Abstract
Older adults represent a rapidly growing segment of the population in developed countries. Advancing age is the most powerful risk factor for the development of cardiovascular disease (CVD), and CVD-related mortality increases markedly in older individuals. Procedures for patients with CVD, including percutaneous coronary intervention, aortic valve replacement and implantable cardioverter defibrillators were all initially validated in younger individuals but are increasingly being applied in older adults who for the most part have been significantly understudied in clinical trials. While advanced age alone is not a contraindication to these procedures, with the advent of less invasive methods to manage CVD including percutaneous techniques to treat both coronary artery disease and valvular heart disease, future research will need to weigh the potential harms of intervention in a population of older adults with multiple medical comorbidities and complex physiologic phenotypes against outcomes that include preventing functional decline and improving quality of life.
Keywords: aging, aortic valve replacement, cardiovascular disease, implantable cardioverter defibrillator, percutaneous coronary intervention
The developed world is aging, and this trend is expected to continue into the foreseeable future [1,201]; for example, in the USA, the percentage of the population ≥75 years old is expected to double from 6 to 12% by the year 2050 [202]. Although definitions vary and in the absence of consensus make comparisons between studies difficult, ‘older adults’ has been used to refer to all individuals ≥65 years of age, and has been further divided into the ‘young old’ (65–74 years), ‘middle old’ (75–84 years) and ‘oldest old’ (≥85 years) [2]. Cardiovascular disease (CVD) is the leading cause of mortality in older adults in the USA and Europe, and advancing age is the most powerful risk factor for the development of CVD as well as CVD-related mortality [1,3].
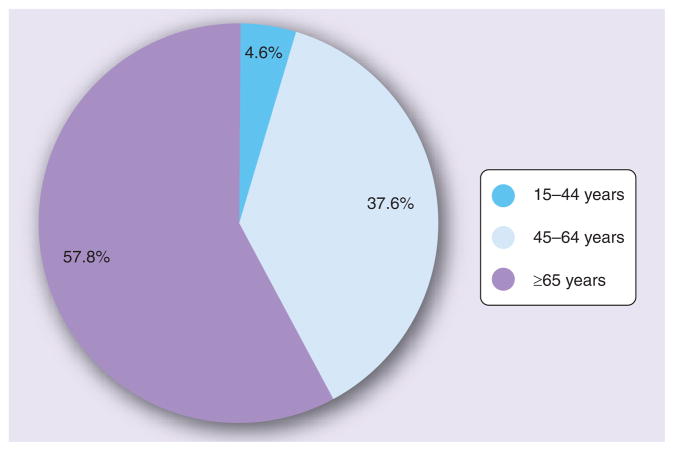
The majority of cardiac procedures, including percutaneous coronary intervention (PCI), coronary artery bypass grafting (CABG), valve replacement, pacemakers and implantable cardioverter defibrillators (ICDs) are performed in patients ≥65 years of age (Figure 1) [3]. In addition, most procedures are being applied with increasing frequency in this population [4–6], which may in part reflect the development of less-invasive approaches that have the potential for reduced procedural and postprocedural complications. For example, PCI now requires smaller sheaths for arterial access, and bleeding complications appear to be declining [7]. ICDs used to require a thoracotomy, are currently placed transvenously, and in the future may be implanted subcutaneoustly [8]. Minimally invasive and transcatheter aortic valve replacement (AVR) has been employed as an alternative to full sternotomy in a number of patients [9,10], allowing for faster recovery times.
Figure 1. Inpatient procedures including percutaneous coronary intervention, coronary artery bypass grafting, implantable cardioverter defibrillators and pacemaker valves by age in the USA in 2006.
Data taken from [3].
Despite such advances, the risks of adverse outcomes with these interventions are still highest in patients of advanced age [11–13]. For example, age-related physiologic changes make this population more prone to bleeding than younger patients [13,14], which can complicate periprocedural management (such as anticoagulant use for patients with ST-elevation myocardial infarction) as well as long-term outcomes. Older adults are also at increased risk of postoperative and postprocedural renal failure, stroke, respiratory failure and infection [11,15,16]. Competing life-limiting comorbidities, such as cancer and chronic lung disease, may attenuate the expected mortality benefit from performing interventions. In addition, in light of the competing risks and benefits, quality of life and functional capacity have assumed an increasingly important role as outcomes for therapeutic interventions in this population, although they have not been widely incorporated into clinical trials.
This review will focus on three procedures that have changed dramatically over the past two decades: PCI, AVR and ICDs. They currently represent approximately 60% of all cardiac procedures performed in patients ≥65 years of age [3], and with changing demographics their numbers are likely to increase significantly in the near future. We will discuss trends in utilization, data on outcomes (where available) and areas for future research and when possible, will address the unique challenges in applying these technologies to the oldest old populations.
Percutaneous coronary intervention
Coronary artery disease (CAD) is the leading cause of death in older adults, and the prevalence and complications of CAD continue to increase with advancing age [3]. The advent of PCI, which refers to any catheter-based procedure (typically balloon angioplasty or more recently stenting) involving the coronary arteries, has significantly changed the management and outcomes of CAD in older adults. The number of PCI procedures in the USA tripled between 1995 and 2005 [3], largely owing to the advent of intracoronary stents. Similar trends have been described in Europe [17]. PCI has become the most frequently utilized intervention for patients with acute coronary syndromes (ACS) including ST-elevation myocardial infarction, non-ST-elevation myocardial infarction and unstable angina [3,4], and rates of alternative therapies including CABG and fibrinolysis have concomitantly decreased [3,4]. CABG has remained important in the management of older adults with complex multivessel disease, and techniques such as off-pump surgery have been developed with the goal of reducing postoperative complications in selected patients [18,19]. CABG may also provide more durable relief of angina and reduce the need for re-intervention compared with PCI, albeit with a higher procedure-related stroke risk [20]; however, a full comparison of these therapies is beyond the scope of this article.
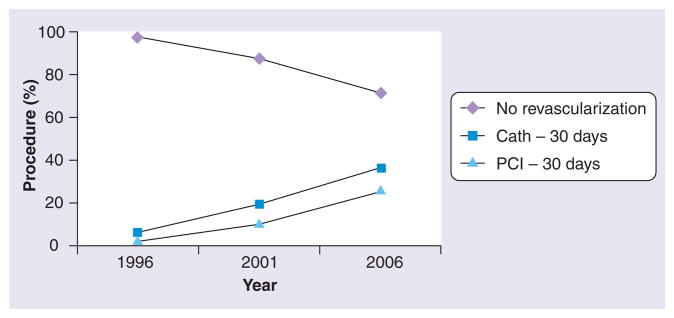
Several studies in the last two decades found that utilization of reperfusion therapy for ACS was lower in patients of advanced age [12,21,22]. Recent data, however, indicate that this trend is changing [3,4,23]. In the US Worcester Heart Attack Study, the percentage of patients ≥75 years of age hospitalized for acute myocardial infarction (AMI) who underwent PCI increased from 5% in 1999 to 19% in 2005 [4]. A Canadian study of nearly 30,000 patients ≥80 years of age admitted with AMI between 1996 and 2007 found that the use of PCI increased from 2 to 25% (Figure 2) [23]. In this group, 1-year mortality improved over time but was still high (47 vs 41%), underscoring the poor prognosis of a significant subgroup of this population.
Figure 2. Trend in revascularization strategies for patients hospitalized with acute myocardial infarction in Canada from 1996 to 2006.
Cath: Cardiac catheterization; PCI: Percutaneous coronary intervention.
Data taken from [21].
Whether the increased application of PCI to older adults with ACS leads to improved outcomes is a matter of debate, as no definitive evidence from a randomized trial exists [24]. In general, patients of advanced age with ACS undergoing PCI have higher rates of postprocedural bleeding, renal failure, vascular complications, stroke, periprocedural AMI and early mortality compared with younger individuals [24–26]. While there is no absolute contraindication to PCI, it is generally thought that the risk outweighs the benefit in the setting of active bleeding, hemorrhagic stroke, advanced dementia and in patients who have contraindications to antiplatelet therapy [27,28]. Bleeding rates and vascular complications after PCI are higher in older women than men, although the exact mechanism is unclear [7,29]. While bleeding complications are highest in older adults, they appear to be decreasing over time, which may be related to smaller sheath sizes and safer antithrombotic regimens [7,30].
Although the risks of intervening in older adults remain higher than in younger individuals, subgroup analyses of several studies suggest that there may still be a benefit to intervening in selected patients (Table 1) [17,23,31]; for example, in the Treat Angina with Aggrastat and Determine Cost of Therapy with Invasive or Conservative Strategy – Thrombolysis In Myocardial Infarction (TACTICS-TIMI) 18 trial, which enrolled patients with non-ST-elevation myocardial infarction across nine countries to an early invasive versus conservative strategy, there was a relative risk reduction of 56% in death or MI in patients ≥75 years of age at 6 months in the invasive arm, which was significantly greater than in younger individuals [31]. This trial and others excluded prior gastrointestinal bleeding, stroke or advanced renal insufficiency, all of which are more common in older adults; the application of clinical trial evidence to this population is therefore somewhat limited. The decision to perform PCI remains a very individualized one (Table 2), and the risks of adverse events (most notably bleeding and renal failure) must be carefully weighed against the potential benefits.
Table 1.
Reported outcomes of older adults in selected randomized trials of percutaneous coronary intervention.
| Trial (condition) | Strategy | Exclusions | Older adults | Outcome in older adults | Ref. |
|---|---|---|---|---|---|
| FRISC II (UA/NSTEMI) | Early angiography ± revascularization vs medical therapy | Age ≥ 75 years Creatinine >1.7 mg/dl High bleeding risk PCI within 6 months Osteoporosis |
53% 65–74 years | 1-year death or nonfatal MI: significant benefit with early angiography in patients 65–74 years (RR = 0.63) | [96] |
| TACTICS-TIMI 18 (UA/NSTEMI) | Early angiography ± revascularization vs medical therapy | Creatinine >2.5 mg/dl Prior GI bleeding CVA within 1 year Cardiogenic shock |
43% ≥ 65 years | 30-day mortality, nonfatal MI or rehospitalization: significant benefit with early angiography in patients ≥ 65 years (OR = 0.59) | [31] |
| GUSTO IIb (STEMI) | PCI vs fibrinolysis (TPA) | Creatinine >2.0 mg/dl Active bleeding Prior CVA On Coumadin® |
26% >70 years | 30-day mortality: trend towards benefit with PCI in patients >70 years | [97] |
| DANAMI-2 (STEMI) | PCI vs fibrinolysis (alteplase) | Creatinine >2.8 mg/dl On metformin Cardiogenic shock Prior CABG Life expectancy <1 year |
50% >63 years 25% >73 years |
30-day mortality, nonfatal MI or stroke: significant benefit with PCI in patients >63 years (OR = 0.54) | [98] |
| SHOCK (STEMI + shock) | Emergent PCI vs initial medical stabilization | Noncardiogenic shock Severe systemic illness Severe valvular disease |
19% ≥75 years | 30-day mortality: trend towards harm with PCI in patients ≥ 75 years | [99] |
Revascularization = PCI or CABG where appropriate.
CABG: Coronary artery bypass grafting; CVA: Cerebrovascular accident (stroke/transient ischemic attack); DANAMI: Danish Multicenter Randomized Study on Thrombolytic Therapy Versus Acute Coronary Angioplasty in Acute Myocardial Infarction; FRISC II: Framingham and Fast Revascularization During Instability in Coronary Artery Disease II; GI: Gastrointestinal; GUSTO IIb: Global Use of Strategies to Open Occluded Coronary Arteries IIb; MI: Myocardial infarction; NSTEMI: Non-ST elevation myocardial infarction; OR: Odds ratio; PCI: Percutaneous coronary intervention; RR: Relative risk; SHOCK: Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock; STEMI: ST elevation myocardial infarction; TACTICS-TIMI: Treat Angina with Aggrastat and Determine Cost of Therapy with Invasive or Conservative Strategy – Thrombolysis In Myocardial Infarction; TPA: Tissue plasminogen activator; UA: Unstable angina.
Table 2.
Factors influencing decision-making for interventions in older adults.
| Intervention | Complications (%) | Cardiac measures | Geriatric measures | Ref. |
|---|---|---|---|---|
| PCI | Mortality <1 Myocardial infarction 8–18 Bleeding 5–20 Renal failure 3–6 Stroke <1 |
Coronary anatomy Clinical presentation (e.g., ACS vs stable angina, shock etc.) |
Functional status (ADLs, IADLs, submaximal exercise such as hall walk) Quality of life (e.g., Seattle Angina Questionnaire, KCCQ), felt restrictions living status (independent vs long-term care) Frailty Sarcopenia and muscle function Cognition including memory and executive function Time tradeoff |
[92,100–109] |
| AVR | Mortality 4–10 Infection 6 Respiratory failure 5–20 Renal failure 5–24 Stroke 1–9 |
Aortic valve gradient Left ventricular function NYHA class |
[5,15,38, 39,92,108] | |
| ICD | Infection 1–2 Bleeding 1–3 Recurrent, inappropriate ICD shocks 10–24 Anxiety 13–38 |
Left ventricular function NYHA class Dyssynchrony (for ICD + CRT) |
[75,76,92, 109–111] |
ACS: Acute coronary syndromes; ADL: Activity of daily living; AVR: Aortic valve replacement; CRT: Cardiac resynchronization therapy; IADL: Instrumental activity of daily living; ICD: Implantable cardioverter defibrillator; KCCQ: Kansas City Cardiomyopathy Questionnaire; NYHA: New York Heart Association; PCI: Percutaneous coronary intervention.
Aortic valve replacement
Aortic stenosis (AS) is the most prevalent valvular pathology in older adults [32]; it is infrequent in younger patients except in the presence of a bicuspid aortic valve. Exact population estimates of AS vary, but prevalence appears to increase with advancing age [33,34]. In subjects ≥65 years of age enrolled in the Cardiovascular Health Study, the prevalence of AS was 2% [33], while for subjects aged 75–86 years in the Helsinki Aging study, AS was present in over 13% of participants [34]. Once moderate AS is present, the disease progresses on average by a decrease in valve area of 0.1 cm2 per year and an increase in mean pressure gradient of 7 mmHg per year, though there is marked variability among individual patients [35].
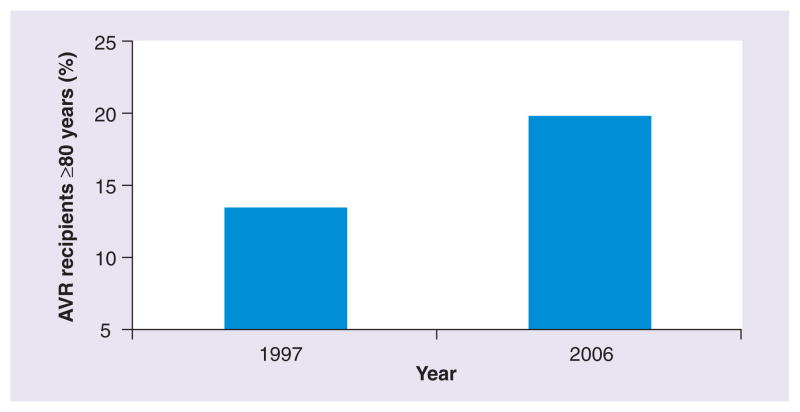
After the onset of symptoms, which are classically manifested by angina, syncope or heart failure, the mean survival for individuals with severe AS is 2–3 years with a high risk of sudden death [35]. AVR is the standard of care in patients who are considered an acceptable operative risk. Since its first use in 1960 [36], several advances have been made in operative and postoperative management that have significantly reduced procedure-related morbidity and mortality [5]. Concurrently, older patients who were once considered too high risk, especially those ≥80 years of age, are now being operated on routinely [5,15,37]. Analysis of over 100,000 patients undergoing isolated AVR in the North American Society of Thoracic Surgeons (STS) database found that between 1997 and 2006 the mean age increased, and despite an increase in comorbidities (e.g., diabetes, hypertension, cerebrovascular disease and renal failure), overall operative mortality fell by 24% [5]. The proportion of patients 80 years or older increased from 13.4% in 1997 to 19.7% in 2006 (Figure 3) [5]. A number of case series of patients aged ≥80 [15,38,39] and even ≥90 [37] years of age have been published (Table 3), with 5-year survival rates above 50%. In appropriately selected candidates, quality of life outcomes have been impressive; for example, in a case series of patients ≥80 years of age undergoing AVR, postoperative physical and mental health functional scores on the Medical Outcomes Short Form-36 (SF-36) were comparable to the general population of similar age not undergoing surgery [40].
Figure 3. Proportion of aortic valve recipients ≥80 years of age in the Society of Thoracic Surgeons Database in 1997 versus 2006.
AVR: Aortic valve replacement.
Data taken from [5].
Table 3.
Outcomes of selected case series of aortic valve replacement in patients ≥80 years of age.
| Authors (year) | Age range (years) | Patients (n) | 30-day complications (%) | Early mortality (%) | Ref. |
|---|---|---|---|---|---|
| Chiappini et al. (2004) | ≥80 | 115 | 17.3 arrhythmias 3.8 myocardial infarction 5.2 prolonged mechanical ventilation 0.8 stroke |
8.5 (in-hospital) | [39] |
| Collart et al. (2005)† | ≥80 | 215 | 31.2 arrhythmias 5.6 infection 10.7 prolonged mechanical ventilation 8.8 renal failure 6.0 reoperation |
8.8 (in-hospital) | [15] |
| Kolh et al. (2007) | ≥80 | 220 | 24 arrhythmias 21 prolonged mechanical ventilation 5.0 renal failure (dialysis) 1.0 reoperation 1.8 stroke |
10.0 (in-hospital) | [38] |
| Zingone et al. (2009) | ≥80 | 355 | 20.3 prolonged mechanical ventilation 23.7 renal failure 5.1 reoperation 3.7 stroke |
9.3 (in-hospital) | [108] |
| Lisosky et al. (2009) | ≥85 | 156 | 54.6 arrhythmias 6.1 reoperation 4.6 stroke |
10.3 (in-hospital) | [112] |
| Speziale et al. (2010) | ≥90 | 127 | 29.9 arrhythmias 13.4 prolonged mechanical ventilation 18.1 renal failure 2.4 reoperation 8.7 stroke |
13.4 (30-day) | [37] |
74% of cases AVR, remainder involved mitral valve replacement.
AVR: Aortic valve replacement.
While older patients are undergoing AVR with increasing frequency, age remains an important risk factor for mortality as well as postoperative complications including stroke, renal failure, bleeding and prolonged ventilation [11,15,37,38]. In the Society of Thoracic Surgeons (STS) database between 2002 and 2006, overall in-hospital mortality was 3.2%, and age of 80 years (compared with 50 years) was associated with an odds ratio of 3.34 for this outcome [11]. For patients ≥80 years, case series have estimated the operative mortality for AVR at approximately 10%, with combined AVR/CABG conferring a higher risk than isolated AVR [38–41]. Postoperative complication rates vary (Table 3), likely representing heterogeneous patient populations; arrhythmias such as atrial fibrillation are the most common, and are relatively benign; however, other problems such as prolonged mechanical ventilation or stroke can be permanently disabling. Factors that predict operative success (and complications) after AVR in the oldest old population are still being elucidated, and as many studies to date are derived from a single site their generalizability is limited. Geriatric-specific impairments such as frailty are just beginning to be explored, and appear to add important predictive value to traditional risk models [42].
Patients with severe AS who meet guideline-specified criteria for AVR and do not undergo surgery are less well studied, although they represent a significant proportion of the AS population [43,44]. Reasons for not undergoing AVR are likely to be multifactorial and may include patients’ and/or their caregivers’ declining to be considered for surgery, cardiologists’ reluctance to refer for surgery secondary to contraindications, such as life-limiting comorbidities (e.g., prior stroke, dementia or malignancy), and surgeons declining to proceed owing to high operative risk [44]. For patients who are not candidates for AVR, treatment has traditionally been palliative. Studies of medical therapy for severe AS have been disappointing; for example, several well-designed randomized trials of statin therapy (with the goal of inhibiting the inflammatory process) have demonstrated no effect in slowing progression of the disease [45,46].
However, the paradigm for treatment of AS candidates at high-operative risk may be changing with transcatheter aortic valve implantation (TAVI), which theoretically avoids many of the risks associated with open-heart surgery including a sternotomy, aortotomy and exposure to cardiopulmonary bypass [47]. In a study of 50 patients (mean age 82 years) with symptomatic severe AS who were considered too high risk for conventional AVR, this procedure was successful in 86% of patients, and 35 of 43 individuals (81%) who had undergone successful TAVI were alive at 1-year follow-up [47]. Another study of 646 high-risk patients (mean age 81 years) undergoing TAVI found a 97% procedural success rate, and 30-day mortality was 8% [48]. The US-based Placement of Aotric Transcatheter Valve (PARTNER) trial, which randomized 358 nonoperative candidates (mean age 83 years) with severe AS to TAVI versus conventional therapy (including balloon valvuloplasty) found a 20% absolute risk reduction in mortality with TAVI (30.7 vs 50.7%) at 1 year [10]. New York Heart Association (NYHA) class was improved in the surviving TAVI patients at 1 year (compared with controls), although the rate of stroke 30 days post-procedure was higher (5.0 vs 1.1%), which was attributed to the intervention itself [10]. Based on studies to date, other procedure-related adverse events include vascular complications (10–30%), heart block (5–10%) and tamponade (2–5%) [10,48,49]. Operator experience appears to play an important role in procedural success [49]. As this technique evolves and further studies are completed, it may become a more routine option for older adults who are not surgical candidates, or even as a minimally invasive alternative for those deemed an acceptable surgical risk.
Selection for TAVI may be assisted by risk-stratification tools including the STS Cardiac Surgery Risk Model [11,203] and the European System for Cardiac Risk Operative Evaluation (EuroSCORE) [50,204], which were developed with data submitted from surgeons in the USA and Europe respectively. Randomized trials have to date utilized these risk scores in selecting candidates who are ‘high-risk’ or ‘inoperable’ for traditional AVR [10,51]. However, these scores may overestimate surgical mortality in the oldest old population [15]. In addition, they do not provide vital functional information that can be obtained from other geriatric measures, such as frailty or gait speed, which may provide distinctly different information and further improve prediction of outcomes [42,52].
In addition to stratifying patients for mortality and the appropriate intervention, increasing attention is being paid to quality of life measures including physical function [40,53,54], NYHA functional class [10,40,54], depression [53,54] and discharge to the nonhome setting [42,55]. Although the focus of most trials remains mortality, it is recognized that the intervention itself may be debilitating in selected subgroups, especially the frail elderly. Further knowledge regarding these outcomes and the risk factors associated with them will help in providers’ discussions with patients about the potential benefits and risks associated with intervention.
Implantable cardioverter defibrillators
Implantable cardioverter defibrillators are designed to deliver an electrical shock to terminate a potentially fatal rhythm disturbance, such as sustained ventricular tachycardia or ventricular fibrillation. In the 1980s the first ICDs were placed in three patients with refractory malignant arrhythmias and required a thoracotomy for implantation [56]. Over time, less invasive (transvenous) systems were developed, although overall utilization remained low. Until the early part of the last decade, ICDs were used mainly for secondary prevention of sudden cardiac death (SCD) in patients with a prior history of life-threatening arrhythmia [57–59]. Two major randomized trials, Multicenter Automatic Defibrillator Implantation II (MADIT II) [60] and Sudden Cardiac Death in Heart Failure (SCD-HeFT) [61], changed this paradigm by demonstrating the effectiveness of ICDs in the primary prevention of SCD in patients with a depressed left ventricular ejection fraction (LVEF). Based largely on these results, both US and European guidelines have recommended placement of an ICD for primary prevention of SCD in patients with congestive heart failure and LVEF <35% who have a life expectancy of greater than 1 year [62,63].
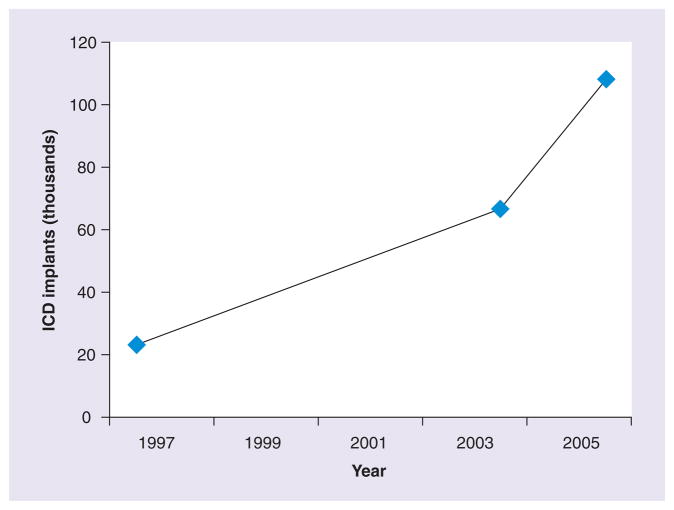
After the publication of MADIT II and SCD-HeFT, the volume of ICDs implanted increased markedly, in both the young and older population (Figure 4) [6,16,64–66]. Between 1997 and 2004, the number of ICDs placed in the USA increased from 22,922 to 66,545 [6]. In 2006, the first year of the Centers for Medicare Services-mandated National ICD Registry, the number had increased to 108,341 [64]. Data from Europe are similar, and although regional variations exist, there has been a 75% increase in the number if ICDs placed in Western Europe between 2004 and 2008 [66]. Recent studies have shown that a significant proportion of ICDs are placed in older adults, including the oldest old population, with estimates of the proportion of US ICD recipients ≥80 years of age ranging between 12 and 18% [16,65]. With the changing demographics in the USA and Europe, this proportion is likely to increase in the future.
Figure 4. Number of implantable cardioverter defibrillators placed in the USA from 1997 to 2006.
ICD: Implantable cardioverter defibrillator.
An additional procedure of increasing frequency in older adults with heart failure has been the placement of biventricular pacemakers for cardiac resynchronization therapy (CRT). These devices, which can be combined with defibrillator capabilities (known as CRT-D), have been shown to reduce hospitalization and mortality in eligible individuals (LVEF <35%, QRS >120 ms, NYHA class III or IV) [67]. CRT-D devices constitute over 50% of defibrillator implants in patients >80 years of age, and represent a greater proportion of defibrillator-capable devices than in younger individuals [65]. They are more technically challenging to place than conventional ICDs, as they require the insertion of a pacing lead in the coronary sinus, and in addition to longer procedure times they can carry an increased risk of complications.
A question remains as to whether patients ≥80 years of age derive a survival benefit from ICDs similar to that seen in younger patients [68,69]. The mean ages in MADIT II and SCD-HeFT were 64 and 60 years, respectively, and the rate of comorbidities common in older adults including advanced renal insufficiency and cerebrovascular disease was low [60,61]. The subgroup of patients in MADIT II >70 years of age (35% of the population) randomized to ICD appeared to have a reduction in mortality compared with non-ICD patients, while no such effect was seen in the subgroup of patients ≥65 years of age (34% of the population) in SCD-HeFT. While it has been argued that older adult ICD recipients have improved survival compared with non-ICD matched controls [70,71], there is general consensus that large-scale outcomes data are lacking in the oldest old population [69]. Placement of the device for primary prevention is contraindicated if life expectancy is less than 1 year [62], although in practice this if often a difficult determination. A single-center case series of 107 consecutive patients ≥80 years of age undergoing ICD implantation found that 1-year mortality was approximately 20%, while another retrospective study of 225 patients ≥80 years found that 16.4% died within a year [72,73]. Median survival was 4.2 and 3.6 years, respectively, and was worse in both studies in patients with low ejection fraction. Future research with large datasets including national ICD registries [74,75] may help in better defining predictors of early mortality after implantation.
Complications, which include bleeding, infection and cardiac perforation, range from 5 to 10% in reported registries of older adults [16,76] and appear to increase with advancing age [16]. Advancing age has been associated with a decreasing likelihood of SCD compared with death from other causes [65,77], and older ICD recipients are considerably more likely to die of nonarrhythmic causes than arrhythmia within the first year of implantation [78]. Other quality-of-life considerations, including inappropriate shocks (which may constitute up to half of all therapies delivered) and changing the mode of death (reducing the likelihood of a painless sudden death) are increasingly being emphasized as important aspects of device therapy that should be discussed with patients prior to implantation [79].
In the absence of a definitive randomized trial in patients ≥80 years of age, the decision to place an ICD for primary prevention needs to take into account comorbid conditions (such as malignancy and advanced chronic obstructive pulmonary disease) that may limit the mortality benefit derived from an appropriate ICD discharge, as well as patient preferences concerning aggressiveness of therapy at the end of life. In eligible patients who do not wish to receive ICD shocks but desire an improved quality of life, a CRT pacing only device (without defibrillator capability) may be considered [80].
Novel approaches for study
Inclusion of frail older adults in clinical trials
Aging is associated with the development of comorbid conditions and age-related impairments in multiple organ systems that are independently predictive of morbidity and mortality [81,82]. These include comorbidities such as chronic renal disease, anemia and cognitive dysfunction, as well as frailty (defined as a physiologic state of increased vulnerability to stressors that results from decreased physiologic reserves) [81], sarcopenia (age-related loss in skeletal muscle mass) and functional disability. While certain factors (such as advanced renal disease) frequently serve as exclusion criteria for clinical trials, others (such as frailty) are often not formally measured prior to enrollment or as an outcome.
An emerging area of research is focusing on pre-procedural measurement of geriatric syndromes, such as frailty and their association with outcomes after cardiac interventions [42,83,84]. For example, a recent study of patients undergoing cardiac surgery found that slow gait speed, an indicator of frailty, provided incremental benefit to standard risk measures (in this case the STS score) in predicting postoperative morbidity and mortality [42]. This measure, as well as formal assessments of cognition and muscle strength, are relatively simple to perform and may play an increasing future role in risk stratification prior to major interventions.
Incorporation of functional & quality of life outcomes
Patient-centered decision-making involves the recentering of care to explicitly incorporate patients’ perspectives [85], including full disclosure of diagnostic information as well as incorporating patient preferences into the therapeutic plan. In older adults this decision-making should ideally incorporate age-related impairments (which include cognitive impairment, falls, incontinence, low BMI, dizziness, vision and hearing impairment, physical function impairment and muscle strength) [86,87] and their effect on outcomes. While half of all patients over 65 years of age have one or more of these conditions [86], they are often under-recognized [87]. Research is beginning to examine the association of specific impairments with adverse outcomes after cardiac interventions; for example, patients with impairments in ADLs, cognition or ambulation are more likely to die after cardiac surgery [42,54] and are more likely to be discharged to long-term institutional care [52]. Further research is necessary to better elucidate the relationship between specific geriatric impairments and functional and mortality outcomes.
The major outcome in most clinical trials of cardiac interventions has been mortality, and reductions in this end point serve as a strong incentive for widespread adoption of new therapies. This was seen relatively recently with ICDs for primary prevention of SCD; the number of devices increased fivefold in a 10-year period during which several major randomized trials, predominantly focused on mortality, were published [6,64]. Given the association of age with increasing mortality, the benefit derived from the aforementioned interventions may be attenuated owing to competing causes of death in the oldest old population; thus, quality-of-life considerations play an increasingly important role. Using the example of ICDs, while the device may terminate a potential arrhythmia, there is also the possibility of inappropriate shocks, hospitalizations for shocks (both appropriate and inappropriate) and device-related complications such as infection [61]. In addition, as the likelihood of SCD, which is often painless, is reduced with an ICD, patients are more likely to die of other symptomatic heart failure-related etiologies such as progressive pump failure or pulmonary edema [88], or alternately other noncardiac illnesses.
The concept of time tradeoff, where patients are asked how many years they would be willing to give up in order to spend their remaining years in their current health, may have some utility in ICD discussions [89]; patients may want to avoid hospitalizations for shocks, device-related complications or decompensated heart failure, even though there is a theoretical mortality benefit. Research has shown that patients often know little about the risks and benefits of ICDs; they frequently overestimate their potential for life-saving therapy, and know little about adverse events, such as inappropriate shocks [79]. Informed discussions at the time of evaluation for device placement in older adults are thus critically important.
As quality of life becomes an increasingly important goal of therapeutic interventions for older adults with CVD, a greater understanding of the process of accessing choices and choosing between treatment options is likely to become important. Indeed, providing choices to patients has become a central tenet of good quality clinical care. Accordingly, rather than focusing on the multiple measures that are employed to define quality of life and the various domains that are hypothesized to define a good quality of life, focusing on the process of how individuals access choices and choose among them [90,91] may provide important guidance for clinicians in understanding how best to apply the multitude of cardiovascular procedures that are increasingly being offered to older adults. Such an approach may prove essential in evaluating the optimal approach for an individual with a complex physiologic state, in whom treating the host is preferable to treating the disease.
Directions for clinical trials
Recent editorials have brought to light the under-representation of older adults (particularly the oldest old population) with multiple medical comorbidities in most major clinical trials [92,93]. Although the rationale for this phenomenon may have been to isolate and treat a single pathologic mechanism, or minimize the risk of doing harm in those with relative contraindications, the realities of an aging population make further study imperative.
One potential mechanism to study older adults with multiple comorbidities in clinical trials may be to investigate multifaceted interventions that address several domains simultaneously. For example, a study to evaluate the effectiveness of TAVI in frail older adults with AS may incorporate the procedure itself, as well as referral to a structured rehabilitation program. A study of CRT therapy in patients with heart failure may also include a multidisciplinary team approach to management, with identification of geriatric impairments and interventions to reduce heart failure-related hospitalization. A trial of PCI for stable angina may also incorporate therapy for depression, which co-occurs commonly with angina in older adults [94,95]. Such approaches inherently embrace the complexity of CVD in older adults, recognizing that even the most effective procedures need to be applied in the context of care that is directed at multiple causal or contributing factors to the overall clinicalphenotype.
Conclusion & future perspective
The aging of the developed world has led to an increase in the global burden of CVD, and this trend will continue into the foreseeable future. Cardiac interventions are increasingly being applied in older adults, which reflects these changing demographics as well as the decreased invasiveness of procedures over time. In the oldest old population, there is little evidence from randomized trials – registry data may provide vital information to inform clinical decisions. Considerations such as functional capacity and quality of life, are increasingly being recognized as important goals of therapy. Future studies should incorporate specific measures relevant to an aging population both in the selection of candidates and in the evaluation of outcomes. Finally, in light of the inherent heterogeneity of aging individuals, a simple, straightforward and widely relevant approach to determining the application of the growing number of cardiovascular procedures in older adults is not likely. Rather, individualized, patient-centric care that assists patients, family members and caregivers in accessing the growing available choices and choosing among them, is likely to be extremely useful.
Executive summary.
The majority of cardiac interventions are performed in patients ≥65 years of age.
Utilization of three procedures – percutaneous coronary intervention (PCI), aortic valve replacement and implantable cardioverter defibrillators (ICD) – has increased substantially over time in this population.
Less-invasive approaches may partially account for increased utilization.
Outcomes data are lacking in the oldest old population (≥85 years of age).
Percutaneous coronary intervention
Percutaneous coronary intervention has become the most common intervention for treatment of acute coronary syndromes.
PCI rates have traditionally been lower in older adults; however, this trend is changing.
Complication rates (e.g., bleeding, renal failure, stroke and mortality) increase with advancing age, although overall complications appear to be decreasing over time.
The benefits of PCI are unclear in patients with comorbidities (advanced renal insufficiency, active bleeding, stroke and dementia) that served as exclusion criteria for most clinical trials.
Aortic valve replacement
While the average age and comorbidities of aortic valve replacement recipients have increased over the past decade, mortality has declined.
Octogenarians are now operated on routinely.
Assessment of functional measures (e.g., gait speed) may be helpful in preoperative risk stratification.
Transcatheter aortic valve implantation has shown early promise and may become a routine alternative for patients deemed high surgical risk.
Implantable cardioverter defibrillators
Based on several randomized studies, ICDs were approved for the primary prevention of sudden cardiac death in patients with symptomatic heart failure and ejection fraction <35%.
Most patients in these trials were relatively young with few comorbidities.
The use of ICDs has increased significantly, and by current estimates the proportion of device recipients ≥80 years of age is as high as 18% and growing.
Comorbid conditions, overall life expectancy and individual preferences must be considered in patients of very advanced age who meet criteria for an ICD.
Future perspective
Future clinical trials should include more older adults, with a wide range of comorbidities, to reflect real-world practice.
Outcomes other than mortality, including quality of life and prevention of functional decline, will be important to evaluate.
Investigating multifaceted interventions related to both cardiovascular disease and aging-related impairments may be beneficial.
A greater understanding of the process of accessing choices and choosing between treatment options is likely to become increasingly important.
Footnotes
Financial & competing interests disclosure
Dr John Dodson is supported by a grant from the NIH/National Institute on Aging T32AG019134 – 10. Dr Mathew Maurer is supported by a grant from the NIH/National Institute on Aging K24AG036778 – 01A1. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
•of interest
• •of considerable interest
- 1.Gershlick AH. Managing myocardial infarction in the elderly: time to bury inappropriate concerns instead. Eur Heart J. 2009;30(8):887–889. doi: 10.1093/eurheartj/ehp117. [DOI] [PubMed] [Google Scholar]
- 2.Harris T, Kovar MG, Suzman R, Kleinman JC, Feldmam JJ. Longitudinal study of physical ability in the oldest-old. Am J Public Health. 1989;79(6):698–702. doi: 10.2105/ajph.79.6.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 4.Wasser J, Goldberg RJ, Spencer FA, Yarzebski J, Gore JM. Multidecade-long trends (1986–2005) in the utilization of coronary reperfusion and revascularization treatment strategies in patients hospitalized with acute myocardial infarction. Coron Artery Dis. 2009;20(1):71–80. doi: 10.1097/MCA.0b013e32831bb4aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5••.Brown JM, O’Brien SM, Wu C, Sikora JAH, Griffith BP, Gammie JS. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the society of thoracic surgeons national database. J Thorac Cardiovasc Surg. 2009;137(1):82–90. doi: 10.1016/j.jtcvs.2008.08.015. Describes major trends in comorbidities and outcomes among surgical aortic valve recipients. [DOI] [PubMed] [Google Scholar]
- 6.Zhan C, Baine W, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med. 2008;23:13–19. doi: 10.1007/s11606-007-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmed B, Piper WD, Malenka D, et al. Significantly improved vascular complications among women undergoing percutaneous coronary intervention. Circ Cardiovasc Interv. 2009;2(5):423–429. doi: 10.1161/CIRCINTERVENTIONS.109.860494. [DOI] [PubMed] [Google Scholar]
- 8.Bardy GH, Smith WM, Hood MA, et al. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363(1):36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 9.Bakir I, Casselman FP, Wellens F, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg. 2006;81(5):1599–1604. doi: 10.1016/j.athoracsur.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 10.Leon MB, Smith CR, Mack M, et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363(17):1597–1607. doi: 10.1056/NEJMoa1008232. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien SM, Shahian DM, Filardo G, et al. The Society Thoracic Surgeons cardiac surgery risk models: part 2 – isolated valve surgery. Ann Thorac Surg. 2008;788(Suppl 1):S23–S42. doi: 10.1016/j.athoracsur.2009.05.056. [DOI] [PubMed] [Google Scholar]
- 12.Hasdai D, Holmes DR, Criger DA, Topol EJ, Califf RM, Harrington RA. Age and outcome after acute coronary syndromes without persistent ST-segment elevation. Am Heart J. 2000;139(5):858–866. doi: 10.1016/s0002-8703(00)90018-8. [DOI] [PubMed] [Google Scholar]
- 13••.Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part II: ST-segment-elevation myocardial infarction: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2570–2589. doi: 10.1161/CIRCULATIONAHA.107.182616. Highlights lower rates of reperfusion in older adults with ST elevation myocardial infarction, as well as increased adverse outcomes such as bleeding. [DOI] [PubMed] [Google Scholar]
- 14.Dangas GD, Singh HS. Primary percutaneous coronary intervention in octogenarians: navigate with caution. Heart. 2010;96(11):813–814. doi: 10.1136/hrt.2009.191916. [DOI] [PubMed] [Google Scholar]
- 15.Collart F, Feier H, Kerbaul F, et al. Valvular surgery in octogenarians: operative risks factors, evaluation of Euroscore and long term results. Eur J Cardiothorac Surg. 2005;27(2):276–280. doi: 10.1016/j.ejcts.2004.10.041. [DOI] [PubMed] [Google Scholar]
- 16.Swindle JP, Rich MW, McCann P, Burroughs TE, Hauptman PJ. Implantable cardiac device procedures in older patients: use and in-hospital outcomes. Arch Intern Med. 2010;170(7):631–637. doi: 10.1001/archinternmed.2010.30. [DOI] [PubMed] [Google Scholar]
- 17.Schiele F, Meneveau N, Seronde MF, et al. Changes in management of elderly patients with myocardial infarction. Eur Heart J. 2009;30(8):987–994. doi: 10.1093/eurheartj/ehn601. [DOI] [PubMed] [Google Scholar]
- 18.LaPar DJ, Bhamidipati CM, Reece TB, Cleveland JC, Kron IL, Ailawadi G. Is off-pump coronary artery bypass grafting superior to conventional bypass in octogenarians? J Thorac Cardiovasc Surg. 2011;141(1):81–90. doi: 10.1016/j.jtcvs.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puskas JD, Kilgo PD, Kutner M, Pusca SV, Lattouf O, Guyton RA. Off-pump techniques disproportionately benefit women and narrow the gender disparity in outcomes after coronary artery bypass surgery. Circulation. 2007;116(Suppl I):192–199. doi: 10.1161/CIRCULATIONAHA.106.678979. [DOI] [PubMed] [Google Scholar]
- 20.Bravata DM, Gienger AL, McDonald KM, et al. Systematic review: the comparative effectiveness of percutaneous coronary interventions and coronary artery bypass graft surgery. Ann Intern Med. 2007;147(10):703–716. doi: 10.7326/0003-4819-147-10-200711200-00185. [DOI] [PubMed] [Google Scholar]
- 21.Eagle KA, Goodman SG, Avezum Á, Budaj A, Sullivan CM, López-Sendón J. Practice variation and missed opportunities for reperfusion in ST-segment-elevation myocardial infarction: findings from the global registry of acute coronary events (GRACE) Lancet. 2002;359(9304):373–377. doi: 10.1016/S0140-6736(02)07595-5. [DOI] [PubMed] [Google Scholar]
- 22.Berger AK, Schulman KA, Gersh BJ, et al. Primary coronary angioplasty vs thrombolysis for the management of acute myocardial infarction in elderly patients. JAMA. 1999;282(4):341–348. doi: 10.1001/jama.282.4.341. [DOI] [PubMed] [Google Scholar]
- 23••.Pagé M, Doucet M, Eisenberg MJ, Behlouli H, Pilote L. Temporal trends in revascularization and outcomes after acute myocardial infarction among the very elderly. CMAJ. 2010;182(13):1415–1420. doi: 10.1503/cmaj.092053. Describes increased use of invasive strategy over time for patients ≥80 years with myocardial infarction in a nationally representative population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merchant FM, Weiner RB, Rao SR, et al. In-hospital outcomes of emergent and elective percutaneous coronary intervention in octogenarians. Coron Artery Dis. 2009;20(2):118–123. doi: 10.1097/MCA.0b013e3283292ae1. [DOI] [PubMed] [Google Scholar]
- 25.Avezum A, Makdisse M, Spencer F, et al. Impact of age on management and outcome of acute coronary syndrome: observations from the global registry of acute coronary events (GRACE) Am Heart J. 2005;149(1):67–73. doi: 10.1016/j.ahj.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Claessen BEPM, Kikkert WJ, Engstrom AE, et al. Primary percutaneous coronary intervention for ST elevation myocardial infarction in octogenarians: trends and outcomes. Heart. 2010;96(11):843–847. doi: 10.1136/hrt.2009.185678. [DOI] [PubMed] [Google Scholar]
- 27.Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (writing committee to revise the (1999) guidelines for the management of patients with acute myocardial infarction) Circulation. 2004;110(5):588–636. doi: 10.1161/01.CIR.0000134791.68010.FA. [DOI] [PubMed] [Google Scholar]
- 28.Alexander KP, Newby LK, Armstrong PW, et al. Acute coronary care in the elderly, part I: non-ST-segment-elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569. doi: 10.1161/CIRCULATIONAHA.107.182615. [DOI] [PubMed] [Google Scholar]
- 29.Piper WD, Malenka DJ, Ryan TJ, et al. Predicting vascular complications in percutaneous coronary interventions. Am Heart J. 2003;145(6):1022–1029. doi: 10.1016/S0002-8703(03)00079-6. [DOI] [PubMed] [Google Scholar]
- 30.Singh M, Mathew V, Garratt KN, et al. Effect of age on the outcome of angioplasty for acute myocardial infarction among patients treated at the Mayo Clinic. Am J Med. 2000;108(3):187–192. doi: 10.1016/s0002-9343(99)00429-5. [DOI] [PubMed] [Google Scholar]
- 31.Bach RG, Cannon CP, Weintraub WS, et al. The effect of routine, early invasive management on outcome for elderly patients with non-ST-segment elevation acute coronary syndromes. Ann Intern Med. 2004;141(3):186–195. doi: 10.7326/0003-4819-141-3-200408030-00007. [DOI] [PubMed] [Google Scholar]
- 32.Carabello BA, Paulus WJ. Aortic stenosis. Lancet. 2009;373(9667):956–966. doi: 10.1016/S0140-6736(09)60211-7. [DOI] [PubMed] [Google Scholar]
- 33.Novaro GM, Katz R, Aviles RJ, et al. Clinical factors, but not C-reactive protein, predict progression of calcific aortic-valve disease: The Cardiovascular Health Study. J Am Coll Cardiol. 2007;50(20):1992–1998. doi: 10.1016/j.jacc.2007.07.064. [DOI] [PubMed] [Google Scholar]
- 34.Iivanainen AM, Lindroos M, Tilvis R, Heikkilä J, Kupari M. Natural history of aortic valve stenosis of varying severity in the elderly. Am J Cardiol. 1996;78(1):97–101. doi: 10.1016/s0002-9149(96)00235-4. [DOI] [PubMed] [Google Scholar]
- 35.Bonow RO, Carabello BA, Chatterjee K, et al. 2008 focused update incorporated into the ACC/AHA (2006) guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the (1998) Guidelines for the Management of Patients with Valvular Heart Disease), Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2008;118(15):e523–e661. doi: 10.1161/CIRCULATIONAHA.108.190748. [DOI] [PubMed] [Google Scholar]
- 36.Harken DE, Soroff MS, Taylor MC. Partial and complete prostheses in aortic insufficiency. J Thorac Cardiovasc Surg. 1960;40:744–762. [PubMed] [Google Scholar]
- 37.Speziale G, Nasso G, Barattoni MC, et al. Operative and middle-term results of cardiac surgery in nonagenarians: a bridge toward routine practice. Circulation. 2010;121(2):208–213. doi: 10.1161/CIRCULATIONAHA.108.807065. [DOI] [PubMed] [Google Scholar]
- 38.Kolh P, Kerzmann A, Honore C, Comte L, Limet R. Aortic valve surgery in octogenarians: predictive factors for operative and long-term results. Eur J Cardiothorac Surg. 2007;31(4):600–606. doi: 10.1016/j.ejcts.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Chiappini B, Camurri N, Loforte A, Di Marco L, Di Bartolomeo R, Marinelli G. Outcome after aortic valve replacement in octogenarians. Ann Thorac Surg. 2004;78(1):85–89. doi: 10.1016/j.athoracsur.2003.12.060. [DOI] [PubMed] [Google Scholar]
- 40.Sundt TM, Bailey MS, Moon MR, et al. Quality of life after aortic valve replacement at the age of >80 years. Circulation. 2000;102(Suppl III):III-70–III-74. doi: 10.1161/01.cir.102.suppl_3.iii-70. [DOI] [PubMed] [Google Scholar]
- 41.Melby SJ, Zierer A, Kaiser SP, et al. Aortic valve replacement in octogenarians: risk factors for early and late mortality. Ann Thorac Surg. 2007;83(5):1651–1657. doi: 10.1016/j.athoracsur.2006.09.068. [DOI] [PubMed] [Google Scholar]
- 42.Afilalo J, Eisenberg MJ, Morin J, et al. Gait speed as an incremental predictor of mortality and major morbidity in elderly patients undergoing cardiac surgery. J Am Coll Cardiol. 2010;56(20):1668–1676. doi: 10.1016/j.jacc.2010.06.039. [DOI] [PubMed] [Google Scholar]
- 43.Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg. 2006;82(6):2111–2115. doi: 10.1016/j.athoracsur.2006.07.048. [DOI] [PubMed] [Google Scholar]
- 44.Schueler R, Hammerstingl C, Sinning J, Nickenig G, Omran H. Prognosis of octogenarians with severe aortic valve stenosis at high risk for cardiovascular surgery. Heart. 2010;96(22):1831–1836. doi: 10.1136/hrt.2010.202663. [DOI] [PubMed] [Google Scholar]
- 45.Cowell SJ, Newby DE, Prescott RJ, et al. A randomized trial of intensive lipid-lowering therapy in calcific aortic stenosis. N Engl J Med. 2005;352(23):2389–2397. doi: 10.1056/NEJMoa043876. [DOI] [PubMed] [Google Scholar]
- 46.Chan KL, Teo K, Dumesnil JG, Ni A, Tam J for the ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121(2):306–314. doi: 10.1161/CIRCULATIONAHA.109.900027. [DOI] [PubMed] [Google Scholar]
- 47.Webb JG, Pasupati S, Humphries K, et al. Percutaneous transarterial aortic valve replacement in selected high-risk patients with aortic stenosis. Circulation. 2007;116(7):755–763. doi: 10.1161/CIRCULATIONAHA.107.698258. [DOI] [PubMed] [Google Scholar]
- 48.Piazza N, Grube E, Gerckens U, et al. Procedural and 30-day outcomes following transcatheter valve implantation using the third generation (18 Fr) CoreValve ReValving system: results from the multicentre, expanded evaluation registry 1-year following CE mark approval. EuroIntevention. 2008;4:242–249. doi: 10.4244/eijv4i2a43. [DOI] [PubMed] [Google Scholar]
- 49.Himbert D, Descoutures F, Al-Attar N, et al. Results of transfemoral or transapical aortic valve implantation following a uniform assessment in high-risk patients with aortic stenosis. J Am Coll Cardiol. 2009;54(4):303–311. doi: 10.1016/j.jacc.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 50.Nashef SAM, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European System for Cardiac Operative Risk Evaluation (EuroSCORE) Eur J Cardiothorac Surg. 1999;16(1):9–13. doi: 10.1016/s1010-7940(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 51.Piazza N, Wenaweser P, van Gameren M, et al. Relationship between the logistic EuroSCORE and the society of thoracic surgeons predicted risk of mortality score in patients implanted with the CoreValve ReValving system – a Bern-Rotterdam study. Am Heart J. 2010;159(2):323–329. doi: 10.1016/j.ahj.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 52.Lee DH, Buth KJ, Martin B, Yip AM, Hirsch GM. Frail patients are at increased risk for mortality and prolonged institutional care after cardiac surgery. Circulation. 2010;2121(8):973–978. doi: 10.1161/CIRCULATIONAHA.108.841437. [DOI] [PubMed] [Google Scholar]
- 53.Krane M, Deutsch M, Bleiziffer S, et al. Quality of life among patients undergoing transcatheter aortic valve implantation. Am Heart J. 2010;160(3):451–457. doi: 10.1016/j.ahj.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 54.Ussia GP, Mulè M, Barbanti M, et al. Quality of life assessment after percutaneous aortic valve implantation. Eur Heart J. 2009;30(14):1790–1796. doi: 10.1093/eurheartj/ehp171. [DOI] [PubMed] [Google Scholar]
- 55.Thourani VH, Myung R, Kilgo P, et al. Long-term outcomes after isolated aortic valve replacement in octogenarians: a modern perspective. Ann Thorac Surg. 2008;86(5):1458–1465. doi: 10.1016/j.athoracsur.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 56.Mirowski M, Reid PR, Mower MM, et al. Termination of malignant ventricular arrhythmias with an implanted automatic defibrillator in human beings. N Engl J Med. 1980;303:322–324. doi: 10.1056/NEJM198008073030607. [DOI] [PubMed] [Google Scholar]
- 57.The Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med. 1997;337(22):1576–1584. doi: 10.1056/NEJM199711273372202. [DOI] [PubMed] [Google Scholar]
- 58.Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS): a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation. 2000;101(11):1297–1302. doi: 10.1161/01.cir.101.11.1297. [DOI] [PubMed] [Google Scholar]
- 59.Kuck K, Cappato R, Siebels J, Ruppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest: the Cardiac Arrest Study Hamburg (CASH) Circulation. 2000;102(7):748–754. doi: 10.1161/01.cir.102.7.748. [DOI] [PubMed] [Google Scholar]
- 60.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 61.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 62.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (writing committee to revise the ACC/AHA/NASPE (2002) Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices) developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;51(21):e1–e62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 63.Dickstein K, Cohen-Solal A, Filippatos G, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2008;29(19):2388–2442. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 64.Hammill SC, Stevenson LW, Kadish AH, et al. Review of the Registry’s first year, data collected, and future plans. Heart Rhythm. 2007;4(9):1260–1263. doi: 10.1016/j.hrthm.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 65.Epstein AE, Kay GN, Plumb VJ, et al. Implantable cardioverter-defibrillator prescription in the elderly. Heart Rhythm. 2009;6(8):1136–1143. doi: 10.1016/j.hrthm.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 66.van Veldhuisen DJ, Maass AH, Priori SG, et al. Implementation of device therapy (cardiac resynchronization therapy and implantable cardioverter defibrillator) for patients with heart failure in Europe: Changes from 2008 to 2009. Eur J Heart Fail. 2004;11(12):1143–1151. doi: 10.1093/eurjhf/hfp149. [DOI] [PubMed] [Google Scholar]
- 67.Bristow MR, Saxon LA, Boehmer J, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 68.Santangeli P, Di Biase L, Dello Russo A, et al. Meta-analysis: age and effectiveness of prophylactic implantable cardioverter-defibrillators. Ann Intern Med. 2010;153(9):592–599. doi: 10.7326/0003-4819-153-9-201011020-00009. [DOI] [PubMed] [Google Scholar]
- 69.Heidenreich PA, Tsai V. Is anyone too old for an implantable cardioverter-defibrillator? Circ Cardiovasc Qual Outcomes. 2009;2(1):6–8. doi: 10.1161/CIRCOUTCOMES.108.842369. [DOI] [PubMed] [Google Scholar]
- 70.Groeneveld PW, Farmer SA, Suh JJ, Matta MA, Yang F. Outcomes and costs of implantable cardioverter-defibrillators for primary prevention of sudden cardiac death among the elderly. Heart Rhythm. 2008;5(5):646–653. doi: 10.1016/j.hrthm.2008.01.038. [DOI] [PubMed] [Google Scholar]
- 71.Hernandez AF, Fonarow GC, Hammill BG, et al. Clinical effectiveness of implantable cardioverter-defibrillators among Medicare beneficiaries with heart failure. Circ Heart Fail. 2010;3:7–13. doi: 10.1161/CIRCHEARTFAILURE.109.884395. [DOI] [PubMed] [Google Scholar]
- 72.Koplan BA, Epstein LM, Albert CM, Stevenson WG. Survival in octogenarians receiving implantable defibrillators. Am Heart J. 2006;152(4):714–719. doi: 10.1016/j.ahj.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 73.Ertel D, Phatak K, Makati K, et al. Predictors of early mortality in patients age 80 and older receiving implantable defibrillators. Pacing Clin Electrophysiol. 2010;33(8):981–987. doi: 10.1111/j.1540-8159.2010.02729.x. [DOI] [PubMed] [Google Scholar]
- 74.Maisel WH. Pacemaker and ICD generator reliability: meta-analysis of device registries. JAMA. 2006;295(16):1929–1934. doi: 10.1001/jama.295.16.1929. [DOI] [PubMed] [Google Scholar]
- 75.Curtis JP, Luebbert JJ, Wang Y, et al. Association of physician certification and outcomes among patients receiving an implantable cardioverter-defibrillator. JAMA. 2009;301(16):1661–1670. doi: 10.1001/jama.2009.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reynolds MR, Cohen DJ, Kugelmass AD, et al. The frequency and incremental cost of major complications among Medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47(12):2493–2497. doi: 10.1016/j.jacc.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Krahn AD, Connolly SJ, Roberts RS, Gent M. Diminishing proportional risk of sudden death with advancing age: implications for prevention of sudden death. Am Heart J. 2004;147(5):837–840. doi: 10.1016/j.ahj.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 78.Healey JS, Hallstrom AP, Kuck K, et al. Role of the implantable defibrillator among elderly patients with a history of life-threatening ventricular arrhythmias. Eur Heart J. 2007;28(14):1746–1749. doi: 10.1093/eurheartj/ehl438. [DOI] [PubMed] [Google Scholar]
- 79••.Stevenson LW, Desai AS. Selecting patients for discussion of the ICD as primary prevention for sudden death in heart failure. J Card Fail. 2006;812(6):407–412. doi: 10.1016/j.cardfail.2006.06.001. Highlights need to discuss benefits of implantable cardioverter defibrillators in context of patient preferences and potential risks. [DOI] [PubMed] [Google Scholar]
- 80.Hauptman PJ, Havranek EP. Integrating palliative care into heart failure care. Arch Intern Med. 2005;165(4):374–378. doi: 10.1001/archinte.165.4.374. [DOI] [PubMed] [Google Scholar]
- 81.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–M157. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 82.Gill TM, Baker DI, Gottschalk M, Peduzzi PN, Allore H, Byers A. A program to prevent functional decline in physically frail, elderly persons who live at home. N Engl J Med. 2002;347(14):1068–1074. doi: 10.1056/NEJMoa020423. [DOI] [PubMed] [Google Scholar]
- 83.Dasgupta M, Rolfson DB, Stolee P, Borrie MJ, Speechley M. Frailty is associated with postoperative complications in older adults with medical problems. Arch Gerontol Geriatr. 2009;48(1):78–83. doi: 10.1016/j.archger.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 84.Fukuse T, Satoda N, Hijiya K, Fujinaga T. Importance of a comprehensive geriatric assessment in prediction of complications following thoracic surgery in elderly patients. Chest. 2005;127(3):886–891. doi: 10.1378/chest.127.3.886. [DOI] [PubMed] [Google Scholar]
- 85.Laine C, Davidoff F. Patient-centered medicine. JAMA. 1996;275(2):152–156. [PubMed] [Google Scholar]
- 86.Cigolle CT, Langa KM, Kabeto MU, Tian Z, Blaum CS. Geriatric conditions and disability: the Health and Retirement Study. Ann Intern Med. 2007;147(3):156–164. doi: 10.7326/0003-4819-147-3-200708070-00004. [DOI] [PubMed] [Google Scholar]
- 87.Chaudhry SI, McAvay G, Ning Y, Allore HG, Newman AB, Gill TM. Geriatric impairments and disability: the Cardiovascular Health Study. J Am Geriatr Soc. 2010;58(9):1686–1692. doi: 10.1111/j.1532-5415.2010.03022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hohnloser SH, Kuck KH, Dorian P, et al. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N Engl J Med. 2004;351(24):2481–2488. doi: 10.1056/NEJMoa041489. [DOI] [PubMed] [Google Scholar]
- 89.Stevenson LW, Lewis E. Mapping the journey. J Am Coll Cardiol. 2006;47(8):1612–1614. doi: 10.1016/j.jacc.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 90.Gurland BJ, Cheng H, Maurer MS. Helath-related restrictions of choices and choosing: implications for quality of life and clinical interventions. Patient Related Outcome Measures. 2010;1:73–80. doi: 10.2147/prom.s11842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gurland BJ, Gurland RV. The choices, choosing model of quality of life: description and rationale. Int J Geriat Psychiatry. 2009;24(1):90–95. doi: 10.1002/gps.2110. [DOI] [PubMed] [Google Scholar]
- 92.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304(17):1950–1951. doi: 10.1001/jama.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee PY, Alexander KP, Hammill BG, Pasquali SK, Peterson ED. Representation of elderly persons and women in published randomized trials of acute coronary syndromes. JAMA. 2001;286(6):708–713. doi: 10.1001/jama.286.6.708. [DOI] [PubMed] [Google Scholar]
- 94.Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: Results from the world health surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- 95.Hammond AJ, Yu S, Esa K, et al. Factors associated with persistent risk of depression in older people following discharge from an acute cardiac unit. Int Psychogeriatr. 2008;20(4):738–751. doi: 10.1017/S1041610208007138. [DOI] [PubMed] [Google Scholar]
- 96.Wallentin L, Lagerqvist B, Husted S, Kontny F, Ståhle E, Swahn E. Outcome at 1 year after an invasive compared with a non-invasive strategy in unstable coronary-artery disease: the FRISC II invasive randomised trial. Lancet. 2000;356(9223):9–16. doi: 10.1016/s0140-6736(00)02427-2. [DOI] [PubMed] [Google Scholar]
- 97.Global Use of Strategies to Open Occluded Coronary Arteries in Acute Coronary Syndromes (GUSTO IIb) Angioplasty Substudy Investigators. A clinical trial comparing primary coronary angioplasty with tissue plasminogen activator for acute myocardial infarction. N Engl J Med. 1997;336:1621–1628. doi: 10.1056/NEJM199706053362301. [DOI] [PubMed] [Google Scholar]
- 98.Andersen HR, Nielsen TT, Rasmussen K, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349(8):733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 99.Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med. 1999;341:625–634. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- 100.Weintraub WS, Mahoney EM, Ghazzal ZMB, et al. Trends in outcome and costs of coronary intervention in the 1990s. Am J Cardiol. 2001;88(5):497–503. doi: 10.1016/s0002-9149(01)01726-x. [DOI] [PubMed] [Google Scholar]
- 101.Wang TY, Peterson ED, Dai D, et al. Patterns of cardiac marker surveillance after elective percutaneous coronary intervention and implications for the use of periprocedural myocardial infarction as a quality metric: a report from the national cardiovascular data registry (NCDR) J Am Coll Cardiol. 2008;51(21):2068–2074. doi: 10.1016/j.jacc.2008.01.054. [DOI] [PubMed] [Google Scholar]
- 102.Stone GW, Mehran R, Dangas G, Lansky AJ, Kornowski R, Leon MB. Differential impact on survival of electrocardiographic Q-wave versus enzymatic myocardial infarction after percutaneous intervention: a device-specific analysis of 7147 patients. Circulation. 2001;104(6):642–647. doi: 10.1161/hc3101.093902. [DOI] [PubMed] [Google Scholar]
- 103.Kinnaird T, Anderson R, Hill J, Thomas M. Bleeding during percutaneous intervention: tailoring the approach to minimise risk. Heart. 2009;95(1):15–19. doi: 10.1136/hrt.2007.131284. [DOI] [PubMed] [Google Scholar]
- 104.Mehta RH, Sadiq I, Goldberg RJ, et al. Effectiveness of primary percutaneous coronary intervention compared with that of thrombolytic therapy in elderly patients with acute myocardial infarction. Am Heart J. 2004;147(2):253–259. doi: 10.1016/j.ahj.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 105.Batchelor WB, Anstrom KJ, Muhlbaier LH, et al. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: results in 7,472 octogenarians. J Am Coll Cardiol. 2000;36(3):723–730. doi: 10.1016/s0735-1097(00)00777-4. [DOI] [PubMed] [Google Scholar]
- 106.Hassani S, Wolfram RM, Kuchulakanti PK, et al. Percutaneous coronary intervention with drug-eluting stents in octogenarians: characteristics, clinical presentation, and outcomes. Catheter Cardiovasc Interv. 2006;68(1):36–43. doi: 10.1002/ccd.20768. [DOI] [PubMed] [Google Scholar]
- 107.Peterson ED, Alexander KP, Malenka DJ, et al. Multicenter experience in revascularization of very elderly patients. Am Heart J. 2004;148(3):486–492. doi: 10.1016/j.ahj.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 108.Zingone B, Gatti G, Rauber E, et al. Early and late outcomes of cardiac surgery in octogenarians. Ann Thorac Surg. 2009;87(1):71–78. doi: 10.1016/j.athoracsur.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 109.Lee DS, Krahn AD, Healey JS, et al. Evaluation of early complications related to de novo cardioverter defibrillator implantation: insights from the Ontario ICD database. J Am Coll Cardiol. 2010;55(8):774–782. doi: 10.1016/j.jacc.2009.11.029. [DOI] [PubMed] [Google Scholar]
- 110.Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97(8):1255–1261. doi: 10.1016/j.amjcard.2005.11.048. [DOI] [PubMed] [Google Scholar]
- 111.Sears SF, Conti JB. Quality of life and psychological functioning of ICD patients. Heart. 2002;87(5):488–493. doi: 10.1136/heart.87.5.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Likosky DS, Sorensen MJ, Dacey LJ, et al. Long-term survival of the very elderly undergoing aortic valve surgery. Circulation. 2009;120(Suppl 1):S127–S133. doi: 10.1161/CIRCULATIONAHA.108.842641. [DOI] [PubMed] [Google Scholar]
Websites
- 201.United Nations world population prospects: the 2006 revision. www.un.org/esa/population/publications/wpp2006/WPP2006_Highlights_rev.pdf. 202. National center for health statistics www.cdc.gov/NCHS.
- 203.The Society of Thoracic Surgeons. www.sts.org.
- 204.European System for Cardiac Operative Risk Evaluation (EuroSCORE) doi: 10.1016/s1010-7940(99)00134-7. www.euroscore.org. [DOI] [PubMed]