 3.2 ng/dL (P < .001), whereas no significant change was observed with PLB (20.3 ± 4.2 to 13.6 ± 4.5 ng/dL). Plasma angiotensin II levels (mean ± SD) at baseline and the end of infusion were 29.2 ± 5.2 and 23.0 ± 3.5 ng/L, respectively (P = .086 by 2-tailed t test, P = .69 by signed rank test). Plasma angiotensin II levels in the PLB group at baseline and at the end of infusion were 23.3 ± 2.2 and 19.8 ± 8.2 ng/L, respectively (not significant).
Overall, in this clinical trial, heart rate increased approximately 25% in cohort 1 (10 ng/kg/min), 30% in cohort 2 (25 ng/kg/min), 15% in cohort 3 (17.5 ng/kg/min), and 25% in cohort 4 (17.5 ng/kg/min) during the 8-hour period after the beginning of infusion.
DISCUSSION
In this first-in-human clinical trial on CD-NP, the favorable cGMP-stimulating, natriuretic, and renal-preserving effects of CD-NP were observed in the absence of excessive hypotension in healthy subjects. Moreover, CD-NP suppressed plasma aldosterone versus baseline, which is particularly desirable in novel cardiovascular therapeutics for treating heart failure and for reducing ventricular remodeling post myocardial infarction.15 Thus, the favorable in vivo responses observed in preclinical studies on CD-NP3,13 were indeed translated to human subjects.
The last drug approved for the treatment of AHF was the native and endogenous cardiac natriuretic peptide BNP in 2001, which when compared with nitroglycerin was superior in reducing cardiac filling pressures.16 The renal actions of BNP in AHF have been controversial, with reports of improved or worsened renal function, the latter attributed to excessive hypotension induced by this potent arterial vasodilator.17,18 More recently, when recombinant BNP was infused in 2 clinical trials at lower doses so as to avoid hypotension, renal function improved.19,20
In recognition of the favorable renal actions of BNP when infused at low concentrations with or without bolus administration so as to avoid excessive hypotension, we designed CD-NP. This peptide was engineered to reduce cardiac filling pressures in part by the venodilating properties of CNP, which is associated with less hypotension.3,9 Most important, the addition of the C-terminus of DNP to the open C-terminus of CNP also transformed CNP into a natriuretic and diuretic peptide, which in vivo and in vitro proved a strong activator of cGMP.3,13,14
In this first-in-class (ie, designer natriuretic peptides) peptide therapeutic, we observed for the first time that CD-NP is capable of activating natriuretic peptide receptors linked to cGMP as demonstrated by the increase in both plasma cGMP and urinary cGMP excretion. Thus, this designer peptide is capable in humans in vivo to interact with human natriuretic peptide receptors relying on cGMP as a second messenger to demonstrate ligand–receptor interactions. This observation also demonstrates that the use of the canine proved predictive of the actions of CD-NP in humans, which may be helpful information when considering animal species for further evaluation of CD-NP.
CD-NP induced natriuresis and diuresis in the absence of a change in creatinine clearance in normal humans. It is likely that the mild decrease in MAP in the CD-NP group is only of statistical but not clinical significance. The natriuretic response in the absence of a change in creatinine clearance would indicate a CD-NP–mediated reduction in sodium reabsorption within the human nephron. Considering studies in the canine, we speculate that the reduction in sodium reabsorption occurs at the level of the proximal and distal tubule. Further in-depth renal studies are warranted to determine the exact nephron segments involved and the response of the renal circulation.
A hallmark of the natriuretic peptides, especially ANP and BNP, is the suppression of aldosterone thought secondary to activation of the NPR-A in the adrenal gland. In the current human study in normal humans with physiological concentrations of aldosterone, CD-NP but not placebo reduced aldosterone. Further investigations will need to address the question of CD-NP–mediated reduction of aldosterone in states in which aldosterone is activated, such as heart failure. Nonetheless, the ability to suppress aldosterone in the setting of natriuresis and diuresis is a feature that distinguishes CD-NP from conventional diuretics, which are associated with activation of renin–angiotensin–aldosterone system.21
CD-NP in normal subjects appeared safe. In the double-blind study (phase 2), orthostatic hypotension was noted in 3 subjects and was thought related to reduced intravascular volume secondary to natriuresis and diuresis. We cannot exclude as well that this may have been related to venodilatation and a reduction in venous return. There were no sustained blood pressure–reducing properties of CD-NP in the current study, and as in studies in the dog, the observed reductions in blood pressure were minimal. The significance of an increase in heart rate observed in this study remains to be determined.
In summary, we report the rapid translation of a novel designer natriuretic peptide (CD-NP) to a first-in-human study, which establishes the concept that CD-NP safely activates the cGMP pathway in normal humans, enhancing sodium excretion and suppressing aldosterone with minimal blood pressure–lowering effects. These studies support the further clinical development of CD-NP for heart failure that is currently underway worldwide.
Acknowledgments
We gratefully acknowledge the statistical expertise of David O. Hodge and Joshua P. Slusser of the Division of Biostatistics at Mayo Clinic, Rochester, Minnesota, in performing an independent analysis of the data from the first-in-human clinical trial on CD-NP.
Financial disclosure: Supported by the National Institutes of Health (R01 HL36634, P01 HL76611, and R01 HL83231) and the Mayo Foundation (Dr Burnett). Dr Lee was a recipient of a Canadian Institutes of Health Research Clinical Research Initiative Fellowship Award (2006), a 2007 Research Fellowship Award from the Heart Failure Society of America, and the American Society for Clinical Pharmacology and Therapeutics 2007 Young Investigator Award. Mayo Clinic has licensed CD-NP to Nile Therapeutics, Inc. Dr Burnett and Dr Lisy were co-inventors of CD-NP. Dr Burnett chairs the Scientific Advisory Board of Nile Therapeutics. CD-NP is being developed through a joint partnership between Nile Therapeutics, Inc, and Mayo Clinic. The first-in-human clinical trial was jointly funded by Mayo Foundation and Nile Therapeutics.
REFERENCES
- 1.Letts G, Loscalzo J. Frontiers in nephrology: targeting inflammation using novel nitric oxide donors. J Am Soc Nephrol. 2007;18:2863–2869. doi: 10.1681/ASN.2007030321. [DOI] [PubMed] [Google Scholar]
- 2.Lee CYW, Burnett JC., Jr. Natriuretic peptides and therapeutic applications. Heart Fail Rev. 2007;12:131–142. doi: 10.1007/s10741-007-9016-3. [DOI] [PubMed] [Google Scholar]
- 3.Lisy O, Huntley BK, McCormick DJ, Kurlansky PA, Burnett JC., Jr. Design, synthesis, and actions of a novel chimeric natriuretic peptide: CD-NP. J Am Coll Cardiol. 2008;52:60–68. doi: 10.1016/j.jacc.2008.02.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heart Failure Society of America HFSA 2006 comprehensive heart failure practice guidelines. J Card Fail. 2006;12:e1–e122. doi: 10.1016/j.cardfail.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 5.Tawaragi Y, Fuchimura K, Tanaka S, et al. Gene and precursor structures of human C-type natriuretic peptide. Biochem Biophys Res Commun. 1991;175:645–651. doi: 10.1016/0006-291x(91)91614-i. [DOI] [PubMed] [Google Scholar]
- 6.Suga S, Nakao K, Itoh H, et al. Endothelial production of C-type natriuretic peptide and its marked augmentation by transforming growth factor-beta: possible existence of “vascular natriuretic peptide system.”. J Clin Invest. 1992;90:1145–1149. doi: 10.1172/JCI115933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stingo AJ, Clavell AL, Heublein DM, et al. Presence of C-type natriuretic peptide in cultured human endothelial cells and plasma. Am J Physiol. 1992;263:H1318–H1321. doi: 10.1152/ajpheart.1992.263.4.H1318. [DOI] [PubMed] [Google Scholar]
- 8.Hunt PJ, Richards AM, Espiner EA, Nicholls MG, Yandle TG. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J Clin Endocrinol Metab. 1994;78:1428–1435. doi: 10.1210/jcem.78.6.8200946. [DOI] [PubMed] [Google Scholar]
- 9.Wei CM, Aarhus LL, Miller VM, Burnett JC., Jr. Action of C-type natriuretic peptide in isolated canine arteries and veins. Am J Physiol. 1993;264:H71–H73. doi: 10.1152/ajpheart.1993.264.1.H71. [DOI] [PubMed] [Google Scholar]
- 10.Schweitz H, Vigne P, Moinier D, Frelin C, Lazdunski M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps) J Biol Chem. 1992;267:13928–13932. [PubMed] [Google Scholar]
- 11.Suga S, Nakao K, Hosoda K, et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology. 1992;130:229–239. doi: 10.1210/endo.130.1.1309330. [DOI] [PubMed] [Google Scholar]
- 12.Protter AA, Wallace AM, Ferraris VA, Weishaar RE. Relaxant effect of human brain natriuretic peptide on human artery and vein tissue. Am J Hypertens. 1996;9:432–436. doi: 10.1016/0895-7061(95)00435-1. [DOI] [PubMed] [Google Scholar]
- 13.Lee CY, Boerrigter G, Harty GJ, Lisy O, Burnett JC., Jr. Pharmacodynamic profile of a novel chimeric natriuretic peptide, CD-NP, compared with C-type natriuretic peptide [abstract] Circulation. 2007;116(suppl II):II 550. [Google Scholar]
- 14.Lee CY, Sandberg SM, Chen HH, Lisy O, Burnett JC., Jr. Renal cyclic GMP stimulating actions of a novel chimeric natriuretic peptide CD-NP in isolated glomeruli: evidence for NPR-A activation [abstract] J Card Fail. 2008;14:S11. [Google Scholar]
- 15.Kasama S, Furuya M, Toyama T, Ichikawa S, Kurabayashi M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J. 2008;29:1485–1494. doi: 10.1093/eurheartj/ehn206. [DOI] [PubMed] [Google Scholar]
- 16.Publication Committee for the VMAC Investigators (Vasodilation in the Management of Acute CHF) Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial [published correction appears in JAMA. 2002;1288:1577] JAMA. 2002;287:1531–1540. doi: 10.1001/jama.287.12.1531. [DOI] [PubMed] [Google Scholar]
- 17.Burnett JC, Jr, Korinek J. The tumultuous journey of nesiritide: past, present, and future. Circ Heart Fail. 2008;1:6–8. doi: 10.1161/CIRCHEARTFAILURE.108.776294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Riter HG, Redfield MM, Burnett JC, Chen HH. Nonhypotensive low-dose nesiritide has differential renal effects compared with standard-dose nesiritide in patients with acute decompensated heart failure and renal dysfunction [letter] J Am Coll Cardiol. 2006;47:2334–2335. doi: 10.1016/j.jacc.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Mentzer RM, Jr, Oz MC, Sladen RN, et al. Effects of perioperative nesiritide in patients with left ventricular dysfunction undergoing cardiac surgery: the NAPA Trial. J Am Coll Cardiol. 2007;49:716–726. doi: 10.1016/j.jacc.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC., Jr. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardiopulmonary-bypass surgery: a double-blind placebo-controlled pilot study. Circulation. 2007;116:I-134–I-138. doi: 10.1161/CIRCULATIONAHA.106.697250. [DOI] [PubMed] [Google Scholar]
- 21.Cataliotti A, Boerriqter G, Costello-Boerriqter LC, et al. Brain natriuretic peptide enhances renal actions of furosemide and suppresses furosemide-induced aldosterone activation in experimental heart failure. Circulation. 2004;109:1680–1685. doi: 10.1161/01.CIR.0000124064.00494.21. [DOI] [PubMed] [Google Scholar]


